Abstract
Mastocytosis is a heterogeneous disease characterized by the expansion and accumulation of neoplastic mast cells in various tissues. Diffuse cutaneous mastocytosis (DCM) is a rare and most severe form of cutaneous mastocytosis, which typically occurs in childhood. There have been reports of a familial DCM with specific gene mutations, indicating both sporadic and hereditary factors involved in its pathogenesis. DCM is associated with severe MC mediator-related symptoms and an increased risk of anaphylaxis. The diagnosis is based on the appearance of skin lesions, which typically show generalized thickening, erythroderma, blistering dermographism, and a positive Darier’s sign. Recognition, particularly in infants, is challenging due to DCMs resemblance to other bullous skin disorders. Therefore, in unclear cases, a skin biopsy is crucial. Treatment focuses on symptom management, mainly including antihistamines and mast cell stabilizers. In extremely severe cases, systemic steroids, tyrosine kinase inhibitors, phototherapy, or omalizumab may be considered. Patients should be equipped with an adrenaline autoinjector. Herein, we conducted a comprehensive review of literature data on DCM since 1962, which could help to better understand both the management and prognosis of DCM, which depends on the severity of skin lesions, intensity of mediator-related symptoms, presence of anaphylaxis, and treatment response.
1. Introduction
Mastocytosis is a rare condition characterized by an abnormal accumulation of neoplastic mast cells (MCs) in various tissues, mostly including the skin, bone marrow (BM), spleen, liver, gastrointestinal tract, and lymph nodes [1,2,3]. In 2016, the World Health Organization (WHO) categorized the disease into three primary clinical variants: cutaneous mastocytosis (CM), systemic mastocytosis (SM), and the locally aggressive disease known as MC sarcoma (MCS) [4]. The current updated classification of mastocytosis is presented in Table 1 [1,2,3,4,5].

Table 1.
Updated Classification of Mastocytosis.
In contrast to adults, pediatric patients usually suffer from CM and only rarely from SM [6,7,8]. CM is diagnosed on the basis of the typical morphology of skin lesions and the absence of signs or criteria of SM [9]. According to the EU/US consensus group, the diagnostic criteria of CM include the presence of typical skin lesions of mastocytosis together with the Darier’s sign, which is a major CM criterion, and one or two of the minor criteria—increased numbers of MCs in biopsy sections of lesional skin and an activating KIT mutation at codon 816 in lesional skin [6,9]. The Darier’s sign is characterized by reddening and urticarial swelling of the lesion after its mechanical irritation [6,8]. CM is subdivided into three main subtypes: the most common form, namely maculopapular CM (MPCM, also referred to as urticaria pigmentosa, with two variants—monomorphic MPCM and polymorphic MPCM); mastocytoma of the skin; and diffuse CM (DCM); which is the least common form of the disease [6,7,8,10,11].
DCM represents the most severe clinical manifestation of CM and is characterized by an extensive infiltration of MCs throughout the entire skin in the absence of macroscopic individualized cutaneous lesions [6,8]. This condition is typically observed in early childhood and may persist into adulthood in some cases [8]. The frequency of DCM ranges from 2% to 11% of all forms of pediatric CM [10,11,12,13,14,15,16]. In the largest systemic review of 1747 cases of pediatric mastocytosis, skin involvement presenting as DCM included 5.2% of patients [7].
The pathogenesis of mastocytosis is closely associated with gain-of-function somatic mutations in the KIT gene, resulting in the steam cell factor (SCF)-independent activation and phosphorylation of the KIT receptor, which drives differentiation, survival, and accumulation of MCs in various organs [1,17,18]. A KIT mutation involving codon 816 in exon 17 (most commonly the KIT D816V mutation) is found in the majority of adult patients with SM [1,17]. In contrast, pediatric patients, who mostly present with CM, may exhibit a mutation of codon 816 in exon 17 in approximately 42% (including the KIT D816V mutation in 36%), as well as different somatic or germline KIT mutations, mainly in exons 8, 9, and 11 (approximately 44%), or have no KIT mutation (wild-type genotype, approximately 14%) [16,18]. The clinical presentation of mastocytosis is related to the release of MC mediators, which results in a variety of clinical symptoms, ranging from mild symptoms such as flushing, pruritus, dyspnea, abdominal pain, vomiting, and diarrhea to potentially life-threatening conditions such as hypotension and anaphylactic shock [6,10,11,19]. Upon activation, MCs promptly initiate degranulation, leading to the release of preformed mediators stored in cytoplasmic granules, including histamine, serotonin, heparin, chymase, chondroitin sulfate, carboxypeptidase, tryptase, and TNF-α [20]. This initial phase is followed by a de novo synthesis of membrane lipid-derived mediators, principally prostaglandin D2 (PGD2), cysteinyl leukotrienes (LTC4, D4, and E4), and platelet-activating factor (PAF). As MC activation continues, a set of cytokines, both pro-inflammatory and anti-inflammatory, is also newly synthesized. This includes TNF-α, GM-CSF, IL-1, IL-3, IL-4, IL-5, IL-6, IL-13, IL-1RA, chemokines such as IL-8, CCL-2, CC-3, CCL-5, and CXCL-8, along with growth factors like transforming growth factor-beta 1 (TGF-β1), SCF, fibroblast growth factor (FGF), nerve growth factor (NGF), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and interferons [20,21]. In recent years, the role of some cytokines and chemokines in inducing MC mediator-related symptoms has been examined in humans with mastocytosis [22,23,24,25,26,27]. It has been found that IL-6 may act as an autocrine growth factor for neoplastic MCs; IL-31 is associated with itching of the skin; and oncostatin-M and monocyte chemoattractant protein-1 (MCP-1/CCL2) impact BM remodeling and modulate the BM microenvironment [22,23,24,26,27]. Moreover, it has been shown that blistering within the lamina lucida (junctional) is attributed to serine proteases released from MCs, which infiltrate the upper dermis [28]. Activation of the MCs in patients with mastocytosis leads to the elicitation of multiple mediators, which may be provoked by numerous triggers [20,29]. Most often, MCs can be activated by Immunoglobulin E (IgE)-mediated mechanisms (such as Hymenoptera venoms, plants, food, and some drugs, e.g., nonsteroidal anti-inflammatory drugs [NSAIDs], quinolones, neuromuscular blocking agents [NMBAs], and radio-contrast media [RCM], among others). Non-IgE-mediated mechanisms include activation of the complement system (e.g., C5aReceptor/CD88 mediate a reaction to polyethyleneglycol [PEG] and polysorbate included in vaccines), direct MCs degranulation mediated by MAS-related G Protein-Coupled Receptor-X2 (MRGPRX2) activated by neuropeptides and some drugs (e.g., opiates, quinolones, vancomycin, NMBAs, RCM), activation pathways through Toll-like receptors (involved in response to bacteria, parasites, viruses), and stimulation by physical factors (heat, cold, pressure, stress, physical exertion, the friction of mastocytosis skin lesions), among others [20,30,31,32,33]. However, in a subset of patients, the mechanism and triggers of MC activation and anaphylaxis remain unknown. In contrast to adults in whom hymenoptera venom is the most common trigger of MC activation, most reported cases of children with mastocytosis who suffer from anaphylaxis are idiopathic or induced by drugs and food [12,29,30,34].
2. Genetic Background of Diffuse Cutaneous Mastocytosis
According to current knowledge, genetic drivers, epigenetics, and both hormonal and metabolic factors involved in the pathogenesis of pediatric mastocytosis are poorly understood [1,3,8]. However, numerous studies show that children with DCM can carry different somatic mutations in exon 17 of KIT (D816V, D816Y, or D816I), as well as mutations in other exons (Del419, K509I, or internal tandem duplication A502_Y503dup) [18,35,36,37,38,39,40,41]. Familial mastocytosis is a specific form of the disease in which mostly germline KIT mutations are detected in affected family members [42,43,44,45,46,47,48,49,50]. Interestingly, familial mastocytosis associated with deafness has also been reported [51]. Familial DCM has been reported in three families in which germline mutations, including the A533D and the p.S451C KIT mutations, were found [52,53,54]. Additionally, rare cases of DCM associated with gastrointestinal stromal tumors (GIST) and tuberous sclerosis have been described [36,49,50,55,56]. It has been found that patients with DCM and coexisting GIST systematically show germline KIT mutations such as S476I and Del419Asp [6,49,55]. Of note, some children with DCM may suffer from a biological variant of SM known as well-differentiated SM (WDSM), in which BM MCs have a mature, apparently normal morphology, usually lacking CD25 and CD2 expression, and often display an aberrant expression of CD30 in the absence of KIT codon 816 mutations [56,57]. Interestingly, the K509I KIT mutation located at exon 9 of the gene has been reported in some patients with WDSM presenting as DCM [36,56]. Altogether, these findings reflect the complex genetic background of DCM and highlight a potential hereditary component in a significant fraction of cases.
3. Clinical Presentation of Diffuse Cutaneous Mastocytosis
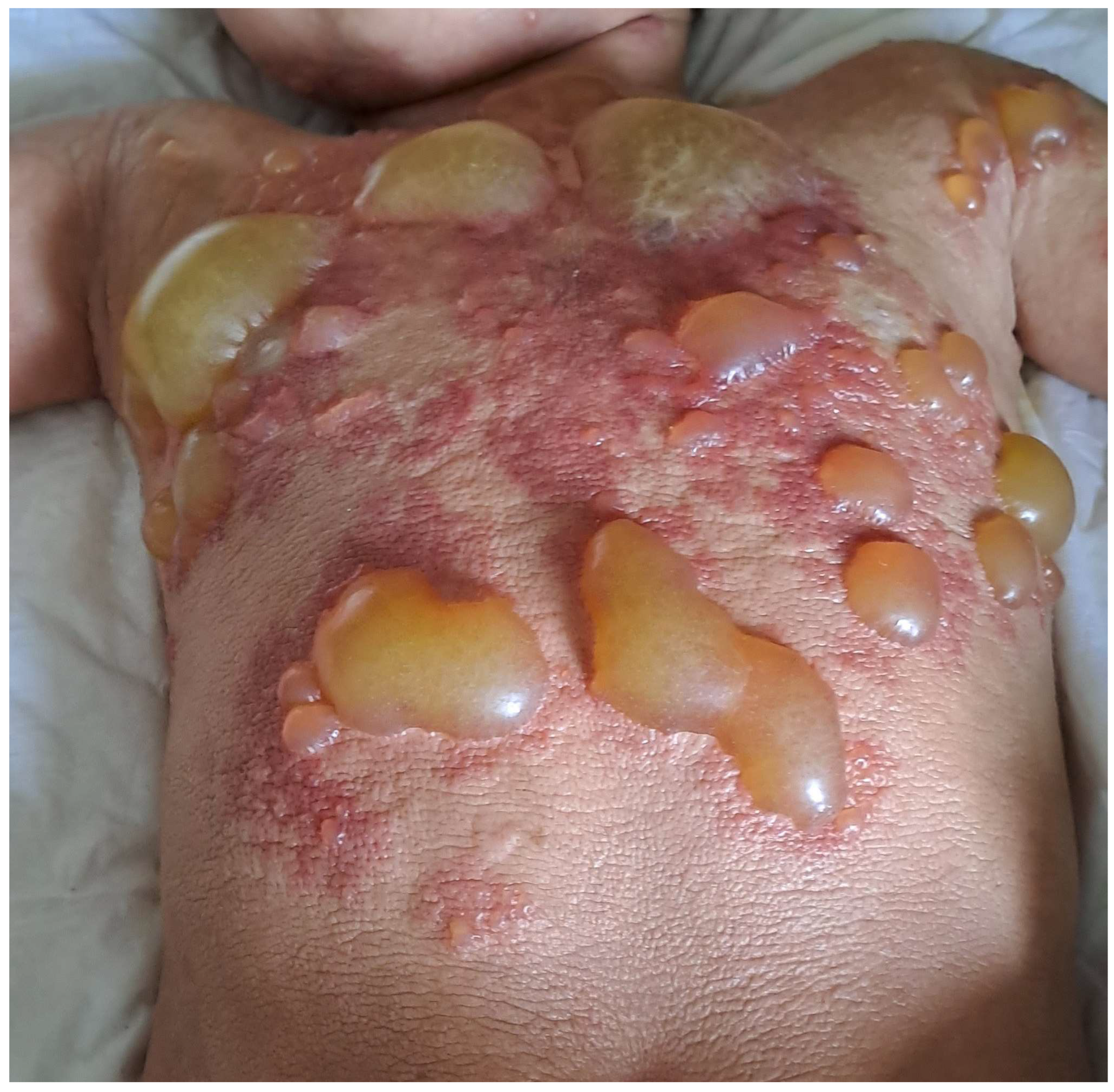
DCM manifests as generalized erythema, usually with pachydermia (thickened skin), associated with pronounced dermographism or a positive Darier’s sign, which are evident after minimal mechanical irritation of the skin [6]. Extensive blistering is a typical feature of DCM in the infantile period [6,19,40,58,59] (Figure 1). It is worth pointing out here that the diagnosis of DCM should be established only in children with generalized thickened and darker than normal skin, but not in those with extensive, confluent MPCM or bullous lesions [6]. The current classification of CM does not distinguish bullous mastocytosis as a separate form of the disease because blistering may also occur in MPCM and mastocytoma [6]. In infants, DCM may present with large hemorrhagic blisters and with small vesicular lesions; however, both variants may coexist [8,19,59]. The hemorrhagic nature of bullous lesions, as well as prolonged bleeding from skin wounds in some children, may be related to the local release of heparin from dermal MCs [6]. Papules can be present in pachydermatous skin areas. Occasionally, DCM may present as generalized erythema with pseudoxanthomatous or tumor-like lesions [6,11,59,60]. Clinical manifestations of DCM tend to evolve with age [6,8]. Extensive blistering is predominantly observed in infancy and usually ceases within 2 or 3 years of age (Figure 1), while diffuse pachyderma with slight brown or yellow discoloration and a leather-like skin appearance develop with time. Children with DCM usually present with numerous symptoms associated with the release of MC mediators, such as itching, flushing, hypotension, headache, abdominal cramping, or diarrhea [13,14,28,40,58,61]. It has also been found that baseline serum tryptase levels are significantly higher in patients with DCM than in patients with other forms of CM [10,13,14]. Moreover, children with DCM are at a higher risk of developing severe MC mediator-related symptoms, such as sudden hypotension or anaphylactic shock [10,13,59]. In a study analyzing 10 pediatric patients with DCM, three episodes of anaphylaxis were observed in patients with basal tryptase levels of 22, 103, and 2.7 ng/mL, respectively. These severe reactions were provoked by clindamycin, ketamine, magnetic resonance imaging-contrast medium, and an unidentified trigger [59]. In other studies, anaphylaxis in children with DCM was provoked by an unknown factor, either after a meal or during a peripheral intravenous placement [52,62,63]. The presence of severe MC mediator-related symptoms in children with DCM may be primarily attributed to the extensive and substantial infiltration of MCs throughout the skin. Furthermore, numerous studies have shown that rubbing and scratching of the skin, sudden temperature changes (such as hot baths or exposure to heat), teething, viral infections, or vaccinations may induce blistering or provoke other MC mediator-related symptoms [13,28,40,58,59]. A brief review of case reports on DCM, published since 1962, is presented in Table 2 [28,35,36,38,39,40,41,52,53,54,58,60,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81].
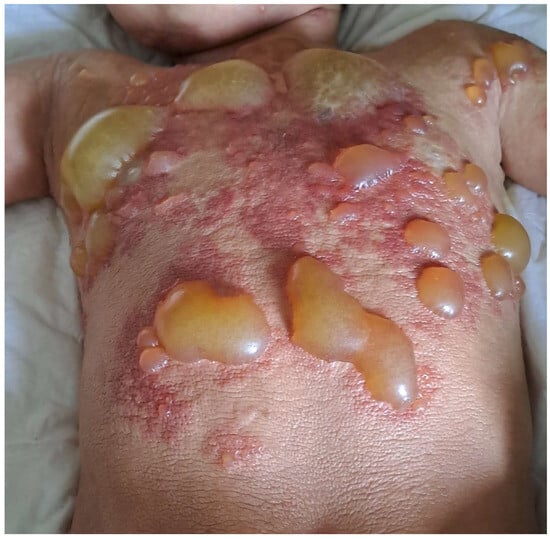
Figure 1.
DCM with extensive blistering in an infant.

Table 2.
A brief literature review of case reports on DCM.
As mentioned above, the familial occurrence of DCM has also been reported [52,53,54]. Interestingly, five patients with DCM in three generations within a single family have been described [53]. In all these cases, the symptoms started during infancy and initially presented as diffuse thickening of the skin, blistering, pruritus, and dermographism. DNA sequencing revealed a germline mutation in the transmembrane domain of the KIT gene (the A533D mutation) in all five family members [53]. Another report of familial DCM concerned a 35-year-old man who was suffering from generalized bullous skin lesions until the age of 2 and his 8-year-old son, who experienced nearly identical symptoms [54]. In both cases, genetic examination revealed a germline mutation in the p.S451C domain of the KIT gene [54]. Recently, another familial DCM, associated with germline KIT A533D (an autosomal dominant gain-of-function germline KIT variant: c.1598C > A, p.Ala533Asp), has been reported in a six-month-old boy with bullous lesions and fever and in his mother, who had a history of blistering in childhood and carried the same mutation [52].
4. Diagnostics and Differential Diagnosis
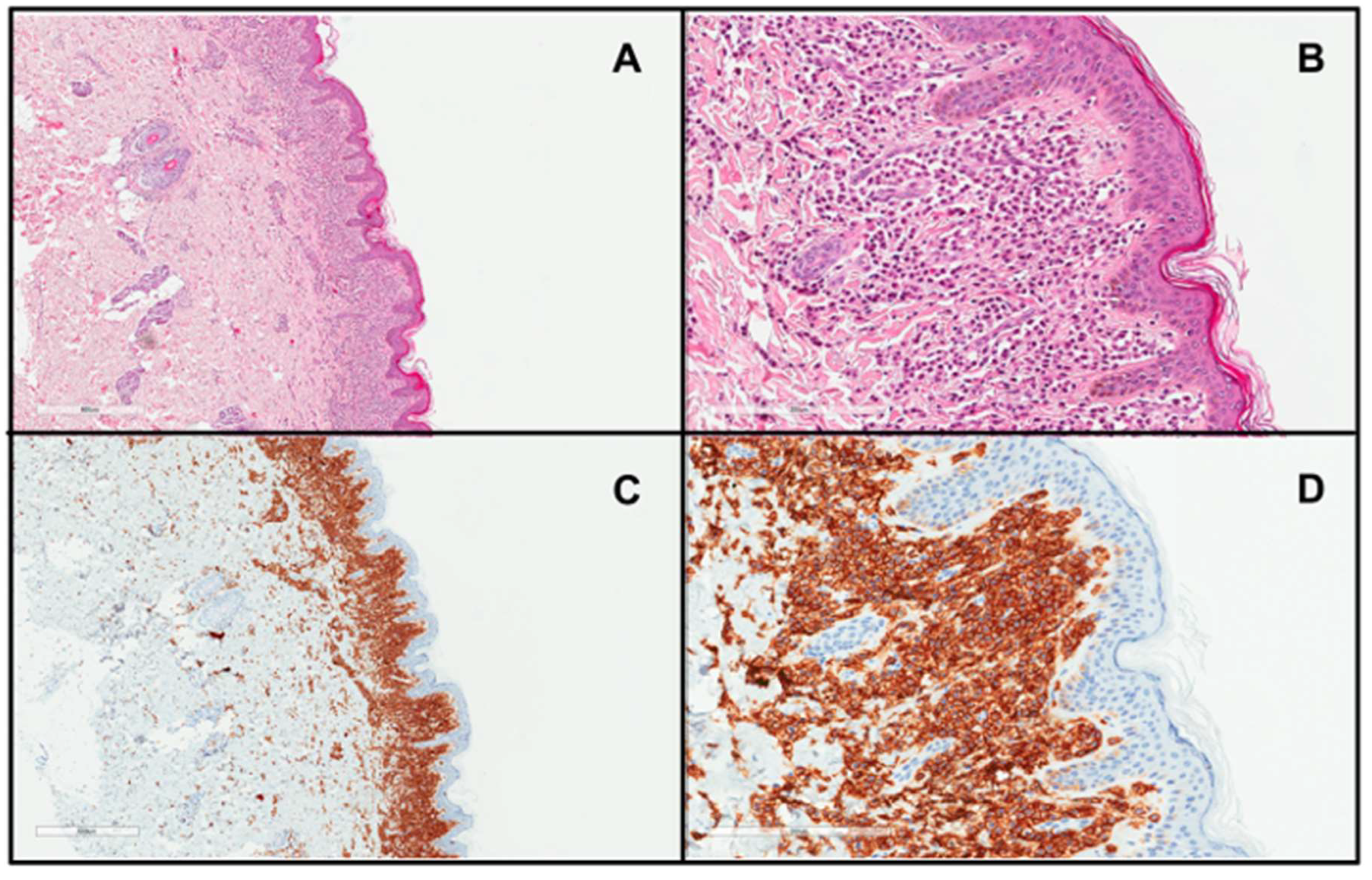
The diagnosis of DCM is mainly based on the clinical presentation, which includes thickening of the entire skin, erythroderma with blistering, prominent dermographism, or a positive Darier’s sign usually associated with the presence of MC mediator-related symptoms [6,8,61]. In children with DCM, the Darier’s sign should be elicited with caution, particularly in infants, because hard stroking of the skin may lead to flushing or hypotension due to massive MC degranulation [6,28,82]. In unclear cases, lesional skin biopsy and immunohistochemistry should be conducted with antibodies against tryptase and/or CD117, which is the gold-standard [6,8,83] (Figure 2). MC infiltration in the dermis (Figure 2) may be accompanied by subepidermal edema, causing vesiculobullous lesions [28,58]. Moreover, molecular analysis of KIT mutations in lesional skin may be used to confirm the diagnosis of CM [7]. The determination of baseline serum tryptase levels is also considered a valuable tool for diagnosing and monitoring DCM patients [8,61,83]. However, it is important to note that elevated tryptase levels can strongly suggest a diagnosis of mastocytosis but do not conclusively confirm it. There are many other conditions presenting with elevated basal serum tryptase levels, such as hereditary alpha-tryptasemia, allergies, chronic eosinophilic leukemia, and some nephropathies [20,82]. It is worth pointing out here that children with DCM commonly have elevated serum tryptase levels even in the absence of an underlying SM in many cases, which is due to the extensive MC burden in the entire skin [14,15,28,59]. In all DCM children, a physical examination including inspection of the skin, abdominal palpation, abdominal ultrasound, serum chemistry, and a complete blood count with differential are also recommended [8,83]. Further diagnostic evaluations, including BM studies, are indicated only in selected cases with highly suspected systemic involvement and are not universally recommended [8,19,59,61,83].
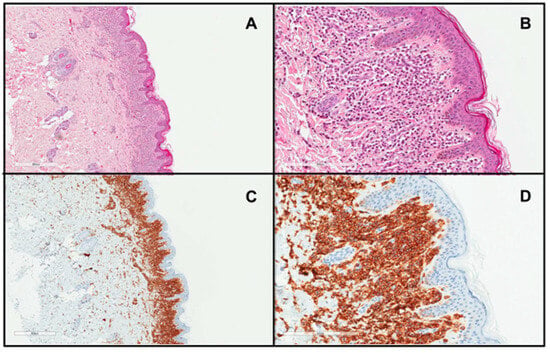
Figure 2.
Skin biopsy from a patient with DCM showing a marked infiltrate of round to polygonal MCs occupying the whole papillary dermis ((A), H&E stain ×4; (B), H&E stain ×20; (C), CD117 stain ×4; (D), CD117 stain ×20)).
DCM, particularly in the infantile period, often represents a diagnostic challenge due to a wide spectrum of diseases that may resemble DCM, encompassing mainly staphylococcal scalded skin syndrome (SSSS), epidermolysis bullosa (EB), impetigo bullosa (IB), erythema multiforme (EM), atopic dermatitis, Langerhans cell histiocytosis, linear IgA bullous dermatosis, and incontinentia pigmenti [28,59,69,76,81,84,85,86,87,88]. It is widely believed that misdiagnosis of DCM can be attributed to at least two factors: the absence of maculopapular or plaque lesions, which are most typical for CM, and the rare occurrence of DCM [59]. Table 3 provides a brief summary of the clinical characteristics of diseases that should be considered in the differential diagnosis of DCM in children [28,59,69,76,81,84,85,86,87,88].

Table 3.
Skin diseases mimicking DCM.
5. Treatment
Treatment of DCM predominantly focuses on trigger avoidance and the management of MC mediator-related symptoms [8,40,58,83]. Parents and caregivers should be informed that friction, rubbing, heat exposure, sudden temperature changes, teething, fever, and vaccines may provoke exacerbations of skin lesions, blistering, or anaphylaxis [8,30,58,82,89,90]. Avoidance of skin irritation is one of the most important rules in the skin care of children with DCM.
5.1. Topical Therapy
In children with blistering and denuded skin areas, topical antibiotics or antiseptics are indicated to prevent skin infections [8,30]. Mupirocine ointment or fucidic acid creams are commonly used [30]. To reduce blistering and pruritus, topical mild- or medium-potency corticosteroids in short-term therapy may be considered [41,81,83]. Therapy with mometasone furoate 0.1% cream applied once daily to the lesions of DCM infants showed essential improvement in erythema and a decrease in bullae formation [40]. However, topical corticosteroids should be used cautiously, in short-term therapy, and for limited skin areas to avoid side effects, particularly skin atrophy and adrenal suppression therapy [30,41,81]. Supportive topical treatment, which includes creams containing disodium cromoglycate (0.2% to 4%), should only be used for intact skin [41,81,91].
5.2. Oral Therapy
First-line therapy includes antihistamines, which block H1 receptors, and MC stabilizers [8,30,83,92]. Flushing, blistering, and itching may be reduced mainly by using second-generation antihistamines (cetirizine, loratadine, desloratadine, fexofenadine, levocetirizine, rupatadine, and bilastine) and MC stabilizers (e.g., ketotifen and sodium cromoglycate) [8,77]. First-generation antihistamines (chlorpheniramine, diphenhydramine, hydroxyzine, and azelastine) are also used in children, mostly in those with very severe pruritus, as they reduce itching more effectively than second-generation antihistamines [30,92]. In unresponsive cases, second-generation antihistamines’ dose can be increased up to four times the standard dose for age [8,30,92]. To reduce gastrointestinal symptoms, H2 antagonists, proton pump inhibitors, and oral MC stabilizers are recommended [8,30,58,83,93]. If MC mediator-related symptoms persist, an add-on of antileukotrienes may be considered [92]. In severe blistering unresponsive to standard antimediator therapy (H1-antihistamines, cromolyn sodium, H2-antihistamines, proton pump inhibitors, and leukotriene antagonists), oral steroids in short-term therapy may be applied [8,30]. Oral steroids are very effective, but in long-term use, are associated with serious side effects like growth retardation, skin changes, muscle weakness, and obesity, among others [83,92].
As patients with DCM are at a higher risk of anaphylaxis, adequate training on adrenaline autoinjector (epinephrine) administration is crucial [30]. A typical dosage of adrenaline in children is 0.01 mg/kg [30,94]. In Europe, prefilled autoinjectors are available, offering a dose of 0.15 mg for children ranging from 7.5 to 30 kg, with variations based on the autoinjector’s license [30,94]. Whenever needed (e.g., anaphylaxis), adrenaline should be administered via intramuscular injection into the mid-outer thigh and, if necessary, can be repeated every 5 to 15 min (with the maximum dosage being 0.5 mg) [94]. In the event of cardiovascular or respiratory reactions, accompanying measures include the administration of high-flow oxygen, patient positioning (e.g., Trendelenburg position with elevation of the lower limbs for improving/preventing hypotension), and the use of inhaled adrenaline or beta-agonists such as salbutamol [94]. Tyrosine kinase inhibitors may be considered only in life-threatening cases; generally, they are reserved for SM [8,58,61,83]. A successful treatment with imatinib has been reported in two infants with DCM carrying an exon 8 KIT mutation (Del419) who underwent antimediator treatment, which failed to prevent severe relapses [95]. In both cases, imatinib treatment was started with an initial dosage of 200 mg/day and resulted in rapid improvement and eventual remission. Therefore, imatinib was gradually tapered and discontinued, with no relapses observed during the 6-month follow-up period [95]. Moreover, successful therapy with imatinib was achieved in three members of a family (a father and two daughters) who were diagnosed with WDSM associated with the K509I germline KIT mutation. Two of these patients (the father and one daughter) fulfilled WHO diagnostic criteria for MCL, while the remaining daughter had ISM. In addition, all three patients presented with DCM and showed concomitant GIST; noteworthy, imatinib rapidly induced a complete remission of mastocytosis in all three cases [57]. Similarly, an additional few WDSM cases presenting with DCM showing a complete or near-complete response to imatinib have been reported in the last decade [45,48]. Another therapeutic option for mastocytosis patients, in whom the disease is associated with severe MC mediator-related symptoms and anaphylaxis, may be omalizumab, a monoclonal anti-IgE antibody [96]. Up until now, treatment with omalizumab was reported only in one patient with DCM in whom the therapy was proven to be effective and safe [96]. This patient received monthly subcutaneous injections of 150 mg of omalizumab for three months and remained asymptomatic within the first month following treatment initiation [96].
5.3. Phototherapy
In selected cases of CM with severe, recurrent, or persistent MC-mediator release symptoms, refractory to standard antimediator therapy, UVA1 or narrow-band (NB)-UVB phototherapy may be considered, as these therapies have fewer side effects than photochemotherapy (PUVA) and UVA1 has the ability to reach deeper layers of the skin [58,72,97]. PUVA is generally not recommended in children with CM due to the risk of potent side effects of this therapy (mainly the risk of skin cancers, melanoma, cataracts, and hepatotoxicity of psoralen) and the tendency to spontaneously regression of skin lesions around puberty [8].
6. Discussion
The rare occurrence of DCM results in a lack of large cohorts of patients, which significantly limits the experience in the diagnosis and management of this rare and severe form of CM, even among the most reference or excellence centers for mastocytosis. In a recent large cohort French study on pediatric mastocytosis, DCM was reported in 15 (5.5%) of 272 children; noteworthy, mastocytosis was congenital in more than half of the cases, but there were no cases of familial mastocytosis [14]. The majority (87%) of these children had MC mediator-related symptoms, and the mean baseline serum tryptase was 23.99 ng/mL (range: 2–60 ng/mL). In a Polish study of 102 patients with childhood-onset mastocytosis, 7 children (6.9%) had DCM, 6 of them presented with blistering, and all had MC mediator-related symptoms, as well as basal serum tryptase over 20 ng/mL [12]. Generally, these results are in line with the data reported by other centers [13,15,19].
Much more diverse are the data on the risk of anaphylaxis among children with DCM, which has been reported with a frequency ranging from 0% to 50% [10,12,19,29,59,98] commonly without a known triggering factor [29,52,59,62]. Importantly, there was no anaphylaxis provoked by vaccination in any of the 13 children with DCM diagnosed by the National Institute of Health in the US [89]; in contrast, one child with DCM from Italy had bullous lesions and bronchospasm after a hexavalent vaccine (diphtheria, tetanus, pertussis, inactivated poliovirus, Haemophilus influenza type B, hepatitis B) [90].
Another issue that deserves discussion is the clinical course of DCM in children. Although some studies have shown no evidence of a complete regression in children with DCM [99], others have revealed an overall rate of regression (complete and partial) of up to 94% [7]. Moreover, a spontaneous decrease in skin involvement has been recently reported in 5 of 12 patients with DCM [15]. Interestingly, this study also showed that a decrease in both skin involvement and serum tryptase levels was systematically observed in patients with sporadic DCM, whereas those with familial DCM displayed no decrease in cutaneous lesions and stable serum tryptase levels [15]. The detailed analysis of the tendency to spontaneous remission in 7 children with DCM who were followed-up for 10 years performed by the Polish group shows that extension, elevation of skin lesions, blistering, and serum tryptase level decreased significantly with time [100]. However, none of these patients experienced a complete or major regression of skin lesions; 6 of 7 patients had a partial regression and 1 exhibited no regression. A complete regression of MC mediator-related symptoms was reported only in one child. The tendency to spontaneous regression was lower in children with DCM than in those with MPCM [100]. Altogether, these findings suggest that spontaneous remission occurs in a significant number of children with DCM, but not in all.
Another important point for discussion concerns the assessment of the true frequency of an underlying SM in patients who present with skin lesions corresponding to DCM. In three series of 15, 10, and 8 children with DCM, respectively, none of them developed SM [14,19,59]. Results of a long-term follow-up of pediatric mastocytosis show that none of the 15 patients with childhood-onset DCM progressed to SM for at least 8 years of the disease duration [14]. However, it has also been shown that children with DCM-like skin lesions may suffer from both WDSM and aggressive SM (ASM) [14,56]. In a French population of children with mastocytosis, 3 of 610 patients had congenital ASM with the KIT D816V mutation, and all of them presented with skin lesions corresponding to DCM [14]. Also, a few case studies present children in whom the initial diagnosis was DCM, but further diagnostic procedures revealed SM [68,71,101,102,103,104]. In some children with DCM, a progression to SM over time was also observed [11,13,19,37]. Despite the intensive treatment and fatal outcome of DCM due to the severe course of the disease, infectious complications or comorbidities have been occasionally reported [35,39,41,64,69,75]. Taking all of the above into consideration, the long-term prognosis is good for the majority of children with DCM. Nevertheless, one should keep in mind that children with this form of CM are at risk of severe, life-threatening MC mediator-related symptoms, infections due to extensive blistering, and may develop SM. Therefore, a multidisciplinary approach is highly recommended.
7. Conclusions and Future Perspectives
The rare occurrence of DCM and fragmented and sometimes incompatible data on the incidence, symptomatology, and evolution of this disorder indicate the need for collecting data in various centers using a uniform classification, terminology, and grading system for assessing MC mediator-related symptoms and response/spontaneous regression criteria (complete, major, partial, or no regression) depending on the percentage of improvement [6,9]. It is crucial for the analysis of data obtained from distinct countries to appropriately interpret the tendency towards spontaneous regression and the assessment of treatment response. Currently, the standard method of documenting skin involvement is photography. In the future, 3D total body photography may be applied for assessing skin lesions in patients with CM in a more precise way [105]. Moreover, new diagnostic tools, such as reflectance confocal microscopy and two-photon fluorescence lifetime imaging, may become useful in children in whom non-invasive procedures are of major importance [106,107].
Regarding new options of treatment, the determination of the genetic status of the patient has proved to be an essential issue in the era of targeted therapies and personalized management strategies. An important aspect is to select those patients with the D816V KIT mutation who may respond to treatment with tyrosine kinase inhibitors (e.g., midostaurin and avapritinib), as well as identify those who have no KIT codon 816 mutations or exhibit other KIT mutations and may be sensitive to imatinib [57,95]. Nevertheless, tyrosine kinase inhibitors, which reduce MC burden, may be considered only in selected DCM patients with the most severe course of the disease who do not respond to intensive antimediator approaches [95]. In children with DCM, who usually display severe MC mediator-related symptoms, new drugs capable of reducing the pathogenic MC activity may turn out to be more effective and less toxic than MC depleters, such as tyrosine kinase inhibitors. MC activation antagonists, including omalizumab, for which only a few uncontrolled studies showing response in children with CM have been reported so far, or other monoclonal antibodies that engage inhibitory receptors (e.g., lirentelimab), might prove to be effective and become true therapeutic options in the near future [96,108,109]. Currently, early clinical trials of new drugs believed to reduce pathogenic MC activity are ongoing, which provides hope for patients with various MC disorders.
Author Contributions
Conceptualization, M.L.; writing—original draft preparation, A.R.; writing—review and editing, A.R., M.L., I.A.-T., H.Ł.-U., M.S., R.J.N. and C.M.-C.; funding acquisition, R.J.N.; supervision, M.L. and I.A.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Medical University of Gdańsk.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thanks to Karolina Lange for the linguistic proofreading of the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Valent, P.; Akin, C.; Sperr, W.R.; Horny, H.-P.; Arock, M.; Metcalfe, D.D.; Galli, S.J. New Insights into the Pathogenesis of Mastocytosis: Emerging Concepts in Diagnosis and Therapy. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 361–386. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A. Systemic Mastocytosis in Adults: 2023 Update on Diagnosis, Risk Stratification and Management. Am. J. Hematol. 2023, 98, 1097–1116. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Hartmann, K.; Alvarez-Twose, I.; Brockow, K.; Hermine, O.; Niedoszytko, M.; Schwaab, J.; Lyons, J.J.; Carter, M.C.; et al. Updated Diagnostic Criteria and Classification of Mast Cell Disorders: A Consensus Proposal. Hemasphere 2021, 5, e646. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 Updated WHO Classification and Novel Emerging Treatment Concepts. Blood 2017, 129, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Escribano, L.; Grattan, C.; Brockow, K.; Carter, M.C.; Alvarez-Twose, I.; Matito, A.; Broesby-Olsen, S.; Siebenhaar, F.; Lange, M.; et al. Cutaneous Manifestations in Patients with Mastocytosis: Consensus Report of the European Competence Network on Mastocytosis; The American Academy of Allergy, Asthma & Immunology; And the European Academy of Allergology and Clinical Immunology. J. Allergy Clin. Immunol. 2016, 137, 35–45. [Google Scholar] [CrossRef]
- Méni, C.; Bruneau, J.; Georgin-Lavialle, S.; Le Saché De Peufeilhoux, L.; Damaj, G.; Hadj-Rabia, S.; Fraitag, S.; Dubreuil, P.; Hermine, O.; Bodemer, C. Paediatric Mastocytosis: A Systematic Review of 1747 Cases. Br. J. Dermatol. 2015, 172, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Hartmann, K.; Carter, M.C.; Siebenhaar, F.; Alvarez-twose, I.; Torrado, I.; Brockow, K.; Renke, J.; Irga-jaworska, N.; Plata-nazar, K.; et al. Molecular Background, Clinical Features and Management of Pediatric Mastocytosis: Status 2021. Int. J. Mol. Sci. 2021, 22, 2586. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Escribano, L.; Födinger, M.; Hartmann, K.; Brockow, K.; Castells, M.; Sperr, W.R.; Kluin-Nelemans, H.C.; Hamdy, N.A.T.; et al. Standards and Standardization in Mastocytosis: Consensus Statements on Diagnostics, Treatment Recommendations and Response Criteria. Eur. J. Clin. Investig. 2007, 37, 435–453. [Google Scholar] [CrossRef]
- Lange, M.; Niedoszytko, M.; Renke, J.; Gleń, J.; Nedoszytko, B. Clinical Aspects of Paediatric Mastocytosis: A Review of 101 Cases. J. Eur. Acad. Dermatol. Venereol. 2011, 27, 97–102. [Google Scholar] [CrossRef]
- Hannaford, R.; Rogers, M. Presentation of Cutaneous Mastocytosis in 173 Children. Australas. J. Dermatol. 2001, 42, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Zawadzka, A.; Schrörs, S.; Słomka, J.; Ługowska-Umer, H.; Nedoszytko, B.; Nowicki, R. The Role of Serum Tryptase in the Diagnosis and Monitoring of Pediatric Mastocytosis: A Single-Center Experience. Adv. Dermatol. Alergol. 2017, 4, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Twose, I.; Vañõ-Galván, S.; Sánchez-Muñoz, L.; Morgado, J.M.; Matito, A.; Torrelo, A.; Jaén, P.; Schwartz, L.B.; Orfao, A.; Escribano, L. Increased Serum Baseline Tryptase Levels and Extensive Skin Involvement Are Predictors for the Severity of Mast Cell Activation Episodes in Children with Mastocytosis. Allergy 2012, 67, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Polivka, L.; Rossignol, J.; Neuraz, A.; Condé, D.; Agopian, J.; Méni, C.; Garcelon, N.; Dubreuil, P.; Maouche-Chrétien, L.; Hadj-Rabia, S.; et al. Criteria for the Regression of Pediatric Mastocytosis: A Long-Term Follow-Up. J. Allergy Clin. Immunol. Pract. 2021, 9, 1695–1704.e5. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, T.; Rabenhorst, A.; Schick, T.; Preussner, L.M.; Förster, A.; Valent, P.; Horny, H.-P.; Sotlar, K.; Hartmann, K. Large Maculopapular Cutaneous Lesions Are Associated with Favorable Outcome in Childhood-Onset Mastocytosis. J. Allergy Clin. Immunol. 2015, 136, 1581–1590.e3. [Google Scholar] [CrossRef]
- Méni, C.; Georgin-Lavialle, S.; Le Saché de Peufeilhoux, L.; Jais, J.P.; Hadj-Rabia, S.; Bruneau, J.; Fraitag, S.; Hanssens, K.; Dubreuil, P.; Hermine, O.; et al. Paediatric Mastocytosis: Long-Term Follow-up of 53 Patients with Whole Sequencing of KIT. A Prospective Study. Br. J. Dermatol. 2018, 179, 925–932. [Google Scholar] [CrossRef]
- Valent, P.; Hartmann, K.; Bonadonna, P.; Niedoszytko, M.; Triggiani, M.; Arock, M.; Brockow, K. Mast Cell Activation Syndromes: Collegium Internationale Allergologicum Update 2022. Int. Arch. Allergy Immunol. 2022, 183, 693–705. [Google Scholar] [CrossRef]
- Bodemer, C.; Hermine, O.; Palmérini, F.; Yang, Y.; Grandpeix-Guyodo, C.; Leventhal, P.S.; Hadj-Rabia, S.; Nasca, L.; Georgin-Lavialle, S.; Cohen-Akenine, A.; et al. Pediatric Mastocytosis Is a Clonal Disease Associated with D816V and Other Activating C-KIT Mutations. J. Investig. Dermatol. 2010, 130, 804–815. [Google Scholar] [CrossRef]
- Heide, R.; Zuidema, E.; Beishuizen, A.; Den Hollander, J.C.; Van Gysel, D.; Seyger, M.M.B.; Pasmans, S.G.M.A.; Kakourou, T.; Oranje, A.P. Clinical Aspects of Diffuse Cutaneous Mastocytosis in Children: Two Variants. Dermatology 2009, 219, 309–315. [Google Scholar] [CrossRef]
- Gülen, T. A Puzzling Mast Cell Trilogy: Anaphylaxis, MCAS, and Mastocytosis. Diagnostics 2023, 13, 3307. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast Cells as a Unique Hematopoietic Lineage and Cell System: From Paul Ehrlich’s Visions to Precision Medicine Concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Valent, P. KIT D816V and the Cytokine Storm in Mastocytosis: Production and Role of Interleukin-6. Haematologica 2020, 105, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Tobío, A.; Bandara, G.; Morris, D.A.; Kim, D.-K.; O’Connell, M.P.; Komarow, H.D.; Carter, M.C.; Smrz, D.; Metcalfe, D.D.; Olivera, A. Oncogenic D816V-KIT Signaling in Mast Cells Causes Persistent IL-6 Production. Haematologica 2020, 105, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Gleń, J.; Zabłotna, M.; Nedoszytko, B.; Sokołowska-Wojdyło, M.; Rębała, K.; Ługowska-Umer, H.; Niedoszytko, M.; Górska, A.; Sikorska, M.; et al. Interleukin-31 Polymorphisms and Serum IL-31 Level in Patients with Mastocytosis: Correlation with Clinical Presentation and Pruritus. Acta Derm. Venereol. 2017, 97, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ługowska-Umer, H.; Zabłotna, M.; Lange, M.; Niedoszytko, M.; Nowicki, R.; Nedoszytko, B. -2518A/G Polymorphism of Monocyte Chemotactic Protein 1 (MCP-1/CCL2) Is Associated with Cutaneous Mastocytosis. Adv. Dermatol. Alergol. 2021, 38, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Hoermann, G.; Cerny-Reiterer, S.; Perné, A.; Klauser, M.; Hoetzenecker, K.; Klein, K.; Müllauer, L.; Gröger, M.; Nijman, S.M.B.; Klepetko, W.; et al. Identification of Oncostatin M as a STAT5-Dependent Mediator of Bone Marrow Remodeling in KIT D816V-Positive Systemic Mastocytosis. Am. J. Pathol. 2011, 178, 2344–2356. [Google Scholar] [CrossRef] [PubMed]
- Greiner, G.; Witzeneder, N.; Berger, A.; Schmetterer, K.; Eisenwort, G.; Schiefer, A.-I.; Roos, S.; Popow-Kraupp, T.; Müllauer, L.; Zuber, J.; et al. CCL2 Is a KIT D816V–Dependent Modulator of the Bone Marrow Microenvironment in Systemic Mastocytosis. Blood 2017, 129, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Kleewein, K.; Lang, R.; Diem, A.; Vogel, T.; Pohla-Gubo, G.; Bauer, J.W.; Hintner, H.; Laimer, M. Diffuse Cutaneous Mastocytosis Masquerading as Epidermolysis Bullosa. Pediatr. Dermatol. 2011, 28, 720–725. [Google Scholar] [CrossRef]
- Brockow, K.; Jofer, C.; Behrendt, H.; Ring, J. Anaphylaxis in Patients with Mastocytosis: A Study on History, Clinical Features and Risk Factors in 120 Patients. Allergy 2008, 63, 226–232. [Google Scholar] [CrossRef]
- Brockow, K.; Plata-Nazar, K.; Lange, M.; Nedoszytko, B.; Niedoszytko, M.; Valent, P. Mediator-Related Symptoms and Anaphylaxis in Children with Mastocytosis. Int. J. Mol. Sci. 2021, 22, 2684. [Google Scholar] [CrossRef]
- Rama, T.A.; Castells, M. Triggers of Anaphylaxis in Mastocytosis Patients: Evidence of the Current Drug-Avoidance Recommendation. Curr. Treat. Options Allergy 2023, 10, 442–457. [Google Scholar] [CrossRef]
- Gülen, T. Management of Mediator Symptoms, Allergy, and Anaphylaxis in Mastocytosis. Immunol. Allergy. Clin. N. Am. 2023, 43, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Couto, S.; Rama, T.; Martins, C.; Miguel Borrego, L. Mast Cells and Mast Cell Activation Syndrome: What’s New? Mastócitos e Síndrome de Ativação Mastocitária: O Que Há de Novo? Arq. Asma Alerg. Imunol. 2023, 7, 69–77. [Google Scholar] [CrossRef]
- González de Olano, D.; De La Hoz Caballer, B.; Núñez López, R.; Sánchez Muñoz, L.; Cuevas Agustín, M.; Diéguez, M.C.; Álvarez Twose, I.; Castells, M.C.; Escribano Mora, L. Prevalence of Allergy and Anaphylactic Symptoms in 210 Adult and Pediatric Patients with Mastocytosis in Spain: A Study of the Spanish Network on Mastocytosis (REMA). Clin. Exp. Allergy 2007, 37, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Shapiro, N.; Bhutada, A.; Rastogi, S. C-KIT-Positive Fatal Diffuse Cutaneous Mastocytosis with Systemic Manifestations in a Neonate. J. Pediatr. Hematol. Oncol. 2019, 41, e338–e340. [Google Scholar] [CrossRef] [PubMed]
- Otani, I.M.; Carroll, R.W.; Yager, P.; Kroshinsky, D.; Murphy, S.; Hornick, J.L.; Akin, C.; Castells, M.; Walter, J.E. Diffuse Cutaneous Mastocytosis with Novel Somatic KIT Mutation K509I and Association with Tuberous Sclerosis. Clin. Case Rep. 2018, 6, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.J.; Tharp, M.D. Comparison of Lesional Skin C-KIT Mutations with Clinical Phenotype in Patients with Mastocytosis. Clin. Exp. Dermatol. 2018, 43, 416–422. [Google Scholar] [CrossRef]
- Olteanu, E.-G.; Bataneant, M.; Puiu, M.; Chirita-Emandi, A. When Mast Cells Run Amok: A Comprehensive Review and Case Study on Severe Neonatal Diffuse Cutaneous Mastocytosis. Genes 2023, 14, 2021. [Google Scholar] [CrossRef]
- Shah, P.Y.; Sharma, V.; Worobec, A.S.; Metcalfe, D.D.; Zwick, D.C. Congenital Bullous Mastocytosis with Myeloproliferative Disorder and C-Kit Mutation. J. Am. Acad. Dermatol. 1998, 39, 119–121. [Google Scholar] [CrossRef]
- Jenkinson, H.A.; Lundgren, A.D.; Carter, M.C.; Diaz, L.Z.; Levy, M.L. Management of a Neonate with Diffuse Cutaneous Mastocytosis: Case Report and Literature Review. Pediatr. Dermatol. 2019, 36, 486–489. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liu, X.; Kang, L.; Liu, X. Genotypic and Phenotypic Characteristics of Chinese Neonates with Cutaneous Mastocytosis: A Case Report and Literature Review. J. Int. Med. Res. 2020, 48, 0300060520952621. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.F.; Parsley, W.M.; Cotter, P.G. Familial Urticaria Pigmentosa. Arch. Dermatol. 1986, 122, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Wöhrl, S.; Moritz, K.B.; Bracher, A.; Fischer, G.; Stingl, G.; Loewe, R. A C-Kit Mutation in Exon 18 in Familial Mastocytosis. J. Investig. Dermatol. 2013, 133, 839–841. [Google Scholar] [CrossRef]
- Zanotti, R.; Simioni, L.; Garcia-Montero, A.C.; Perbellini, O.; Bonadonna, P.; Caruso, B.; Jara-Acevedo, M.; Bonifacio, M.; De Matteis, G. Somatic D816V KIT Mutation in a Case of Adult-Onset Familial Mastocytosis. J. Allergy Clin. Immunol. 2013, 131, 605–607. [Google Scholar] [CrossRef]
- de Melo Campos, P.; Machado-Neto, J.A.; Scopim-Ribeiro, R.; Visconte, V.; Tabarroki, A.; Duarte, A.S.S.; Barra, F.F.C.; Vassalo, J.; Rogers, H.J.; Lorand-Metze, I.; et al. Familial Systemic Mastocytosis with Germline KIT K509I Mutation Is Sensitive to Treatment with Imatinib, Dasatinib and PKC412. Leuk. Res. 2014, 38, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Smith, M.L.; Schultheis, B.; Fitzgibbon, J.; Lister, T.A.; Melo, J.V.; Cross, N.C.P.; Cavenagh, J.D. A Novel K509I Mutation of KIT Identified in Familial Mastocytosis—In Vitro and in Vivo Responsiveness to Imatinib Therapy. Leuk. Res. 2006, 30, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Arock, M.; Sotlar, K.; Akin, C.; Broesby-Olsen, S.; Hoermann, G.; Escribano, L.; Kristensen, T.K.; Kluin-Nelemans, H.C.; Hermine, O.; Dubreuil, P.; et al. KIT Mutation Analysis in Mast Cell Neoplasms: Recommendations of the European Competence Network on Mastocytosis. Leukemia 2015, 29, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.C.; Bai, Y.; Kirshenbaum, A.S.; Fischer, E.R.; Simakova, O.; Bandara, G.; Scott, L.M.; Wisch, L.B.; Cantave, D.; Carter, M.C.; et al. Mastocytosis Associated with a Rare Germline KIT K509I Mutation Displays a Well-Differentiated Mast Cell Phenotype. J. Allergy Clin. Immunol. 2014, 134, 178. [Google Scholar] [CrossRef]
- Ke, H.; Kazi, J.U.; Zhao, H.; Sun, J. Germline Mutations of KIT in Gastrointestinal Stromal Tumor (GIST) and Mastocytosis. Cell Biosci. 2016, 6, 55. [Google Scholar] [CrossRef]
- Hartmann, K.; Wardelmann, E.; Ma, Y.; Merkelbach–Bruse, S.; Preussner, L.M.; Woolery, C.; Baldus, S.E.; Heinicke, T.; Thiele, J.; Buettner, R.; et al. Novel Germline Mutation of KIT Associated With Familial Gastrointestinal Stromal Tumors and Mastocytosis. Gastroenterology 2005, 129, 1042–1046. [Google Scholar] [CrossRef]
- Trevisan, G.; Pauluzzi, P.; Gatti, A.; Semeraro, A. Familial Mastocytosis Associated with Neurosensory Deafness. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Wangberg, H.; Willis, M.J.H.; Lindsey, D.; Schmidgal, E.C.; White, A.A. A Familial Case of Diffuse Cutaneous Mastocytosis. J. Allergy Clin. Immunol. Pract. 2023, 11, 3802–3803. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Boxer, M.; Drummond, A.; Ogston, P.; Hodgins, M.; Burden, A.D. A Germline Mutation in KIT in Familial Diffuse Cutaneous Mastocytosis. J. Med. Genet. 2004, 41, e88. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Lin, Z.M.; Zhang, J.; Yin, J.H.; Yang, Y. A New Germline Mutation in KIT Associated with Diffuse Cutaneous Mastocytosis in a Chinese Family. Clin. Exp. Dermatol. 2014, 39, 146–149. [Google Scholar] [CrossRef]
- Peters, F.; Fiebig, B.; Lundberg, P.; Jaspers, N.I.; Holzapfel, B.; Ghadimi, M.P.H.; Drebber, U.; Tuchscherer, A.; Ullrich, R.; Hartmann, K.; et al. Detection of the Germline KIT S476I Mutation in a Kindred with Familial Mastocytosis Associated with Gastrointestinal Stromal Tumors. J. Allergy Clin. Immunol. Pract. 2021, 9, 2123–2125.e1. [Google Scholar] [CrossRef]
- Alvarez-Twose, I.; Jara-Acevedo, M.; Morgado, J.M.; García-Montero, A.; Sánchez-Muñoz, L.; Teodósio, C.; Matito, A.; Mayado, A.; Caldas, C.; Mollejo, M.; et al. Clinical, Immunophenotypic, and Molecular Characteristics of Well-Differentiated Systemic Mastocytosis. J. Allergy Clin. Immunol. 2016, 137, 168–178.e1. [Google Scholar] [CrossRef]
- Alvarez-Twose, I.; Matito, A.; Morgado, J.M.; Sánchez-Muñoz, L.; Jara-Acevedo, M.; García-Montero, A.; Mayado, A.; Caldas, C.; Teodósio, C.; Muñoz-González, J.I.; et al. Imatinib in Systemic Mastocytosis: A Phase IV Clinical Trial in Patients Lacking Exon 17 KIT Mutations and Review of the Literature. Oncotarget 2017, 8, 68950–68963. [Google Scholar] [CrossRef]
- Hosking, A.M.; Makdisi, J.; Ortenzio, F.; de Feraudy, S.; Smith, J.; Linden, K. Diffuse Cutaneous Mastocytosis: Case Report and Literature Review. Pediatr. Dermatol. 2018, 35, e348–e352. [Google Scholar] [CrossRef]
- Lange, M.; Niedoszytko, M.; Nedoszytko, B.; Łata, J.; Trzeciak, M.; Biernat, W. Diffuse Cutaneous Mastocytosis: Analysis of 10 Cases and a Brief Review of the Literature. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1565–1571. [Google Scholar] [CrossRef]
- Willemze, R.; Ruiter, D.J.; Scheffer, E.; van Vloten, W.A. Diffuse Cutaneous Mastocytosis with Multiple Cutaneous Mastocytomas. Br. J. Dermatol. 1980, 102, 601–607. [Google Scholar] [CrossRef]
- Renke, J.; Irga-Jaworska, N.; Lange, M. Pediatric and Hereditary Mastocytosis. Immunol. Allergy Clin. N. Am. 2023, 43, 665–679. [Google Scholar] [CrossRef]
- Park, M.-N.; Kim, G.-A.; Chey, M.J.; Shim, G.H. A Case of Diffuse Cutaneous Mastocytosis in a Newborn. Korean J. Perinatol. 2014, 25, 105. [Google Scholar] [CrossRef]
- Orkin, M.; Good, R.A.; Clawson, C.C.; Fisher, I.; Windhorst, D.B. Bullous Mastocytosis. Arch. Derm. 1970, 101, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Kukita, A. A Fatal Case of Purely Cutaneous Form of Diffuse Mastocytosis. Proc. XII Int. Cong Dermatol. 1962, 2, 1558–1561. [Google Scholar]
- Allison, J. Skin Mastocytosis Presenting as a Neonatal Bullous Eruption. Australas. J. Dermatol. 1967, 9, 83–85. [Google Scholar] [CrossRef]
- Klaber, M.; Pegum, J.S. Diffuse Cutaneous Mastocytosis in Mother and Daughter. Proc. R. Soc. Med. 1976, 69, 16. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.V.; Cook, L.J.; Lake, H.J.; Shuster, S. Diffuse Cutaneous Mastocytosis: A Report of Neonatal Onset. Acta Derm. Venereol. 1979, 59, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Olgun, N.; Oren, H.; Oren, B.; Irken, G.; Polat, M.; Cevik, N. Diffuse Erythrodermie Cutaneous M Astocytosis with Bone Marrow Infiltration. Dermatology 1993, 187, 127–129. [Google Scholar] [CrossRef]
- Murphy, M.; Walsh, D.; Drumm, B.; Watson, R. Bullous Mastocytosis: A Fatal Outcome. Pediatr. Dermatol. 1999, 16, 452–455. [Google Scholar] [CrossRef]
- Enomoto, U.; Kusakabe, H.; Matsumura, T.; Kuno, T.; Tamai, H.; Kiyokane, K. Diffuse Cutaneous Mastocytosis Responding to Cyproheptadine. Clin. Exp. Dermatol. 1999, 24, 16–18. [Google Scholar] [CrossRef]
- Waxtein, L.M.; Elisa Vega-Memije, M.; Cortés-Franco, R.; Dominguez-Soto, L. Diffuse Cutaneous Mastocytosis with Bone Marrow Infiltration in a Child: A Case Report. Pediatr. Dermatol. 2000, 17, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Kinsler, V.A.; Hawk, J.L.M.; Atherton, D.J. Diffuse Cutaneous Mastocytosis Treated with Psoralen Photochemotherapy: Case Report and Review of the Literature. Br. J. Dermatol. 2005, 152, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; von Komorowski, G.; Scheurlen, W.; Dorn-Beineke, A.; Back, W.; Bayerl, C. Neonatal Mastocytosis with Pachydermic Bullous Skin without C-Kit 816 Mutation. Dermatology 2006, 212, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.K.; Bhatti, A.; Barnes, C. Diffuse Cutaneous Mastocytosis in Fraternal Twins. Int. J. Dermatol. 2009, 48, 170–172. [Google Scholar] [CrossRef]
- Ghiasi, M.; Ghanadan, A.; Jesri, S.B.; Sotudeh, S.; Ramyar, A. Diffuse Cutaneous Mastocytosis: Report of a Severe Case with Fatal Outcome. Dermatol. Online J. 2011, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Kokubo, T.; Akaishi, M.; Iida, K.; Korematsu, S. Neonatal Onset Diffuse Cutaneous Mastocytosis: A Case Report and Review of the Literature. Pediatr. Dermatol. 2011, 28, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Wawrzycki, B.; Pietrzak, A.; Chodorowska, G.; Kanitakis, J. Diffuse Cutaneous Bullous Mastocytosis in a Newborn. Dermatol. Ther. 2013, 26, 176–179. [Google Scholar] [CrossRef]
- Gupta, M.; Akin, C.; Sanders, G.M.; Chan, M.P.; Ross, C.W.; Castells, M.C. Blisters, Vaccines, and Mast Cells: A Difficult Case of Diffuse Cutaneous Mastocytosis. J. Allergy Clin. Immunol. Pract. 2019, 7, 1370–1372. [Google Scholar] [CrossRef]
- Cardoso, J.M.; Cabral, C.A.S.; Lellis, R.F.; Ravelli, F.N. Bullous Congenital Diffuse Cutaneous Mastocytosis. An. Bras. Dermatol. 2020, 95, 255–256. [Google Scholar] [CrossRef]
- Turnbull, L.; Calhoun, D.A.; Agarwal, V.; Drehner, D.; Chua, C. Congenital Mastocytosis: Case Report and Review of the Literature. Cureus 2020, 12, e10565. [Google Scholar] [CrossRef]
- Rayinda, T.; Mira Oktarina, D.A.; Danarti, R. Diffuse Cutaneous Mastocytosis Masquerading as Linear IgA Bullous Dermatosis of Childhood. Dermatol. Rep. 2021, 13, 9021. [Google Scholar] [CrossRef]
- Özdemir, Ö. Cutaneous Mastocytosis in Childhood: An Update from the Literature. J. Clin. Pract. Res. 2023, 45, 311–320. [Google Scholar] [CrossRef]
- Tiano, R.; Krase, I.Z.; Sacco, K. Updates in Diagnosis and Management of Paediatric Mastocytosis. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 158–163. [Google Scholar] [CrossRef]
- Golitz, L.E.; Weston, W.L.; Lane, A.T. Bullous Mastocytosis: Diffuse Cutaneous Mastocytosis with Extensive Blisters Mimicking Scalded Skin Syndrome or Erythema Multiforme. Pediatr. Dermatol. 1984, 1, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Oranje, A.P.; Soekanto, W.; Sukardi, A.; Vuzevski, V.D.; Willigen, A.V.D.; Afiani, H.M. Diffuse Cutaneous Mastocytosis Mimicking Staphylococcal Scalded-Skin Syndrome: Report of Three Cases. Pediatr. Dermatol. 1991, 8, 147–151. [Google Scholar] [CrossRef]
- Salas-Alanis, J.C.; Rosales-Mendoza, C.E.; Ocampo-Candiani, J. Bullous Mastocytosis Mimicking Congenital Epidermolysis Bullosa. Case Rep. Dermatol. 2014, 6, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J. Bullous Cutaneous Mastocytosis, a Rarely Reported Disease in Asian Children. Asian Pac. J. Allergy Immunol. 2014, 32, 354–357. [Google Scholar] [CrossRef]
- Jezierska, M.; Stefanowicz, J.; Romanowicz, G.; Kosiak, W.; Lange, M. Langerhans Cell Histiocytosis in Children—A Disease with Many Faces. Recent Advances in Pathogenesis, Diagnostic Examinations and Treatment. Adv. Dermatol. Alergol. 2018, 35, 6–17. [Google Scholar] [CrossRef]
- Abuhay, H.; Clark, A.S.; Carter, M.C. Occurrence of Unexpected Adverse Reactions to Vaccines in Children with Mastocytosis. J. Pediatr. Res. 2020, 7, 81–86. [Google Scholar] [CrossRef]
- Parente, R.; Pucino, V.; Magliacane, D.; Petraroli, A.; Loffredo, S.; Marone, G.; Triggiani, M. Evaluation of Vaccination Safety in Children with Mastocytosis. Pediatr. Allergy Immunol. 2017, 28, 93–95. [Google Scholar] [CrossRef]
- González-González, O.; Leal, E.; Martín-Martínez, M.; Bautista, L.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Guiding Clinical Prescription of Topical Extemporaneous Formulations of Sodium Cromoglycate Based on Pharmaceutical Performance. Pharmaceutics 2023, 15, 1609. [Google Scholar] [CrossRef] [PubMed]
- Siebenhaar, F.; Akin, C.; Bindslev-Jensen, C.; Maurer, M.; Broesby-Olsen, S. Treatment Strategies in Mastocytosis. Immunol. Allergy Clin. N. Am. 2014, 34, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Sandru, F.; Petca, R.C.; Costescu, M.; Dumitrașcu, M.C.; Popa, A.; Petca, A.; Miulescu, R.G. Cutaneous Mastocytosis in Childhood—Update from the Literature. J. Clin. Med. 2021, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Brockow, K.; Fernández Rivas, M.; Santos, A.F.; Zolkipli, Z.Q.; Bellou, A.; Beyer, K.; et al. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014, 69, 1026–1045. [Google Scholar] [CrossRef] [PubMed]
- Morren, M.-A.; Hoppé, A.; Renard, M.; Debiec Rychter, M.; Uyttebroeck, A.; Dubreuil, P.; Martin, L. Imatinib Mesylate in the Treatment of Diffuse Cutaneous Mastocytosis. J. Pediatr. 2013, 162, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.D.M.; Olynyc, T.; Chapdelaine, H.; Segal, L.; Miedzybrodzki, B.; Ben-Shoshan, M. Effective Management of Severe Cutaneous Mastocytosis in Young Children with Omalizumab (Xolair®). Clin. Exp. Dermatol. 2018, 43, 573–576. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Bettolini, L.; Tonon, F.; Rossi, M.; Venturini, M. The Realistic Positioning of UVA1 Phototherapy after 25 Years of Clinical Experience and the Availability of New Biologics and Small Molecules: A Retrospective Clinical Study. Front. Med. 2023, 10, 1295145. [Google Scholar] [CrossRef]
- Czarny, J.; Żuk, M.; Żawrocki, A.; Plata-Nazar, K.; Biernat, W.; Niedoszytko, M.; Ługowska-Umer, H.; Nedoszytko, B.; Wasąg, B.; Nowicki, R.; et al. New Approach to Paediatric Mastocytosis: Implications of KIT D816V Mutation Detection in Peripheral Blood. Acta Derm. Venereol. 2020, 100, adv00149. [Google Scholar] [CrossRef]
- Le, M.; Miedzybrodzki, B.; Olynych, T.; Chapdelaine, H.; Ben-Shoshan, M. Natural History and Treatment of Cutaneous and Systemic Mastocytosis. Postgrad. Med. 2017, 129, 896–901. [Google Scholar] [CrossRef]
- Czarny, J.; Renke, J.; Żawrocki, A.; Nowicki, R.J.; Lange, M. Natural Evolution in Pediatric Cutaneous Mastocytosis: 10-year Follow-up. Int. J. Dermatol. 2021, 60, 1253–1257. [Google Scholar] [CrossRef]
- Huang, A.; Fiadorchanka, N.; Brar, K.; Balderacchi, J.L.; Glick, S.A. In Utero Presentation of Aggressive Systemic Mastocytosis in a Neonate. Br. J. Dermatol. 2017, 177, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
- Angus, J.; Leach, I.H.; Grant, J.; Ravenscroft, J.C. Systemic Mastocytosis with Diffuse Cutaneous Involvement and Haematological Disease Presenting in Utero Treated Unsuccessfully with Vincristine. Clin. Exp. Dermatol. 2008, 33, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.; Lacson, P. Mast Cell Leukemia as Urticaria Pigmentosa: Report of a Case. Pediatrics 1957, 19, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Meneghini, C.L.; Angelini, G. Systemic Mastocytosis with Diffuse Crocodile-like Pachydermic Skin, Pedunculated Pseudofibromas and Comedones. Br. J. Dermatol. 1980, 103, 329–334. [Google Scholar] [CrossRef]
- Rayner, J.E.; Laino, A.M.; Nufer, K.L.; Adams, L.; Raphael, A.P.; Menzies, S.W.; Soyer, H.P. Clinical Perspective of 3D Total Body Photography for Early Detection and Screening of Melanoma. Front. Med. 2018, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Al-Muriesh, M.; Abdul-Fattah, B.; Gao, Y.; Tao, J.; Yang, J.; Huang, C. Reflectance Confocal Microscopy as a Diagnostic Tool for Mastocytoma in Children. J. Am. Acad. Dermatol. 2020, 83, 1781–1784. [Google Scholar] [CrossRef] [PubMed]
- Kröger, M.; Scheffel, J.; Nikolaev, V.V.; Shirshin, E.A.; Siebenhaar, F.; Schleusener, J.; Lademann, J.; Maurer, M.; Darvin, M.E. In Vivo Non-Invasive Staining-Free Visualization of Dermal Mast Cells in Healthy, Allergy and Mastocytosis Humans Using Two-Photon Fluorescence Lifetime Imaging. Sci. Rep. 2020, 10, 14930. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Altrichter, S.; Bonnekoh, H.; Hawro, T.; Hawro, M.; Michaelis, E.G.; Kantor, A.M.; Chang, A.T.; Youngblood, B.A.; Singh, B.; et al. Safety and Efficacy of Lirentelimab in Patients with Refractory Indolent Systemic Mastocytosis: A First-in-Human Clinical Trial. Br. J. Dermatol. 2023, 189, 511–519. [Google Scholar] [CrossRef]
- Metz, M.; Kolkhir, P.; Altrichter, S.; Siebenhaar, F.; Levi-Schaffer, F.; Youngblood, B.A.; Church, M.K.; Maurer, M. Mast Cell Silencing: A Novel Therapeutic Approach for Urticaria and Other Mast Cell-mediated Diseases. Allergy 2023, 79, 37–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).