Anti-Inflammatory Effect of Synaptamide in Ischemic Acute Kidney Injury and the Role of G-Protein-Coupled Receptor 110
Abstract
1. Introduction
2. Results
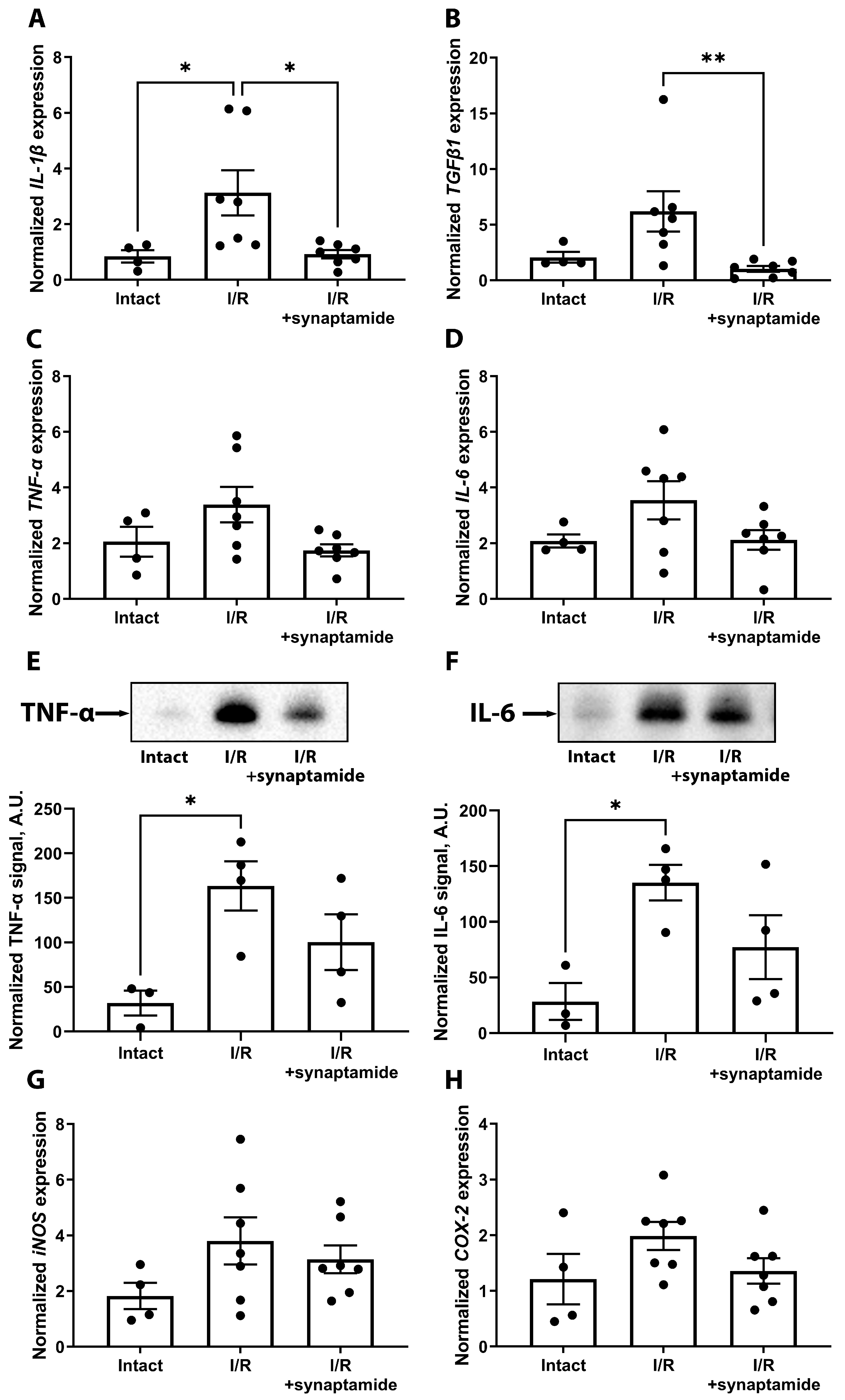
2.1. Synaptamide Mitigates Inflammation in Kidney Tissue after Ischemic Injury
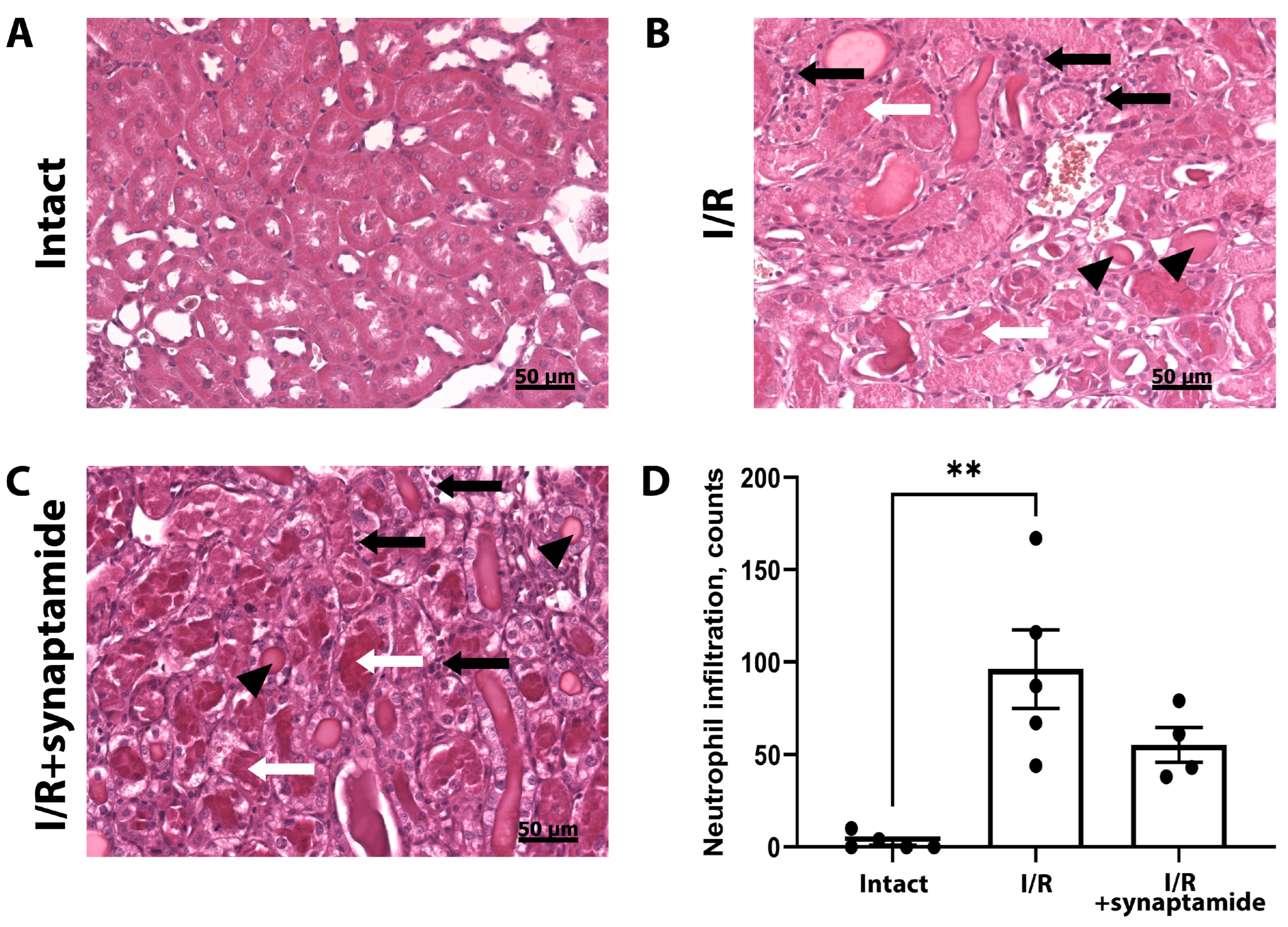
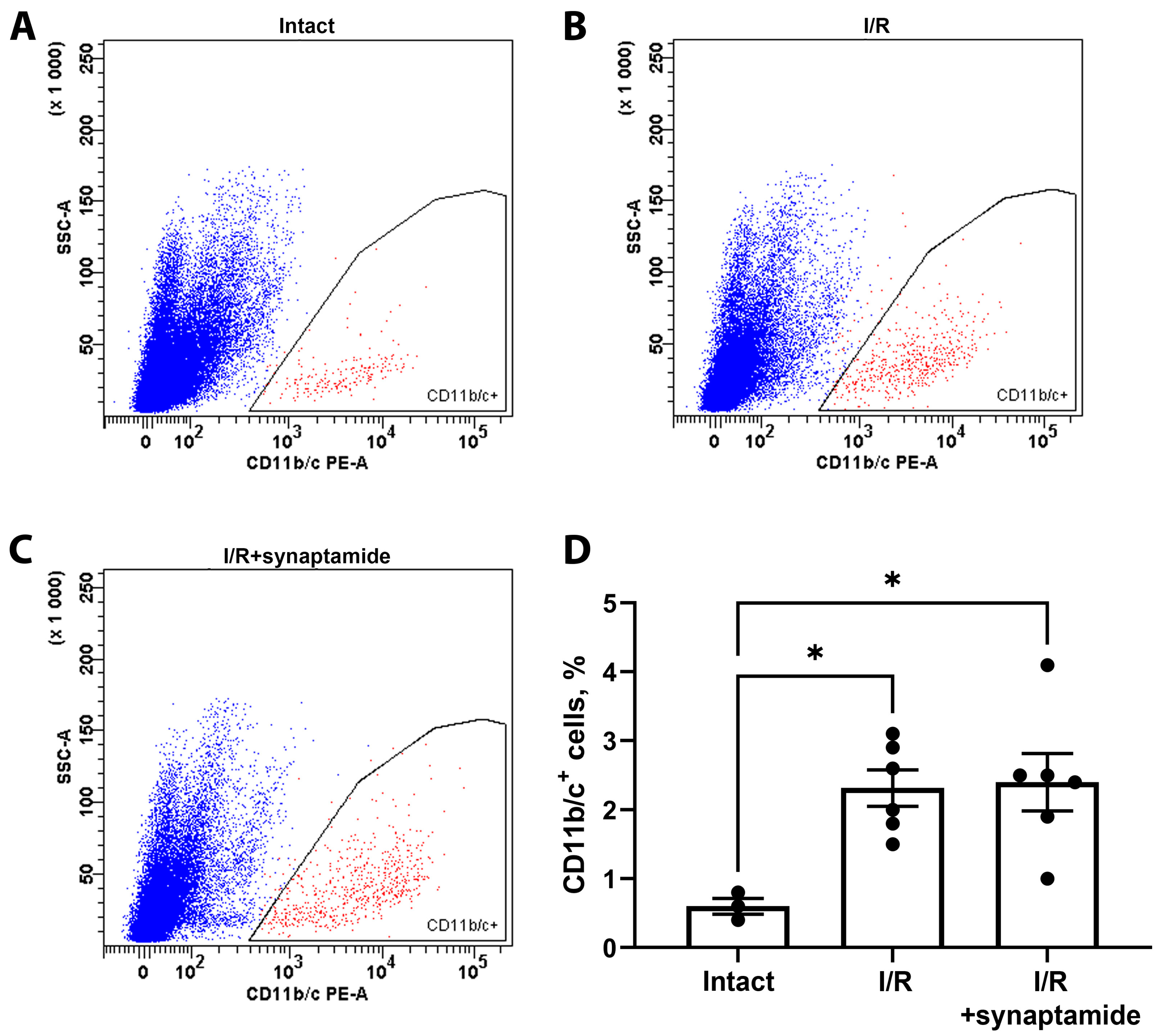
2.2. The Effects of Synaptamide on Leukocyte Infiltration in Kidney Tissue after Ischemic Injury
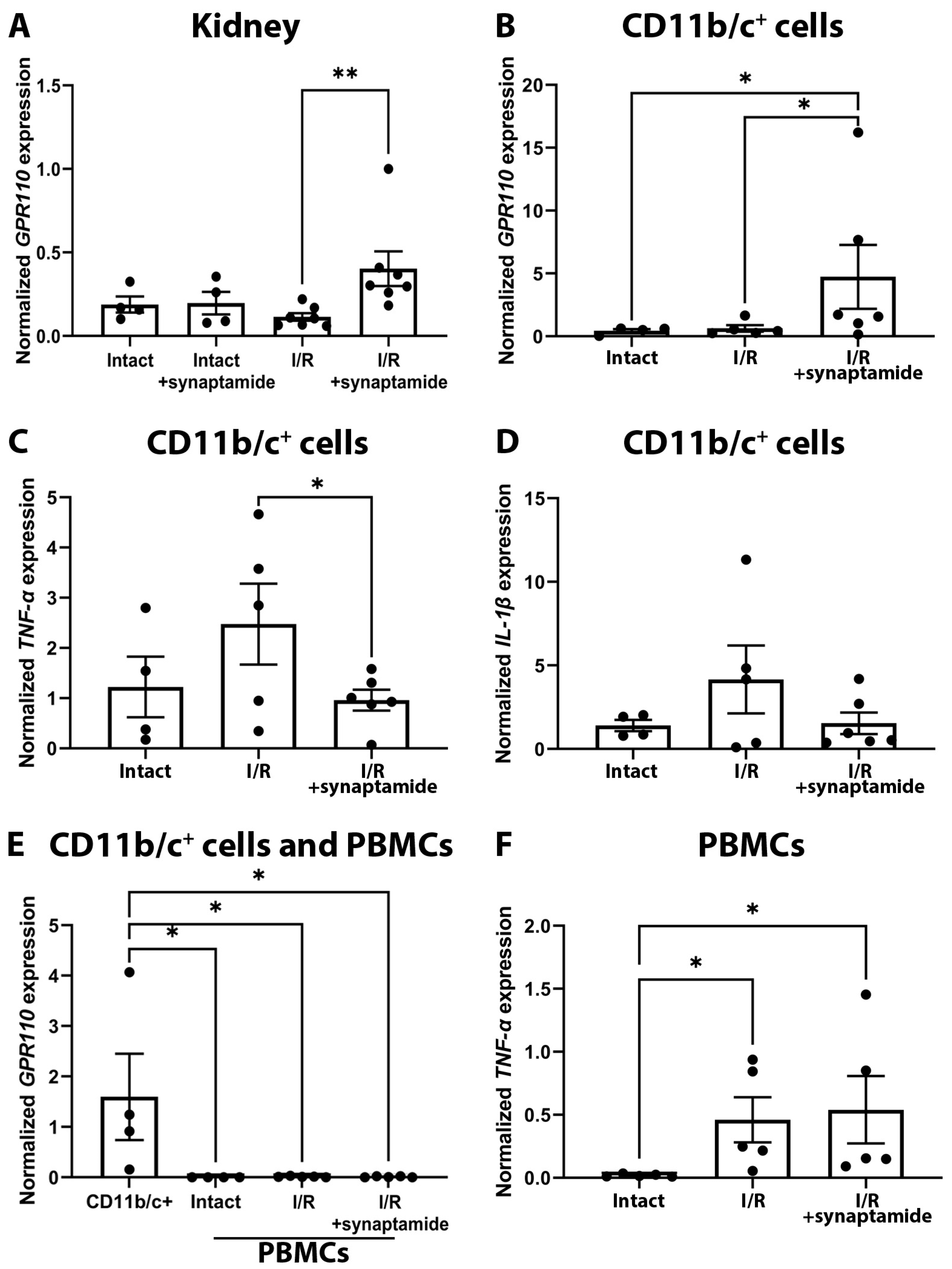
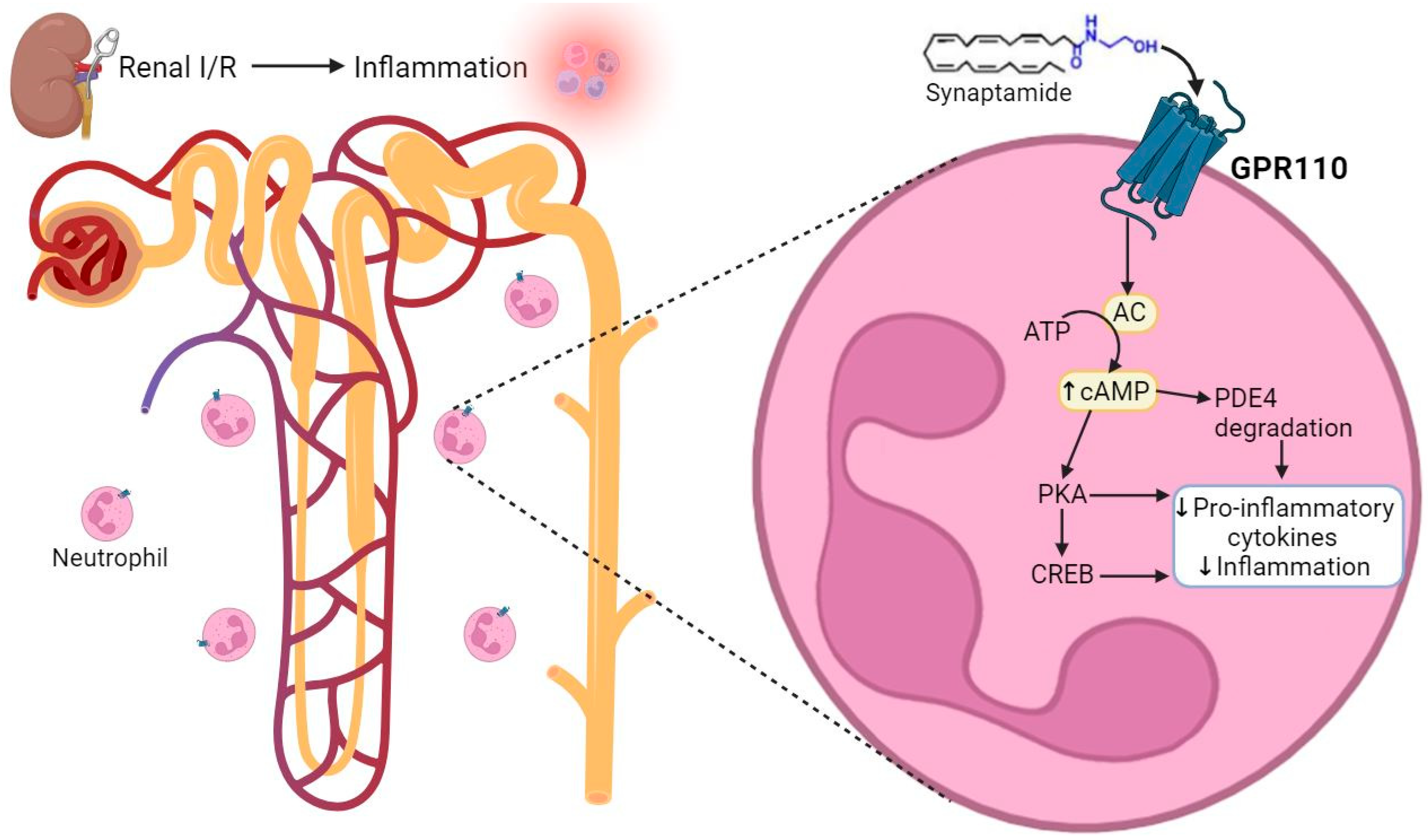
2.3. Synaptamide Exerts Its Anti-Inflammatory Effects through GPR110 in Neutrophils
2.4. Effects of Synaptamide on AKI Severity
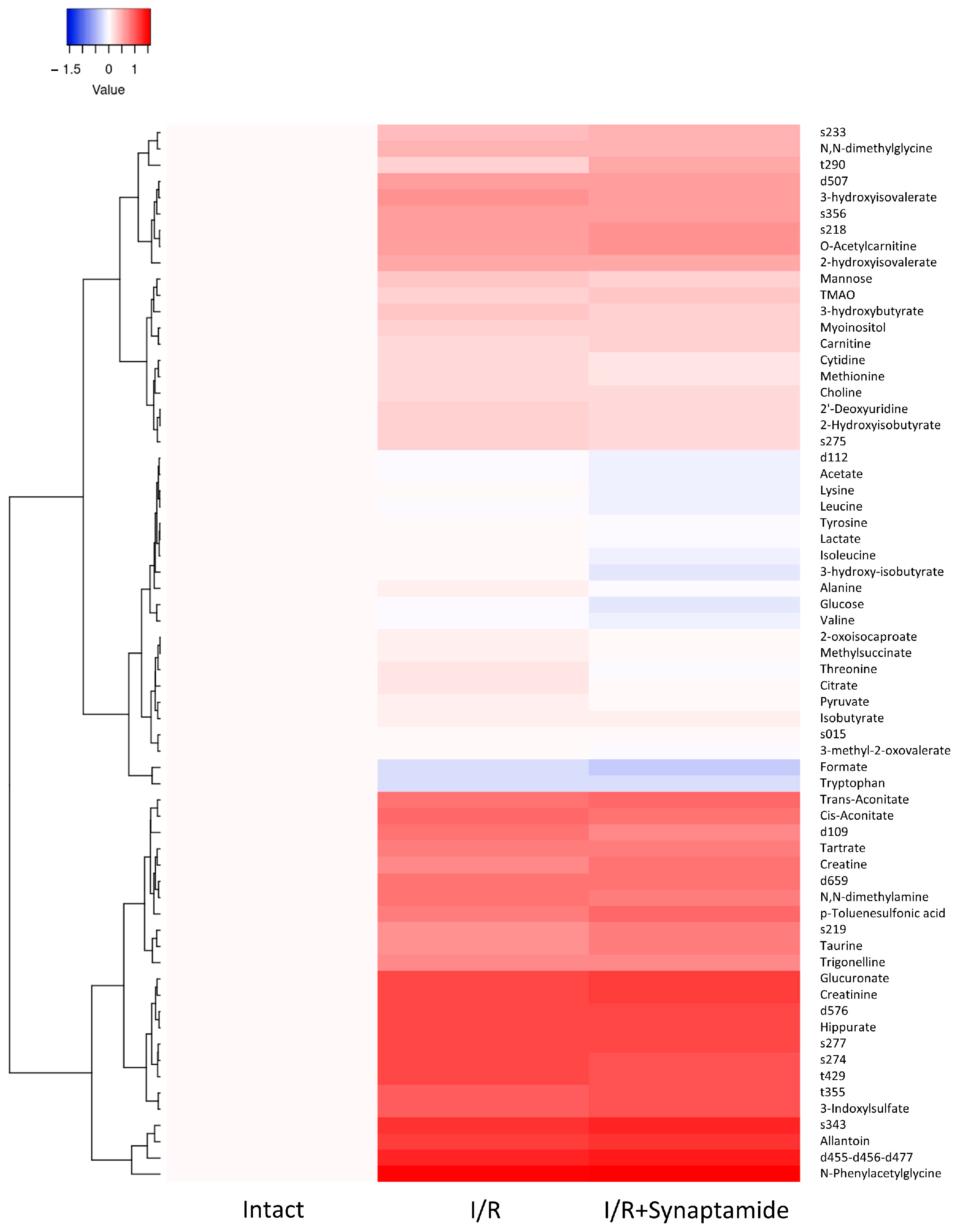
2.5. Synaptamide Affects Serum Metabolic Profile after Ischemic Injury
3. Discussion
4. Materials and Methods
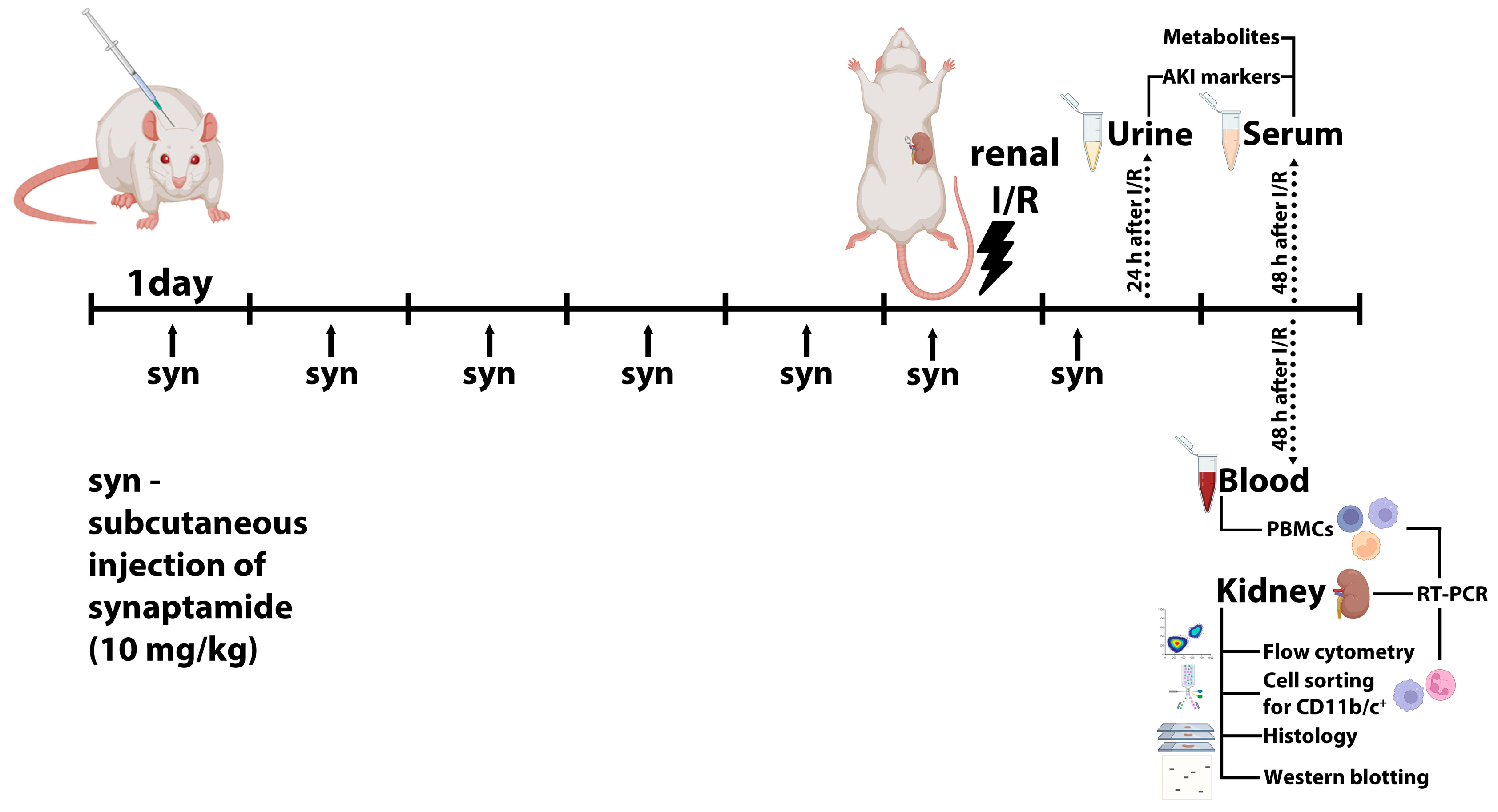
4.1. Animals
4.2. Renal I/R
4.3. Flow Cytometry and Cell Sorting
4.4. Real-Time PCR
4.5. Serum Analysis
4.6. Western Blotting
4.7. Histology
4.8. Quantification of Serum Metabolite Level
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute Kidney Injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-Y.; Kim, J.-S.; Jeong, K.-H.; Kim, S.-K. Acute Kidney Injury: Biomarker-Guided Diagnosis and Management. Medicina 2022, 58, 340. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular Pathophysiology of Ischemic Acute Kidney Injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Holderied, A.; Kumar, S.V.; Anders, H.-J. Targeting Inflammation in So-Called Acute Kidney Injury. Semin. Nephrol. 2016, 36, 17–30. [Google Scholar] [CrossRef]
- Zuk, A.; Bonventre, J.V. Acute Kidney Injury. Annu. Rev. Med. 2016, 67, 293–307. [Google Scholar] [CrossRef]
- Jang, H.R.; Rabb, H. Immune Cells in Experimental Acute Kidney Injury. Nat. Rev. Nephrol. 2015, 11, 88–101. [Google Scholar] [CrossRef]
- Awad, A.S.; Rouse, M.; Huang, L.; Vergis, A.L.; Reutershan, J.; Cathro, H.P.; Linden, J.; Okusa, M.D. Compartmentalization of Neutrophils in the Kidney and Lung Following Acute Ischemic Kidney Injury. Kidney Int. 2009, 75, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Rabb, H. The Innate Immune Response in Ischemic Acute Kidney Injury. Clin. Immunol. 2009, 130, 41–50. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Spector, A.A. Synaptamide, Endocannabinoid-like Derivative of Docosahexaenoic Acid with Cannabinoid-Independent Function. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 121–125. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Spector, A.A. N-Docosahexaenoylethanolamine: A Neurotrophic and Neuroprotective Metabolite of Docosahexaenoic Acid. Mol. Asp. Med. 2018, 64, 34–44. [Google Scholar] [CrossRef]
- Meijerink, J.; Poland, M.; Balvers, M.G.J.; Plastina, P.; Lute, C.; Dwarkasing, J.; van Norren, K.; Witkamp, R.F. Inhibition of COX-2-Mediated Eicosanoid Production Plays a Major Role in the Anti-Inflammatory Effects of the Endocannabinoid N-Docosahexaenoylethanolamine (DHEA) in Macrophages. Br. J. Pharmacol. 2015, 172, 24–37. [Google Scholar] [CrossRef]
- Park, T.; Chen, H.; Kim, H.-Y. GPR110 (ADGRF1) Mediates Anti-Inflammatory Effects of N-Docosahexaenoylethanolamine. J. Neuroinflamm. 2019, 16, 225. [Google Scholar] [CrossRef]
- Naeim, F. Chapter 2—Principles of Immunophenotyping. In Hematopathology; Naeim, F., Rao, P.N., Grody, W.W., Eds.; Academic Press: Oxford, UK, 2008; pp. 27–55. ISBN 9780123706072. [Google Scholar]
- Yakupova, E.I.; Maleev, G.V.; Krivtsov, A.V.; Plotnikov, E.Y. Macrophage Polarization in Hypoxia and Ischemia/reperfusion: Insights into the Role of Energetic Metabolism. Exp. Biol. Med. 2022, 247, 15353702221080130. [Google Scholar] [CrossRef]
- Spitzer, J.A.; Zhang, P. Protein Tyrosine Kinase Activity and the Influence of Gender in Phagocytosis and Tumor Necrosis Factor Secretion in Alveolar Macrophages and Lung-Recruited Neutrophils. Shock 1996, 6, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Andrianova, N.V.; Zorov, D.B.; Plotnikov, E.Y. Targeting Inflammation and Oxidative Stress as a Therapy for Ischemic Kidney Injury. Biochemistry (Moscow) 2020, 85, 1591–1602. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, Y.; Ding, G.; Heiney, K.M.; Huang, S.; Zhang, A. Role of COX-2/mPGES-1/prostaglandin E2 Cascade in Kidney Injury. Mediat. Inflamm. 2015, 2015, 147894. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Cheungpasitporn, W.; Crowson, C.S.; Matteson, E.L. Individual Non-Steroidal Anti-Inflammatory Drugs and Risk of Acute Kidney Injury: A Systematic Review and Meta-Analysis of Observational Studies. Eur. J. Intern. Med. 2015, 26, 285–291. [Google Scholar] [CrossRef]
- Watson, J.E.; Kim, J.S.; Das, A. Emerging Class of Omega-3 Fatty Acid Endocannabinoids & Their Derivatives. Prostaglandins Other Lipid Mediat. 2019, 143, 106337. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Fredman, G.; Krishnamoorthy, S.; Agrawal, N.; Irimia, D.; Piomelli, D.; Serhan, C.N. Decoding Functional Metabolomics with Docosahexaenoyl Ethanolamide (DHEA) Identifies Novel Bioactive Signals. J. Biol. Chem. 2011, 286, 31532–31541. [Google Scholar] [CrossRef]
- Kelly, K.J.; Williams, W.W., Jr.; Colvin, R.B.; Meehan, S.M.; Springer, T.A.; Gutierrez-Ramos, J.C.; Bonventre, J.V. Intercellular Adhesion Molecule-1-Deficient Mice Are Protected against Ischemic Renal Injury. J. Clin. Investig. 1996, 97, 1056–1063. [Google Scholar] [CrossRef]
- Solez, K.; Morel-Maroger, L.; Sraer, J.-D. The Morphology of “Acute Tubular Necrosis” in Man: Analysis of 57 Renal Biopsies and a Comparison with the Glycerol Model. Medicine 1979, 58, 362. [Google Scholar] [CrossRef]
- Awad, A.S.; Ye, H.; Huang, L.; Li, L.; Foss, F.W., Jr.; Macdonald, T.L.; Lynch, K.R.; Okusa, M.D. Selective Sphingosine 1-Phosphate 1 Receptor Activation Reduces Ischemia-Reperfusion Injury in Mouse Kidney. Am. J. Physiol. Renal Physiol. 2006, 290, F1516–F1524. [Google Scholar] [CrossRef]
- Li, L.; Huang, L.; Sung, S.-S.J.; Vergis, A.L.; Rosin, D.L.; Rose, C.E., Jr.; Lobo, P.I.; Okusa, M.D. The Chemokine Receptors CCR2 and CX3CR1 Mediate Monocyte/macrophage Trafficking in Kidney Ischemia-Reperfusion Injury. Kidney Int. 2008, 74, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, A.; Tyrtyshnaia, A.; Ivashkevich, D.; Ermolenko, E.; Dyuizen, I.; Manzhulo, I. Synaptamide Modulates Astroglial Activity in Mild Traumatic Brain Injury. Mar. Drugs 2022, 20, 538. [Google Scholar] [CrossRef] [PubMed]
- Starinets, A.; Tyrtyshnaia, A.; Kipryushina, Y.; Manzhulo, I. Analgesic Activity of Synaptamide in a Rat Sciatic Nerve Chronic Constriction Injury Model. Cells Tissues Organs 2022, 211, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Starinets, A.; Tyrtyshnaia, A.; Manzhulo, I. Anti-Inflammatory Activity of Synaptamide in the Peripheral Nervous System in a Model of Sciatic Nerve Injury. Int. J. Mol. Sci. 2023, 24, 6273. [Google Scholar] [CrossRef] [PubMed]
- Manzhulo, I.; Manzhulo, O.; Tyrtyshnaia, A.; Ponomarenko, A.; Konovalova, S.; Ermolenko, E.; Milkina, E.; Starinets, A. Modulation of Hippocampal Astroglial Activity by Synaptamide in Rats with Neuropathic Pain. Brain Sci. 2021, 11, 1561. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Chen, H.; Kevala, K.; Lee, J.-W.; Kim, H.-Y. N-Docosahexaenoylethanolamine Ameliorates LPS-Induced Neuroinflammation via cAMP/PKA-Dependent Signaling. J. Neuroinflamm. 2016, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Starinets, A.; Ponomarenko, A.; Tyrtyshnaia, A.; Manzhulo, I. Synaptamide Modulates Glial and Neurotransmitter Activity in the Spinal Cord during Neuropathic Pain. J. Chem. Neuroanat. 2023, 134, 102361. [Google Scholar] [CrossRef]
- Tajra, L.C.; Martin, X.; Margonari, J.; Blanc-Brunat, N.; Ishibashi, M.; Vivier, G.; Panaye, G.; Steghens, J.P.; Kawashima, H.; Miyasaka, M.; et al. In Vivo Effects of Monoclonal Antibodies against Rat β2 Integrins on Kidney Ischemia-Reperfusion Injury. J. Surg. Res. 1999, 87, 32–38. [Google Scholar] [CrossRef]
- Huang, B.X.; Hu, X.; Kwon, H.-S.; Fu, C.; Lee, J.-W.; Southall, N.; Marugan, J.; Kim, H.-Y. Synaptamide Activates the Adhesion GPCR GPR110 (ADGRF1) through GAIN Domain Binding. Commun. Biol. 2020, 3, 109. [Google Scholar] [CrossRef]
- Farina, F.; Laezza, M.; Fasano, A.; Del Pozzo, G. In Vitro Differentiation of Macrophages from Peripheral Blood Cells of Celiac Patients. Methods Cell Biol. 2023, 179, 103–112. [Google Scholar] [CrossRef]
- Maremonti, F.; Meyer, C.; Linkermann, A. Mechanisms and Models of Kidney Tubular Necrosis and Nephron Loss. J. Am. Soc. Nephrol. 2022, 33, 472–486. [Google Scholar] [CrossRef]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef] [PubMed]
- Tyrtyshnaia, A.; Konovalova, S.; Bondar, A.; Ermolenko, E.; Sultanov, R.; Manzhulo, I. Anti-Inflammatory Activity of N-Docosahexaenoylethanolamine and N-Eicosapentaenoylethanolamine in a Mouse Model of Lipopolysaccharide-Induced Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 10728. [Google Scholar] [CrossRef] [PubMed]
- Snytnikova, O.A.; Khlichkina, A.A.; Sagdeev, R.Z.; Tsentalovich, Y.P. Evaluation of Sample Preparation Protocols for Quantitative NMR-Based Metabolomics. Metabolomics 2019, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Yanshole, V.V.; Melnikov, A.D.; Yanshole, L.V.; Zelentsova, E.A.; Snytnikova, O.A.; Osik, N.A.; Fomenko, M.V.; Savina, E.D.; Kalinina, A.V.; Sharshov, K.A.; et al. Animal Metabolite Database: Metabolite Concentrations in Animal Tissues and Convenient Comparison of Quantitative Metabolomic Data. Metabolites 2023, 13, 1088. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | The Sequence of Forward Primer 5′- to 3′ | The Sequence of Reverse Primer 5′- to 3′ |
|---|---|---|
| C3 | AAGCCCAACACCAGCTACATC | ACTTCTGATCCTGGCATTCTTCT |
| CD11a | TGGCAGATGTGGTTGTAGG | TCTGGAAGCACACCTTGAG |
| CD45 | AAGCAATACCACCACAAGCACAG | TGGAGTACATGAGCCATTGGAGAG |
| CD68 | TTGAACCCGAACAAAACCAAGGTC | GAGAATGTCCACTGTGCTGCTTG |
| CD86 | TCAGATGCTGTTCCTGTGAAGAGG | TGAAGTCGTAGAGCCTGGTTATCC |
| CD163 | ACAACCGATGCTCAGGAAGAGTAG | CAAGCCAGATTTGTCCAGAACCAG |
| CXCL1 | GTGGCAGGGATTCACTTCAAGAAC | GGGACACCCTTTAGCATCTTTTGG |
| COX-2 | TACGTGTTGACGTCCAGATC | TGGAGAAAGCTTCCCAACTT |
| GPR110 | CATACATAGGGCTGGGCGTCTC | TTGCGTGTGTAGGAGGTTTGGC |
| HPRT | CTCATGGACTGATTATGGACAGGAC | GCAGGTCAGCAAAGAACTTATAGCC |
| IL-1b | CACCTCTCAAGCAGAGCACAG | GGGTTCCATGGTGAAGTCAAC |
| Il-6 | CTGGTCTTCTGGAGTTCCGT | TGGTCTTGGTCCTTAGCCAC |
| IL10 | GCTGAAGACCCTCTGGATAC | CCAGGCTTACCTTATTAAAATCATTC |
| iNOS | CCACAATAGTACAATACTACTTGG | ACGAGGTGTTCAGCGTGCTCCACG |
| KIM-1 | AGGAAGCCGCAGAGAAAC | ATAATGATGTACCTGGTGACAAC |
| RPLP0 | CACAGTACCTGCTCAGAACAC | ACCTTGTCTCCAGTCTTTATCAG |
| TGFβ1 | CTACGCCAAAGAAGTCACC | CACTGCTTCCCGAATGTC |
| TNF-α | CAAGGAGGAGAAGTTCCCAA | TGATCTGAGTGTGAGGGTCTG |
| UBC | TCGTACCTTTCTCACCACAGTATCTAG | GAAAACTAAGACCCTCCCCATCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brezgunova, A.A.; Andrianova, N.V.; Saidova, A.A.; Potashnikova, D.M.; Abramicheva, P.A.; Manskikh, V.N.; Mariasina, S.S.; Pevzner, I.B.; Zorova, L.D.; Manzhulo, I.V.; et al. Anti-Inflammatory Effect of Synaptamide in Ischemic Acute Kidney Injury and the Role of G-Protein-Coupled Receptor 110. Int. J. Mol. Sci. 2024, 25, 1500. https://doi.org/10.3390/ijms25031500
Brezgunova AA, Andrianova NV, Saidova AA, Potashnikova DM, Abramicheva PA, Manskikh VN, Mariasina SS, Pevzner IB, Zorova LD, Manzhulo IV, et al. Anti-Inflammatory Effect of Synaptamide in Ischemic Acute Kidney Injury and the Role of G-Protein-Coupled Receptor 110. International Journal of Molecular Sciences. 2024; 25(3):1500. https://doi.org/10.3390/ijms25031500
Chicago/Turabian StyleBrezgunova, Anna A., Nadezda V. Andrianova, Aleena A. Saidova, Daria M. Potashnikova, Polina A. Abramicheva, Vasily N. Manskikh, Sofia S. Mariasina, Irina B. Pevzner, Ljubava D. Zorova, Igor V. Manzhulo, and et al. 2024. "Anti-Inflammatory Effect of Synaptamide in Ischemic Acute Kidney Injury and the Role of G-Protein-Coupled Receptor 110" International Journal of Molecular Sciences 25, no. 3: 1500. https://doi.org/10.3390/ijms25031500
APA StyleBrezgunova, A. A., Andrianova, N. V., Saidova, A. A., Potashnikova, D. M., Abramicheva, P. A., Manskikh, V. N., Mariasina, S. S., Pevzner, I. B., Zorova, L. D., Manzhulo, I. V., Zorov, D. B., & Plotnikov, E. Y. (2024). Anti-Inflammatory Effect of Synaptamide in Ischemic Acute Kidney Injury and the Role of G-Protein-Coupled Receptor 110. International Journal of Molecular Sciences, 25(3), 1500. https://doi.org/10.3390/ijms25031500









