Targeting the Endocannabinoid System Present in the Glioblastoma Tumour Microenvironment as a Potential Anti-Cancer Strategy
Abstract
1. Introduction
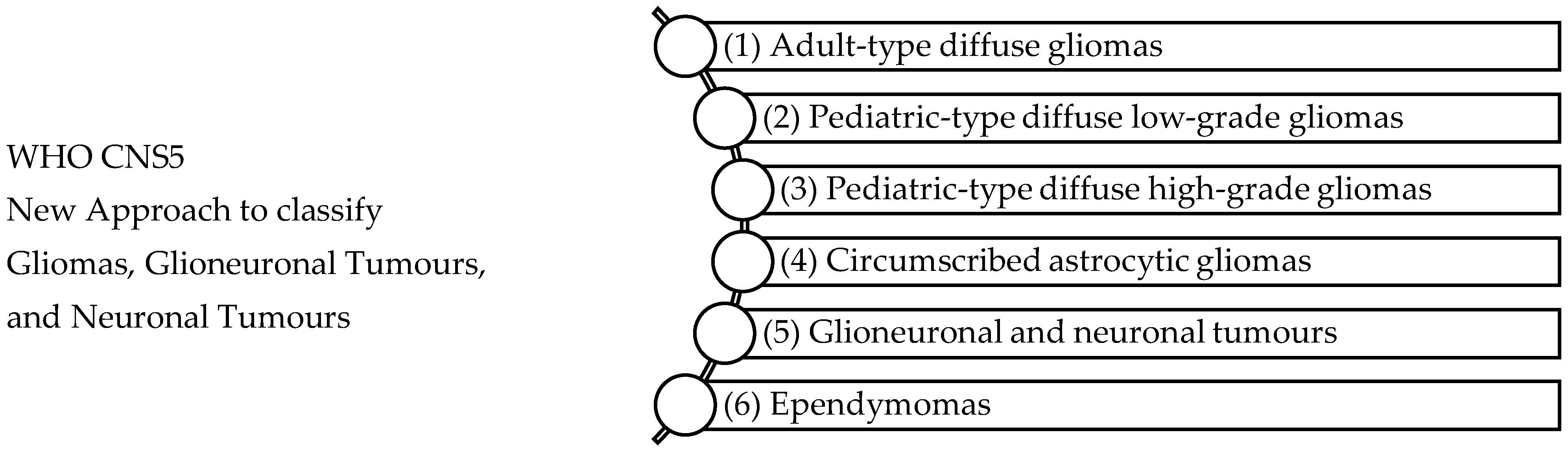
2. Classification of Glioblastoma Tumours
| Landscape of Glioblastoma Tumours | ||
|---|---|---|
| Etiology | While the etiology of most glioblastomas remains unknown, a small population is inherited as part of genetic tumour syndromes. Although environmental factors such as non-ionising radiation from mobile phones and occupational exposures have been investigated as potential causes, the results remain negative or inconclusive. | |
| Epidemiology | GBM occurs mostly in older adults, with peak incidence in patients between the ages 55–85 years old. The male to female incidence ratio of GBM is 1.4, which shows a higher occurrence amongst males compared to females. In the United States the M:F ratio is 1.60:1 and 1.28:1 in Switzerland. A substantial difference in incidence rates of GBM by race and ethnicity have been demonstrated by previous studies. Consistent finding shows that incidence of GBM is the highest amongst the Caucasian population compared to African or Asian populations. While approximately 80% of all malignant brain tumours are gliomas, an estimated 70% of the reported gliomas are GBM. The annual incidence of newly diagnosed cases has been reported to be between 3 and 5 per 100,000 inhabitants. | [49,63,64] |
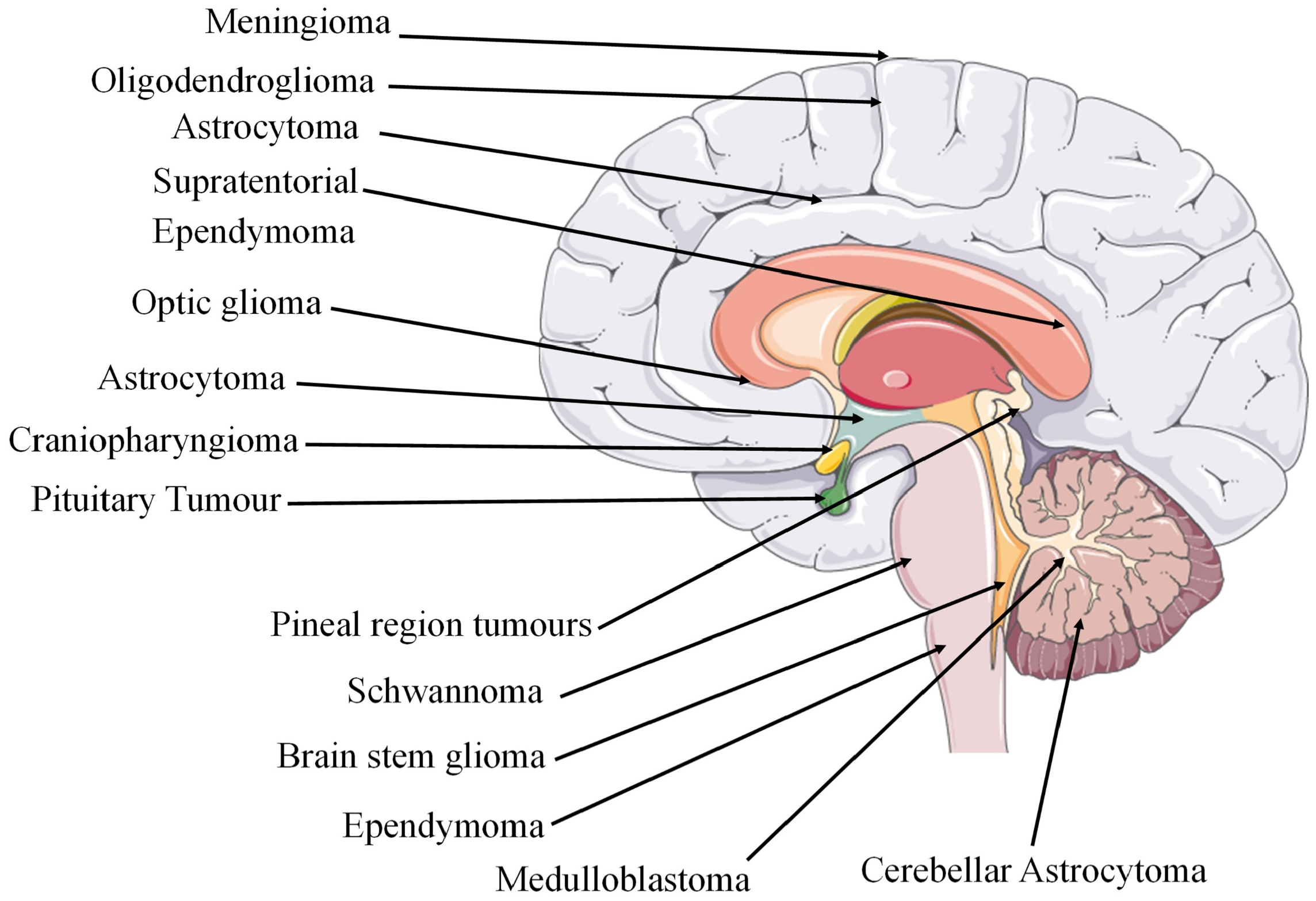
| sLocalisation | GBM is often centred in the subcortical white matter and deeper grey matter of the cerebral hemispheres which affects all cerebral lobes. When tumour infiltration occurs, it extends into the adjacent cortex and into the contralateral hemisphere through the corpus callosum. GBM has also been found to affect the brainstem, cerebellum, and spinal cord. The sites of glioma tumours are shown in Figure 2. | |
| Histopathology | Cytological atypia, high cellularity, and mitotic activity coupled with necrosis are the key features required for histological diagnosis of GBM. | [65] |
| Molecular classification | High cellular proliferation and angiogenesis resulting in rapid tumour growth and necrosis. High migration and invasive properties. Glioma stem-like cells partially account for high resistance to therapy and recurrence rates. | [57] |
| Symptoms | Pain, difficulty communicating, perceived cognition, seizures, weakness, fatigue, and aphasia. | [66] |
| Genomic profiling | The importance of O6-methylguanine-DNA methyltransferase (MGMT), GATA binding protein 6 (GATA6), and caspase-8 (CASP8) gene methylation was demonstrated in a study where high methylation frequency showed a correlation between heterogeneity of GBM epigenome and patient outcome. The promoter region’s methylation is the mechanism that affects gene expression in tumours, and newly identified epigenetically modified genes play a role in glioblastoma genesis and understanding patient outcome and the differences between long term and short-term survivors. Elucidation of the molecular differences of GBM in long-term survivors in a study investigated the genome and transcriptome-wide molecular profiling of GBM samples from 94 patients. The study consisted of 28 long-term survivors (>36 months survival), 20 short-term survivors (<12 months survival) and 46 intermediate survival patients. The gene expression profile of long-term survivors was linked to IDH1/2 mutation and associated with more MGMT-methylated tumours. | [67] |
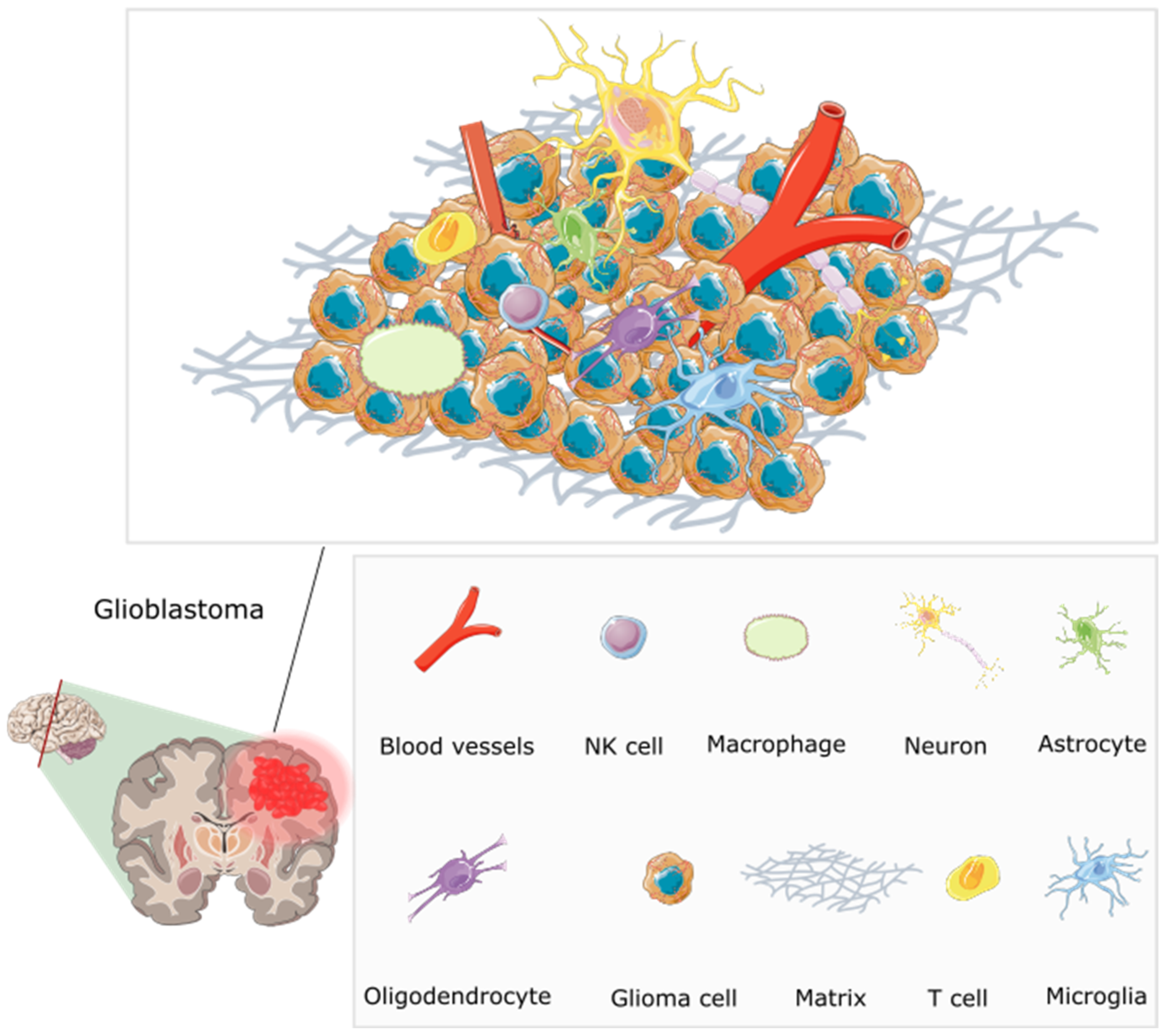
3. GBM Tumour Microenvironment, Macroenvironment and Microbiome
4. Hallmarks of GBM
5. Cannabinoids as a Promising Adjuvant in the Treatment of GBM
6. Current Standard Treatment and Associated Challenges
7. Novel Pharmaceutical Anti-Cancer Strategies to Overcome GBM Treatment Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma |
| CNS | Central nervous system |
| WHO | World health organisation |
| TME | Tumour microenvironment |
| ECS | Endocannabinoid system |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| BBB | Blood brain barrier |
| BTB | Brain tumour barrier |
| ECM | Extracellular matrix |
| MDSC | Myeloid-derived suppressor cells |
| TNFα | Tumour necrosis factor alpha |
| IL-6 | Interleukin 6 |
| IL-17 | Interleukin 17 |
| LPS | Lipopolysaccharide |
| p53 | Tumour protein 53 |
| CB1-R | Cannabinoid receptor type 1 |
| CB2-R | Cannabinoid receptor type 2 |
| FAAH | fatty acid amide hydrolase |
| MAGL | Monoacylglycerol lipase |
| IDH | iso-citrate dehydrogenase |
| ATRX | ATP-de-136 pendent helicase |
| H3K27M | Lys-27-Met mutations in histone 3 |
| cIMPACT NOW | Consortium to 164 Inform Molecular and Practical Approaches to CNS Tumour Taxonomy |
| VEGF | vascular endothelial growth factor |
| LPS | lipopolysaccharide |
| GIT | Gastrointestinal tract |
| MGMT | O6-methylguanine-DNA methyltransferase |
| GATA6 | GATA binding protein 6 |
| CASP8 | caspase-8 |
| PI3K | phosphoinositide 3-kinase |
| Akt | protein kinase |
| MAPK | mitogen-activated protein kinases |
| ERK | extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| p70S6K | 70-kDa ribosomal protein S6 kinase |
| 4EBP1 | Eukaryotic initiation factor 4E-binding protein 1 |
| ROS | reactive oxygen species |
| FAK | focal adhesion kinase |
| ER | endoplasmic reticulum |
| TMZ | temozolomide |
| GSCs | glioblastoma stem cells |
| TIF | therapeutic impact factor |
| HA | hyaluronic acid |
| NPs | nanoparticles |
| FDA | Food and Drug Administration |
| LNCs | lipid nanocapsules |
| BA NPs | betulinic acid nanoparticles |
| PTEe NPs | poly(thioether-ester) nanoparticles |
| CN | cannabis extract |
| Me-PTEe | miniemulsion polymerisation poly(thioether-ester) nanoparticles |
| Se-PTEe | thiol-ene bulk polymerisation polymerisation poly(thioether-ester) nanoparticles |
References
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical Implications of the 2021 Edition of the WHO Classification of Central Nervous System Tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Merve, A.; Millner, T.O.; Marino, S. Integrated Phenotype–Genotype Approach in Diagnosis and Classification of Common Central Nervous System Tumours. Histopathology 2019, 75, 299–311. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.K.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. CIMPACT-NOW Update 3: Recommended Diagnostic Criteria for “Diffuse Astrocytic Glioma, IDH-Wildtype, with Molecular Features of Glioblastoma, WHO Grade IV”. Acta Neuropathol. 2018, 136, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A.; Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; et al. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef] [PubMed]
- Perus, L.J.M.; Walsh, L.A. Microenvironmental Heterogeneity in Brain Malignancies. Front. Immunol. 2019, 10, 2294. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Pillai, P.P. Current Insights on Extracellular Vesicle-Mediated Glioblastoma Progression: Implications in Drug Resistance and Epithelial-Mesenchymal Transition. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2022, 1866, 130065. [Google Scholar] [CrossRef] [PubMed]
- Abels, E.R.; Maas, S.L.N.; Tai, E.; Ting, D.T.; Broekman, M.L.D.; Breakefield, X.O.; El Khoury, J. GlioM&M: Web-Based Tool for Studying Circulating and Infiltrating Monocytes and Macrophages in Glioma. Sci. Rep. 2020, 10, 9898. [Google Scholar] [CrossRef]
- Broekman, M.L.; Maas, S.L.N.; Abels, E.R.; Mempel, T.R.; Krichevsky, A.M.; Breakefield, X.O. Multidimensional Communication in the Microenvirons of Glioblastoma. Nat. Rev. Neurol. 2018, 14, 482–495. [Google Scholar] [CrossRef]
- Allen, B.M.; Hiam, K.J.; Burnett, C.E.; Venida, A.; DeBarge, R.; Tenvooren, I.; Marquez, D.M.; Cho, N.W.; Carmi, Y.; Spitzer, M.H. Systemic Dysfunction and Plasticity of the Immune Macroenvironment in Cancer Models. Nat. Med. 2020, 26, 1125. [Google Scholar] [CrossRef]
- Erdman, S.E.; Poutahidis, T. The Microbiome Modulates the Tumor Macroenvironment. OncoImmunology 2014, 3, e28271. [Google Scholar] [CrossRef]
- Sverdlov, E.D. Multidimensional Complexity of Cancer. Simple Solutions Are Needed. Biochemistry 2016, 81, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Del Bianco, P.; Pinton, L.; Magri, S.; Canè, S.; Masetto, E.; Basso, D.; Padovan, M.; Volpin, F.; d’Avella, D.; Lombardi, G.; et al. Myeloid Diagnostic and Prognostic Markers of Immune Suppression in the Blood of Glioma Patients. Front. Immunol. 2022, 12, 5672. [Google Scholar] [CrossRef] [PubMed]
- Ugel, S.; De Sanctis, F.; Mandruzzato, S.; Bronte, V. Tumor-Induced Myeloid Deviation: When Myeloid-Derived Suppressor Cells Meet Tumor-Associated Macrophages. J. Clin. Investig. 2015, 125, 3365–3376. [Google Scholar] [CrossRef] [PubMed]
- Psaila, B.; Lyden, D. The Metastatic Niche: Adapting the Foreign Soil. Nat. Rev. Cancer 2009, 9, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Lah, T.T.; Novak, M.; Breznik, B. Brain Malignancies: Glioblastoma and Brain Metastases. Semin. Cancer Biol. 2020, 60, 262–273. [Google Scholar] [CrossRef]
- Sceneay, J.; Smyth, M.J.; Möller, A. The Pre-Metastatic Niche: Finding Common Ground. Cancer Metastasis Rev. 2013, 32, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining Tumor Niches. Trends Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Ueda, N.; Tsuboi, K.; Uyama, T. Metabolism of Endocannabinoids and Related N-Acylethanolamines: Canonical and Alternative Pathways. FEBS J. 2013, 280, 1874–1894. [Google Scholar] [CrossRef]
- Khan, M.I.; Sobocinska, A.A.; Czarnecka, A.M.; Król, M.; Botta, B.; Szczylik, C. The Therapeutic Aspects of the Endocannabinoid System (ECS) for Cancer and Their Development: From Nature to Laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Contino, M.; McCormick, P.J. Editorial: The Canonical and Non-Canonical Endocannabinoid System as a Target in Cancer and Acute and Chronic Pain. Front. Pharmacol. 2020, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Biringer, R.G. Endocannabinoid Signaling Pathways: Beyond CB1R and CB2R. J. Cell Commun. Signal. 2021, 15, 335–360. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Makkar, R.; Sehgal, A.; Singh, S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S. Exploration of Multiverse Activities of Endocannabinoids in Biological Systems. Int. J. Mol. Sci. 2022, 23, 5734. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Archambault, A.S.; Lavoie, J.P.C.; Dumais, É.; Di Marzo, V.; Flamand, N. Biosynthesis and Metabolism of Endocannabinoids and Their Congeners from the Monoacylglycerol and N-Acyl-Ethanolamine Families. Biochem. Pharmacol. 2022, 205, 115261. [Google Scholar] [CrossRef] [PubMed]
- Abyadeh, M.; Gupta, V.; Paulo, J.A.; Gupta, V.; Chitranshi, N.; Godinez, A.; Saks, D.; Hasan, M.; Amirkhani, A.; McKay, M.; et al. A Proteomic View of Cellular and Molecular Effects of Cannabis. Biomolecules 2021, 11, 1411. [Google Scholar] [CrossRef]
- Costas-Insua, C.; Guzmán, M. Endocannabinoid Signaling in Glioma. Glia 2022, 71, 127–138. [Google Scholar] [CrossRef]
- Braile, M.; Marcella, S.; Marone, G.; Galdiero, M.R.; Varricchi, G.; Loffredo, S. The Interplay between the Immune and the Endocannabinoid Systems in Cancer. Cells 2021, 10, 1282. [Google Scholar] [CrossRef]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Torres-Suárez, A.I. Insights into the Effects of the Endocannabinoid System in Cancer: A Review. Br. J. Pharmacol. 2018, 175, 2566–2580. [Google Scholar] [CrossRef]
- Rocha, F.C.M.; Dos Santos Júnior, J.G.; Stefano, S.C.; Da Silveira, D.X. Systematic Review of the Literature on Clinical and Experimental Trials on the Antitumor Effects of Cannabinoids in Gliomas. J. Neurooncol. 2014, 116, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, L.; Zhang, X.; Li, L.; Jiang, C.; Qiu, Y.; Huang, R.; Xie, B.; Lin, Z.; Ren, J.; et al. Alteration of Endocannabinoid System in Human Gliomas. J. Neurochem. 2012, 120, 842–849. [Google Scholar] [CrossRef] [PubMed]
- De Jesús, M.L.; Hostalot, C.; Garibi, J.M.; Sallés, J.; Meana, J.J.; Callado, L.F. Opposite Changes in Cannabinoid CB1 and CB2 Receptor Expression in Human Gliomas. Neurochem. Int. 2010, 56, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Held-Feindt, J.; Dörner, L.; Sahan, G.; Mehdorn, H.M.; Mentlein, R. Cannabinoid Receptors in Human Astroglial Tumors. J. Neurochem. 2006, 98, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Sredni, S.T.; Huang, C.C.; Suzuki, M.; Pundy, T.; Chou, P.; Tomita, T. Spontaneous Involution of Pediatric Low-Grade Gliomas: High Expression of Cannabinoid Receptor 1 (CNR1) at the Time of Diagnosis May Indicate Involvement of the Endocannabinoid System. Child’s Nerv. Syst. 2016, 32, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Lorente, M.; Egia, A.; Blázquez, C.; García, S.; Giroux, V.; Malicet, C.; Villuendas, R.; Gironella, M.; González-Feria, L.; et al. The Stress-Regulated Protein P8 Mediates Cannabinoid-Induced Apoptosis of Tumor Cells. Cancer Cell 2006, 9, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Singh Chauhan, B.P.; Sharma, V. Challenges of Cannabinoid Delivery: How Can Nanomedicine Help? Nanomedicine 2020, 15, 2023–2028. [Google Scholar] [CrossRef]
- Ngwa, W.; Kumar, R.; Moreau, M.; Dabney, R.; Herman, A. Nanoparticle Drones to Target Lung Cancer with Radiosensitizers and Cannabinoids. Front. Oncol. 2017, 7, 208. [Google Scholar] [CrossRef]
- Deshpande, A.; Patil, T.S. Nanocarrier Technologies for Enhancing the Solubility and Dissolution Rate of Api. In Medicinal Chemistry with Pharmaceutical Product Development; Apple Academic Press: Cambridge, MA, USA, 2019; pp. 155–234. [Google Scholar] [CrossRef]
- Uddin, M.S.; Al Mamun, A.; Alghamdi, B.S.; Tewari, D.; Jeandet, P.; Sarwar, M.S.; Ashraf, G.M. Epigenetics of Glioblastoma Multiforme: From Molecular Mechanisms to Therapeutic Approaches. Semin. Cancer Biol. 2022, 83, 100–120. [Google Scholar] [CrossRef]
- Shah, J.L.; Li, G.H.; Soltys, S.G. Glioblastoma Multiforme. CyberKnife Stereotact. Radiosurgery Brain 2022, 1, 85–98. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of Its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Yamamoto, K.; Morishita, R.; Hayashi, S.-I.; Matsushita, H.; Nakagami, H.; Moriguchi, A.; Matsumoto, K.; Nakamura, T.; Kaneda, Y.; Ogihara, T. HGF/MET Signaling in Malignant Brain Tumors. Int. J. Mol. Sci. 2020, 21, 7546. [Google Scholar] [CrossRef]
- Jiang, Y.; Uhrbom, L. On the Origin of Glioma. Ups. J. Med. Sci. 2012, 117, 113. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Loras, A.; Gonzalez-Bonet, L.G.; Gutierrez-Arroyo, J.L.; Martinez-Cadenas, C.; Marques-Torrejon, M.A. Neural Stem Cells as Potential Glioblastoma Cells of Origin. Life 2023, 13, 905. [Google Scholar] [CrossRef]
- DeCordova, S.; Shastri, A.; Tsolaki, A.G.; Yasmin, H.; Klein, L.; Singh, S.K.; Kishore, U. Molecular Heterogeneity and Immunosuppressive Microenvironment in Glioblastoma. Front. Immunol. 2020, 11, 1402. [Google Scholar] [CrossRef]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary Brain Tumours in Adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Central Nervous System Tumours; IARC, Ed.; IARC: Lyon, France, 2022; Volume 1, ISBN 978-92-832-4508-7. [Google Scholar]
- Liu, Y.; Lang, F.; Chou, F.J.; Zaghloul, K.A.; Yang, C. Isocitrate Dehydrogenase Mutations in Glioma: Genetics, Biochemistry, and Clinical Indications. Biomedicines 2020, 8, 294. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef]
- Haase, S.; Garcia-Fabiani, M.B.; Carney, S.; Altshuler, D.; Núñez, F.J.; Méndez, F.M.; Núñez, F.; Lowenstein, P.R.; Castro, M.G. Mutant ATRX: Uncovering a New Therapeutic Target for Glioma. Expert Opin. Ther. Targets 2018, 22, 599–613. [Google Scholar] [CrossRef]
- Jiao, Y.; Killela, P.J.; Reitman, Z.J.; Rasheed, B.A.; Heaphy, C.M.; de Wilde, R.F.; Rodriguez, F.J.; Rosemberg, S.; Obashinjo, S.M.; Marie, S.K.N.; et al. Frequent ATRX, CIC, FUBP1 and IDH1 Mutations Refine the Classification of Malignant Gliomas. Oncotarget 2012, 3, 709–722. [Google Scholar] [CrossRef]
- Jenkins, R.B.; Blair, H.; Ballman, K.V.; Giannini, C.; Arusell, R.M.; Law, M.; Flynn, H.; Passe, S.; Felten, S.; Brown, P.D.; et al. A t(1;19)(Q10;P10) Mediates the Combined Deletions of 1p and 19q and Predicts a Better Prognosis of Patients with Oligodendroglioma. Cancer Res. 2006, 66, 9852–9861. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Xie, M.; Zuo, P.; Li, X.; Gu, G.; Li, T.; Jiang, Z.; Wu, Z.; Zhang, J.; et al. Adult Diffuse Intrinsic Pontine Glioma: Clinical, Radiological, Pathological, Molecular Features, and Treatments of 96 Patients. J. Neurosurg. 2022, 137, 1628–1638. [Google Scholar] [CrossRef]
- Onizuka, H.; Masui, K.; Komori, T. Diffuse Gliomas to Date and beyond 2016 WHO Classification of Tumours of the Central Nervous System. Int. J. Clin. Oncol. 2020, 25, 997–1003. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Aldape, K.; Brat, D.J.; Capper, D.; Ellison, D.W.; Hawkins, C.; Paulus, W.; Perry, A.; Reifenberger, G.; Figarella-Branger, D.; et al. Announcing CIMPACT-NOW: The Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy. Acta Neuropathol. 2017, 133, 1–3. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Burger, P.; Ellison, D.W.; Reifenberger, G.; von Deimling, A.; Aldape, K.; Brat, D.; Collins, V.P.; Eberhart, C.; et al. International Society of Neuropathology-Haarlem Consensus Guidelines for Nervous System Tumor Classification and Grading. Brain Pathol. 2014, 24, 429–435. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and Molecular Prognostic Review of Glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non–Contrast-Enhanced Tumor with Survival within Molecular Subgroups of Patients with Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef]
- Alfonso, J.C.L.; Talkenberger, K.; Seifert, M.; Klink, B.; Hawkins-Daarud, A.; Swanson, K.R.; Hatzikirou, H.; Deutsch, A. The Biology and Mathematical Modelling of Glioma Invasion: A Review. J. R. Soc. Interface 2017, 14, 20170490. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The Epidemiology of Glioma in Adults: A “State of the Science” Review. Neuro-Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef]
- Amirian, E.S.; Armstrong, G.N.; Zhou, R.; Lau, C.C.; Claus, E.B.; Barnholtz-Sloan, J.S.; Il’Yasova, D.; Schildkraut, J.; Ali-Osman, F.; Sadetzki, S.; et al. The Glioma International Case-Control Study: A Report from the Genetic Epidemiology of Glioma International Consortium. Am. J. Epidemiol. 2016, 183, 85–91. [Google Scholar] [CrossRef]
- Belden, C.J.; Valdes, P.A.; Ran, C.; Pastel, D.A.; Harris, B.T.; Fadul, C.E.; Israel, M.A.; Paulsen, K.; Roberts, D.W. Genetics of Glioblastoma: A Window into Its Imaging and Histopathologic Variability. Radiographics 2011, 31, 1717–1740. [Google Scholar] [CrossRef]
- Armstrong, T.S.; Dirven, L.; Arons, D.; Bates, A.; Chang, S.M.; Coens, C.; Espinasse, C.; Gilbert, M.R.; Jenkinson, D.; Kluetz, P.; et al. Glioma Patient-Reported Outcome Assessment in Clinical Care and Research: A Response Assessment in Neuro-Oncology Collaborative Report. Lancet Oncol. 2020, 21, e97–e103. [Google Scholar] [CrossRef]
- Skiriute, D.; Vaitkiene, P.; Saferis, V.; Asmoniene, V.; Skauminas, K.; Deltuva, V.P.; Tamasauskas, A. MGMT, GATA6, CD81, DR4, and CASP8 Gene Promoter Methylation in Glioblastoma. BMC Cancer 2012, 12, 218. [Google Scholar] [CrossRef]
- Agravat, R.R. Robust Brain Tumor Segmentation for Overall Survival Prediction. Ph.D. Thesis, Ahmedabad University, Ahmedabad, India, 2021. [Google Scholar]
- Bikfalvi, A.; da Costa, C.A.; Avril, T.; Barnier, J.-V.; Bauchet, L.; Brisson, L.; Cartron, P.F.; Castel, H.; Chevet, E.; Chneiweiss, H.; et al. Challenges in Glioblastoma Research: Focus on the Tumor Microenvironment. Trends Cancer 2023, 9, 9–27. [Google Scholar] [CrossRef]
- Kim, Y.; Jeon, H.; Othmer, H. The Role of the Tumor Microenvironment in Glioblastoma: A Mathematical Model. IEEE Trans. Biomed. Eng. 2017, 64, 519–527. [Google Scholar] [CrossRef]
- Simon, T.; Jackson, E.; Giamas, G. Breaking through the Glioblastoma Micro-Environment via Extracellular Vesicles. Oncogene 2020, 39, 4477–4490. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-Derived Suppressor Cells: Linking Inflammation and Cancer. J. Immunol. 2009, 182, 4499–4506. [Google Scholar] [CrossRef]
- Kamran, N.; Chandran, M.; Lowenstein, P.R.; Castro, M.G. Immature Myeloid Cells in the Tumor Microenvironment: Implications for Immunotherapy. Clin. Immunol. 2018, 189, 34–42. [Google Scholar] [CrossRef]
- Abt, M.C.; Osborne, L.C.; Monticelli, L.A.; Doering, T.A.; Alenghat, T.; Sonnenberg, G.F.; Paley, M.A.; Antenus, M.; Williams, K.L.; Erikson, J.; et al. Commensal Bacteria Calibrate the Activation Threshold of Innate Antiviral Immunity. Immunity 2012, 37, 158–170. [Google Scholar] [CrossRef]
- Ivanov, I.I.; de Llanos Frutos, R.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific Microbiota Direct the Differentiation of IL-17-Producing T-Helper Cells in the Mucosa of the Small Intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef]
- Casanova, J.L.; Abel, L.; Quintana-Murci, L. Human TLRs and IL-1Rs in Host Defense: Natural Insights from Evolutionary, Epidemiological, and Clinical Genetics. Annu. Rev. Immunol. 2011, 29, 447–491. [Google Scholar] [CrossRef]
- Rutkowski, M.R.; Svoronos, N.; Perales-Puchalt, A.; Conejo-Garcia, J.R. The Tumor Macroenvironment; Springer: Berlin/Heidelberg, Germany, 2015; pp. 235–262. [Google Scholar]
- Branton, W.G.; Ellestad, K.K.; Maingat, F.; Wheatley, B.M.; Rud, E.; Warren, R.L.; Holt, R.A.; Surette, M.G.; Power, C. Brain Microbial Populations in HIV/AIDS: α-Proteobacteria Predominate Independent of Host Immune Status. PLoS ONE 2013, 8, e54673. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The Human Tumor Microbiome Is Composed of Tumor Type-Specific Intracellular Bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Ciernikova, S.; Sevcikova, A.; Stevurkova, V.; Mego, M. Tumor Microbiome—An Integral Part of the Tumor Microenvironment. Front. Oncol. 2022, 12, 1063100. [Google Scholar] [CrossRef]
- Zella, D.; Gallo, R.C. Viruses and Bacteria Associated with Cancer: An Overview. Viruses 2021, 13, 1039. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Q.; Liu, W.; Wen, Z.; Li, Y. Precision Strategies for Cancer Treatment by Modifying the Tumor-Related Bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 6183–6197. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.F. Impact of Microbiota on Central Nervous System and Neurological Diseases: The Gut-Brain Axis. J. Neuroinflamm. 2019, 16, 53. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Dees, K.J.; Koo, H.; Humphreys, J.F.; Hakim, J.A.; Crossman, D.K.; Crowley, M.R.; Nabors, L.B.; Benveniste, E.N.; Morrow, C.D.; McFarland, B.C. Human Gut Microbial Communities Dictate Efficacy of Anti-PD-1 Therapy in a Humanized Microbiome Mouse Model of Glioma. Neurooncol. Adv. 2021, 3, vdab023. [Google Scholar] [CrossRef] [PubMed]
- Jobin, C. Precision Medicine Using Microbiota. Science 2018, 359, 32–34. [Google Scholar] [CrossRef] [PubMed]
- Sanegre, S.; Lucantoni, F.; Burgos-Panadero, R.; de La Cruz-Merino, L.; Noguera, R.; Naranjo, T.Á. Integrating the Tumor Microenvironment into Cancer Therapy. Cancers 2020, 12, 1677. [Google Scholar] [CrossRef] [PubMed]
- Obradović, M.M.S.; Hamelin, B.; Manevski, N.; Couto, J.P.; Sethi, A.; Coissieux, M.M.; Münst, S.; Okamoto, R.; Kohler, H.; Schmidt, A.; et al. Glucocorticoids Promote Breast Cancer Metastasis. Nature 2019, 567, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kroemer, G. Cancer Cells Thrive on Stress. Trends Cell Biol. 2019, 29, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Balmain, A.; Barrett, J.C.; Moses, H.; Renan, M.J. How Many Mutations Are Required for Tumorigenesis? Implications from Human Cancer Data. Mol. Carcinog. 1993, 7, 139–146. [Google Scholar] [CrossRef]
- Molecular Oncology: Principles and Recent Advances; Camacho, J., Ed.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; ISBN 1608050165. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Torrisi, F.; Alberghina, C.; D’aprile, S.; Pavone, A.M.; Longhitano, L.; Giallongo, S.; Tibullo, D.; Di Rosa, M.; Zappalà, A.; Cammarata, F.P.; et al. The Hallmarks of Glioblastoma: Heterogeneity, Intercellular Crosstalk and Molecular Signature of Invasiveness and Progression. Biomedicines 2022, 10, 806. [Google Scholar] [CrossRef]
- Mao, H.; Lebrun, D.G.; Yang, J.; Zhu, V.F.; Li, M. Deregulated Signaling Pathways in Glioblastoma Multiforme: Molecular Mechanisms and Therapeutic Targets. Cancer Investig. 2012, 30, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Bastien, J.I.L.; McNeill, K.A.; Fine, H.A. Molecular Characterizations of Glioblastoma, Targeted Therapy, and Clinical Results to Date. Cancer 2015, 121, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, X.; Nie, S.; Liu, J.; Wang, S. Disorders of Cancer Metabolism: The Therapeutic Potential of Cannabinoids. Biomed. Pharmacother. 2023, 157, 113993. [Google Scholar] [CrossRef] [PubMed]
- Dariš, B.; Verboten, M.T.; Knez, Ž.; Ferk, P. Cannabinoids in Cancer Treatment: Therapeutic Potential and Legislation. Bosn. J. Basic Med. Sci. 2019, 19, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Shekher, A.; Puneet; Narula, A.S.; Abrahamse, H.; Gupta, S.C. Cannabis and Its Constituents for Cancer: History, Biogenesis, Chemistry and Pharmacological Activities. Pharmacol. Res. 2021, 163, 105302. [Google Scholar] [CrossRef]
- Massi, P.; Vaccani, A.; Ceruti, S.; Colombo, A.; Abbracchio, M.P.; Parolaro, D. Antitumor Effects of Cannabidiol, a Nonpsychoactive Cannabinoid, on Human Glioma Cell Lines. J. Pharmacol. Exp. Ther. 2004, 308, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarivelo, V.; Sihag, J.; Flamand, N. Role of the Endocannabinoid System in the Adipose Tissue with Focus on Energy Metabolism. Cells 2021, 10, 1279. [Google Scholar] [CrossRef]
- Doherty, G.J.; de Paula, B.H.R. Cannabinoids in Glioblastoma Multiforme—Hype or Hope? Br. J. Cancer 2021, 124, 1341–1343. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Grajkowska, W.; Gabrusiewicz, K.; Kaminska, B.; Konarska, L. Distinctive Pattern of Cannabinoid Receptor Type II (CB2) Expression in Adult and Pediatric Brain Tumors. Brain Res. 2007, 1137, 161–169. [Google Scholar] [CrossRef]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as Therapeutic Agents in Cancer: Current Status and Future Implications. Oncotarget 2014, 5, 5852. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of Inflammation by Cannabinoids, the Endocannabinoids 2-Arachidonoyl-Glycerol and Arachidonoyl-Ethanolamide, and Their Metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- Shevyrin, V.A.; Morzherin, Y.Y. Cannabinoids: Structures, Effects, and Classification. Russ. Chem. Bull. 2015, 64, 1249–1266. [Google Scholar] [CrossRef]
- Bow, E.W.; Rimoldi, J.M. The Structure–Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation. Perspect. Med. Chem. 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Su, M.K.; Seely, K.A.; Moran, J.H.; Hoffman, R.S. Metabolism of Classical Cannabinoids and the Synthetic Cannabinoid JWH-018. Clin. Pharmacol. Ther. 2015, 97, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Bardhi, K.; Coates, S.; Watson, C.J.W.; Lazarus, P. Cannabinoids and Drug Metabolizing Enzymes: Potential for Drug-Drug Interactions and Implications for Drug Safety and Efficacy. Expert Rev. Clin. Pharmacol. 2022, 15, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid Formulations and Delivery Systems: Current and Future Options to Treat Pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef]
- Bloom, A.S.; Edgemond, W.S.; Moldvan, J.C. Nonclassical and Endogenous Cannabinoids: Effects on the Ordering of Brain Membranes. Neurochem. Res. 1997, 22, 563–568. [Google Scholar] [CrossRef]
- Pop, E. Cannabinoids, Endogenous Ligands and Synthetic Analogs. Curr. Opin. Chem. Biol. 1999, 3, 418–425. [Google Scholar] [CrossRef]
- Zendulka, O.; Dovrtělová, G.; Nosková, K.; Turjap, M.; Šulcová, A.; Hanuš, L.; Juřica, J. Cannabinoids and Cytochrome P450 Interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef]
- Shevyrin, V.; Melkozerov, V.; Endres, G.W.; Shafran, Y.; Morzherin, Y. On a New Cannabinoid Classification System: A Sight on the Illegal Market of Novel Psychoactive Substances. Cannabis Cannabinoid Res. 2016, 1, 186–194. [Google Scholar] [CrossRef]
- Shim, J.-Y.; Collantes, E.R.; Welsh, W.J.; Howlett, A.C. Unified Pharmacophoric Model for Cannabinoids and Aminoalkylindoles. In Molecular Modeling and Prediction of Bioactivity; Springer: Boston, MA, USA, 2000; pp. 201–206. [Google Scholar] [CrossRef]
- Mardal, M.; Gracia-Lor, E.; Leibnitz, S.; Castiglioni, S.; Meyer, M.R. Toxicokinetics of New Psychoactive Substances: Plasma Protein Binding, Metabolic Stability, and Human Phase I Metabolism of the Synthetic Cannabinoid WIN 55,212-2 Studied Using in Vitro Tools and LC-HR-MS/MS. Drug Test. Anal. 2016, 8, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S.H. Eicosanoid Mediation of Cannabinoid Actions. Bioorg. Med. Chem. 2019, 27, 2718–2728. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S.H.; Zurier, R.B. Cannabinoids, Endocannabinoids, and Related Analogs in Inflammation. AAPS J. 2009, 11, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, D.G. A Personal Retrospective: Elevating Anandamide (AEA) by Targeting Fatty Acid Amide Hydrolase (FAAH) and the Fatty Acid Binding Proteins (FABPs). Front. Pharmacol. 2016, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Ghebreselasie, K.; Marnett, L.J. Chemical Stability of 2-Arachidonylglycerol under Biological Conditions. Chem. Phys. Lipids 2002, 119, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [PubMed]
- Ellert-Miklaszewska, A.; Ciechomska, I.A.; Kaminska, B. Cannabinoid Signaling in Glioma Cells. In Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Downer, E.J.; Gowran, A.; Murphy, Á.C.; Campbell, V.A. The Tumour Suppressor Protein, P53, Is Involved in the Activation of the Apoptotic Cascade by Δ9-Tetrahydrocannabinol in Cultured Cortical Neurons. Eur. J. Pharmacol. 2007, 564, 57–65. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Malik, A.; Mukhtar, H. Cannabinoid Receptor Agonist-Induced Apoptosis of Human Prostate Cancer Cells LNCaP Proceeds through Sustained Activation of ERK1/2 Leading to G 1 Cell Cycle Arrest. J. Biol. Chem. 2006, 281, 39480–39491. [Google Scholar] [CrossRef]
- Galanti, G.; Fisher, T.; Kventsel, I.; Shoham, J.; Gallily, R.; Mechoulam, R.; Lavie, G.; Amariglio, N.; Rechavi, G.; Toren, A. Δ9-Tetrahydrocannabinol Inhibits Cell Cycle Progression by Downregulation of E2F1 in Human Glioblastoma Multiforme Cells. Acta. Oncol. 2008, 47, 1062–1070. [Google Scholar] [CrossRef]
- Irrera, N.; D’ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-Caryophyllene Inhibits Cell Proliferation through a Direct Modulation of CB2 Receptors in Glioblastoma Cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, C.; González-Feria, L.; Álvarez, L.; Haro, A.; Casanova, M.L.; Guzmán, M. Cannabinoids Inhibit the Vascular Endothelial Growth Factor Pathway in Gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.M.; Cosgrave, J.M.; Gallagher, W.M.; Perry, A.S. Plant-Derived Cannabinoids as Anticancer Agents. Trends Cancer 2022, 8, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Ramer, R. Cannabinoids as Anticancer Drugs: Current Status of Preclinical Research. Br. J. Cancer 2022, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kolbe, M.R.; Hohmann, T.; Hohmann, U.; Ghadban, C.; Mackie, K.; Zöller, C.; Prell, J.; Illert, J.; Strauss, C.; Dehghani, F. THC Reduces Ki67-Immunoreactive Cells Derived from Human Primary Glioblastoma in a GPR55-Dependent Manner. Cancers 2021, 13, 1064. [Google Scholar] [CrossRef] [PubMed]
- Oesch, S.; Gertsch, J. Cannabinoid Receptor Ligands as Potential Anticancer Agents—High Hopes for New Therapies? J. Pharm. Pharmacol. 2010, 61, 839–853. [Google Scholar] [CrossRef]
- Mangal, N.; Erridge, S.; Habib, N.; Sadanandam, A.; Reebye, V.; Sodergren, M.H. Cannabinoids in the Landscape of Cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2507–2534. [Google Scholar] [CrossRef]
- Cherkasova, V.; Wang, B.; Gerasymchuk, M.; Fiselier, A.; Kovalchuk, O.; Kovalchuk, I. Use of Cannabis and Cannabinoids for Treatment of Cancer. Cancers 2022, 14, 5142. [Google Scholar] [CrossRef]
- Worster, B.; Hajjar, E.R.; Handley, N. Cannabis Use in Patients with Cancer: A Clinical Review. JCO Oncol. Pract. 2022, 18, 743–749. [Google Scholar] [CrossRef]
- Klimkiewicz, A.; Jasinska, A. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Psychiatria 2017, 15, 88–92. [Google Scholar] [CrossRef]
- Sánchez, C.; Galve-Roperh, I.; Canova, C.; Brachet, P.; Guzmán, M. Δ9-Tetrahydrocannabinol Induces Apoptosis in C6 Glioma Cells. FEBS Lett. 1998, 436, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Allister, S.D.; Chan, C.; Taft, R.J.; Luu, T.; Abood, M.E.; Moore, D.H.; Aldape, K.; Yount, G. Cannabinoids Selectively Inhibit Proliferation and Induce Death of Cultured Human Glioblastoma Multiforme Cells. J. Neurooncol. 2005, 74, 31–40. [Google Scholar] [CrossRef] [PubMed]
- End, D.W.; Thoursen, K.; Dewey, W.L.; Carchman, R.A. A Comparative Study of the Disposition of Tetrahydrocannabinol in Neuroblastoma and Glioma Cells in Tissue Culture: Relation to Cellular Impairment. Mol. Pharmacol. 1977, 13, 864–871. [Google Scholar] [PubMed]
- Velasco, G.; Galve-Roperh, I.; Sánchez, C.; Blázquez, C.; Guzmán, M. Hypothesis: Cannabinoid Therapy for the Treatment of Gliomas? Neuropharmacology 2004, 47, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, M.; Laezza, C.; Gazzerro, P.; Pentimalli, F. Endocannabinoids as Emerging Suppressors of Angiogenesis and Tumor Invasion (Review). Oncol. Rep. 2007, 17, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Carkaci-Salli, N.; Raup-Konsavage, W.M.; Karelia, D.; Sun, D.; Jiang, C.; Lu, J.; Vrana, K.E. Cannabinoids as Potential Cancer Therapeutics: The Concentration Conundrum. Cannabis Cannabinoid Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Choi, N.; Kim, K.; Koo, H.J.; Choi, J.; Kim, H.N. Chemoresistance of Cancer Cells: Requirements of Tumor Microenvironment-Mimicking in Vitro Models in Anti-Cancer Drug Development. Theranostics 2018, 8, 5259–5275. [Google Scholar] [CrossRef]
- Fisher, T.; Golan, H.; Schiby, G.; Prichen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In Vitro and in Vivo Efficacy of Non-Psychoactive Cannabidiol in Neuroblastoma. Curr. Oncol. 2016, 23, S15–S22. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Sandalcioglu, I.E.; Karsak, M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, Í.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid Action Induces Autophagy-Mediated Cell Death through Stimulation of ER Stress in Human Glioma Cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef]
- Ciechomska, I.A.; Gabrusiewicz, K.; Szczepankiewicz, A.A.; Kaminska, B. Endoplasmic Reticulum Stress Triggers Autophagy in Malignant Glioma Cells Undergoing Cyclosporine A-Induced Cell Death. Oncogene 2012, 32, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Valenti, M.; Solinas, M.; Parolaro, D. Molecular Mechanisms Involved in the Antitumor Activity of Cannabinoids on Gliomas: Role for Oxidative Stress. Cancers 2010, 2, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Q.; Li, Q.; Zhang, Z.; Gao, J.; Fan, C.; Sun, B.; Ni, Q. Cannabinoid WIN 55,212-2 Inhibits Human Glioma Cell Growth by Triggering ROS-Mediated Signal Pathways. BioMed Res. Int. 2021, 2021, 6612592. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, C.; Casanova, M.L.; Planas, A.; Gómez del Pulgar, T.; Villanueva, C.; Fernández-Aceñero, M.J.; Aragonés, J.; Huffman, J.W.; Jorcano, J.L.; Guzmán, M. Inhibition of Tumor Angiogenesis by Cannabinoids. FASEB J. 2003, 17, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Widmer, M.; Hanemann, C.O.; Zajicek, J. High Concentrations of Cannabinoids Activate Apoptosis in Human U373MG Glioma Cells. J. Neurosci. Res. 2008, 86, 3212–3220. [Google Scholar] [CrossRef]
- Peeri, H.; Shalev, N.; Vinayaka, A.C.; Nizar, R.; Kazimirsky, G.; Namdar, D.; Anil, S.M.; Belausov, E.; Brodie, C.; Koltai, H. Specific Compositions of Cannabis Sativa Compounds Have Cytotoxic Activity and Inhibit Motility and Colony Formation of Human Glioblastoma Cells In Vitro. Cancers 2021, 13, 1720. [Google Scholar] [CrossRef]
- De los Reyes Corrales, T.; Losada-Pérez, M.; Casas-Tintó, S. JNK Pathway in CNS Pathologies. Int. J. Mol. Sci. 2021, 22, 3883. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Teixeira, N.A.; Correia-da-Silva, G. Cannabinoids as Modulators of Cell Death: Clinical Applications and Future Directions; Springer: Cham, Switzerland, 2017; pp. 63–88. [Google Scholar]
- Rodriguez-Almaraz, J.E.; Butowski, N. Therapeutic and Supportive Effects of Cannabinoids in Patients with Brain Tumors (CBD Oil and Cannabis). Curr. Treat. Options Oncol. 2023, 24, 30–44. [Google Scholar] [CrossRef]
- Ganesh, A.N.; McLaughlin, C.K.; Duan, D.; Shoichet, B.K.; Shoichet, M.S. A New Spin on Antibody-Drug Conjugates: Trastuzumab-Fulvestrant Colloidal Drug Aggregates Target HER2-Positive Cells. ACS Appl. Mater. Interfaces 2017, 9, 12195–12202. [Google Scholar] [CrossRef]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sánchez, C.; Velasco, G.; González-Feria, L. A Pilot Clinical Study of Δ9-Tetrahydrocannabinol in Patients with Recurrent Glioblastoma Multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef]
- Johnson, E.C.; Hatoum, A.S.; Deak, J.D.; Polimanti, R.; Murray, R.M.; Edenberg, H.J.; Gelernter, J.; Di Forti, M.; Agrawal, A. The Relationship between Cannabis and Schizophrenia: A Genetically Informed Perspective. Addiction 2021, 116, 3227–3234. [Google Scholar] [CrossRef]
- Marijuana and Madness; Castle, D., Murray, R.M., D’Souza, D.C., Eds.; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Hindley, G.; Beck, K.; Borgan, F.; Ginestet, C.E.; McCutcheon, R.; Kleinloog, D.; Ganesh, S.; Radhakrishnan, R.; D’Souza, D.C.; Howes, O.D. Psychiatric Symptoms Caused by Cannabis Constituents: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2020, 7, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.; Shahinas, J. Dosage, Efficacy and Safety of Cannabidiol Administration in Adults: A Systematic Review of Human Trials. J. Clin. Med. Res. 2020, 12, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Sawtelle, L.; Holle, L.M. Use of Cannabis and Cannabinoids in Patients with Cancer. Ann. Pharmacother. 2021, 55, 870–890. [Google Scholar] [CrossRef] [PubMed]
- Hostiuc, S.; Moldoveanu, A.; Negoi, I.; Drima, E. The Association of Unfavorable Traffic Events and Cannabis Usage: A Meta-Analysis. Front. Pharmacol. 2018, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Winterstein, A.G. Potential Adverse Drug Events and Drug–Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J. Clin. Med. 2019, 8, 989. [Google Scholar] [CrossRef] [PubMed]
- Buchtova, T.; Lukac, D.; Skrott, Z.; Chroma, K.; Bartek, J.; Mistrik, M. Drug–Drug Interactions of Cannabidiol with Standard-of-Care Chemotherapeutics. Int. J. Mol. Sci. 2023, 24, 2885. [Google Scholar] [CrossRef] [PubMed]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Kazazi-Hyseni, F.; Beijnen, J.H.; Schellens, J.H.M. Bevacizumab. Oncologist 2010, 15, 819. [Google Scholar] [CrossRef]
- Shih, T.; Lindley, C. Bevacizumab: An Angiogenesis Inhibitor for the Treatment of Solid Malignancies. Clin. Ther. 2006, 28, 1779–1802. [Google Scholar] [CrossRef] [PubMed]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma Multiforme: An Overview of Emerging Therapeutic Targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.F.; Carter, T.J.; Ottaviani, D.; Mulholland, P. Harnessing the Immune System in Glioblastoma. Br. J. Cancer 2018, 119, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Li, J.; Han, L. Receptor-Mediated Cascade Targeting Strategies for the Application to Medical Diagnoses and Therapeutics of Glioma. J. Nanopart. Res. 2022, 24, 106. [Google Scholar] [CrossRef]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Frosina, G. Limited Advances in Therapy of Glioblastoma Trigger Re-Consideration of Research Policy. Crit. Rev. Oncol. Hematol. 2015, 96, 257–261. [Google Scholar] [CrossRef]
- Torres, S.; Lorente, M.; Rodríguez-Fornés, F.; Hernández-Tiedra, S.; Salazar, M.; García-Taboada, E.; Barcia, J.; Guzmán, M.; Velasco, G. A Combined Preclinical Therapy of Cannabinoids and Temozolomide against Glioma. Mol. Cancer Ther. 2011, 10, 90–103. [Google Scholar] [CrossRef]
- López-Valero, I.; Torres, S.; Salazar-Roa, M.; García-Taboada, E.; Hernández-Tiedra, S.; Guzmán, M.; Sepúlveda, J.M.; Velasco, G.; Lorente, M. Optimization of a Preclinical Therapy of Cannabinoids in Combination with Temozolomide against Glioma. Biochem. Pharmacol. 2018, 157, 275–284. [Google Scholar] [CrossRef]
- Twelves, C.; Sabel, M.; Checketts, D.; Miller, S.; Tayo, B.; Jove, M.; Brazil, L.; Short, S.C.; McBain, C.; Haylock, B.; et al. A Phase 1b Randomised, Placebo-Controlled Trial of Nabiximols Cannabinoid Oromucosal Spray with Temozolomide in Patients with Recurrent Glioblastoma. Br. J. Cancer 2021, 124, 1379–1387. [Google Scholar] [CrossRef]
- Vermersch, P. Sativex® (Tetrahydrocannabinol + Cannabidiol), an Endocannabinoid System Modulator: Basic Features and Main Clinical Data. Expert Rev. Neurother. 2011, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Zamberletti, E. Alterations of THC and CBD Ratios and Impact on Cognition. In Cannabis Use, Neurobiology, Psychology, and Treatment; Academic Press: Cambridge, MA, USA, 2023; pp. 181–191. [Google Scholar] [CrossRef]
- Wolf, K.J.; Chen, J.; Coombes, J.D.; Aghi, M.K.; Kumar, S. Dissecting and Rebuilding the Glioblastoma Microenvironment with Engineered Materials. Nat. Rev. Mater. 2019, 4, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Giesexs, A.; Westphal, M. Glioma Invasion in the Central Nervous System. Neurosurgery 1996, 39, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Budday, S.; Nay, R.; de Rooij, R.; Steinmann, P.; Wyrobek, T.; Ovaert, T.C.; Kuhl, E. Mechanical Properties of Gray and White Matter Brain Tissue by Indentation. J. Mech. Behav. Biomed. Mater. 2015, 46, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug Transport across the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959. [Google Scholar] [CrossRef]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The Blood-Brain Barrier: An Engineering Perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef]
- Banks, W.A.; Greig, N.H. Small Molecules as Central Nervous System Therapeutics: Old Challenges, New Directions, and a Philosophic Divide. Future Med. Chem. 2019, 11, 489–493. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood-Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef] [PubMed]
- van Solinge, T.S.; Nieland, L.; Chiocca, E.A.; Broekman, M.L.D. Advances in Local Therapy for Glioblastoma—Taking the Fight to the Tumour. Nat. Rev. Neurol. 2022, 18, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Johanson, C.E.; Duncan, J.A.; Stopa, E.G.; Baird, A. Enhanced Prospects for Drug Delivery and Brain Targeting by the Choroid Plexus–CSF Route. Pharm. Res. 2005, 22, 1011–1037. [Google Scholar] [CrossRef] [PubMed]
- Graff, C.L.; Pollack, G.M. (Section B: Integrated Function of Drug Transporters In Vivo) Drug Transport at the Blood-Brain Barrier and the Choroid Plexus. Curr. Drug Metab. 2005, 5, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.C.; Appleton, I.; Darlington, C.L.; Smith, P.F. Cannabinoid CB1 Receptor Protein Expression in the Rat Choroid Plexus: A Possible Involvement of Cannabinoids in the Regulation of Cerebrospinal Fluid. Neurosci. Lett. 2004, 364, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, K.; Wang, D.; Yao, S.; Lu, L.; Wang, H.; Song, J.; Zhou, J.; Fan, X.; Wang, Y.; et al. Core-Shell Lipoplexes Inducing Active Macropinocytosis Promote Intranasal Delivery of c-Myc SiRNA for Treatment of Glioblastoma. Acta Biomater. 2022, 138, 478–490. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Torres-Suárez, A.I. Glioblastoma Multiforme and Lipid Nanocapsules: A Review. J. Biomed. Nanotechnol. 2014, 11, 1283–1311. [Google Scholar] [CrossRef]
- Tosi, G.; Bortot, B.; Ruozi, B.; Dolcetta, D.; Vandelli, M.A.; Forni, F.; Severini, G.M. Potential Use of Polymeric Nanoparticles for Drug Delivery Across the Blood-Brain Barrier. Curr. Med. Chem. 2013, 20, 2212–2225. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Chen, X.; Du, X.S.; Zhang, J.L.; Liu, G.; Zhang, W.G. Application of Iron Oxide Nanoparticles in Glioma Imaging and Therapy: From Bench to Bedside. Nanoscale 2016, 8, 7808–7826. [Google Scholar] [CrossRef]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging Concepts in Dendrimer-Based Nanomedicine: From Design Principles to Clinical Applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef]
- Pandey, N.; Anastasiadis, P.; Carney, C.P.; Kanvinde, P.P.; Woodworth, G.F.; Winkles, J.A.; Kim, A.J. Nanotherapeutic Treatment of the Invasive Glioblastoma Tumor Microenvironment. Adv. Drug Deliv. Rev. 2022, 188, 114415. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.F.; Young, J.S.; Amara, D.; Berger, M.S.; Raleigh, D.R.; Aghi, M.K.; Butowski, N.A. Mouse Models of Glioblastoma for the Evaluation of Novel Therapeutic Strategies. Neuro-Oncol. Adv. 2021, 3, vdab100. [Google Scholar] [CrossRef] [PubMed]
- Rybin, M.J.; Ivan, M.E.; Ayad, N.G.; Zeier, Z. Organoid Models of Glioblastoma and Their Role in Drug Discovery. Front. Cell. Neurosci. 2021, 15, 605255. [Google Scholar] [CrossRef] [PubMed]
- Park, T.E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-Enhanced Blood-Brain Barrier Chip Recapitulates Human Barrier Function and Shuttling of Drugs and Antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef]
- Jampílek, J.; Král’ová, K. Natural Biopolymeric Nanoformulations for Brain Drug Delivery. In Nanocarriers for Brain Targeting; Apple Academic Press: Cambridge, MA, USA, 2019; pp. 131–204. [Google Scholar] [CrossRef]
- Ndemazie, N.B.; Inkoom, A.; Morfaw, E.F.; Smith, T.; Aghimien, M.; Ebesoh, D.; Agyare, E. Multi-Disciplinary Approach for Drug and Gene Delivery Systems to the Brain. AAPS PharmSciTech 2022, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Hanuš, L. Cannabidiol: An Overview of Some Chemical and Pharmacological Aspects. Part I: Chemical Aspects. Chem. Phys. Lipids 2002, 121, 35–43. [Google Scholar] [CrossRef]
- Fairbairn, J.W.; Liebmann, J.A.; Rowan, M.G. The Stability of Cannabis and Its Preparations on Storage. J. Pharm. Pharmacol. 1976, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Martin-Sabroso, C.; Torres-Suárez, A.I. Stability Characteristics of Cannabidiol for the Design of Pharmacological, Biochemical and Pharmaceutical Studies. J. Chromatogr. B 2020, 1150, 122188. [Google Scholar] [CrossRef]
- FDA and Cannabis: Research and Drug Approval Process|FDA. Available online: https://www.fda.gov/news-events/public-health-focus/fda-and-cannabis-research-and-drug-approval-process (accessed on 30 June 2023).
- Hasan, N.; Imran, M.; Sheikh, A.; Saad, S.; Chaudhary, G.; Jain, G.K.; Kesharwani, P.; Ahmad, F.J. Cannabis as a Potential Compound against Various Malignancies, Legal Aspects, Advancement by Exploiting Nanotechnology and Clinical Trials. J. Drug Target. 2022, 30, 709–725. [Google Scholar] [CrossRef]
- Regina Lazzarotto Rebelatto, E.; Schneider Rauber, G.; Caon, T. An update of nano-based drug delivery systems for cannabinoids: Biopharmaceutical aspects & therapeutic applications. Int. J. Pharm. 2023, 635, 122727. [Google Scholar] [CrossRef]
- Kebede, L.; Masoomi Dezfooli, S.; Seyfoddin, A. Medicinal Cannabis Pharmacokinetics and Potential Methods of Delivery. Pharm. Dev. Technol. 2022, 27, 202–214. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Suganthy, N.; Chau, T.P.; Sharma, A.; Unpaprom, Y.; Ramaraj, R.; Karuppusamy, I.; Brindhadevi, K. Cannabinoids as Anticancer and Neuroprotective Drugs: Structural Insights and Pharmacological Interactions—A Review. Process. Biochem. 2021, 111, 9–31. [Google Scholar] [CrossRef]
- Li, X.; Shen, L.; Hua, T.; Liu, Z.J. Structural and Functional Insights into Cannabinoid Receptors. Trends Pharmacol. Sci. 2020, 41, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Basagni, F.; Rosini, M.; Decker, M. Functionalized Cannabinoid Subtype 2 Receptor Ligands: Fluorescent, PET, Photochromic and Covalent Molecular Probes. ChemMedChem 2020, 15, 1374–1389. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; Bialer, M. Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Franco, V.; Gershkovich, P.; Perucca, E.; Bialer, M. The Interplay between Liver First-Pass Effect and Lymphatic Absorption of Cannabidiol and Its Implications for Cannabidiol Oral Formulations. Clin. Pharmacokinet. 2020, 59, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.R.; Alghalayini, A.; Valenzuela, S.M. Current Challenges and Opportunities for Improved Cannabidiol Solubility. Int. J. Mol. Sci. 2023, 24, 14514. [Google Scholar] [CrossRef]
- de Medeiros Ramalho, Í.M.; Pereira, D.T.; Galvão, G.B.L.; Freire, D.T.; Amaral-Machado, L.; do Nascimento Alencar, É.; do Egito, E.S.T. Current Trends on Cannabidiol Delivery Systems: Where Are We and Where Are We Going? Expert Opin. Drug Deliv. 2021, 18, 1577–1587. [Google Scholar] [CrossRef]
- Kuźmińska, J.; Sobczak, A.; Majchrzak-Celińska, A.; Żółnowska, I.; Gostyńska, A.; Jadach, B.; Krajka-Kuźniak, V.; Jelińska, A.; Stawny, M. Etoricoxib-Cannabidiol Combo: Potential Role in Glioblastoma Treatment and Development of PLGA-Based Nanoparticles. Pharmaceutics 2023, 15, 2104. [Google Scholar] [CrossRef]
- Dasram, M.H.; Walker, R.B.; Khamanga, S.M. Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 13223. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Martín-Banderas, L. Receptor-Targeted Nanoparticles Modulate Cannabinoid Anticancer Activity through Delayed Cell Internalization. Sci. Rep. 2022, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Blanco, J.; Sebastián, V.; Benoit, J.P.; Torres-Suárez, A.I. Lipid Nanocapsules Decorated and Loaded with Cannabidiol as Targeted Prolonged Release Carriers for Glioma Therapy: In Vitro Screening of Critical Parameters. Eur. J. Pharm. Biopharm. 2019, 134, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Gao, L.; Tan, Y.; Cai, J.; Ye, Z.; Chen, A.T.; Xu, Y.; Zhao, L.; Tong, S.; et al. Betulinic Acid Self-Assembled Nanoparticles for Effective Treatment of Glioblastoma. J. Nanobiotechnol. 2022, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Freire, N.F.; Feuser, P.E.; Ambel, E.M.T.; Cordani, M.; De Pieri, E.; Machado-de-Ávila, R.A.; Zielinski, A.A.F.; Sayer, C.; de Araújo, P.H.H.; Díez, G.V.; et al. Preparation and Characterization of Full-Spectrum Cannabis Extract Loaded Poly(Thioether-Ester) Nanoparticles: In Vitro Evaluation of Their Antitumoral Efficacy. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 658, 130676. [Google Scholar] [CrossRef]


 allows for targeted cellular internalization via adsorption-driven processes. (b) Carrier-mediated transport. This mechanism leverages the inherent selectivity of endothelial transporters, akin to molecular gatekeepers, for targeted nanoparticle internalisation. Functioning like protein shuttles, these transporters recognize specific ligands displayed on nanoparticle surfaces
allows for targeted cellular internalization via adsorption-driven processes. (b) Carrier-mediated transport. This mechanism leverages the inherent selectivity of endothelial transporters, akin to molecular gatekeepers, for targeted nanoparticle internalisation. Functioning like protein shuttles, these transporters recognize specific ligands displayed on nanoparticle surfaces  , facilitating their entry across the BBB endothelium. This selective binding triggers conformational changes in the transporter, creating a transient tunnel
, facilitating their entry across the BBB endothelium. This selective binding triggers conformational changes in the transporter, creating a transient tunnel  for the nanoparticle to bypass the tight junctions and enter the brain parenchyma. This enables precise delivery of encapsulated drugs directly to neuronal targets, bypassing the restrictive diffusion limitations and efflux pumps that pose significant challenges for conventional drug delivery. (c) Pinocytosis transport. Specific surface features on the nanoparticles are recognized by receptors, triggering membrane invagination and vesicle formation. This targeted transport offers advantages like enhanced uptake and potential for organelle-specific delivery, while presenting challenges like non-specificity and saturation. (d) Efflux pump mechanism. These pumps, embedded in the endothelial membrane, recognize specific chemical features on the nanoparticle surface, triggering conformational changes that propel the nanoparticle out of the cell. The membrane-bound ATP-binding cassette
for the nanoparticle to bypass the tight junctions and enter the brain parenchyma. This enables precise delivery of encapsulated drugs directly to neuronal targets, bypassing the restrictive diffusion limitations and efflux pumps that pose significant challenges for conventional drug delivery. (c) Pinocytosis transport. Specific surface features on the nanoparticles are recognized by receptors, triggering membrane invagination and vesicle formation. This targeted transport offers advantages like enhanced uptake and potential for organelle-specific delivery, while presenting challenges like non-specificity and saturation. (d) Efflux pump mechanism. These pumps, embedded in the endothelial membrane, recognize specific chemical features on the nanoparticle surface, triggering conformational changes that propel the nanoparticle out of the cell. The membrane-bound ATP-binding cassette  transporters efflux mechanism utilizes conformational changes within the transporter protein to generate a directional transport pathway, safeguarding the brain from potentially harmful substances. This efflux mechanism presents a formidable challenge for nanoparticle delivery, hindering their ability to reach their target sites within the brain. However, understanding the selectivity and mechanisms of efflux pumps can guide the design of nanoparticles with optimized surface properties, enabling them to evade detection and bypass this cellular blockade. (e) Receptor-mediated endocytosis. Nanoparticles functionalized with transferrin or LDL ligands
transporters efflux mechanism utilizes conformational changes within the transporter protein to generate a directional transport pathway, safeguarding the brain from potentially harmful substances. This efflux mechanism presents a formidable challenge for nanoparticle delivery, hindering their ability to reach their target sites within the brain. However, understanding the selectivity and mechanisms of efflux pumps can guide the design of nanoparticles with optimized surface properties, enabling them to evade detection and bypass this cellular blockade. (e) Receptor-mediated endocytosis. Nanoparticles functionalized with transferrin or LDL ligands  engage in specific, high-affinity interactions with cognate receptors on the endothelial cell surface. This ligand-receptor binding triggers clathrin-mediated pit formation
engage in specific, high-affinity interactions with cognate receptors on the endothelial cell surface. This ligand-receptor binding triggers clathrin-mediated pit formation  , resulting in the encapsulation of the nanoparticle within a membranous vesicle. This vesicle then undergoes scission and vesicular transport, delivering the nanoparticle directly into the brain parenchyma. Within the brain parenchyma, these nanoparticle-laden vesicles fuse with early endosomes
, resulting in the encapsulation of the nanoparticle within a membranous vesicle. This vesicle then undergoes scission and vesicular transport, delivering the nanoparticle directly into the brain parenchyma. Within the brain parenchyma, these nanoparticle-laden vesicles fuse with early endosomes  , initiating the intracellular sorting process. Early endosomes contain specific receptors and sorting molecules that recognize the nanoparticle’s surface ligands and direct its trafficking towards distinct intracellular compartments. The acidic lumen of the early endosome triggers ligand dissociation from the nanoparticle, allowing for potential ligand recycling and further processing of the nanoparticle. Redrawn with permission from Ndemazie, N.B., Multi-Disciplinary Approach for Drug and Gene Delivery Systems to the Brain. AAPS; published by PharmSciTech, 2022. “Methods of transporting materials across the BBB” by Ndemazie, et al. is licensed under CC BY 4.0 [206]. http://creativecommons.org/licenses/by/4.0/ (accessed on 22 July 2023).
, initiating the intracellular sorting process. Early endosomes contain specific receptors and sorting molecules that recognize the nanoparticle’s surface ligands and direct its trafficking towards distinct intracellular compartments. The acidic lumen of the early endosome triggers ligand dissociation from the nanoparticle, allowing for potential ligand recycling and further processing of the nanoparticle. Redrawn with permission from Ndemazie, N.B., Multi-Disciplinary Approach for Drug and Gene Delivery Systems to the Brain. AAPS; published by PharmSciTech, 2022. “Methods of transporting materials across the BBB” by Ndemazie, et al. is licensed under CC BY 4.0 [206]. http://creativecommons.org/licenses/by/4.0/ (accessed on 22 July 2023).
 allows for targeted cellular internalization via adsorption-driven processes. (b) Carrier-mediated transport. This mechanism leverages the inherent selectivity of endothelial transporters, akin to molecular gatekeepers, for targeted nanoparticle internalisation. Functioning like protein shuttles, these transporters recognize specific ligands displayed on nanoparticle surfaces
allows for targeted cellular internalization via adsorption-driven processes. (b) Carrier-mediated transport. This mechanism leverages the inherent selectivity of endothelial transporters, akin to molecular gatekeepers, for targeted nanoparticle internalisation. Functioning like protein shuttles, these transporters recognize specific ligands displayed on nanoparticle surfaces  , facilitating their entry across the BBB endothelium. This selective binding triggers conformational changes in the transporter, creating a transient tunnel
, facilitating their entry across the BBB endothelium. This selective binding triggers conformational changes in the transporter, creating a transient tunnel  for the nanoparticle to bypass the tight junctions and enter the brain parenchyma. This enables precise delivery of encapsulated drugs directly to neuronal targets, bypassing the restrictive diffusion limitations and efflux pumps that pose significant challenges for conventional drug delivery. (c) Pinocytosis transport. Specific surface features on the nanoparticles are recognized by receptors, triggering membrane invagination and vesicle formation. This targeted transport offers advantages like enhanced uptake and potential for organelle-specific delivery, while presenting challenges like non-specificity and saturation. (d) Efflux pump mechanism. These pumps, embedded in the endothelial membrane, recognize specific chemical features on the nanoparticle surface, triggering conformational changes that propel the nanoparticle out of the cell. The membrane-bound ATP-binding cassette
for the nanoparticle to bypass the tight junctions and enter the brain parenchyma. This enables precise delivery of encapsulated drugs directly to neuronal targets, bypassing the restrictive diffusion limitations and efflux pumps that pose significant challenges for conventional drug delivery. (c) Pinocytosis transport. Specific surface features on the nanoparticles are recognized by receptors, triggering membrane invagination and vesicle formation. This targeted transport offers advantages like enhanced uptake and potential for organelle-specific delivery, while presenting challenges like non-specificity and saturation. (d) Efflux pump mechanism. These pumps, embedded in the endothelial membrane, recognize specific chemical features on the nanoparticle surface, triggering conformational changes that propel the nanoparticle out of the cell. The membrane-bound ATP-binding cassette  transporters efflux mechanism utilizes conformational changes within the transporter protein to generate a directional transport pathway, safeguarding the brain from potentially harmful substances. This efflux mechanism presents a formidable challenge for nanoparticle delivery, hindering their ability to reach their target sites within the brain. However, understanding the selectivity and mechanisms of efflux pumps can guide the design of nanoparticles with optimized surface properties, enabling them to evade detection and bypass this cellular blockade. (e) Receptor-mediated endocytosis. Nanoparticles functionalized with transferrin or LDL ligands
transporters efflux mechanism utilizes conformational changes within the transporter protein to generate a directional transport pathway, safeguarding the brain from potentially harmful substances. This efflux mechanism presents a formidable challenge for nanoparticle delivery, hindering their ability to reach their target sites within the brain. However, understanding the selectivity and mechanisms of efflux pumps can guide the design of nanoparticles with optimized surface properties, enabling them to evade detection and bypass this cellular blockade. (e) Receptor-mediated endocytosis. Nanoparticles functionalized with transferrin or LDL ligands  engage in specific, high-affinity interactions with cognate receptors on the endothelial cell surface. This ligand-receptor binding triggers clathrin-mediated pit formation
engage in specific, high-affinity interactions with cognate receptors on the endothelial cell surface. This ligand-receptor binding triggers clathrin-mediated pit formation  , resulting in the encapsulation of the nanoparticle within a membranous vesicle. This vesicle then undergoes scission and vesicular transport, delivering the nanoparticle directly into the brain parenchyma. Within the brain parenchyma, these nanoparticle-laden vesicles fuse with early endosomes
, resulting in the encapsulation of the nanoparticle within a membranous vesicle. This vesicle then undergoes scission and vesicular transport, delivering the nanoparticle directly into the brain parenchyma. Within the brain parenchyma, these nanoparticle-laden vesicles fuse with early endosomes  , initiating the intracellular sorting process. Early endosomes contain specific receptors and sorting molecules that recognize the nanoparticle’s surface ligands and direct its trafficking towards distinct intracellular compartments. The acidic lumen of the early endosome triggers ligand dissociation from the nanoparticle, allowing for potential ligand recycling and further processing of the nanoparticle. Redrawn with permission from Ndemazie, N.B., Multi-Disciplinary Approach for Drug and Gene Delivery Systems to the Brain. AAPS; published by PharmSciTech, 2022. “Methods of transporting materials across the BBB” by Ndemazie, et al. is licensed under CC BY 4.0 [206]. http://creativecommons.org/licenses/by/4.0/ (accessed on 22 July 2023).
, initiating the intracellular sorting process. Early endosomes contain specific receptors and sorting molecules that recognize the nanoparticle’s surface ligands and direct its trafficking towards distinct intracellular compartments. The acidic lumen of the early endosome triggers ligand dissociation from the nanoparticle, allowing for potential ligand recycling and further processing of the nanoparticle. Redrawn with permission from Ndemazie, N.B., Multi-Disciplinary Approach for Drug and Gene Delivery Systems to the Brain. AAPS; published by PharmSciTech, 2022. “Methods of transporting materials across the BBB” by Ndemazie, et al. is licensed under CC BY 4.0 [206]. http://creativecommons.org/licenses/by/4.0/ (accessed on 22 July 2023).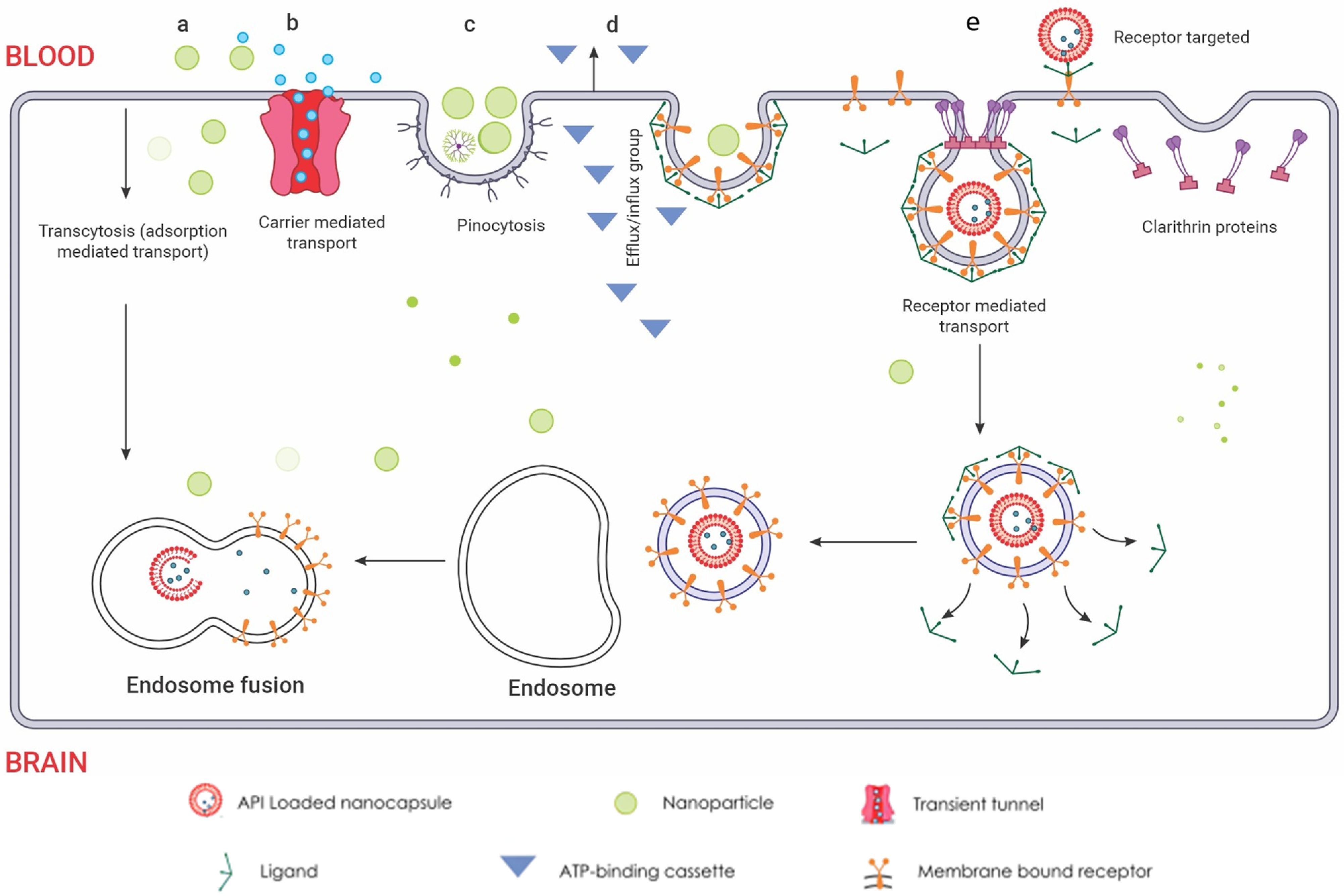
| Cell Type | Associated Tumours | |
|---|---|---|
| Astrocyte |  | Astrocytoma Pilocytic astrocytoma (grade I) Diffuse astrocytoma with IDH mutations (grade II) Anaplastic astrocytoma (grade III) |
| Oligodendrocyte |  | Oligodendrogliomas |
| Ependymal cells |  | Ependymomas |
| Microglia |  | Glioblastomas IDH-Wildtype (grade IV astrocytoma) [46] |
| Neural stem cells |  | |
| Molecular genotypic key markers explained | |
| IDH | IDH enzymes are involved in the citric acid cycle. Mutations in the genes coding for IDH1 and IDH2 are often found in gliomas and result in a neomorphic enzyme activity that produces 2-hydroxyglutarate, an oncometabolite [50]. This accumulation can lead to a hypermethylation phenotype and alterations in cell differentiation, contributing to oncogenesis. |
| p53 Mutations | The TP53 gene, which encodes the p53 protein, role in regulating the cell cycle and maintaining genomic stability [51]. Mutations in p53 are one of the most common genetic alterations in human cancers, leading to loss of function and allowing cells to proliferate unchecked. |
| ATRX | ATRX mutations are often present in gliomas and are associated with alternative lengthening of telomeres, a telomerase-independent mechanism for telomere maintenance [52]. These mutations can lead to genomic instability and have been linked with a specific subtype of gliomas that have a particular molecular signature and prognosis [53]. |
| 1p/19q Chromosomal Deletion | This co-deletion is a hallmark of oligodendrogliomas and is associated with a better response to chemotherapy and radiotherapy, as well as a more favourable prognosis [54]. The loss of these chromosome arms is believed to lead to the loss of tumour suppressor genes, although the precise mechanism by which this improves treatment response is not fully understood. |
| H3K27M | H3K27M mutations are characteristic of diffuse intrinsic pontine gliomas and some midline gliomas [55]. This mutation results in a gain-of-function that affects the methylation status of histone H3, thereby altering gene expression. These mutations are associated with a more aggressive disease course and have been recognized as a distinct entity in the WHO classification of tumours of the central nervous system. |
| Classical Cannabinoids |
| Classical cannabinoids are the most well-known group of cannabinoids, and they are found in the cannabis plant. They have a highly lipophilic structure and poor water-solubility due to their characteristic tricyclic terpenophenolic structure [109]. This lipophilicity facilitates easy passage through the lipid bilayers of cell membranes, influencing their absorption and distribution. Classical cannabinoids are extensively metabolised in the liver, primarily by cytochrome P450 enzymes, leading to a variety of metabolites, some of which are active and contribute to its pharmacological effects [110,111]. The high lipophilicity and poor water solubility of classical cannabinoids pose challenges in formulating them for aqueous-based delivery systems [112]. Techniques like nanoemulsions, liposomes, or microencapsulation may be employed to enhance solubility and bioavailability. Examples: THC, CBD, CBN |
| Non-Classical Cannabinoids |
| Non-classical cannabinoids, often synthetic cannabinoids that are not found in the cannabis plant, can be designed to have specific physicochemical properties [113]. They may be more potent and selective for cannabinoid receptors than classical cannabinoids [114]. They may be designed to have increased metabolic stability, thereby prolonging their duration of action [115]. However, their synthetic nature might lead to unpredictable metabolism and potential toxic metabolites. Formulation strategies would depend on the specific properties of the compound. Solubility enhancement and targeted delivery systems could be key considerations. Examples: CP 47497, CP 55940 |
| Aminoalkylindoles |
| Aminoalkylindoles have a simpler, more stable structure compared to classical cannabinoids. The aminoalkylindole chemical class can be subdivided into four groups: naphthoylindoles, phenylacetylindoles, benzoylindoles, and naphthylmethylindoles [116]. This influences their interaction with cannabinoid receptors, making them more selective for cannabinoid receptors [117]. These compounds generally have high lipophilicity and may show significant brain penetration due to their ability to cross the blood–brain barrier efficiently. Similar to classical cannabinoids, addressing solubility and stability issues is critical [118]. There’s also a need to consider the potential for rapid onset of action due to efficient CNS penetration. Examples: WIN-55212-2, JWH-018 |
| Eicosanoids |
| Endocannabinoids including anandamide and 2-AG are derived from fatty acids, making them lipophilic structures [119,120]. This allows easier cellular uptake and interaction with cannabinoid receptors [121]. Endocannabinoids are rapidly metabolised in the body, which can limit their therapeutic use unless modifications or delivery systems are employed to stabilise them [122]. Enhancing stability and prolonging the duration of action are primary goals. Techniques might include the use of enzyme inhibitors to prevent rapid degradation or using advanced delivery systems to target specific tissues. Examples: Anandamide, 2-AG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasram, M.H.; Naidoo, P.; Walker, R.B.; Khamanga, S.M. Targeting the Endocannabinoid System Present in the Glioblastoma Tumour Microenvironment as a Potential Anti-Cancer Strategy. Int. J. Mol. Sci. 2024, 25, 1371. https://doi.org/10.3390/ijms25031371
Dasram MH, Naidoo P, Walker RB, Khamanga SM. Targeting the Endocannabinoid System Present in the Glioblastoma Tumour Microenvironment as a Potential Anti-Cancer Strategy. International Journal of Molecular Sciences. 2024; 25(3):1371. https://doi.org/10.3390/ijms25031371
Chicago/Turabian StyleDasram, Mendhi Henna, Pavesan Naidoo, Roderick B. Walker, and Sandile M. Khamanga. 2024. "Targeting the Endocannabinoid System Present in the Glioblastoma Tumour Microenvironment as a Potential Anti-Cancer Strategy" International Journal of Molecular Sciences 25, no. 3: 1371. https://doi.org/10.3390/ijms25031371
APA StyleDasram, M. H., Naidoo, P., Walker, R. B., & Khamanga, S. M. (2024). Targeting the Endocannabinoid System Present in the Glioblastoma Tumour Microenvironment as a Potential Anti-Cancer Strategy. International Journal of Molecular Sciences, 25(3), 1371. https://doi.org/10.3390/ijms25031371








