Thermodynamic Characteristics and Selectivity of the Liquid-Phase Adsorption of Aromatic Compounds on Hypercrosslinked Polystyrene Networks with Ultimate-High Crosslinking Densities by Data of Liquid Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology and Textural Characteristics
2.1.1. N2 and Ar Adsorption at 77 K
2.1.2. Benzene Vapor Adsorption at 293 K
2.2. Test Adsorbates
2.3. Thermodynamic Characteristics of Adsorption on Hypercrosslinked Polystyrenes vs. Crosslinking Degree
2.3.1. Henry Adsorption Constants and Gibbs Energies of Adsorption
2.3.2. Enthalpies and Entropies of Adsorption
2.4. Thermodynamic Characteristics of Adsorption on Hypercrosslinked Polystyrenes vs. Liquid Phase Composition
2.4.1. Henry Adsorption Constants and Gibbs Energies of Adsorption
2.4.2. Enthalpies and Entropies of Adsorption
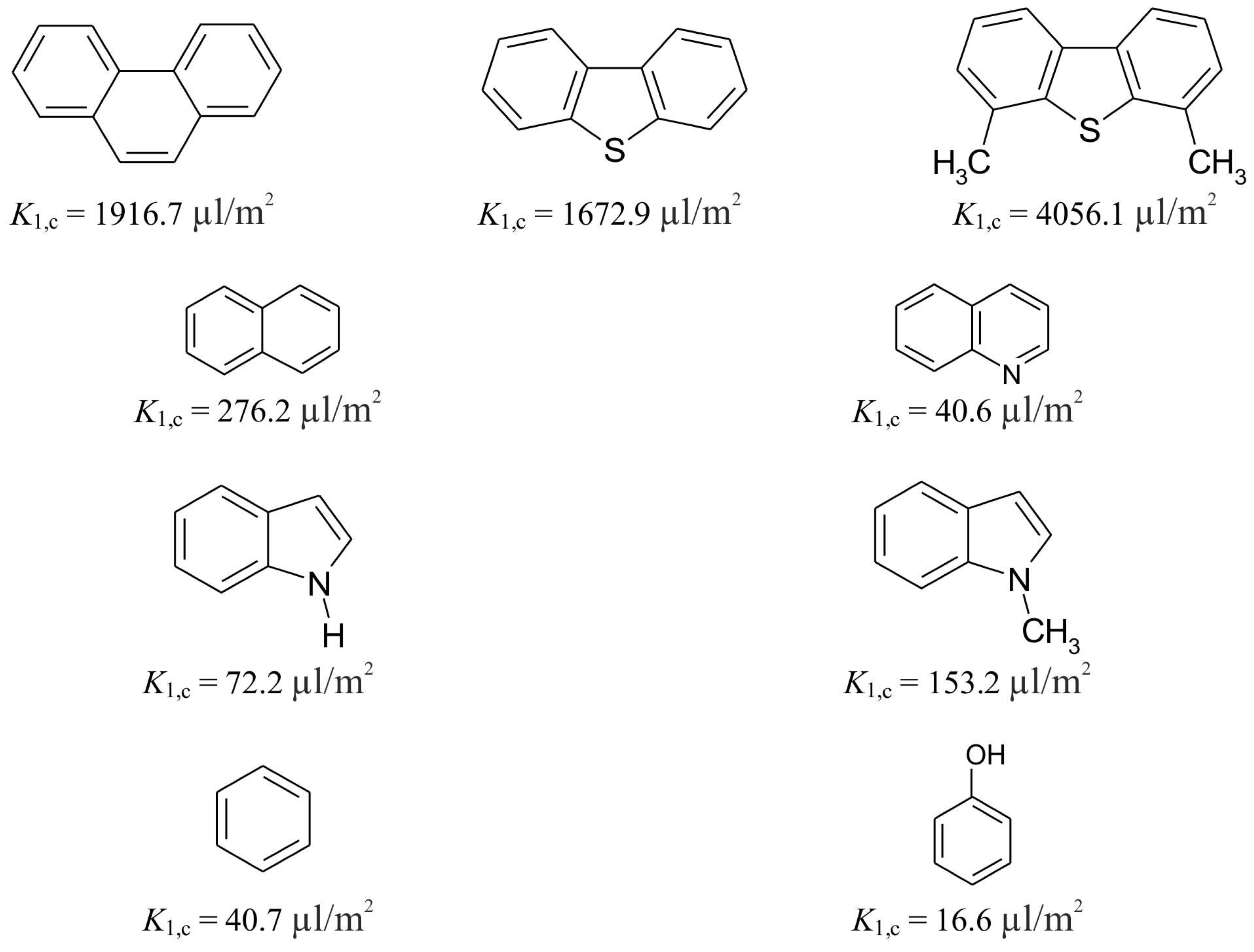
2.5. Structural Selectivity of Hypercrosslinked Polystyrenes and Retention Mechanism
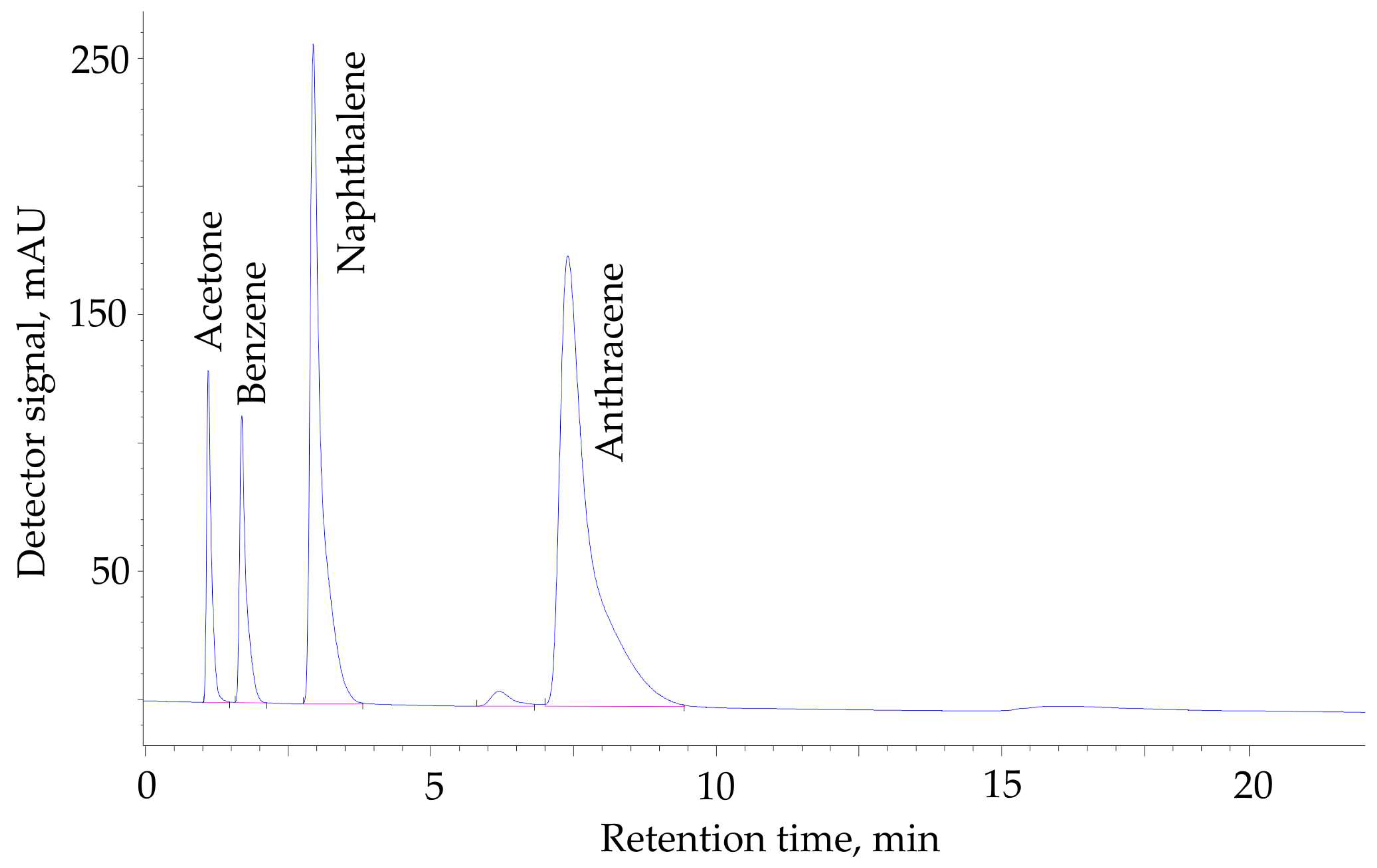
2.6. Selectivity and Throughput of HPLC Separation by Hypercrosslinked Polystyrenes
3. Materials and Methods
3.1. Synthesis of Hypercrosslinked Polystyrenes
3.2. Characterization of Hypercrosslinked Polystyrenes
3.3. Chromatographic Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazakevich, Y.; LoBrutto, R. HPLC Theory and Practice. In HPLC for Pharmaceutical Scientists; Kazakevich, Y., LoBrutto, R., Eds.; Wiley: Hoboken, NJ, USA, 2007; pp. 1–532. [Google Scholar]
- Nölting, B. Liquid chromatography of biomolecules. In Methods in Modern Biophysics; Nölting, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 23–35. [Google Scholar]
- Žuvela, P.; Skoczylas, M.; Liu, J.J.; Bączek, T.; Kaliszan, R.; Wong, M.W.; Buszewski, B. Column characterization and selection systems in reversed-phase high-performance liquid chromatography. Chem. Rev. 2019, 119, 3674–3729. [Google Scholar] [CrossRef]
- Wu, D.; Nedev, G.K.; Lucy, C.A. Retention mechanism of hypercrosslinked polystyrene silica hybrid phase in normal phase chromatography. J. Chromatogr. A 2014, 1370, 50–55. [Google Scholar] [CrossRef]
- Arrua, R.D.; Peristyy, A.; Nesterenko, P.N.; Das, A.; D’Alessandro, D.M.; Hilder, E.F. UiO-66@SiO2 core-shell microparticles as stationary phases for the separation of small organic molecules. Analyst 2017, 412, 517–524. [Google Scholar] [CrossRef]
- Rafferty, J.L.; Siepmann, J.I.; Schure, M.R. Mobile phase effects in reversed-phase liquid chromatography: A comparison of acetonitrile/water and methanol/water solvents as studied by molecular simulation. J. Chromatogr. A 2011, 1218, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Kot, D.; Zou, M.; Brunnengräber, K.; Arndt, J.-H.; Macko, T.; Etzold, B.J.M.; Brüll, R. Porous graphite as stationary phase for the chromatographic separation of polymer additives–determination of adsorption capability by Raman spectroscopy and physisorption. J. Chromatogr. A 2020, 1625, 461302. [Google Scholar] [CrossRef] [PubMed]
- Finsy, V.; Calero, S.; García-Pérez, E.; Merkling, P.J.; Vedts, G.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Low-coverage adsorption properties of the metal–organic framework MIL-47 studied by pulse chromatography and Monte Carlo simulations. Phys. Chem. Chem. Phys. 2009, 11, 3515–3521. [Google Scholar] [CrossRef] [PubMed]
- Yadrova, A.A.; Shafigulin, R.V.; Bulanova, A.V. Chromatographic behavior of benzimidazole derivatives on hypercrosslinked polystyrene by reverse-phase HPLC. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 482–493. [Google Scholar] [CrossRef]
- Davankov, V.A.; Tsyurupa, M.P. Hypercrosslinked Polymeric Networks and Adsorbing Materials: Synthesis, Properties, Structure, and Applications. In Comprehensive Analytical Chemistry; Barcelo, D., Ed.; Elsevier: New York, NY, USA, 2011; Volume 56, pp. 3–638. [Google Scholar]
- Davankov, V.A.; Sychov, C.S.; Ilyin, M.M.; Sochilina, K.O. Hypercrosslinked polystyrene as a novel type of high-performance liquid chromatography column packing material. Mechanisms of retention. J. Chromatogr. A 2003, 987, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sychov, C.S.; Ilyin, M.M.; Davankov, V.A.; Sochilina, K.O. Elucidation of retention mechanisms on hypercrosslinked polystyrene used as column packing material for high-performance liquid chromatography. J. Chromatogr. A 2004, 1030, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Oro, N.E.; Lucy, C.A. Comparison of hypercrosslinked polystyrene columns for the separation of nitrogen group-types in petroleum using high performance liquid chromatography. J. Chromatogr. A 2010, 1217, 6178–6185. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Davankov, V.A. Hypercrosslinked polymers: Basic principle of preparing the new class of polymeric materials. React. Funct. Polym. 2002, 53, 193–203. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Blinnikova, Z.K.; Davankov, V.A. Hypercrosslinked polystyrene networks with ultimate degrees of crosslinking and their sorption activity. Russ. J. Phys. Chem. A 2010, 84, 1767–1771. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Blinnikova, Z.K.; Il’in, M.M.; Davankov, V.A.; Parenago, O.O.; Pokrovskii, O.I.; Usovich, O.I. Monodisperse microbeads of hypercrosslinked polystyrene for liquid and supercritical fluid chromatography. Russ. J. Phys. Chem. A 2015, 89, 2064–2071. [Google Scholar] [CrossRef]
- Davankov, V.A.; Tsyurupa, M.P.; Blinnikova, Z.K.; Popov, A.Y. On the Mechanism of Swelling of Gel-Type, Macroporous and Hypercrosslinked Copolymers of Styrene. Polym. Sci. Ser. A 2021, 63, 260–266. [Google Scholar] [CrossRef]
- Davankov, V.A.; Tsyurupa, M.P. Structure and properties of hypercrosslinked polystyrene—The first representative of a new class of polymer networks. React. Polym. 1990, 13, 27–42. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Blinnikova, Z.K.; Borisov, Y.A.; Ilyin, M.M.; Klimova, T.P.; Mitsen, K.V.; Davankov, V.A. Physicochemical and adsorption properties of hypercross-linked polystyrene with ultimate cross-linking density. J. Sep. Sci. 2014, 37, 803–810. [Google Scholar] [CrossRef]
- Tsyrupa, M.P.; Blinnikova, Z.K.; Proskurina, N.A.; Pastukhov, A.V.; Pavlova, L.A.; Davankov, V.A. Hypercrosslinked polystyrene: The first nanoporous polymeric material. Nanotechnol. Russ. 2009, 4, 665–675. [Google Scholar] [CrossRef]
- Joseph, R.; Ford, W.T.; Zhang, S.; Tsyurupa, M.P.; Pastukhov, A.V.; Davankov, V.A. Solid-state 13C-NMR analysis of hypercrosslinked polystyrene. J. Polym. Sci. A Polym. Chem. 1997, 35, 695–701. [Google Scholar] [CrossRef]
- Pastukhov, A.V.; Tsyurupa, M.P.; Davankov, V.A. Hypercrosslinked polystyrene: A polymer in a non-classical physical state. J. Polym. Sci. B Polym. Phys. 1999, 37, 2324–2333. [Google Scholar] [CrossRef]
- Shantarovich, V.P.; Suzuki, T.; He, C.; Davankov, V.A.; Pastukhov, A.V.; Tsyurupa, M.P.; Kondo, K.; Ito, Y. Positron annihilation study of hyper-cross-linked polystyrene networks. Macromolecules 2002, 35, 9723–9729. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Davankov, V.A. Porous structure of hypercrosslinked polystyrene: State-of-the-art mini-review. React. Funct. Polym. 2006, 66, 768–779. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Papkov, M.A.; Davankov, V.A. Internal stresses in hypercrosslinked polystyrene: Polarized optical studies. Russ. J. Phys. Chem. A 2009, 51, 81–86. [Google Scholar] [CrossRef]
- Babushkina, T.A.; Klimova, T.P.; Davankov, V.A.; Tsyurupa, M.P.; Pastukhov, A.V.; Blinnikova, Z.K. 1H NMR cryoporosimetry of swollen hypercrosslinked polymers. Russ. J. Phys. Chem. A 2010, 84, 460–465. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Blinnikova, Z.K.; Davidovich, Y.A.; Lyubimov, S.E.; Naumkin, A.V.; Davankov, V.A. On the nature of “functional groups” in non-functionalized hypercrosslinked polystyrenes. React. Funct. Polym. 2012, 72, 973–982. [Google Scholar] [CrossRef]
- Tsyurupa, M.P.; Borisov, Y.A.; Blinnikova, Z.K.; Platonova, N.P.; Ul’yanov, A.V.; Buryak, A.K.; Davankov, V.A. On the origin of absorbance band around 1700 cm–1 in FTIR spectra of hypercrosslinked polystyrene. Prot. Met. Phys. Chem. Surf. 2014, 50, 59–63. [Google Scholar] [CrossRef]
- Ferrante, F.; Lo Celso, F.; Duca, D. Construction and characterization of models of hypercrosslinked polystyrene. Colloid Polym. Sci. 2012, 290, 1443–1450. [Google Scholar] [CrossRef]
- Glagolev, M.K.; Lazutin, A.A.; Vasilevskaya, V.V.; Khokhlov, A.R. Influence of cross-linking rate on the structure of hypercrosslinked networks: Multiscale computer simulation. Polymer 2016, 86, 168–175. [Google Scholar] [CrossRef]
- Saifutdinov, B.R.; Davankov, V.A.; Il’in, M.M.; Tsyurupa, M.P.; Blinnikova, Z.K. Selective adsorption of organic compounds from solutions on hyper-cross-linked polystyrenes with ultimate degrees of cross linking. Prot. Met. Phys. Chem. Surf. 2015, 51, 957–963. [Google Scholar] [CrossRef]
- Glagolev, M.K.; Lazutin, A.A.; Vasilevskaya, V.V. Macroscopic properties of hypercrosslinked polystyrene networks: An atomistic and coarse-grained molecular dynamics simulation. Macromol. Symp. 2015, 348, 14–24. [Google Scholar] [CrossRef]
- Saifutdinov, B.R.; Davankov, V.A.; Il’in, M.M. Thermodynamics of the sorption of 1,3,4-oxadiazole and 1,2,4,5-tetrazine derivatives from solutions on hypercrosslinked polystyrene. Russ. J. Phys. Chem. A 2014, 88, 358–364. [Google Scholar] [CrossRef]
- Saifutdinov, B.R.; Davankov, V.A.; Petukhova, G.A.; Tsyurupa, M.P.; Blinnikova, Z.K.; Il’in, M.M. Thermodynamics of liquid-phase adsorption on hypercrosslinked polystyrene networks with ultimate degrees of crosslinking. Dokl. Phys. Chem. 2015, 462, 135–139. [Google Scholar] [CrossRef]
- Saifutdinov, B.R. The effect of the nature of binary water–organic solvents on thermodynamic characteristics of adsorption of aromatic compounds on hyper-cross-linked polystyrene. Prot. Met. Phys. Chem. Surf. 2016, 52, 979–985. [Google Scholar] [CrossRef]
- Saifutdinov, B.R.; Davankov, V.A.; Il’in, M.M. Thermodynamic characteristics of the adsorption of 1,3,4-oxadizoles and 1,2,4,5-tetrazines from methanol and water–methanol solutions onto hypercrosslinked polystyrene. Russ. J. Phys. Chem. A 2017, 91, 577–580. [Google Scholar] [CrossRef]
- Sewell, P.A. Theory of Liquid Chromatography. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 779–787. [Google Scholar] [CrossRef]
- Davankov, V.A.; Blinnikova, Z.K.; Popov, A.Y.; Davidovich, Y.A.; Tsyurupa, M.P. Revisiting the problem of adsorption/desorption isotherms on hypercrosslinked polymers. Polym. Sci. Ser. A 2023, 65, 15–21. [Google Scholar] [CrossRef]
- Fenelonov, V.B. The Introduction to Physical Chemistry of Supramolecular Structure Formation of Adsorbents and Catalysts, 1st ed.; SB RAS: Novosibirsk, Russia, 2004; pp. 1–442. [Google Scholar]
- Bering, B.P.; Dubinin, M.M.; Serpinsky, V.V. Theory of volume filling for vapor adsorption. J. Colloid Interface Sci. 1966, 21, 378–393. [Google Scholar] [CrossRef]
- Abbott, L.J.; Colina, C.M. Formation of microporosity in hyper-cross-linked polymers. Macromolecules 2014, 47, 5409–5415. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Burlington, MA, USA, 2011; pp. 151–168. [Google Scholar]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Introduction to Modern Liquid Chromatography, 3rd ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 253–302. [Google Scholar]
- Zenkevich, I.G.; Deruish, A.; Nikitina, D.A. Features of the dependence of the retention indices of sorbates in reversed-phase high-performance liquid chromatography on the content of organic solvents in the eluent. Russ. J. Phys. Chem. A 2023, 97, 1007–1017. [Google Scholar] [CrossRef]
- Rogozhin, S.V.; Davankov, V.A.; Tsyurupa, M.P. SU Patent No. 299165, 24 April 1973.
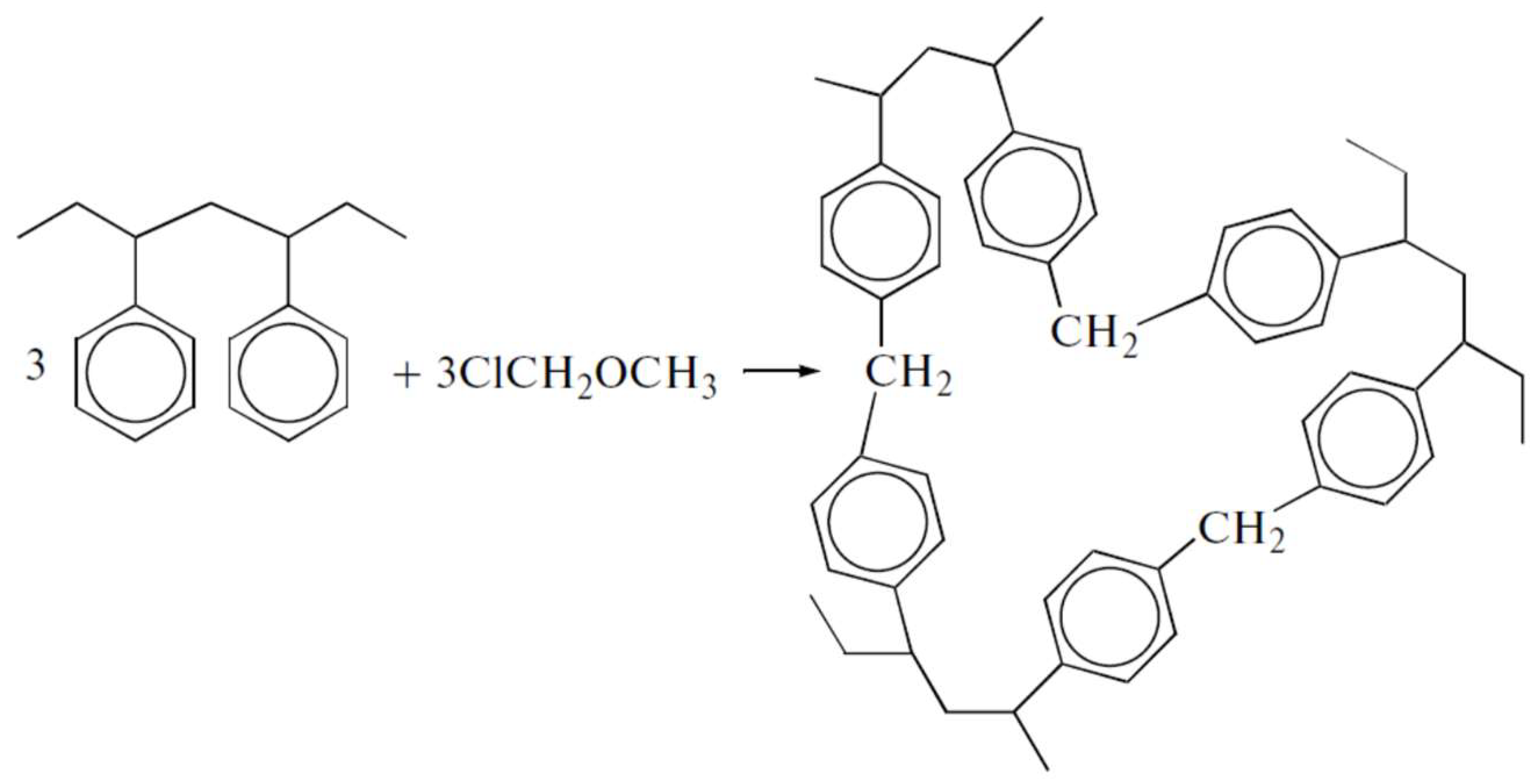
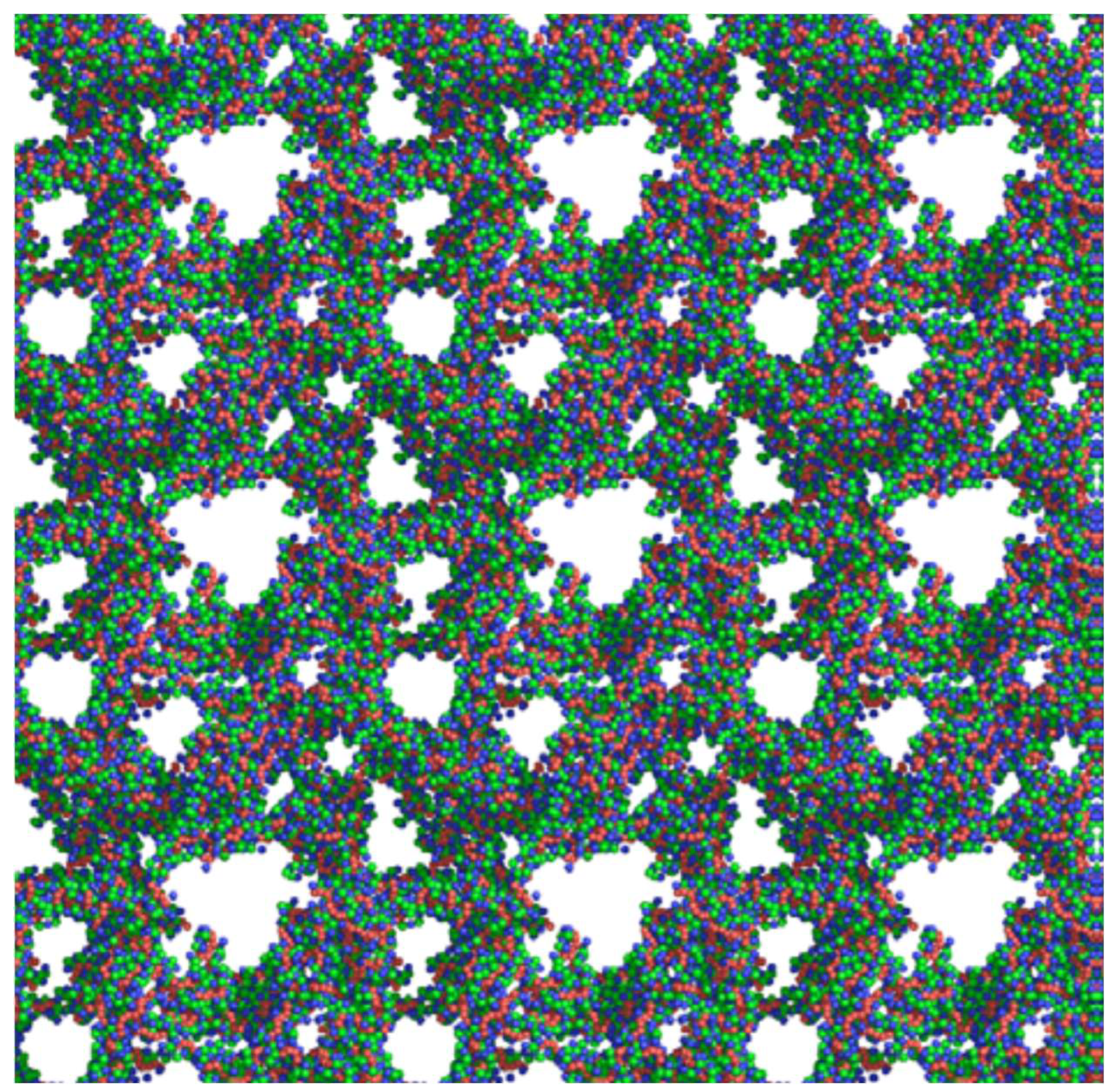
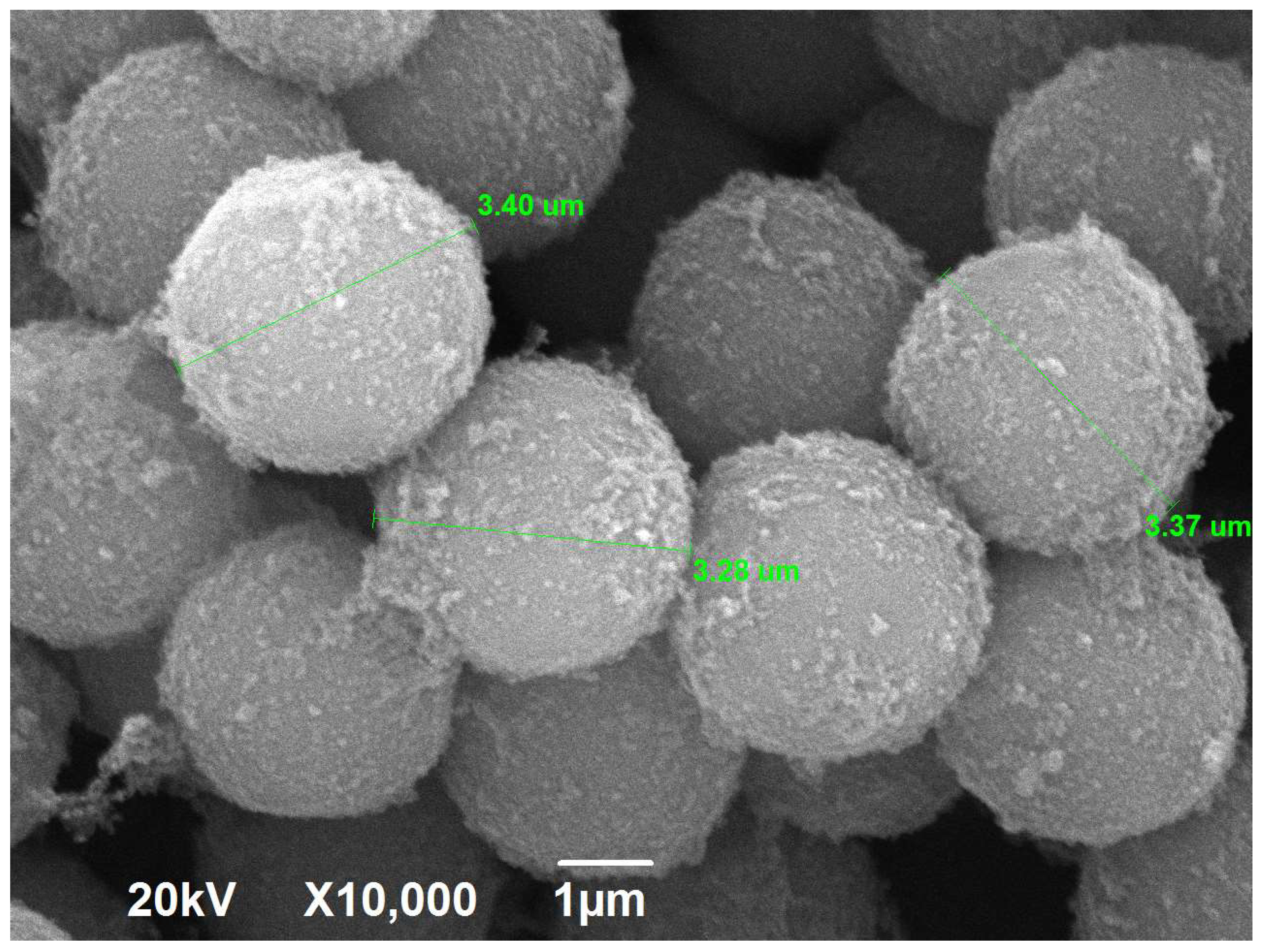
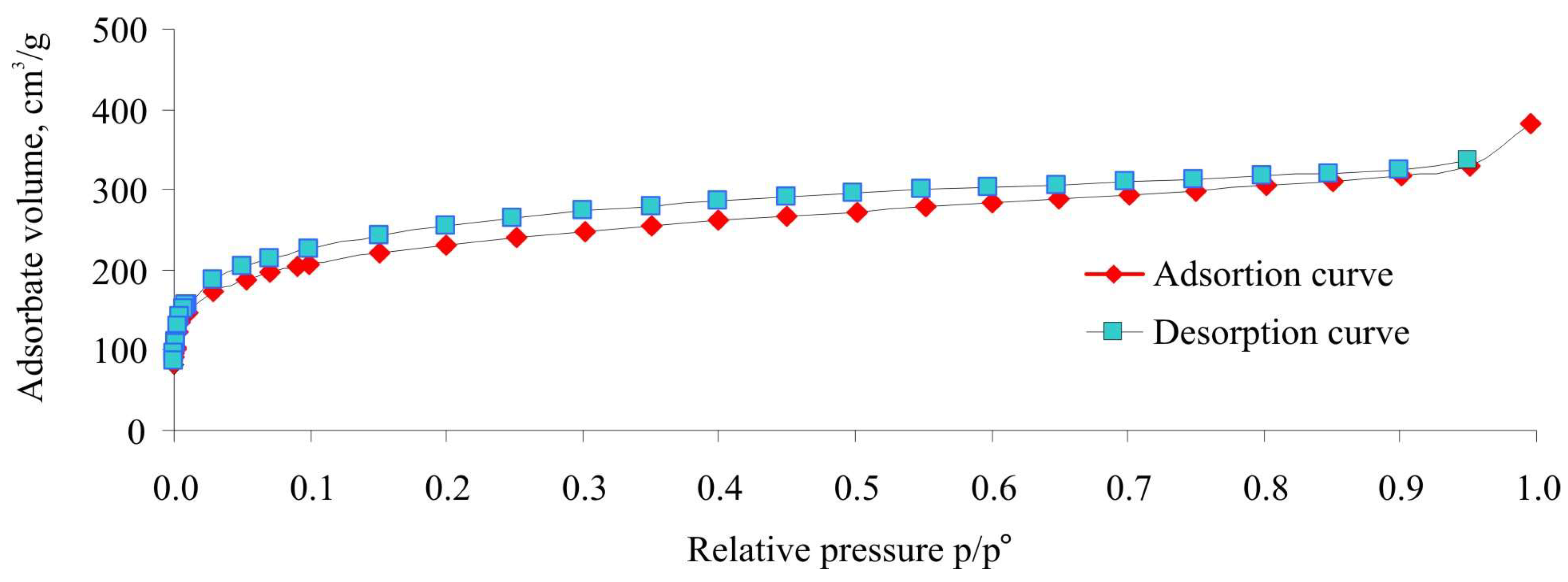
| Textural Characteristics | X = 100% | X = 200% | X = 300% | X = 400% | X = 500% |
|---|---|---|---|---|---|
| SN2mic, a m2/g | 953 | 1044 | 811 | 1015 | 673 |
| SAr, b m2/g | 1050 | 1554 | 880 | 1100 | 684 |
| SN2(Langmuir), c m2/g | 1101 | 1189 | 873 | 1083 | 730 |
| SN2(BET), d m2/g | 765 | 829 | 605 | 751 | 509 |
| r(BJH), e nm | 1.5 | 1.5 | 1.5 | 1.7 | 1.7 |
| Textural Characteristics | X = 100% | X = 200% | X = 300% | X = 400% | X = 500% |
|---|---|---|---|---|---|
| W0, a cm3/g | 0.260 | 0.300 | 0.232 | 0.272 | 0.242 |
| E0, b kJ/mole | 12.13 | 14.03 | 12.92 | 12.53 | 12.08 |
| x0, c nm | 0.82 | 0.71 | 0.77 | 0.80 | 0.83 |
| Vmes, d cm3/g | 0.600 | 0.580 | 0.435 | 0.382 | 0.409 |
| VS, e cm3/g | 0.860 | 0.880 | 0.667 | 0.657 | 0.651 |
| Smes(BET), f m2/g | 980 | 1030 | 720 | 770 | 640 |
| Smes(γ), g m2/g | 330 | 400 | 315 | 320 | 370 |
| No. | Structural Formula | No. | Structural Formula | No. | Structural Formula |
|---|---|---|---|---|---|
| 1 | 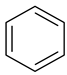 Benzene | 7 | 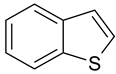 Benzothiophene | 13 | 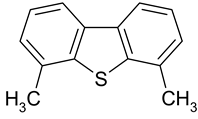 4,6-Dimethyldibenzothiophene |
| 2 | 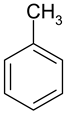 Toluene | 8 | 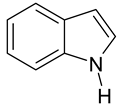 Indole | 14 | 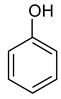 Phenol |
| 3 | 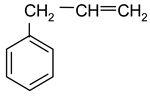 Allylbenzene | 9 | 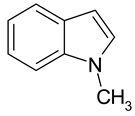 1-Methylindole | 15 | 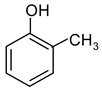 o-Cresol |
| 4 | 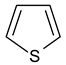 Thiophene | 10 | 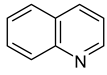 Quinoline | 16 | 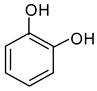 Catechol |
| 5 |  Pyridine | 11 | 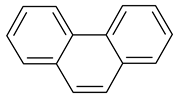 Phenantrene | 17 | 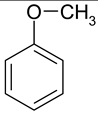 Anisole |
| 6 |  Naphthalene | 12 | 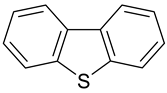 Dibenzothiophene | 18 |  Guaiacol |
| No. | X = 100% | X = 200% | X = 300% | X = 400% | X = 500% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| K1,c | −ΔaG* | K1,c | −ΔaG* | K1,c | −ΔaG* | K1,c | −ΔaG* | K1,c | −ΔaG* | |
| 1 | 11.9 | 6.7 | 6.7 | 5.1 | 12.2 | 6.7 | 7.8 | 5.5 | 11.7 | 6.6 |
| 2 | 17.2 | 7.6 | 9.4 | 6.0 | 17.2 | 7.6 | 10.8 | 6.4 | 15.8 | 7.4 |
| 3 | 62.4 | 11.1 | 34.1 | 9.5 | 63.6 | 11.1 | 38.1 | 9.8 | 51.4 | 10.6 |
| 4 | 10.5 | 6.3 | 6.0 | 4.8 | 11.3 | 6.5 | 7.4 | 5.4 | 11.1 | 6.5 |
| 5 | 2.2 | 2.1 | 1.5 | 1.2 | 3.8 | 3.6 | 2.0 | 1.8 | 3.2 | 3.1 |
| 6 | 37.1 | 9.7 | 20.6 | 8.1 | 39.6 | 9.9 | 24.3 | 8.6 | 34.5 | 9.5 |
| 7 | 33.1 | 9.4 | 18.5 | 7.8 | 36.2 | 9.6 | 22.4 | 8.3 | 32.2 | 9.3 |
| 8 | 11.3 | 6.5 | 7.0 | 5.2 | 14.3 | 7.1 | 8.8 | 5.9 | 13.6 | 7.0 |
| 9 | 23.3 | 8.5 | 13.0 | 6.9 | 24.7 | 8.6 | 15.1 | 7.3 | 22.2 | 8.3 |
| 10 | 7.3 | 5.3 | 4.8 | 4.2 | 11.3 | 6.5 | 6.1 | 4.9 | 9.1 | 5.9 |
| 11 | 122.3 | 12.9 | 68.9 | 11.4 | 135.7 | 13.2 | 78.6 | 11.7 | 106.0 | 12.5 |
| 12 | 116.8 | 12.8 | 66.0 | 11.2 | 129.4 | 13.1 | 76.4 | 11.6 | 103.4 | 12.5 |
| 13 | 242.0 | 14.7 | 127.2 | 13.0 | 237.2 | 14.7 | 131.8 | 13.1 | 167.7 | 13.8 |
| 14 | 3.6 | 3.5 | 2.5 | 2.4 | 5.0 | 4.3 | 3.3 | 3.2 | 5.1 | 4.4 |
| 15 | 5.7 | 4.7 | 3.7 | 3.5 | 7.5 | 5.4 | 4.8 | 4.2 | 7.1 | 5.3 |
| 16 | 1.5 | 1.1 | 0.9 | –0.2 | 1.6 | 1.3 | 1.4 | 0.9 | 1.8 | 1.5 |
| 17 | 13.0 | 6.9 | 7.2 | 5.3 | 13.7 | 7.0 | 8.5 | 5.7 | 12.6 | 6.8 |
| 18 | 5.5 | 4.6 | 3.1 | 3.1 | 6.2 | 4.9 | 4.4 | 4.0 | 6.2 | 4.9 |
| No. | X = 100% | X = 200% | X = 300% | X = 400% | X = 500% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| −ΔaH* | −ΔaS* | −ΔaH* | −ΔaS* | −ΔaH* | −ΔaS* | −ΔaH* | −ΔaS* | −ΔaH* | −ΔaS* | |
| 1 | 8.2 | 4.84 | 8.4 | 10.3 | 9.7 | 9.2 | 8.6 | 9.4 | 9.1 | 7.7 |
| 2 | 8.7 | 3.4 | 9.1 | 9.6 | 10.6 | 9.2 | 9.3 | 9.0 | 9.8 | 7.3 |
| 3 | 12.4 | 4.0 | 13.2 | 11.7 | 13.9 | 8.6 | 13.3 | 11.0 | 13.4 | 8.9 |
| 4 | 8.3 | 6.2 | 8.6 | 11.7 | 9.5 | 9.4 | 8.5 | 9.8 | 8.7 | 7.0 |
| 5 | – | – | – | – | 8.9 | 16.5 | – | – | – | – |
| 6 | 10.9 | –3.6 | 11.5 | 10.5 | 12.1 | 6.8 | 11.4 | 8.7 | 11.9 | 7.5 |
| 7 | 10.7 | –4.0 | 11.2 | 10.4 | 13.7 | 12.4 | 11.2 | 8.8 | 11.5 | 6.7 |
| 8 | 9.8 | –10.2 | 10.5 | 16.3 | 13.1 | 18.4 | 10.7 | 14.9 | 10.7 | 11.5 |
| 9 | 10.1 | –5.0 | 10.6 | 11.6 | 12.5 | 12.1 | 10.4 | 9.8 | 12.2 | 12.1 |
| 10 | 2.7 | –8.2 | 3.2 | –3.1 | 8.2 | 5.4 | 4.5 | –1.2 | 5.5 | –1.2 |
| 11 | 14.0 | 3.5 | 14.8 | 10.6 | 16.4 | 9.9 | 14.5 | 8.6 | 15.4 | 8.8 |
| 12 | 13.6 | 2.6 | 14.5 | 10.2 | 16.1 | 9.5 | 14.9 | 10.0 | 15.0 | 7.9 |
| 13 | 14.5 | –0.7 | 15.4 | 7.4 | 16.5 | 5.5 | 15.7 | 8.2 | 14.3 | 1.7 |
| 14 | 5.5 | 6.5 | 6.4 | 12.3 | 9.2 | 15.3 | 8.0 | 15.0 | 6.4 | 6.3 |
| 15 | 6.4 | 5.2 | 7.3 | 11.8 | 9.8 | 13.6 | 8.2 | 12.3 | 7.0 | 5.2 |
| 16 | 8.7 | – | 2.5 | 8.3 | – | – | – | – | – | – |
| 17 | 8.4 | 4.8 | 8.8 | 10.7 | 10.8 | 11.7 | 9.5 | 11.6 | 8.4 | 5.0 |
| 18 | 7.0 | 7.5 | 5.3 | 6.8 | 7.8 | 8.9 | 8.7 | 14.5 | 6.0 | 3.4 |
| No. | 40:60 | 50:50 | 60:40 | 70:30 | ||||
|---|---|---|---|---|---|---|---|---|
| K1,c | −ΔaG* | K1,c | −ΔaG* | K1,c | −ΔaG* | K1,c | −ΔaG* | |
| 1 | 41.9 | 10.0 | 21.0 | 8.2 | 12.2 | 6.7 | 7.4 | 5.4 |
| 2 | 71.5 | 11.5 | 31.8 | 9.3 | 17.2 | 7.6 | 9.8 | 6.1 |
| 3 | 431.8 | 16.3 | 142.0 | 13.3 | 63.6 | 11.1 | 31.2 | 9.2 |
| 4 | 34.9 | 9.5 | 18.6 | 7.9 | 11.3 | 6.5 | 7.2 | 5.3 |
| 5 | 6.5 | 5.0 | 4.5 | 4.0 | 3.8 | 3.6 | 3.1 | 3.0 |
| 6 | 206.5 | 14.3 | 78.9 | 11.7 | 39.6 | 9.9 | 21.2 | 8.2 |
| 7 | 183.1 | 14.0 | 70.0 | 11.4 | 36.2 | 9.6 | 19.9 | 8.0 |
| 8 | 57.9 | 10.9 | 25.2 | 8.7 | 14.3 | 7.1 | 8.4 | 5.7 |
| 9 | 119.6 | 12.8 | 46.8 | 10.3 | 24.7 | 8.6 | 13.6 | 7.0 |
| 10 | 30.9 | 9.2 | 16.3 | 7.5 | 11.3 | 6.5 | 8.1 | 5.6 |
| 11 | 1011.8 | 18.6 | 309.3 | 15.4 | 135.7 | 13.2 | 64.8 | 11.2 |
| 12 | 933.6 | 18.4 | 290.3 | 15.2 | 129.4 | 13.1 | 62.7 | 11.1 |
| 13 | 2366.6 | 20.9 | 598.8 | 17.2 | 237.2 | 14.7 | 103.5 | 12.5 |
| 14 | 13.5 | 7.0 | 7.3 | 5.3 | 5.0 | 4.3 | 3.3 | 3.2 |
| 15 | 24.8 | 8.6 | 12.0 | 6.7 | 7.5 | 5.4 | 4.7 | 4.1 |
| 16 | 2.8 | 2.8 | 1.8 | 1.7 | 1.6 | 1.3 | 1.3 | 0.6 |
| 17 | 51.7 | 10.6 | 23.3 | 8.5 | 13.7 | 7.0 | 8.0 | 5.6 |
| 18 | 17.6 | 7.7 | 9.3 | 6.0 | 6.2 | 4.9 | 4.2 | 3.9 |
| No. | 40:60 | 50:50 | 60:40 | 70:30 | ||||
|---|---|---|---|---|---|---|---|---|
| −ΔaH* | −ΔaS* | −ΔaH* | −ΔaS* | −ΔaH* | −ΔaS* | −ΔaH* | −ΔaS* | |
| 1 | 7.1 | –9.1 | 9.0 | 2.6 | 9.7 | 9.2 | 9.4 | 12.4 |
| 2 | 7.0 | –13.7 | 9.3 | 0.2 | 10.6 | 9.2 | 10.3 | 12.9 |
| 3 | 11.2 | –15.6 | 13.6 | 0.9 | 13.9 | 8.6 | 14.2 | 15.5 |
| 4 | 7.7 | –5.6 | 9.3 | 4.4 | 9.5 | 9.4 | 9.2 | 12.2 |
| 5 | – | – | – | – | 8.9 | 16.5 | – | – |
| 6 | 9.1 | –16.2 | 11.9 | 0.6 | 12.1 | 6.8 | 12.2 | 12.3 |
| 7 | – | – | 11.6 | 0.6 | 13.7 | 12.4 | 11.9 | 11.9 |
| 8 | – | – | 11.8 | 9.9 | 13.1 | 18.4 | 10.4 | 14.3 |
| 9 | – | – | 11.4 | 3.4 | 12.5 | 12.1 | 10.7 | 11.4 |
| 10 | – | – | 5.7 | –5.7 | 8.2 | 5.4 | 8.2 | 7.9 |
| 11 | 11.8 | –20.8 | 14.4 | –3.0 | 16.4 | 9.9 | 16.0 | 14.8 |
| 12 | 12.1 | –19.3 | 14.4 | –2.7 | 16.1 | 9.5 | 15.7 | 14.2 |
| 13 | 12.6 | –25.5 | 15.3 | –5.7 | 16.5 | 5.5 | 16.6 | 13.0 |
| 14 | – | – | 7.8 | 7.6 | 9.2 | 15.3 | 7.5 | 13.2 |
| 15 | – | – | 8.2 | 4.7 | 9.8 | 13.6 | 8.2 | 12.5 |
| 16 | 0.4 | –7.4 | 0.0 | –5.1 | – | – | – | – |
| 17 | – | – | 9.4 | 2.9 | 10.8 | 11.7 | 8.6 | 9.2 |
| 18 | – | – | 6.2 | 0.6 | 7.8 | 8.9 | 6.9 | 9.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saifutdinov, B.R.; Buryak, A.K. Thermodynamic Characteristics and Selectivity of the Liquid-Phase Adsorption of Aromatic Compounds on Hypercrosslinked Polystyrene Networks with Ultimate-High Crosslinking Densities by Data of Liquid Chromatography. Int. J. Mol. Sci. 2024, 25, 1551. https://doi.org/10.3390/ijms25031551
Saifutdinov BR, Buryak AK. Thermodynamic Characteristics and Selectivity of the Liquid-Phase Adsorption of Aromatic Compounds on Hypercrosslinked Polystyrene Networks with Ultimate-High Crosslinking Densities by Data of Liquid Chromatography. International Journal of Molecular Sciences. 2024; 25(3):1551. https://doi.org/10.3390/ijms25031551
Chicago/Turabian StyleSaifutdinov, Bulat R., and Aleksey K. Buryak. 2024. "Thermodynamic Characteristics and Selectivity of the Liquid-Phase Adsorption of Aromatic Compounds on Hypercrosslinked Polystyrene Networks with Ultimate-High Crosslinking Densities by Data of Liquid Chromatography" International Journal of Molecular Sciences 25, no. 3: 1551. https://doi.org/10.3390/ijms25031551
APA StyleSaifutdinov, B. R., & Buryak, A. K. (2024). Thermodynamic Characteristics and Selectivity of the Liquid-Phase Adsorption of Aromatic Compounds on Hypercrosslinked Polystyrene Networks with Ultimate-High Crosslinking Densities by Data of Liquid Chromatography. International Journal of Molecular Sciences, 25(3), 1551. https://doi.org/10.3390/ijms25031551






