Mannose Ligands for Mannose Receptor Targeting
Abstract
1. Introduction
2. Structure and Function of MR
3. Mannose-Based Glycomimetics as Ligands for MR
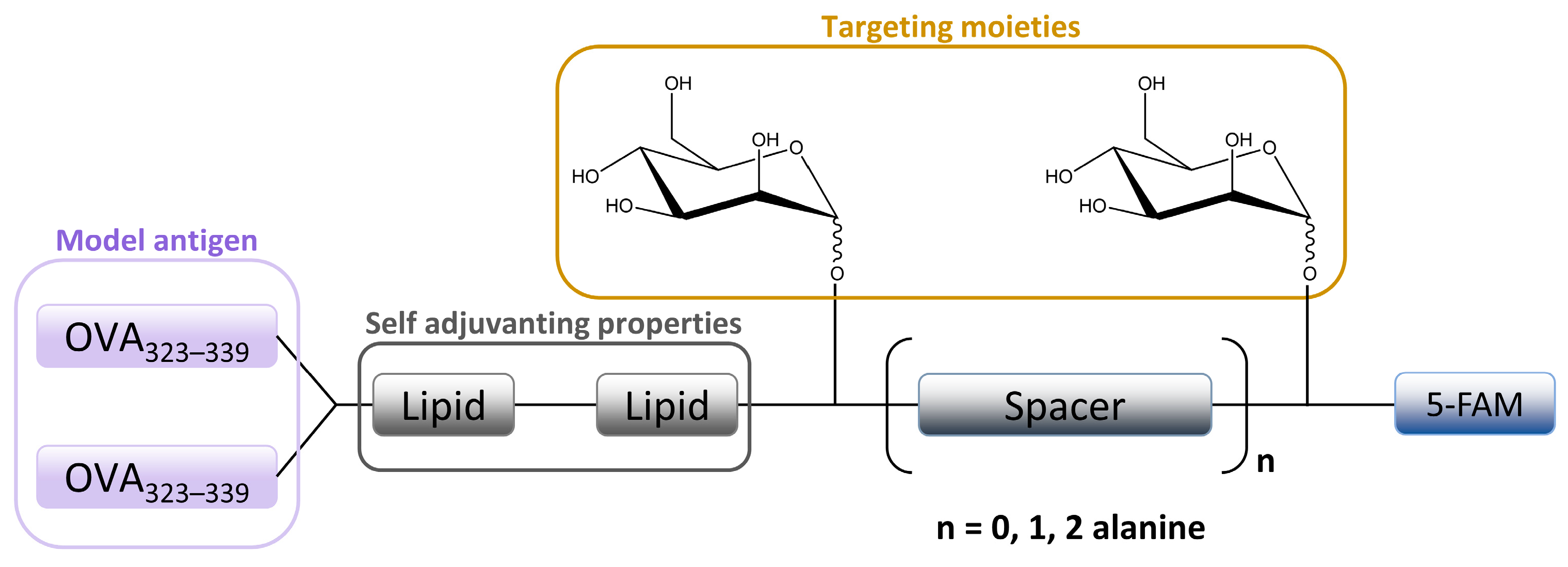
3.1. Mannosylated Peptides and Proteins
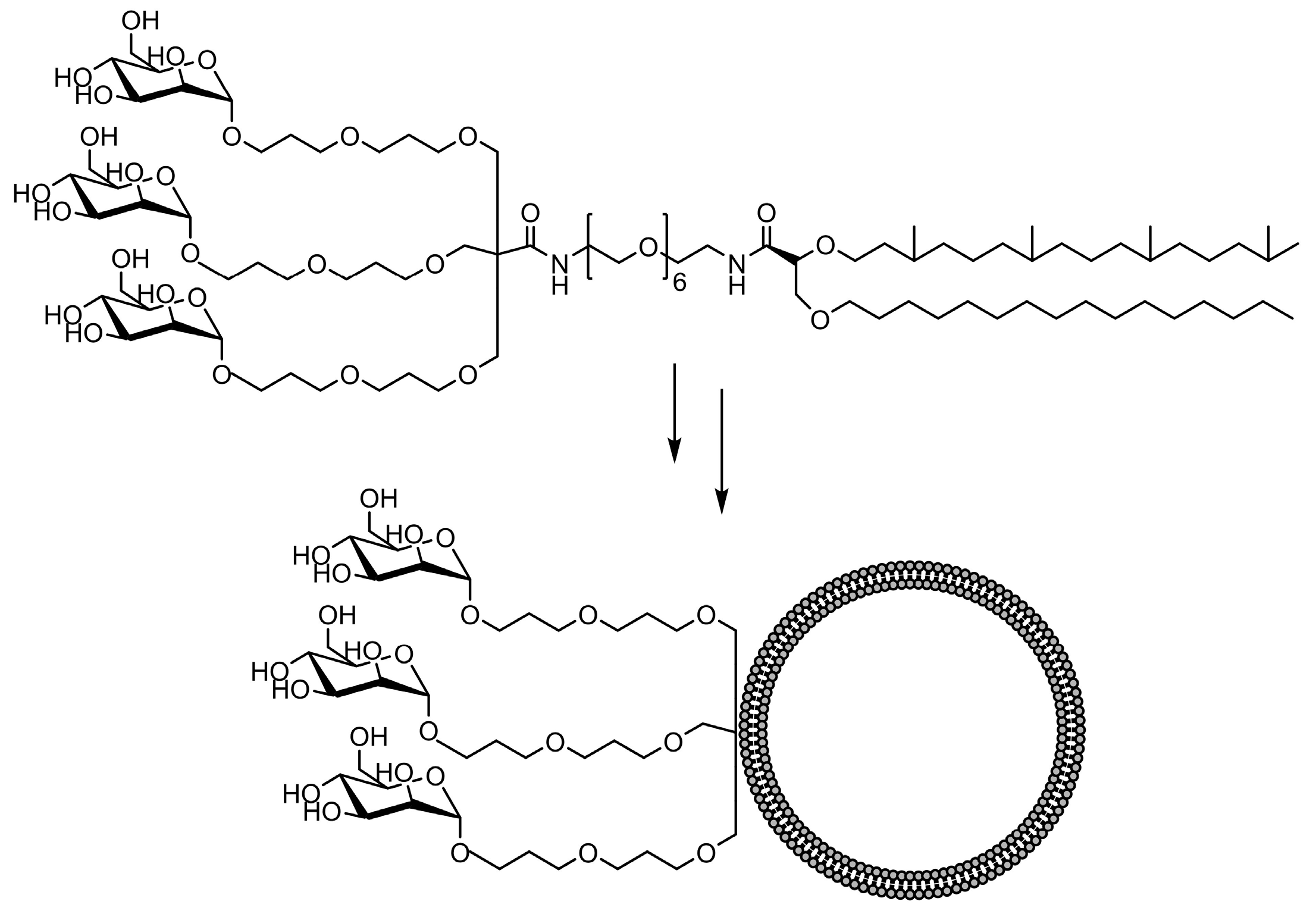
3.2. Mannosylated Lipids and Liposomes
3.3. Mannosylated Nanoparticles (NPs)
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Zande, H.J.P.; Nitsche, D.; Schlautmann, L.; Guigas, B.; Burgdorf, S. The Mannose Receptor: From Endocytic Receptor and Biomarker to Regulator of (Meta)Inflammation. Front. Immunol. 2021, 12, 765034. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D. The Mannose Receptor Ligands and the Macrophage Glycome. Curr. Opin. Struct. Biol. 2022, 75, 102394. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Bezouska, K.; Drickamer, K. Contribution to Ligand Binding by Multiple Carbohydrate-Recognition Domains in the Macrophage Mannose Receptor. J. Biol. Chem. 1992, 267, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Drickamer, K. Structural Requirements for High Affinity Binding of Complex Ligands by the Macrophage Mannose Receptor. J. Biol. Chem. 1993, 268, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Rajaram, M.V.S.; Schlesinger, L.S. Exploitation of the Macrophage Mannose Receptor (CD206) in Infectious Disease Diagnostics and Therapeutics. J. Cytol. Mol. Biol. 2014, 1, 1000003. [Google Scholar] [CrossRef]
- Nguyen, D.G.; Hildreth, J.E.K. Involvement of Macrophage Mannose Receptor in the Binding and Transmission of HIV by Macrophages. Eur. J. Immunol. 2003, 33, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, H.; Jégouzo, S.A.F.; Lasanajak, Y.; Smith, D.F.; Drickamer, K.; Weis, W.I.; Taylor, M.E. Structural Analysis of Carbohydrate Binding by the Macrophage Mannose Receptor CD206. J. Biol. Chem. 2021, 296, 100368. [Google Scholar] [CrossRef]
- Lundquist, J.J.; Toone, E.J. The Cluster Glycoside Effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef]
- Irache, J.M.; Salman, H.H.; Gamazo, C.; Espuelas, S. Mannose-Targeted Systems for the Delivery of Therapeutics. Expert Opin. Drug Deliv. 2008, 5, 703–724. [Google Scholar] [CrossRef]
- Lepenies, B.; Lee, J.; Sonkaria, S. Targeting C-Type Lectin Receptors with Multivalent Carbohydrate Ligands. Adv. Drug Deliv. Rev. 2013, 65, 1271–1281. [Google Scholar] [CrossRef]
- Nahar, U.J.; Toth, I.; Skwarczynski, M. Mannose in Vaccine Delivery. J. Control. Release 2022, 351, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, B.; Stephenson, R.; Toth, I. Targeting the Mannose Receptor with Mannosylated Subunit Vaccines. Curr. Med. Chem. 2014, 21, 3405–3418. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S. Mannosylated Constructs as a Platform for Cell-Specific Delivery of Bioactive Agents. Crit. Rev. Ther. Drug Carrier Syst. 2018, 35, 157–194. [Google Scholar] [CrossRef] [PubMed]
- Al-Barwani, F.; Young, S.L.; Baird, M.A.; Larsen, D.S.; Ward, V.K. Mannosylation of Virus-Like Particles Enhances Internalization by Antigen Presenting Cells. PLoS ONE 2014, 9, e104523. [Google Scholar] [CrossRef] [PubMed]
- Glaffig, M.; Stergiou, N.; Hartmann, S.; Schmitt, E.; Kunz, H. A Synthetic MUC1 Anticancer Vaccine Containing Mannose Ligands for Targeting Macrophages and Dendritic Cells. ChemMedChem 2018, 13, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Boonyarattanakalin, S.; Liu, X.; Michieletti, M.; Lepenies, B.; Seeberger, P.H. Chemical Synthesis of All Phosphatidylinositol Mannoside (PIM) Glycans from Mycobacterium Tuberculosis. J. Am. Chem. Soc. 2008, 130, 16791–16799. [Google Scholar] [CrossRef] [PubMed]
- Del Bino, L.; Romano, M.R. Role of Carbohydrate Antigens in Antifungal Glycoconjugate Vaccines and Immunotherapy. Drug Discov. Today Technol. 2020, 38, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Hirosue, S.; Raczy, M.M.; Bonilla-Ramirez, L.; Jeanbart, L.; Wang, R.; Kwissa, M.; Franetich, J.-F.; Broggi, M.A.S.; Diaceri, G.; et al. Antigens Reversibly Conjugated to a Polymeric Glyco-Adjuvant Induce Protective Humoral and Cellular Immunity. Nat. Mater. 2019, 18, 175–185. [Google Scholar] [CrossRef]
- Surasi, D.S.; O’Malley, J.; Bhambhvani, P. 99mTc-Tilmanocept: A Novel Molecular Agent for Lymphatic Mapping and Sentinel Lymph Node Localization. J. Nucl. Med. Technol. 2015, 43, 87–91. [Google Scholar] [CrossRef]
- Bellato, F.; Feola, S.; Dalla Verde, G.; Bellio, G.; Pirazzini, M.; Salmaso, S.; Caliceti, P.; Cerullo, V.; Mastrotto, F. Mannosylated Polycations Target CD206+ Antigen-Presenting Cells and Mediate T-Cell-Specific Activation in Cancer Vaccination. Biomacromolecules 2022, 23, 5148–5163. [Google Scholar] [CrossRef]
- Kvakova, K.; Ondra, M.; Schimer, J.; Petrik, M.; Novy, Z.; Raabova, H.; Hajduch, M.; Cigler, P. Visualization of Sentinel Lymph Nodes with Mannosylated Fluorescent Nanodiamonds. Adv. Funct. Mater. 2022, 32, 2109960. [Google Scholar] [CrossRef]
- Chae, J.; Hyun Kang, S.; Kim, J.; Choi, Y.; Hyuk Kang, S.; Choi, J. Targeted and Efficient Delivery of Rifampicin to Macrophages Involved in Non-Tuberculous Mycobacterial Infection via Mannosylated Solid Lipid Nanoparticles. Nanoscale Adv. 2023, 5, 4536–4545. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, P.; Singh, Y.; Zhang, X.; Szekely, Z.; Stein, S.; Sinko, P.J. Novel Monodisperse PEGtide Dendrons: Design, Fabrication, and Evaluation of Mannose Receptor-Mediated Macrophage Targeting. Bioconjug. Chem. 2013, 24, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, B.; Stephenson, R.J.; Giddam, A.K.; Eskandari, S.; Apte, S.H.; Pattinson, D.J.; Doolan, D.L.; Toth, I. Synthesis of Mannosylated Lipopeptides with Receptor Targeting Properties. Bioconjug. Chem. 2016, 27, 533–548. [Google Scholar] [CrossRef]
- Brimble, M.A.; Kowalczyk, R.; Harris, P.W.R.; Dunbar, P.R.; Muir, V.J. Synthesis of Fluorescein-Labelled O-Mannosylated Peptides as Components for Synthetic Vaccines: Comparison of Two Synthetic Strategies. Org. Biomol. Chem. 2007, 6, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.S.; Lau, C.M.; Barham, W.J.; Onishko, H.M.; Nelson, C.E.; Li, H.; Smith, C.A.; Yull, F.E.; Duvall, C.L.; Giorgio, T.D. Macrophage-Specific RNA Interference Targeting via “Click”, Mannosylated Polymeric Micelles. Mol. Pharm. 2013, 10, 975–987. [Google Scholar] [CrossRef]
- Glass, E.B.; Masjedi, S.; Dudzinski, S.O.; Wilson, A.J.; Duvall, C.L.; Yull, F.E.; Giorgio, T.D. Optimizing Mannose “Click” Conjugation to Polymeric Nanoparticles for Targeted siRNA Delivery to Human and Murine Macrophages. ACS Omega 2019, 4, 16756–16767. [Google Scholar] [CrossRef]
- East, L.; Isacke, C.M. The Mannose Receptor Family. Biochim. Biophys. Acta BBA-Gen. Subj. 2002, 1572, 364–386. [Google Scholar] [CrossRef]
- Ramkumar, T.P.; Hammache, D.; Stahl, P.D. The Macrophage Mannose Receptor and Innate Immunity. In Innate Immunity; Ezekowitz, R.A.B., Hoffmann, J.A., Eds.; Infectious Disease; Humana Press: Totowa, NJ, USA, 2003; pp. 191–204. ISBN 978-1-59259-320-0. [Google Scholar]
- Raposo, C.D.; Canelas, A.B.; Barros, M.T. Human Lectins, Their Carbohydrate Affinities and Where to Find Them. Biomolecules 2021, 11, 188. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; Mckenzie, I.F.C. Role of the Mannose Receptor in the Immune Response. Curr. Mol. Med. 2001, 1, 469–474. [Google Scholar] [CrossRef]
- Taylor, P.R.; Gordon, S.; Martinez-Pomares, L. The Mannose Receptor: Linking Homeostasis and Immunity through Sugar Recognition. Trends Immunol. 2005, 26, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Nour, J.; Moregola, A.; Svecla, M.; Da Dalt, L.; Bellini, R.; Neyrolles, O.; Fadini, G.P.; Rombouts, Y.; Albiero, M.; Bonacina, F.; et al. Mannose Receptor Deficiency Impacts Bone Marrow and Circulating Immune Cells during High Fat Diet Induced Obesity. Metabolites 2022, 12, 1205. [Google Scholar] [CrossRef] [PubMed]
- Rahabi, M.; Jacquemin, G.; Prat, M.; Meunier, E.; AlaEddine, M.; Bertrand, B.; Lefèvre, L.; Benmoussa, K.; Batigne, P.; Aubouy, A.; et al. Divergent Roles for Macrophage C-Type Lectin Receptors, Dectin-1 and Mannose Receptors, in the Intestinal Inflammatory Response. Cell Rep. 2020, 30, 4386–4398.e5. [Google Scholar] [CrossRef] [PubMed]
- Gazi, U.; Martinez-Pomares, L. Influence of the Mannose Receptor in Host Immune Responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Uehara, K.; Harumoto, T.; Makino, A.; Koda, Y.; Iwano, J.; Suzuki, Y.; Tanigawa, M.; Iwai, H.; Asano, K.; Kurihara, K.; et al. Targeted Delivery to Macrophages and Dendritic Cells by Chemically Modified Mannose Ligand-Conjugated siRNA. Nucleic Acids Res. 2022, 50, 4840–4859. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Chieppa, M.; Monti, P.; Piemonti, L. From Pattern Recognition Receptor to Regulator of Homeostasis: The Double-Faced Macrophage Mannose Receptor. Crit. Rev. Immunol. 2004, 24, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, A.; Stahl, P.D.; Rohrer, J. A Di-Aromatic Motif in the Cytosolic Tail of the Mannose Receptor Mediates Endosomal Sorting*. J. Biol. Chem. 2000, 275, 29694–29700. [Google Scholar] [CrossRef]
- Mastrotto, F.; Pirazzini, M.; Negro, S.; Salama, A.; Martinez-Pomares, L.; Mantovani, G. Sulfation at Glycopolymer Side Chains Switches Activity at the Macrophage Mannose Receptor (CD206) In Vitro and In Vivo. J. Am. Chem. Soc. 2022, 144, 23134–23147. [Google Scholar] [CrossRef]
- Martinez-Pomares, L.; Wienke, D.; Stillion, R.; McKenzie, E.J.; Arnold, J.N.; Harris, J.; McGreal, E.; Sim, R.B.; Isacke, C.M.; Gordon, S. Carbohydrate-Independent Recognition of Collagens by the Macrophage Mannose Receptor. Eur. J. Immunol. 2006, 36, 1074–1082. [Google Scholar] [CrossRef]
- Weis, W.I.; Drickamer, K.; Hendrickson, W.A. Structure of a C-Type Mannose-Binding Protein Complexed with an Oligosaccharide. Nature 1992, 360, 127–134. [Google Scholar] [CrossRef]
- Mullin, N.P.; Hall, K.T.; Taylor, M.E. Characterization of Ligand Binding to a Carbohydrate-Recognition Domain of the Macrophage Mannose Receptor. J. Biol. Chem. 1994, 269, 28405–28413. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, V.L.; Lee, Y.C.; Schlesinger, P.H.; Stahl, P.D. L-Fucose-Terminated Glycoconjugates Are Recognized by Pinocytosis Receptors on Macrophages. Proc. Natl. Acad. Sci. USA 1981, 78, 1019–1022. [Google Scholar] [CrossRef]
- Kéry, V.; Křepinský, J.J.F.; Warren, C.D.; Capek, P.; Stahl, P.D. Ligand Recognition by Purified Human Mannose Receptor. Arch. Biochem. Biophys. 1992, 298, 49–55. [Google Scholar] [CrossRef]
- Napper, C.E.; Dyson, M.H.; Taylor, M.E. An Extended Conformation of the Macrophage Mannose Receptor*. J. Biol. Chem. 2001, 276, 14759–14766. [Google Scholar] [CrossRef]
- Hu, Z.; Shi, X.; Yu, B.; Li, N.; Huang, Y.; He, Y. Structural Insights into the pH-Dependent Conformational Change and Collagen Recognition of the Human Mannose Receptor. Structure 2018, 26, 60–71.e3. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, Y.; Cheng, C.; He, Y. Structural Basis of the pH-Dependent Conformational Change of the N-Terminal Region of Human Mannose Receptor/CD206. J. Struct. Biol. 2019, 208, 107384. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.E.; Conary, J.T.; Lennartz, M.R.; Stahl, P.D.; Drickamer, K. Primary Structure of the Mannose Receptor Contains Multiple Motifs Resembling Carbohydrate-Recognition Domains. J. Biol. Chem. 1990, 265, 12156–12162. [Google Scholar] [CrossRef]
- Jordens, R.; Thompson, A.; Amons, R.; Koning, F. Human Dendritic Cells Shed a Functional, Soluble Form of the Mannose Receptor. Int. Immunol. 1999, 11, 1775–1780. [Google Scholar] [CrossRef][Green Version]
- Martínez-Pomares, L.; Mahoney, J.A.; Káposzta, R.; Linehan, S.A.; Stahl, P.D.; Gordon, S. A Functional Soluble Form of the Murine Mannose Receptor Is Produced by Macrophages in Vitro and Is Present in Mouse Serum*. J. Biol. Chem. 1998, 273, 23376–23380. [Google Scholar] [CrossRef]
- Fiani, M.L.; Barreca, V.; Sargiacomo, M.; Ferrantelli, F.; Manfredi, F.; Federico, M. Exploiting Manipulated Small Extracellular Vesicles to Subvert Immunosuppression at the Tumor Microenvironment through Mannose Receptor/CD206 Targeting. Int. J. Mol. Sci. 2020, 21, 6318. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Andersen, M.N.; Grønbæk, H.; Damgaard Sandahl, T.; Møller, H.J. Extracellular Vesicle-Associated Soluble CD163 and CD206 in Patients with Acute and Chronic Inflammatory Liver Disease. Scand. J. Gastroenterol. 2020, 55, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Tornai, D.; Kodys, K.; Adejumo, A.; Lowe, P.; McClain, C.; Mitchell, M.; McCullough, A.; Dasarathy, S.; Kroll-Desrosiers, A.; et al. Biomarkers of Macrophage Activation and Immune Danger Signals Predict Clinical Outcomes in Alcoholic Hepatitis. Hepatology 2019, 70, 1134. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.; Jost, C.; Ilie, I.M.; Schnabl, J.; Buechi, O.; Eapen, R.S.; Truffer, R.; Caflisch, A.; Forrer, P. Thermostable Designed Ankyrin Repeat Proteins (DARPins) as Building Blocks for Innovative Drugs. J. Biol. Chem. 2022, 298, 101403. [Google Scholar] [CrossRef] [PubMed]
- Filatova, L.Y.; Klyachko, N.L.; Kudryashova, E.V. Targeted Delivery of Anti-Tuberculosis Drugs to Macrophages: Targeting Mannose Receptors. Russ. Chem. Rev. 2018, 87, 374. [Google Scholar] [CrossRef]
- Zheng, F.; Asim, M.; Lan, J.; Zhao, L.; Wei, S.; Chen, N.; Liu, X.; Zhou, Y.; Lin, L. Molecular Cloning and Functional Characterization of Mannose Receptor in Zebra Fish (Danio Rerio) during Infection with Aeromonas Sobria. Int. J. Mol. Sci. 2015, 16, 10997–11012. [Google Scholar] [CrossRef]
- Esparza, M.; Palomares, B.; García, T.; Espinosa, P.; Zenteno, E.; Mancilla, R. PstS-1, the 38-kDa Mycobacterium Tuberculosis Glycoprotein, Is an Adhesin, Which Binds the Macrophage Mannose Receptor and Promotes Phagocytosis. Scand. J. Immunol. 2015, 81, 46–55. [Google Scholar] [CrossRef]
- Upham, J.P.; Pickett, D.; Irimura, T.; Anders, E.M.; Reading, P.C. Macrophage Receptors for Influenza A Virus: Role of the Macrophage Galactose-Type Lectin and Mannose Receptor in Viral Entry. J. Virol. 2010, 84, 3730–3737. [Google Scholar] [CrossRef]
- Reading, P.C.; Miller, J.L.; Anders, E.M. Involvement of the Mannose Receptor in Infection of Macrophages by Influenza Virus. J. Virol. 2000, 74, 5190–5197. [Google Scholar] [CrossRef]
- Ezekowitz, R.A.; Sastry, K.; Bailly, P.; Warner, A. Molecular Characterization of the Human Macrophage Mannose Receptor: Demonstration of Multiple Carbohydrate Recognition-like Domains and Phagocytosis of Yeasts in Cos-1 Cells. J. Exp. Med. 1990, 172, 1785–1794. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, G.; Lin, J.; Li, C.; Jiang, N.; Xu, Q.; Wang, Q.; Zhang, J. Role of the Mannose Receptor During Aspergillus Fumigatus Infection and Interaction With Dectin-1 in Corneal Epithelial Cells. Cornea 2016, 35, 267. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y.; Lee, Y.C. Mannose-Receptor Ligands Stimulate Secretion of Lysosomal Enzymes from Rabbit Alveolar Macrophages. J. Biol. Chem. 1987, 262, 7955–7962. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, H.; Lee, G.; Jang, H.; Kim, S.-S.; Yoon, H.; Kang, G.-H.; Hwang, D.-S.; Kim, S.K.; Chung, H.-S.; et al. Phospholipase A2 Inhibits Cisplatin-Induced Acute Kidney Injury by Modulating Regulatory T Cells by the CD206 Mannose Receptor. Kidney Int. 2015, 88, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Thalhammer, T.; Tzakos, A.G.; Stojanovska, L. Targeting Antigens to Dendritic Cell Receptors for Vaccine Development. J. Drug Deliv. 2013, 2013, e869718. [Google Scholar] [CrossRef] [PubMed]
- Chieppa, M.; Bianchi, G.; Doni, A.; Del Prete, A.; Sironi, M.; Laskarin, G.; Monti, P.; Piemonti, L.; Biondi, A.; Mantovani, A.; et al. Cross-Linking of the Mannose Receptor on Monocyte-Derived Dendritic Cells Activates an Anti-Inflammatory Immunosuppressive Program 1. J. Immunol. 2003, 171, 4552–4560. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Pietersz, G.A.; Gordon, S.; Martinez-Pomares, L.; McKenzie, I.F.C. Aldehyde-Mannan Antigen Complexes Target the MHC Class I Antigen-Presentation Pathway. Eur. J. Immunol. 2000, 30, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Gac, S.; Coudane, J.; Boustta, M.; Domurado, M.; Vert, M. Synthesis, Characterisation and In Vivo Behaviour of a Norfloxacin-Poly(L-Lysine Citramide Imide) Conjugate Bearing Mannosyl Residues. J. Drug Target. 1999, 7, 393–406. [Google Scholar] [CrossRef]
- Nishikawa, M.; Takemura, S.; Yamashita, F.; Takakura, Y.; Meijer, D.; Hashida, M.; Swart, P. Pharmacokinetics and In Vivo Gene Transfer of Plasmid DNA Complexed with Mannosylated Poly(L-Lysine) in Mice. J. Drug Target. 2000, 8, 29–38. [Google Scholar] [CrossRef]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose Receptor (CD206) Activation in Tumor-Associated Macrophages Enhances Adaptive and Innate Antitumor Immune Responses. Sci. Transl. Med. 2020, 12, eaax6337. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, S.; Wu, P.; Lyu, B.; Qin, M.; Sun, Y.; Sun, A.; Mu, L.; Xu, F.; Zhang, L.; et al. Tumor Associated Macrophages and TAMs-Based Anti-Tumor Nanomedicines. Adv. Healthc. Mater. 2021, 10, 2100590. [Google Scholar] [CrossRef]
- Zang, X.; Zhang, X.; Hu, H.; Qiao, M.; Zhao, X.; Deng, Y.; Chen, D. Targeted Delivery of Zoledronate to Tumor-Associated Macrophages for Cancer Immunotherapy. Mol. Pharm. 2019, 16, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Zhou, J.; Zhang, X.; Chen, D.; Han, Y.; Chen, X. Dual-Targeting Tumor Cells and Tumor Associated Macrophages with Lipid Coated Calcium Zoledronate for Enhanced Lung Cancer Chemoimmunotherapy. Int. J. Pharm. 2021, 594, 120174. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Mei, X. Synthetic Glycosylated Natural Products Have Satisfactory Activities. Curr. Drug Targets 2014, 15, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G. Application of Glycosylation in Targeted Drug Delivery. Eur. J. Med. Chem. 2019, 182, 111612. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Ramanujam, D.; Vaccarello, P.; Widenmeyer, F.; Feuerherd, M.; Cheng, C.-C.; Bomhard, A.; Abikeeva, T.; Schädler, J.; Sperhake, J.-P.; et al. Trimannose-Coupled antimiR-21 for Macrophage-Targeted Inhalation Treatment of Acute Inflammatory Lung Damage. Nat. Commun. 2023, 14, 4564. [Google Scholar] [CrossRef]
- Godula, K.; Bertozzi, C.R. Density Variant Glycan Microarray for Evaluating Cross-Linking of Mucin-like Glycoconjugates by Lectins. J. Am. Chem. Soc. 2012, 134, 15732–15742. [Google Scholar] [CrossRef]
- Lusvarghi, S.; Ghirlando, R.; Wong, C.-H.; Bewley, C.A. Glycopeptide Mimetics Recapitulate High-Mannose-Type Oligosaccharide Binding and Function. Angew. Chem. Int. Ed. 2015, 54, 5603–5608. [Google Scholar] [CrossRef]
- François-Heude, M.; Méndez-Ardoy, A.; Cendret, V.; Lafite, P.; Daniellou, R.; Ortiz Mellet, C.; García Fernández, J.M.; Moreau, V.; Djedaïni-Pilard, F. Synthesis of High-Mannose Oligosaccharide Analogues through Click Chemistry: True Functional Mimics of Their Natural Counterparts Against Lectins? Chem.—Eur. J. 2015, 21, 1978–1991. [Google Scholar] [CrossRef]
- Cendret, V.; François-Heude, M.; Méndez-Ardoy, A.; Moreau, V.; Fernández, J.M.G.; Djedaïni-Pilard, F. Design and Synthesis of a “Click” High-Mannose Oligosaccharide Mimic Emulating Man8 Binding Affinity towards Con A. Chem. Commun. 2012, 48, 3733–3735. [Google Scholar] [CrossRef]
- Boehnke, N.; Dolph, K.J.; Juarez, V.M.; Lanoha, J.M.; Hammond, P.T. Electrostatic Conjugation of Nanoparticle Surfaces with Functional Peptide Motifs. Bioconjug. Chem. 2020, 31, 2211–2219. [Google Scholar] [CrossRef]
- Sidorov, I.A.; Prabakaran, P.; Dimitrov, D.S. Non-Covalent Conjugation of Nanoparticles to Antibodies via Electrostatic Interactions—A Computational Model. J. Comput. Theor. Nanosci. 2007, 4, 1103–1107. [Google Scholar] [CrossRef]
- Weiss, A.M.; Hossainy, S.; Rowan, S.J.; Hubbell, J.A.; Esser-Kahn, A.P. Immunostimulatory Polymers as Adjuvants, Immunotherapies, and Delivery Systems. Macromolecules 2022, 55, 6913–6937. [Google Scholar] [CrossRef] [PubMed]
- Menon, I.; Zaroudi, M.; Zhang, Y.; Aisenbrey, E.; Hui, L. Fabrication of Active Targeting Lipid Nanoparticles: Challenges and Perspectives. Mater. Today Adv. 2022, 16, 100299. [Google Scholar] [CrossRef]
- Otvos, L.; Urge, L.; Xiang, Z.Q.; Krivulka, G.R.; Nagy, L.; Szendrei, G.I.; Ertl, H.C.J. Glycosylation of Synthetic T Helper Cell Epitopic Peptides Influences Their Antigenic Potency and Conformation in a Sugar Location-Specific Manner. Biochim. Biophys. Acta BBA-Mol. Cell Res. 1994, 1224, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, E. The Role of Glycosylation in Protein Antigenic Properties. Cell. Mol. Life Sci. CMLS 2002, 59, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, A.; Sjöblom, M.; Strindelius, L.; Johansson, T.; Fleckenstein, T.; Chatzissavidou, N.; Lindberg, L.; Ångström, J.; Rova, U.; Holgersson, J. Pichia Pastoris-Produced Mucin-Type Fusion Proteins with Multivalent O-Glycan Substitution as Targeting Molecules for Mannose-Specific Receptors of the Immune System. Glycobiology 2011, 21, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Ahlén, G.; Strindelius, L.; Johansson, T.; Nilsson, A.; Chatzissavidou, N.; Sjöblom, M.; Rova, U.; Holgersson, J. Mannosylated Mucin-Type Immunoglobulin Fusion Proteins Enhance Antigen-Specific Antibody and T Lymphocyte Responses. PLoS ONE 2012, 7, e46959. [Google Scholar] [CrossRef]
- Ribić, R.; Habjanec, L.; Vranešić, B.; Frkanec, R.; Tomić, S. Synthesis and Biological Evaluation of New Mannose Derived Immunomodulating Adamantyltripeptides. Croat. Chem. Acta 2011, 84, 233–244. [Google Scholar] [CrossRef]
- Ribić, R.; Stojković, R.; Milković, L.; Antica, M.; Cigler, M.; Tomić, S. Design, Synthesis and Biological Evaluation of Immunostimulating Mannosylated Desmuramyl Peptides. Beilstein J. Org. Chem. 2019, 15, 1805–1814. [Google Scholar] [CrossRef]
- Maršavelski, A.; Paurević, M.; Ribić, R. Mannosylated Adamantane-Containing Desmuramyl Peptide Recognition by the NOD2 Receptor: A Molecular Dynamics Study. Org. Biomol. Chem. 2021, 19, 7001–7012. [Google Scholar] [CrossRef]
- Moad, G. RAFT Polymerization to Form Stimuli-Responsive Polymers. Polym. Chem. 2016, 8, 177–219. [Google Scholar] [CrossRef]
- Chuang, V.T.G.; Kragh-Hansen, U.; Otagiri, M. Pharmaceutical Strategies Utilizing Recombinant Human Serum Albumin. Pharm. Res. 2002, 19, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Maruyama, T.; Watanabe, H.; Maeda, H.; Nakajou, K.; Iwao, Y.; Ishima, Y.; Katsumi, H.; Hashida, M.; Otagiri, M. Genetically Engineered Mannosylated-Human Serum Albumin as a Versatile Carrier for Liver-Selective Therapeutics. J. Control. Release 2010, 145, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, Y.; Maeda, H.; Ishima, Y.; Minayoshi, Y.; Ichimizu, S.; Kinoshita, R.; Fujita, I.; Kai, T.; Hirata, K.; Nakamura, T.; et al. A Mannosylated, PEGylated Albumin as a Drug Delivery System for the Treatment of Cancer Stroma Cells. Adv. Funct. Mater. 2021, 31, 2104136. [Google Scholar] [CrossRef]
- Pan, Z.; Kang, X.; Zeng, Y.; Zhang, W.; Peng, H.; Wang, J.; Huang, W.; Wang, H.; Shen, Y.; Huang, Y. A Mannosylated PEI–CPP Hybrid for TRAIL Gene Targeting Delivery for Colorectal Cancer Therapy. Polym. Chem. 2017, 8, 5275–5285. [Google Scholar] [CrossRef]
- Kang, X.; Wang, H.; Peng, H.; Chen, B.; Zhang, W.; Wu, A.; Xu, Q.; Huang, Y. Codelivery of Dihydroartemisinin and Doxorubicin in Mannosylated Liposomes for Drug-Resistant Colon Cancer Therapy. Acta Pharmacol. Sin. 2017, 38, 885–896. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Chronopoulou, L.; Haghighi, F.H.; Fratoddi, I.; Palocci, C. Peptide-Based Hydrogels: New Materials for Biosensing and Biomedical Applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef]
- Dowari, P.; Roy, S.; Das, S.; Chowdhuri, S.; Kushwaha, R.; Das, B.K.; Ukil, A.; Das, D. Mannose-Decorated Composite Peptide Hydrogel with Thixotropic and Syneresis Properties and Its Application in Treatment of Leishmaniasis. Chem.—Asian J. 2022, 17, e202200550. [Google Scholar] [CrossRef]
- Zhang, Y.; Kuang, Y.; Gao, Y.; Xu, B. Versatile Small-Molecule Motifs for Self-Assembly in Water and the Formation of Biofunctional Supramolecular Hydrogels. Langmuir 2011, 27, 529–537. [Google Scholar] [CrossRef]
- Huang, H.; Lovell, J.F. Advanced Functional Nanomaterials for Theranostics. Adv. Funct. Mater. 2017, 27, 1603524. [Google Scholar] [CrossRef]
- He, X.; Abrams, S.I.; Lovell, J.F. Peptide Delivery Systems for Cancer Vaccines. Adv. Ther. 2018, 1, 1800060. [Google Scholar] [CrossRef]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The Multirole of Liposomes in Therapy and Prevention of Infectious Diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-S.; Na, K.S.; Hwang, H.; Oh, P.-S.; Kim, D.H.; Lim, S.T.; Sohn, M.-H.; Jeong, H.-J. Effect of Space Length of Mannose Ligand on Uptake of Mannosylated Liposome in RAW 264.7 Cells: In Vitro and In Vivo Studies. J. Biomed. Mater. Res. A 2014, 102, 4545–4553. [Google Scholar] [CrossRef] [PubMed]
- Espuelas, S.; Thumann, C.; Heurtault, B.; Schuber, F.; Frisch, B. Influence of Ligand Valency on the Targeting of Immature Human Dendritic Cells by Mannosylated Liposomes. Bioconjug. Chem. 2008, 19, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Thompson, D.H. Stimuli-Responsive Liposomes for Drug Delivery. WIREs Nanomed. Nanobiotechnology 2017, 9, e1450. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Szoka, F.C. Steric Stabilization of Fusogenic Liposomes by a Low-pH Sensitive PEG−Diortho Ester−Lipid Conjugate. Bioconjug. Chem. 2001, 12, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Pukanud, P.; Peungvicha, P.; Sarisuta, N. Development of Mannosylated Liposomes for Bioadhesive Oral Drug Delivery via M Cells of Peyer’s Patches. Drug Deliv. 2009, 16, 289–294. [Google Scholar] [CrossRef]
- Shibata, H.; Yomota, C.; Okuda, H. Simultaneous Determination of Polyethylene Glycol-Conjugated Liposome Components by Using Reversed-Phase High-Performance Liquid Chromatography with UV and Evaporative Light Scattering Detection. AAPS PharmSciTech 2013, 14, 811–817. [Google Scholar] [CrossRef]
- Ohvo-Rekilä, H.; Ramstedt, B.; Leppimäki, P.; Peter Slotte, J. Cholesterol Interactions with Phospholipids in Membranes. Prog. Lipid Res. 2002, 41, 66–97. [Google Scholar] [CrossRef]
- Engel, A.; Chatterjee, S.K.; Al-arifi, A.; Riemann, D.; Langner, J.; Nuhn, P. Influence of Spacer Length on Interaction of Mannosylated Liposomes with Human Phagocytic Cells. Pharm. Res. 2003, 20, 51–57. [Google Scholar] [CrossRef]
- Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. A Systematic Analysis of Peptide Linker Length and Liposomal Polyethylene Glycol Coating on Cellular Uptake of Peptide-Targeted Liposomes. ACS Nano 2013, 7, 2935–2947. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, W.; Elbahnasawy, M.A.; Bao, X.; Zheng, Q.; Gong, L.; Zhou, Y.; Yang, S.; Fang, A.; Farag, M.M.S.; et al. Optimization of the Linker Length of Mannose-Cholesterol Conjugates for Enhanced mRNA Delivery to Dendritic Cells by Liposomes. Front. Pharmacol. 2018, 9, 980. [Google Scholar] [CrossRef] [PubMed]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth Liposomes: Review of the Basic Science, Rationale, and Clinical Applications, Existing and Potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Blanchfield, J.T.; Toth, I. Modification of Peptides and Other Drugs Using Lipoamino Acids and Sugars. Methods Mol. Biol. Clifton NJ 2005, 298, 45–61. [Google Scholar] [CrossRef]
- Moyle, P.M.; Toth, I. Self-Adjuvanting Lipopeptide Vaccines. Curr. Med. Chem. 2008, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, R.K.; Gupta, G.S. Targeting Cells for Drug and Gene Delivery: Emerging Applicationsof Mannans and Mannan Binding Lectins. JSIR 2009, 68, 465–483. [Google Scholar]
- Keler, T.; Ramakrishna, V.; Fanger, M.W. Mannose Receptor-Targeted Vaccines. Expert Opin. Biol. Ther. 2004, 4, 1953–1962. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, Z.; Zhang, H.; Cui, M.; Wang, M.; Liu, K. Reversing Tumor Immunosuppressive Microenvironment via Targeting Codelivery of CpG ODNs/PD-L1 Peptide Antagonists to Enhance the Immune Checkpoint Blockade-Based Anti-Tumor Effect. Eur. J. Pharm. Sci. 2022, 168, 106044. [Google Scholar] [CrossRef]
- Yuba, E.; Fukaya, Y.; Yanagihara, S.; Kasho, N.; Harada, A. Development of Mannose-Modified Carboxylated Curdlan-Coated Liposomes for Antigen Presenting Cell Targeted Antigen Delivery. Pharmaceutics 2020, 12, 754. [Google Scholar] [CrossRef]
- Hagimori, M.; Chinda, Y.; Suga, T.; Yamanami, K.; Kato, N.; Inamine, T.; Fuchigami, Y.; Kawakami, S. Synthesis of High Functionality and Quality Mannose-Grafted Lipids to Produce Macrophage-Targeted Liposomes. Eur. J. Pharm. Sci. 2018, 123, 153–161. [Google Scholar] [CrossRef]
- Pinheiro do Nascimento, L.; Tsapis, N.; Reynaud, F.; Desmaële, D.; Moine, L.; Vergnaud, J.; Abreu, S.; Chaminade, P.; Fattal, E. Mannosylation of Budesonide Palmitate Nanoprodrugs for Improved Macrophage Targeting. Eur. J. Pharm. Biopharm. 2022, 170, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Su, F.-Y.; Chen, J.; Son, H.-N.; Kelly, A.M.; Convertine, A.J.; West, T.E.; Skerrett, S.J.; Ratner, D.M.; Stayton, P.S. Polymer-Augmented Liposomes Enhancing Antibiotic Delivery against Intracellular Infections. Biomater. Sci. 2018, 6, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yang, Y.; Dong, W.; Gao, Y.; Meng, Y.; Wang, H.; Li, L.; Jin, J.; Ji, M.; Xia, X.; et al. Drug-Free Mannosylated Liposomes Inhibit Tumor Growth by Promoting the Polarization of Tumor-Associated Macrophages. Int. J. Nanomed. 2019, 14, 3203–3220. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yang, Y.; Jin, J.; Ji, M.; Gao, Y.; Feng, Y.; Wang, H.; Chen, X.; Liu, Y. Targeted Delivery of Chlorogenic Acid by Mannosylated Liposomes to Effectively Promote the Polarization of TAMs for the Treatment of Glioblastoma. Bioact. Mater. 2020, 5, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Lu, Y.; Lv, M.; Wang, F.; Lu, X.; Zhu, W.; Wei, J.; Guo, W.; Liu, R.; Li, G.; et al. Targeted Co-Delivery of Resiquimod and a SIRPα Variant by Liposomes to Activate Macrophage Immune Responses for Tumor Immunotherapy. J. Control. Release 2023, 360, 858–871. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, Y.; Xiao, X.; Liu, H.; Zhou, B.; Luo, B.; Saw, P.E.; Jiang, Q. Targeted Imaging of Tumor Associated Macrophages in Breast Cancer. BIO Integr. 2023, 4, 114–124. [Google Scholar] [CrossRef]
- Gao, H.; Gonçalves, C.; Gallego, T.; François-Heude, M.; Malard, V.; Mateo, V.; Lemoine, F.; Cendret, V.; Djedaini-Pilard, F.; Moreau, V.; et al. Comparative Binding and Uptake of Liposomes Decorated with Mannose Oligosaccharides by Cells Expressing the Mannose Receptor or DC-SIGN. Carbohydr. Res. 2020, 487, 107877. [Google Scholar] [CrossRef]
- Mousavifar, L.; Lewicky, J.D.; Taponard, A.; Bagul, R.; Rivat, M.; Abdullayev, S.; Martel, A.L.; Fraleigh, N.L.; Nakamura, A.; Veyrier, F.J.; et al. Synthesis & Evaluation of Novel Mannosylated Neoglycolipids for Liposomal Delivery System Applications. Pharmaceutics 2022, 14, 2300. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Cheng, Y.; Gao, Y. Glycyrrhetinic Acid Liposomes Containing Mannose-Diester Lauric Diacid-Cholesterol Conjugate Synthesized by Lipase-Catalytic Acylation for Liver-Specific Delivery. Molecules 2017, 22, 1598. [Google Scholar] [CrossRef]
- Lai, C.; Duan, S.; Ye, F.; Hou, X.; Li, X.; Zhao, J.; Yu, X.; Hu, Z.; Tang, Z.; Mo, F.; et al. The Enhanced Antitumor-Specific Immune Response with Mannose- and CpG-ODN-Coated Liposomes Delivering TRP2 Peptide. Theranostics 2018, 8, 1723–1739. [Google Scholar] [CrossRef]
- Le Moignic, A.; Malard, V.; Benvegnu, T.; Lemiègre, L.; Berchel, M.; Jaffrès, P.-A.; Baillou, C.; Delost, M.; Macedo, R.; Rochefort, J.; et al. Preclinical Evaluation of mRNA Trimannosylated Lipopolyplexes as Therapeutic Cancer Vaccines Targeting Dendritic Cells. J. Control. Release 2018, 278, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, J.; Lemiègre, L.; Quelen, A.; Malard, V.; Gao, H.; Gonçalves, C.; Berchel, M.; Jaffrès, P.-A.; Pichon, C.; Midoux, P.; et al. Synthesis of a Trimannosylated-Equipped Archaeal Diether Lipid for the Development of Novel Glycoliposomes. Carbohydr. Res. 2016, 435, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Sabnani, M.K.; Mavinkurve, V.; Shmeeda, H.; Mansouri, H.; Bonkoungou, S.; Le, A.D.; Wood, L.M.; Gabizon, A.A.; La-Beck, N.M. Liposome-Induced Immunosuppression and Tumor Growth Is Mediated by Macrophages and Mitigated by Liposome-Encapsulated Alendronate. J. Control. Release 2018, 271, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Lei, Q.; You, X.; Gao, T.; Song, X.; Xia, Y.; Ye, T.; Zhang, L.; Wang, N.; Yu, L. Mannosylated Liposomes Improve Therapeutic Effects of Paclitaxel in Colon Cancer Models. J. Microencapsul. 2017, 34, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Pontani, L.-L.; Jorjadze, I.; Viasnoff, V.; Brujic, J. Biomimetic Emulsions Reveal the Effect of Mechanical Forces on Cell–Cell Adhesion. Proc. Natl. Acad. Sci. USA 2012, 109, 9839–9844. [Google Scholar] [CrossRef]
- Zhang, Q.; Scigliano, A.; Biver, T.; Pucci, A.; Swager, T.M. Interfacial Bioconjugation on Emulsion Droplet for Biosensors. Bioorg. Med. Chem. 2018, 26, 5307–5313. [Google Scholar] [CrossRef]
- Sawant, A.; Kamath, S.; KG, H.; Kulyadi, G.P. Solid-in-Oil-in-Water Emulsion: An Innovative Paradigm to Improve Drug Stability and Biological Activity. AAPS PharmSciTech 2021, 22, 199. [Google Scholar] [CrossRef]
- Dumat, B.; Montel, L.; Pinon, L.; Matton, P.; Cattiaux, L.; Fattaccioli, J.; Mallet, J.-M. Mannose-Coated Fluorescent Lipid Microparticles for Specific Cellular Targeting and Internalization via Glycoreceptor-Induced Phagocytosis. ACS Appl. Bio Mater. 2019, 2, 5118–5126. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Cai, D.; Gao, W.; Li, Z.; Zhang, Y.; Xiao, L.; Xiao, Y. Current Development of Nano-Drug Delivery to Target Macrophages. Biomedicines 2022, 10, 1203. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the Immune System. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in Polymeric Micelles for Drug Delivery and Tumor Targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.G.D.; Reis, B.; Costas, B.; Lima, S.A.C.; Reis, S. Modulation of Macrophages M1/M2 Polarization Using Carbohydrate-Functionalized Polymeric Nanoparticles. Polymers 2021, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Pramudya, I.; Chung, H. Recent Progress of Glycopolymer Synthesis for Biomedical Applications. Biomater. Sci. 2019, 7, 4848–4872. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.; Burda, C. Nanoparticle Mediated Non-Covalent Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Hatami, E.; Mu, Y.; Shields, D.N.; Chauhan, S.C.; Kumar, S.; Cory, T.J.; Yallapu, M.M. Mannose-Decorated Hybrid Nanoparticles for Enhanced Macrophage Targeting. Biochem. Biophys. Rep. 2019, 17, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Mo, C.; Wang, Y.; Wei, D.; Xiao, H. Anti-Tumour Strategies Aiming to Target Tumour-Associated Macrophages. Immunology 2013, 138, 93–104. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, X.; Jia, L.; Prud’homme, R.K.; Szekely, Z.; Sinko, P.J. Optimal Structural Design of Mannosylated Nanocarriers for Macrophage Targeting. J. Control. Release 2014, 194, 341–349. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, X.; Venosa, A.; Lee, I.H.; Myers, D.; Holloway, J.A.; Prud’homme, R.K.; Gao, D.; Szekely, Z.; Laskin, J.D.; et al. A Novel Bivalent Mannosylated Targeting Ligand Displayed on Nanoparticles Selectively Targets Anti-Inflammatory M2 Macrophages. Pharmaceutics 2020, 12, 243. [Google Scholar] [CrossRef]
- Dossou, A.S.; Mantsch, M.E.; Kapic, A.; Burnett, W.L.; Sabnis, N.; Coffer, J.L.; Berg, R.E.; Fudala, R.; Lacko, A.G. Mannose-Coated Reconstituted Lipoprotein Nanoparticles for the Targeting of Tumor-Associated Macrophages: Optimization, Characterization, and In Vitro Evaluation of Effectiveness. Pharmaceutics 2023, 15, 1685. [Google Scholar] [CrossRef]
- Zhu, S.; Niu, M.; O’Mary, H.; Cui, Z. Targeting of Tumor-Associated Macrophages Made Possible by PEG-Sheddable, Mannose-Modified Nanoparticles. Mol. Pharm. 2013, 10, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das, S.; Kumar De, A.; Kar, N.; Bera, T. Amphotericin B-Loaded Mannose Modified Poly(d,l-Lactide-Co-Glycolide) Polymeric Nanoparticles for the Treatment of Visceral Leishmaniasis: In Vitro and In Vivo Approaches. RSC Adv. 2017, 7, 29575–29590. [Google Scholar] [CrossRef]
- Guerrero-Sanchez, C.; O’Brien, L.; Brackley, C.; Keddie, D.J.; Saubern, S.; Chiefari, J. Quasi-Block Copolymer Libraries on Demand via Sequential RAFT Polymerization in an Automated Parallel Synthesizer. Polym. Chem. 2013, 4, 1857–1862. [Google Scholar] [CrossRef]
- Ortega, R.A.; Barham, W.J.; Kumar, B.; Tikhomirov, O.; McFadden, I.D.; Yull, F.E.; Giorgio, T.D. Biocompatible Mannosylated Endosomal-Escape Nanoparticles Enhance Selective Delivery of Short Nucleotide Sequences to Tumor Associated Macrophages. Nanoscale 2015, 7, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, J.L.; Montgomery, K.S.; Cao, B.; Brown, R.; Dibb, N.J.; Nilsson, S.K.; Chiefari, J.; Fuchter, M.J. Glycosylated Nanoparticles Derived from RAFT Polymerization for Effective Drug Delivery to Macrophages. ACS Appl. Bio Mater. 2020, 3, 5775–5786. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lepland, A.; Scodeller, P.; Fontana, F.; Torrieri, G.; Tiboni, M.; Shahbazi, M.; Casettari, L.; Kostiainen, M.A.; Hirvonen, J.; et al. Peptide-Guided Resiquimod-Loaded Lignin Nanoparticles Convert Tumor-Associated Macrophages from M2 to M1 Phenotype for Enhanced Chemotherapy. Acta Biomater. 2021, 133, 231–243. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, J.; Chen, C.; Chen, M.; Sun, P.; Du, W.; Zhang, S.; Liu, Y.; Zhang, R.; Bai, M.; et al. In Situ Mannosylated Nanotrinity-Mediated Macrophage Remodeling Combats Candida Albicans Infection. ACS Nano 2020, 14, 3980–3990. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Mahajan, S.; Prashant, C.K.; Koul, V.; Choudhary, V.; Dinda, A.K. Receptor Specific Macrophage Targeting by Mannose-Conjugated Gelatin Nanoparticles- An In Vitro and In Vivo Study. Curr. Nanosci. 2010, 6, 413–421. [Google Scholar] [CrossRef]
- Fei, Q.; Shalosky, E.M.; Barnes, R.; Shukla, V.C.; Xu, S.; Ballinger, M.N.; Farkas, L.; Lee, R.J.; Ghadiali, S.N.; Englert, J.A. Macrophage-Targeted Lipid Nanoparticle Delivery of microRNA-146a to Mitigate Hemorrhagic Shock-Induced Acute Respiratory Distress Syndrome. ACS Nano 2023, 17, 16539–16552. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.D.; Bagade, S.B.; Bonde, S.C. In-Vitro and Ex-Vivo Characterization of Novel Mannosylated Gelatin Nanoparticles of Linezolid by Quality-by-Design Approach. J. Drug Deliv. Sci. Technol. 2020, 60, 101976. [Google Scholar] [CrossRef]
- Jiang, X.; Du, Z.; Zhang, X.; Zaman, F.; Song, Z.; Guan, Y.; Yu, T.; Huang, Y. Gelatin-Based Anticancer Drug Delivery Nanosystems: A Mini Review. Front. Bioeng. Biotechnol. 2023, 11, 1158749. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, N.; Huang, J.; Pei, Y.; Wang, F.; Tang, K. A Facile Fabrication of Core–Shell Sodium Alginate/Gelatin Beads for Drug Delivery Systems. Polym. Bull. 2019, 76, 87–102. [Google Scholar] [CrossRef]
- Vaghasiya, K.; Ray, E.; Singh, R.; Jadhav, K.; Sharma, A.; Khan, R.; Katare, O.P.; Verma, R.K. Efficient, Enzyme Responsive and Tumor Receptor Targeting Gelatin Nanoparticles Decorated with Concanavalin-A for Site-Specific and Controlled Drug Delivery for Cancer Therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112027. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, H.; Wang, M.; Bai, X.; Cao, J.; Zhang, Z.; Wang, Q. Mannosylated Gelatin Nanoparticles Enhanced Inactivated PRRSV Targeting Dendritic Cells and Increased T Cell Immunity. Vet. Immunol. Immunopathol. 2021, 235, 110237. [Google Scholar] [CrossRef]
- Barros, D.; Costa Lima, S.A.; Cordeiro-da-Silva, A. Surface Functionalization of Polymeric Nanospheres Modulates Macrophage Activation: Relevance in Leishmaniasis Therapy. Nanomedicine 2015, 10, 387–403. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Ezhov, A.A.; Petrov, R.A.; Vigovskiy, M.A.; Grigorieva, O.A.; Belogurova, N.G.; Kudryashova, E.V. Mannosylated Polymeric Ligands for Targeted Delivery of Antibacterials and Their Adjuvants to Macrophages for the Enhancement of the Drug Efficiency. Pharmaceuticals 2022, 15, 1172. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, Q.; Wang, S.; Bharate, P.; Varela-Aramburu, S.; Lu, M.; Seeberger, P.H.; Yin, J. Tumour-Targeted Drug Delivery with Mannose-Functionalized Nanoparticles Self-Assembled from Amphiphilic β-Cyclodextrins. Chem.—Eur. J. 2016, 22, 15216–15221. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and Design of Polymeric Surfactant-Based Drug Delivery Systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, J.; Xie, F.; Bao, W.; Xu, H.; Wang, H.; Xu, Y.; Du, Z. Herceptin-Conjugated Paclitaxel Loaded PCL-PEG Worm-like Nanocrystal Micelles for the Combinatorial Treatment of HER2-Positive Breast Cancer. Biomaterials 2019, 222, 119420. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric Micelles in Drug Delivery: An Insight of the Techniques for Their Characterization and Assessment in Biorelevant Conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Upponi, J.R.; Jerajani, K.; Nagesha, D.K.; Kulkarni, P.; Sridhar, S.; Ferris, C.; Torchilin, V.P. Polymeric Micelles: Theranostic Co-Delivery System for Poorly Water-Soluble Drugs and Contrast Agents. Biomaterials 2018, 170, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of Polymer Micelles for Imaging and Drug Delivery. WIREs Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-H.; Liang, X.; Cai, M.; Yan, L.; Chen, Z.; Guo, L.; Jing, L.; Wang, Y.; Zhou, D. Protein-Crowned Micelles for Targeted and Synergistic Tumor-Associated Macrophage Reprogramming to Enhance Cancer Treatment. Nano Lett. 2022, 22, 4410–4420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zang, X.; Qiao, M.; Zhao, X.; Hu, H.; Chen, D. Targeted Delivery of Dasatinib to Deplete Tumor-Associated Macrophages by Mannosylated Mixed Micelles for Tumor Immunotherapy. ACS Biomater. Sci. Eng. 2020, 6, 5675–5684. [Google Scholar] [CrossRef] [PubMed]
- Heller, P.; Mohr, N.; Birke, A.; Weber, B.; Reske-Kunz, A.; Bros, M.; Barz, M. Directed Interactions of Block Copolypept(o)Ides with Mannose-Binding Receptors: PeptoMicelles Targeted to Cells of the Innate Immune System. Macromol. Biosci. 2015, 15, 63–73. [Google Scholar] [CrossRef]
- Yin, L.; Chen, Y.; Zhang, Z.; Yin, Q.; Zheng, N.; Cheng, J. Biodegradable Micelles Capable of Mannose-Mediated Targeted Drug Delivery to Cancer Cells. Macromol. Rapid Commun. 2015, 36, 483–489. [Google Scholar] [CrossRef]
- Sanhueza, C.; Vergara, D.; Chávez-Aravena, C.; Gálvez-Jiron, F.; Chavez-Angel, E.; Castro-Alvarez, A. Functionalizing Dendrimers for Targeted Delivery of Bioactive Molecules to Macrophages: A Potential Treatment for Mycobacterium Tuberculosis Infection—A Review. Pharmaceuticals 2023, 16, 1428. [Google Scholar] [CrossRef]
- Sheng, K.-C.; Kalkanidis, M.; Pouniotis, D.S.; Esparon, S.; Tang, C.K.; Apostolopoulos, V.; Pietersz, G.A. Delivery of Antigen Using a Novel Mannosylated Dendrimer Potentiates Immunogenicity In Vitro and In Vivo. Eur. J. Immunol. 2008, 38, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Porterfield, J.E.; Smith, E.; Sharma, R.; Kannan, S.; Kannan, R.M. Effect of Mannose Targeting of Hydroxyl PAMAM Dendrimers on Cellular and Organ Biodistribution in a Neonatal Brain Injury Model. J. Control. Release 2018, 283, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, L.; Cabral, P.; Chammas, R. Mannose Receptor 1 Expression Does Not Determine the Uptake of High-Density Mannose Dendrimers by Activated Macrophages Populations. PLoS ONE 2020, 15, e0240455. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yuan, Q.; Bie, J.; Wallace, R.L.; Yannie, P.J.; Wang, J.; Lancina, M.G.; Zolotarskaya, O.Y.; Korzun, W.; Yang, H.; et al. Development of Mannose Functionalized Dendrimeric Nanoparticles for Targeted Delivery to Macrophages: Use of This Platform to Modulate Atherosclerosis. Transl. Res. 2018, 193, 13–30. [Google Scholar] [CrossRef]

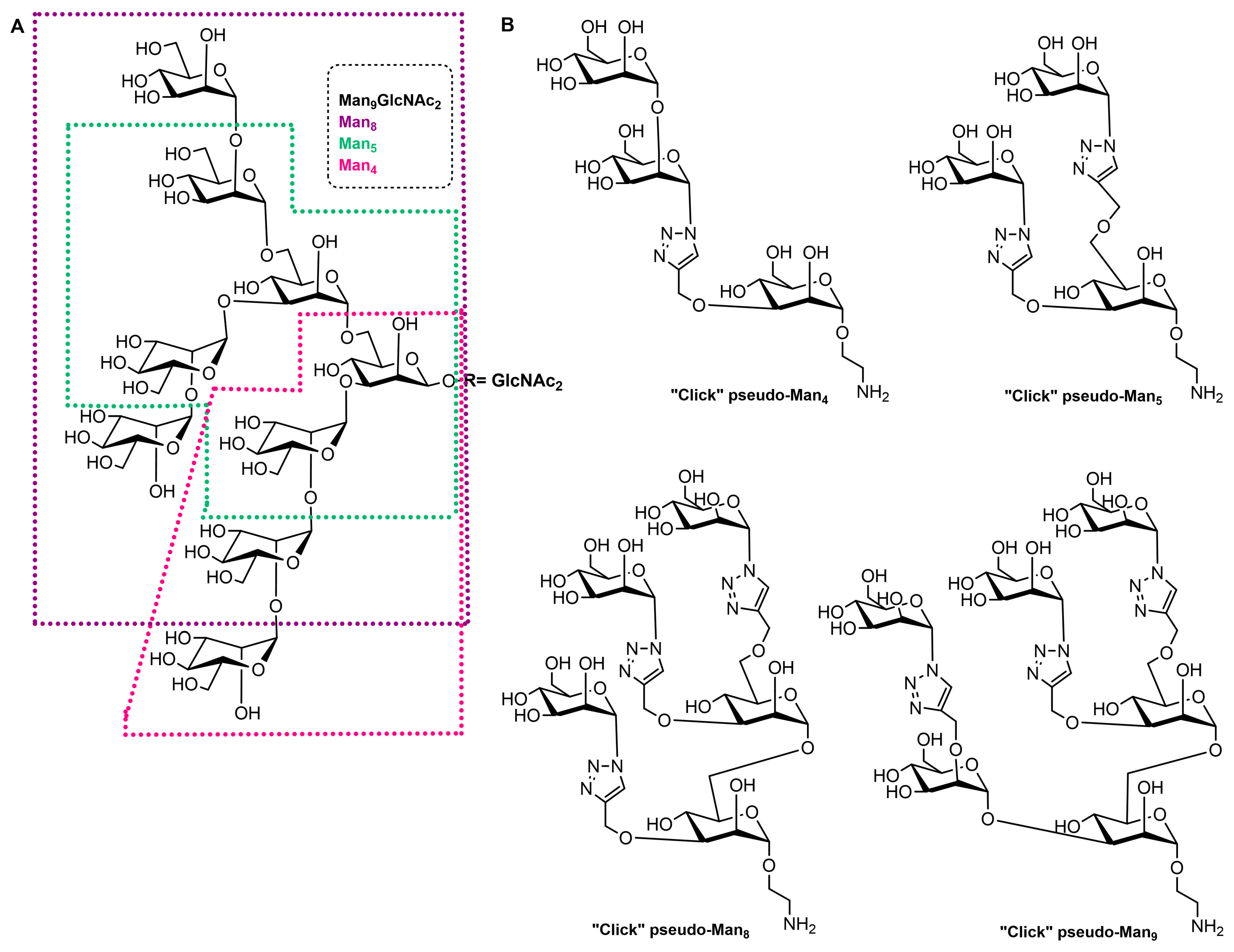
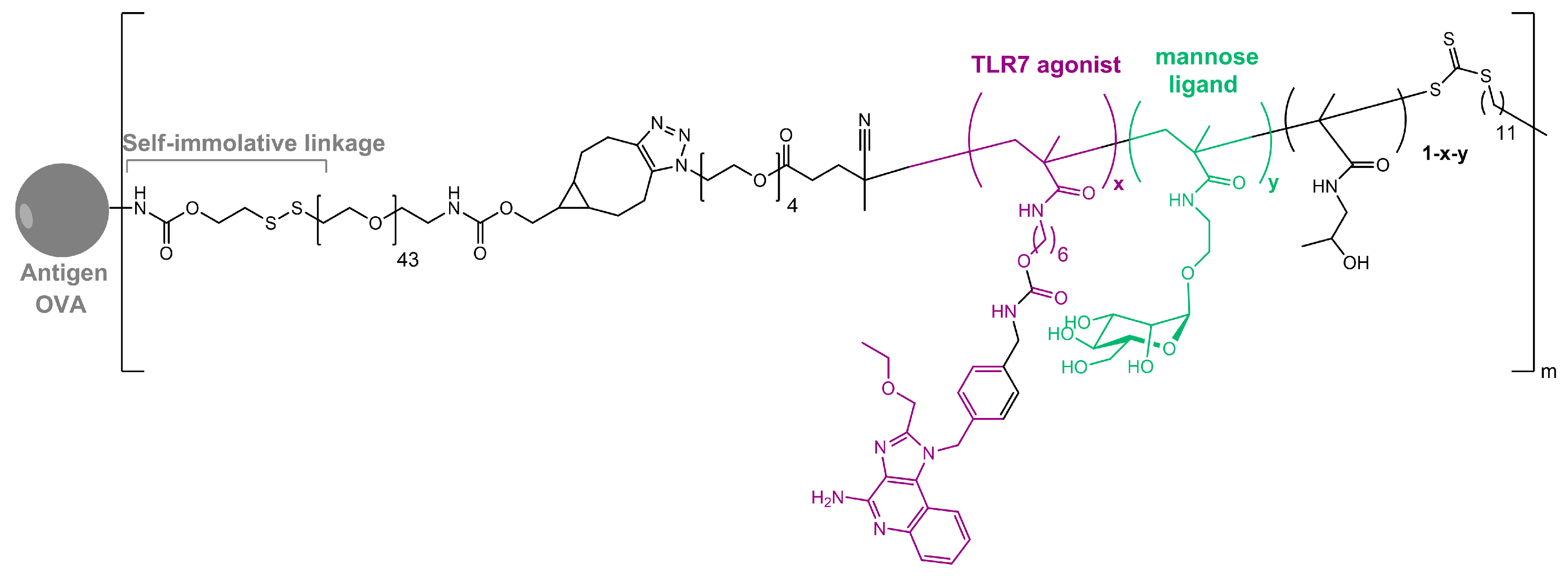
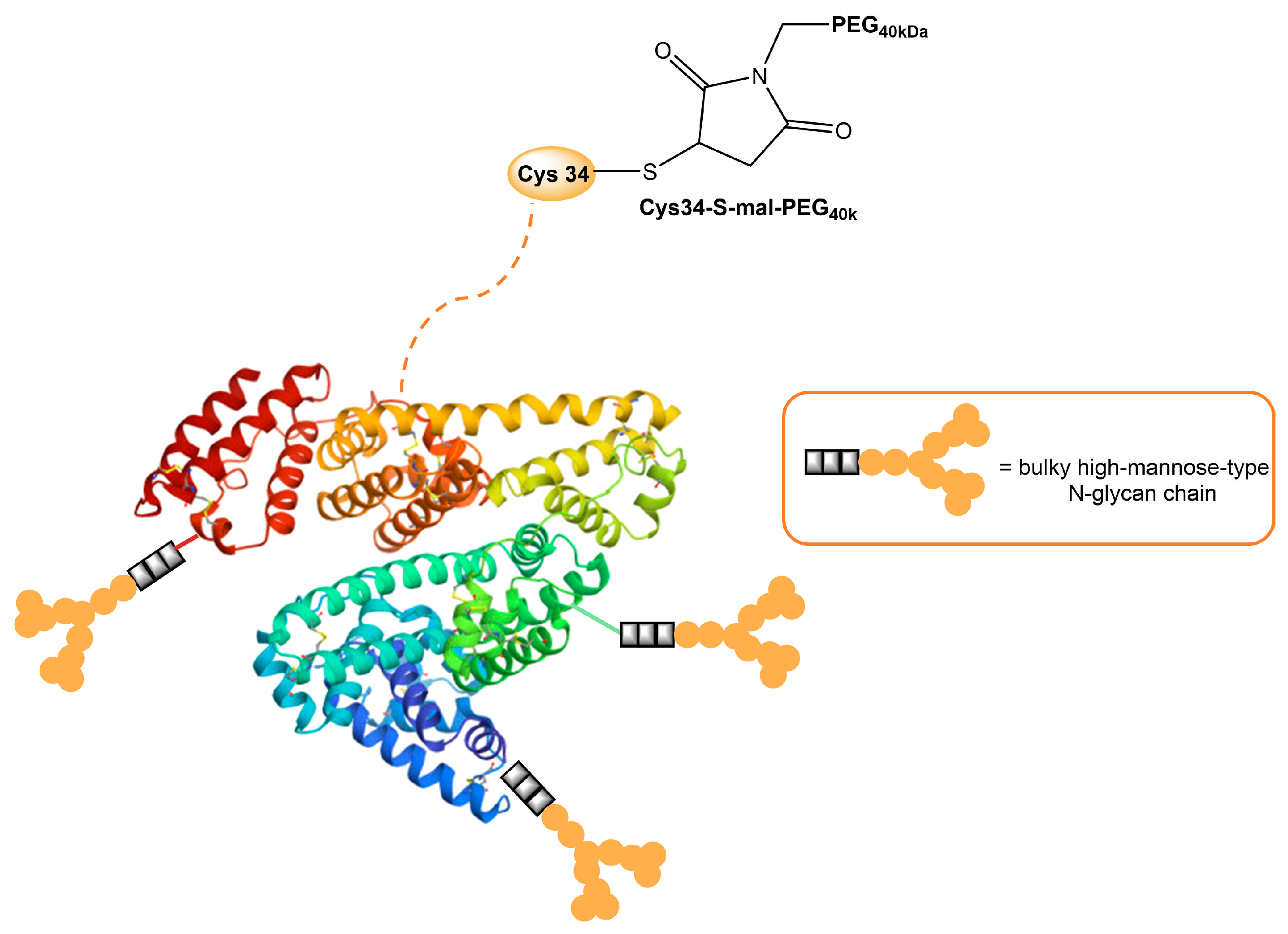
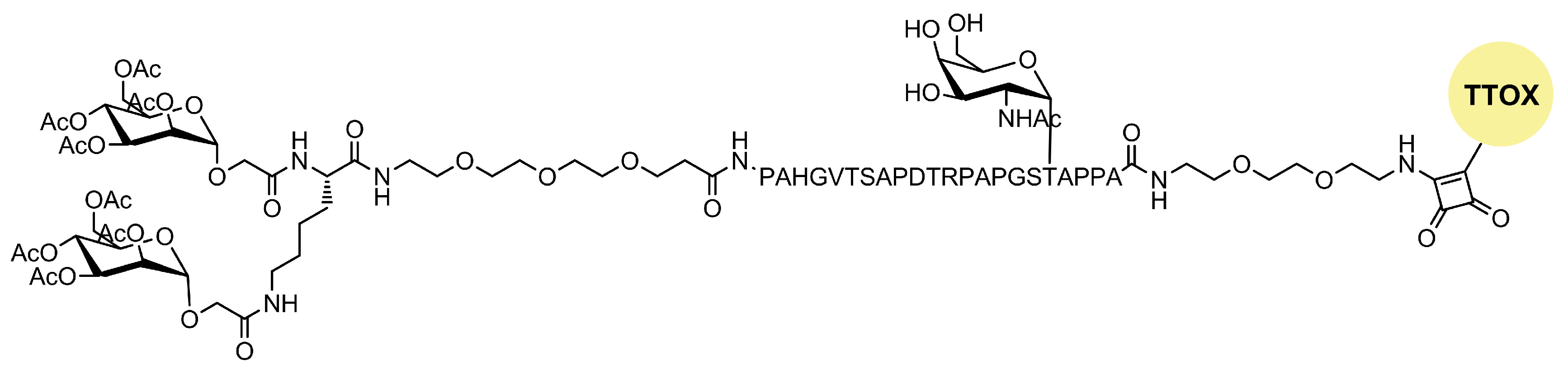
| Mannosylated Carrier System | Advantages |
|---|---|
| Antigens/Proteins/Peptides | Significant enhancement of the cellular and humoral immune response through APC activation and antigen uptake |
| Increased peptide solubility and oral bioavailability | |
| Increased specificity of peptide vaccine candidates toward the desired cellular target | |
| Lipids and liposomes | Increased cellular uptake |
| Promoted cross-presentation of model antigen | |
| Induction of strong antitumor effects in tumor-bearing mice | |
| Improved cytosolic delivery of therapeutics to specific macrophages | |
| Nanoparticles | Improved pharmacokinetic profile |
| Effective internalization in targeted cells | |
| High binding affinity for targeted cells, successful delivery of drugs, and therapeutic mRNA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paurević, M.; Šrajer Gajdošik, M.; Ribić, R. Mannose Ligands for Mannose Receptor Targeting. Int. J. Mol. Sci. 2024, 25, 1370. https://doi.org/10.3390/ijms25031370
Paurević M, Šrajer Gajdošik M, Ribić R. Mannose Ligands for Mannose Receptor Targeting. International Journal of Molecular Sciences. 2024; 25(3):1370. https://doi.org/10.3390/ijms25031370
Chicago/Turabian StylePaurević, Marija, Martina Šrajer Gajdošik, and Rosana Ribić. 2024. "Mannose Ligands for Mannose Receptor Targeting" International Journal of Molecular Sciences 25, no. 3: 1370. https://doi.org/10.3390/ijms25031370
APA StylePaurević, M., Šrajer Gajdošik, M., & Ribić, R. (2024). Mannose Ligands for Mannose Receptor Targeting. International Journal of Molecular Sciences, 25(3), 1370. https://doi.org/10.3390/ijms25031370








