The Aquaporin 3 Polymorphism (rs17553719) Is Associated with Sepsis Survival and Correlated with IL-33 Secretion
Abstract
1. Introduction
2. Results
2.1. Characterization of Sepsis Patients
2.2. Survival Analysis of Sepsis Patients Ranked by AQP3 (rs17553719) Polymorphism
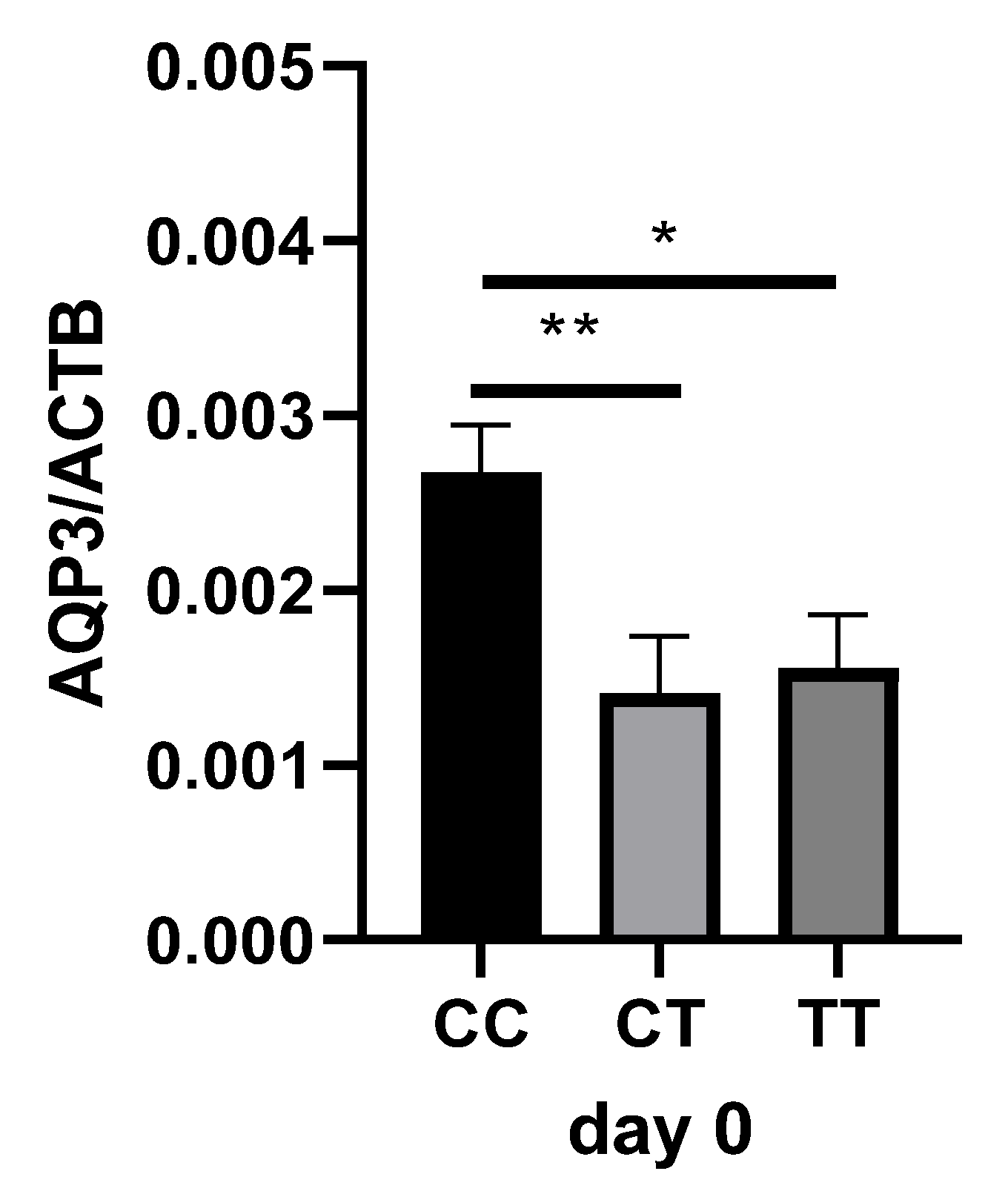
2.3. AQP3 Expression Analysis in Sepsis Patients
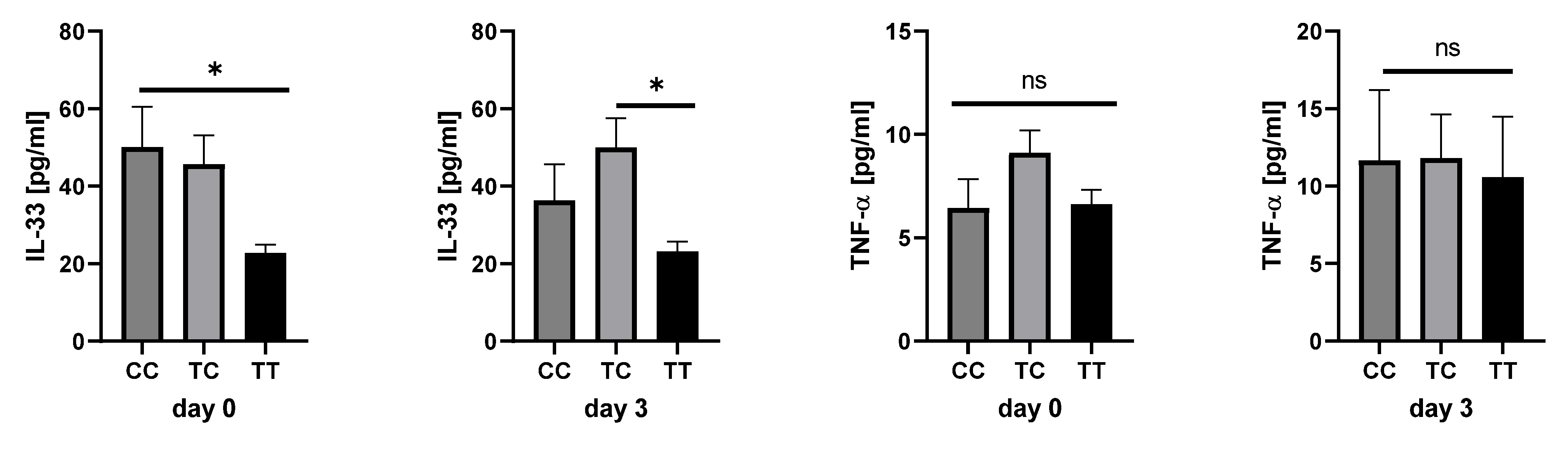
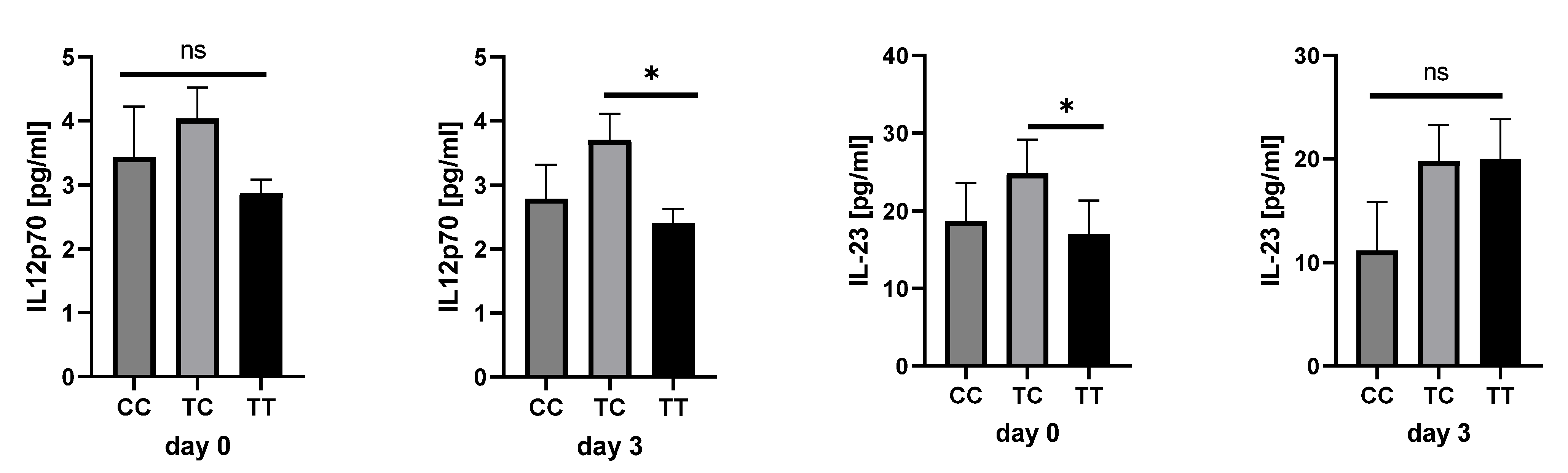
2.4. Cytokine Analysis in Sepsis Patients
3. Discussion
4. Materials and Methods
4.1. Study Design and Cohort
- -
- Age below 18 years at the time of ICU admission;
- -
- Withdrawal or withholding of consent;
- -
- Withdrawal of treatment.
4.2. Collection of Blood Samples
4.3. DNA Genotyping
4.4. RNA Isolation, cDNA Synthesis, and qPCR
4.5. Measurement of Cytokines in Serum
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003, 29, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.; Hammad Altaq, H.; Abdo, T. Management of Sepsis and Septic Shock: What Have We Learned in the Last Two Decades? Microorganisms 2023, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Zumla, A. To have sepsis or to be septic-is the difference between these clinical conditions important? Int. J. Infect. Dis. 2016, 48, 118–119. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Rump, K.; Adamzik, M. Function of aquaporins in sepsis: A systematic review. Cell Biosci. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Garra, S.; Calamita, G.; Soveral, G. The Multifaceted Role of Aquaporin-9 in Health and Its Potential as a Clinical Biomarker. Biomolecules 2022, 12, 897. [Google Scholar] [CrossRef]
- Messerer, D.A.C.; Schmidt, H.; Frick, M.; Huber-Lang, M. Ion and Water Transport in Neutrophil Granulocytes and Its Impairment during Sepsis. Int. J. Mol. Sci. 2021, 22, 1699. [Google Scholar] [CrossRef]
- Agre, P. Membrane water transport and aquaporins: Looking back. Biol. Cell 2005, 97, 355–356. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins in clinical medicine. Annu. Rev. Med. 2012, 63, 303–316. [Google Scholar] [CrossRef]
- Tao, B.; Liu, L.; Wang, N.; Wang, W.; Jiang, J.; Zhang, J. Effects of hydrogen-rich saline on aquaporin 1, 5 in septic rat lungs. J. Surg. Res. 2016, 202, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Mlinarić, M.; Lučić, I.; Milković, L.; da Silva, I.V.; Tartaro Bujak, I.; Musani, V.; Soveral, G.; Čipak Gašparović, A. AQP3-Dependent PI3K/Akt Modulation in Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 8133. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Roles of aquaporin-3 in the epidermis. J. Investig. Dermatol. 2008, 128, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Kan, B.; Yang, B. Aquaporins in Immune System. Adv. Exp. Med. Biol. 2023, 1398, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, S.; Miyamoto, K.; Hatai, M.; Mikasa, Y.; Katsuda, M.; Murata, S.I.; Kondo, T.; Yamaue, H.; Hashimoto, S. Immune Cells Profiles in the Different Sites of COVID-19-Affected Lung Lobes in a Single Patient. Front. Med. 2022, 9, 841170. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Peng, Q.; Tan, Z.; Xu, S.; Wang, Y.; Wu, A.; Xiao, W.; Wang, Q.; Xie, H.; Li, J.; et al. Targeting Aquaporin-3 Attenuates Skin Inflammation in Rosacea. Int. J. Biol. Sci. 2023, 19, 5160–5173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, X.; Zheng, Z.; Lu, B.; Wang, J.; Tan, A.H.; Zhao, M.; Loh, J.T.; Ng, S.W.; Chen, Q.; et al. Host-derived lipids orchestrate pulmonary γδ T cell response to provide early protection against influenza virus infection. Nat. Commun. 2021, 12, 1914. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef]
- Rump, K.; Rahmel, T.; Rustige, A.M.; Unterberg, M.; Nowak, H.; Koos, B.; Schenker, P.; Viebahn, R.; Adamzik, M.; Bergmann, L. The Aquaporin3 Promoter Polymorphism -1431 A/G is Associated with Acute Graft Rejection and Cytomegalovirus Infection in Kidney Recipients Due to Altered Immune Cell Migration. Cells 2020, 9, 1421. [Google Scholar] [CrossRef]
- Ikezoe, K.; Oga, T.; Honda, T.; Hara-Chikuma, M.; Ma, X.; Tsuruyama, T.; Uno, K.; Fuchikami, J.; Tanizawa, K.; Handa, T.; et al. Aquaporin-3 potentiates allergic airway inflammation in ovalbumin-induced murine asthma. Sci. Rep. 2016, 6, 25781. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Teng, W.; Shan, Y.; Yi, S.; Zhu, S.; Li, Y. Role of Aquaporin-3 in Intestinal Injury Induced by Sepsis. Biol. Pharm. Bull. 2019, 42, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, P.; Wu, Y.; Zhu, Y.; Peng, X.Y.; Xiang, X.M.; Xue, M.Y.; Li, Q.H.; Li, J.X.; Yan, Q.G.; et al. Role of AQP3 in the Vascular Leakage of Sepsis and the Protective Effect of Ss-31. J. Cardiovasc. Pharmacol. 2021, 78, 280–287. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Cardoso, C.; Martínez-Banaclocha, H.; Casini, A.; Pelegrín, P.; Soveral, G. Aquaporin-3 is involved in NLRP3-inflammasome activation contributing to the setting of inflammatory response. Cell. Mol. Life Sci. 2021, 78, 3073–3085. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, Y.; Liu, W.; Liu, W.; Dong, J.; Liu, Q.; Hao, H.; Ren, H. Research progress on the activation mechanism of NLRP3 inflammasome in septic cardiomyopathy. Immun. Inflamm. Dis. 2023, 11, e1039. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cai, J.; Tang, Y.; Lu, B. The noncanonical inflammasome-induced pyroptosis and septic shock. Semin. Immunol. 2023, 70, 101844. [Google Scholar] [CrossRef]
- He, W.; Dong, H.; Wu, C.; Zhong, Y.; Li, J. The role of NLRP3 inflammasome in sepsis: A potential therapeutic target. Int. Immunopharmacol. 2023, 115, 109697. [Google Scholar] [CrossRef]
- Adamzik, M.; Frey, U.H.; Mohlenkamp, S.; Scherag, A.; Waydhas, C.; Marggraf, G.; Dammann, M.; Steinmann, J.; Siffert, W.; Peters, J. Aquaporin 5 gene promoter--1364A/C polymorphism associated with 30-day survival in severe sepsis. Anesthesiology 2011, 114, 912–917. [Google Scholar] [CrossRef]
- Rump, K.; Unterberg, M.; Bergmann, L.; Bankfalvi, A.; Menon, A.; Schafer, S.; Scherag, A.; Bazzi, Z.; Siffert, W.; Peters, J.; et al. AQP5-1364A/C polymorphism and the AQP5 expression influence sepsis survival and immune cell migration: A prospective laboratory and patient study. J. Transl. Med. 2016, 14, 321. [Google Scholar] [CrossRef]
- Rump, K.; Spellenberg, T.; von Busch, A.; Wolf, A.; Ziehe, D.; Thon, P.; Rahmel, T.; Adamzik, M.; Koos, B.; Unterberg, M. AQP5-1364A/C Polymorphism Affects AQP5 Promoter Methylation. Int. J. Mol. Sci. 2022, 23, 11813. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Satooka, H.; Watanabe, S.; Honda, T.; Miyachi, Y.; Watanabe, T.; Verkman, A.S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-κB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015, 6, 7454. [Google Scholar] [CrossRef]
- Rabolli, V.; Wallemme, L.; Lo Re, S.; Uwambayinema, F.; Palmai-Pallag, M.; Thomassen, L.; Tyteca, D.; Octave, J.N.; Marbaix, E.; Lison, D.; et al. Critical role of aquaporins in interleukin 1β (IL-1β)-induced inflammation. J. Biol. Chem. 2014, 289, 13937–13947. [Google Scholar] [CrossRef]
- Cleasby, C.; Marshall, T.; Gordon, A.C.; Antcliffe, D.B. The effect of vasopressin and hydrocortisone on cytokine trajectories. Intensive Care Med. 2023, 49, 241–243. [Google Scholar] [CrossRef]
- Gorbacheva, A.M.; Kuprash, D.V.; Mitkin, N.A. Glucocorticoid Receptor Binding Inhibits an Intronic IL33 Enhancer and is Disrupted by rs4742170 (T) Allele Associated with Specific Wheezing Phenotype in Early Childhood. Int. J. Mol. Sci. 2018, 19, 3956. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Lv, R.; Lei, M. IL-33 attenuates mortality by promoting IFN-γ production in sepsis. Inflamm. Res. 2018, 67, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Morrow, K.N.; Coopersmith, C.M.; Ford, M.L. IL-17, IL-27, and IL-33: A Novel Axis Linked to Immunological Dysfunction During Sepsis. Front. Immunol. 2019, 10, 1982. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Zhao, J.; Lei, M.; Xiao, D.; Yu, Y.; Xie, J. IL-33 Attenuates Sepsis by Inhibiting IL-17 Receptor Signaling through Upregulation of SOCS3. Cell Physiol. Biochem. 2017, 42, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Klenke, S.; Renckhoff, K.; Engler, A.; Peters, J.; Frey, U.H. Easy-to-use strategy for reference gene selection in quantitative real-time PCR experiments. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 1353–1366. [Google Scholar] [CrossRef]

| TT Genotype (n = 135) | TC Genotype (n = 112) | CC Genotype (n = 18) | p-Value | |
|---|---|---|---|---|
| Gender male, n (%) | 89 (65.9%) | 66 (59.4%) | 12 (68.8%) | 0.542 |
| Age in years, median [IQR] | 65 [56–73] | 64 [45–71] | 67 [51–80] | 0.726 |
| SOFA score at day 1, median [IQR] | 9 [5–12] | 8 [5–10] | 10.5 [5–14] | 0.212 |
| SAPS-II at ICU admission, median [IQR] | 37 [28–41] | 32 [22–35] | 33 [26–41] | 0.982 |
| Mechanical ventilation, n (%) | 88 (65) | 75 (66.9) | 10 (55.5) | 0.781 |
| Focus of infection, n (%) | 0.458 | |||
| Central nervous system | 1 (0.7%) | 2 (1.8%) | 0 (0%) | |
| Lower respiratory tract | 39 (28.9%) | 41 (36.6%) | 8 (44.4%) | |
| Skin and soft tissue | 7 (5.2%) | 5 (4.5%) | 1 (5.6%) | |
| Genitourinary | 5 (3.7%) | 8 (7.1%) | 0 (0%) | |
| Cardiovascular | 6 (4.4%) | 3 (2.7%) | 0 (0%) | |
| Intra-abdominal | 25 (18.5%) | 11 (9.8%) | 2 (11.1%) | |
| Musculosceletal | 6 (4.4%) | 2 (1.8%) | 0 (0%) | |
| Unknown | 46 (34.1%) | 40 (35.7%) | 7 (38.9%) | |
| Length of stay in ICU, median (days) [IQR] | 8.9 [11.9] | 6.9 [10.2] | 8.9 [11.1] | 0.733 |
| Length of stay in hospital, median (days) [IQR] | 10 [12] | 7 [10] | 9 [23] | 0.428 |
| 30-day survival time, median (days) [IQR] | 30 [3] | 30 [18]] | 11 [27] | 0.003 |
| 30-day survival (yes), n (%) | 103 (76.3%) | 74 (66.1%) | 7 (38.9%) | 0.003 |
| Hydrocortisone administration (yes), n = 181 | 25 (27.2%) | 22 (28.6%) | 5 (41.7%) | 0.580 |
| Co-Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| p-Value | HR | 95% CI | p-Value | HR | 95% CI | |
| AQP3 genotype TT genotype | 0.002 | 0.014 | ||||
| TC genotype | 0.119 | 1.454 | 0.908–2.327 | 0.023 | 2.231 | 1.116–4.459 |
| CC genotype | <0.001 | 3.461 | 1.743–6.873 | 0.012 | 4.232 | 1.374–13.035 |
| Age (per year) | <0.001 | 1.027 | 1.013–1.041 | 0.142 | 1.018 | 0.994–1.041 |
| gender | 0.124 | 1.320 | 0.927–1.880 | 0.540 | 1.239 | 0.623–2.464 |
| IL-33 cutoff at study inclusion | 0.021 | 1.665 | 1.080–2.567 | 0.406 | 0.704 | 0.307–1.613 |
| TNF-α cutoff at study inclusion | 0.004 | 1.842 | 1.209–2.807 | 0.179 | 1.601 | 1.936–7.699 |
| IL-6 at cutoff study inclusion | <0.001 | 2.743 | 1.835–4.100 | <0.001 | 3.861 | 1.936–7.699 |
| SOFA score at study inclusion | <0.001 | 1.262 | 1.164–1.369 | <0.001 | 1.280 | 1.153–1.420 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziehe, D.; Marko, B.; Thon, P.; Rahmel, T.; Palmowski, L.; Nowak, H.; von Busch, A.; Wolf, A.; Witowski, A.; Vonheder, J.; et al. The Aquaporin 3 Polymorphism (rs17553719) Is Associated with Sepsis Survival and Correlated with IL-33 Secretion. Int. J. Mol. Sci. 2024, 25, 1400. https://doi.org/10.3390/ijms25031400
Ziehe D, Marko B, Thon P, Rahmel T, Palmowski L, Nowak H, von Busch A, Wolf A, Witowski A, Vonheder J, et al. The Aquaporin 3 Polymorphism (rs17553719) Is Associated with Sepsis Survival and Correlated with IL-33 Secretion. International Journal of Molecular Sciences. 2024; 25(3):1400. https://doi.org/10.3390/ijms25031400
Chicago/Turabian StyleZiehe, Dominik, Britta Marko, Patrick Thon, Tim Rahmel, Lars Palmowski, Hartmuth Nowak, Alexander von Busch, Alexander Wolf, Andrea Witowski, Jolene Vonheder, and et al. 2024. "The Aquaporin 3 Polymorphism (rs17553719) Is Associated with Sepsis Survival and Correlated with IL-33 Secretion" International Journal of Molecular Sciences 25, no. 3: 1400. https://doi.org/10.3390/ijms25031400
APA StyleZiehe, D., Marko, B., Thon, P., Rahmel, T., Palmowski, L., Nowak, H., von Busch, A., Wolf, A., Witowski, A., Vonheder, J., Ellger, B., Wappler, F., Schwier, E., Henzler, D., Köhler, T., Zarbock, A., Ehrentraut, S. F., Putensen, C., Frey, U. H., ... Rump, K., on behalf of the SepsisDataNet.NRW Research Group. (2024). The Aquaporin 3 Polymorphism (rs17553719) Is Associated with Sepsis Survival and Correlated with IL-33 Secretion. International Journal of Molecular Sciences, 25(3), 1400. https://doi.org/10.3390/ijms25031400







