Targeting Matrix Metalloproteinases and Their Inhibitors in Melanoma
Abstract
1. Introduction
2. Biology and Function of Matrix Metalloproteinases (MMPs)
3. MMPs Endogenous Inhibitors
4. MMPs and TIMPs in Melanoma
MMPs and TIMPs in Melanoma Cell Migration, Tumor Growth, and Metastasis
5. Matrix Metalloproteinases: Characteristics and Roles in Malignant Melanoma Advancement
5.1. MMP-1, and MMP-13 (Collagenases)
5.2. MMP-3 (Stromelysin)
5.3. MMP-2 and MMP-9 (Gelatinases)
5.4. MMP-14 (Membrane-Type MMP)
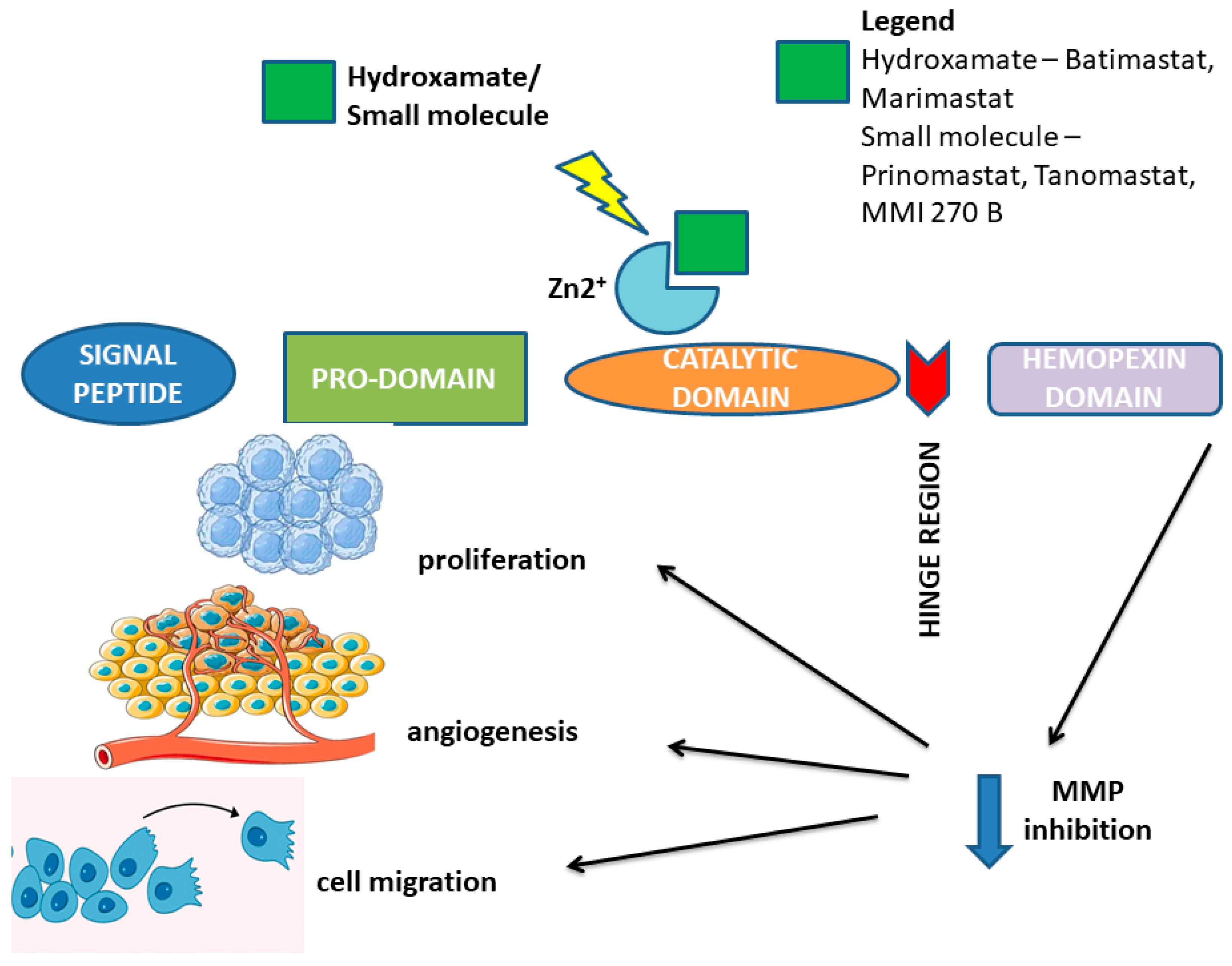
6. Pharmacological Targeting of Matrix Metalloproteinases and Their Tissue Inhibitors in Melanoma
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhanyamraju, P.K.; Patel, T.N. Melanoma therapeutics: A literature review. J. Biomed. Res. 2022, 36, 77. [Google Scholar] [CrossRef]
- Rebecca, V.W.; Somasundaram, R.; Herlyn, M. Pre-clinical modeling of cutaneous melanoma. Nat. Commun. 2020, 11, 2858. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.M.; Soares, P.; Populo, H. Melanoma treatment in review. Immuno. Targets. Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- van der Kooij, M.K.; Speetjens, F.M.; van der Burg, S.H.; Kapiteijn, E. Uveal Versus Cutaneous Melanoma; Same Origin, Very Distinct Tumor Types. Cancers 2019, 11, 845. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.I.; Lin, J.Y. Cutaneous Melanoma-A Review in Detection, Staging, and Management. Hematol. Oncol. Clin. N. Am. 2019, 33, 25–38. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghì, A.; Spandidos, D.A.; McCubrey, J.A.; Candido, S.; Libra, M. Cutaneous melanoma: From pathogenesis to therapy (Review). Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef]
- Vandyck, H.H.; Hillen, L.M.; Bosisio, F.M.; Oord, J.v.D.; Hausen, A.Z.; Winnepenninckx, V. Rethinking the biology of metastatic melanoma: A holistic approach. Cancer Metastasis Rev. 2021, 40, 603–624. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Zhu, S.; Li, S. Recent Progress in Nanomedicine for Melanoma Theranostics With Emphasis on Combination Therapy. Front. Bioeng. Biotechnol. 2021, 9, 661214. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.U.; Papadas, A.; Pagenkopf, A.; Flietner, E.; Morrow, Z.; Chaudhary, S.G.; Asimakopoulos, F. Tumor matrix remodeling and novel immunotherapies: The promise of matrix-derived immune biomarkers. J. Immunother. Cancer 2018, 6, 65. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. 2019, 137, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Napoli, S.; Scuderi, C.; Gattuso, G.; Di Bella, V.; Candido, S.; Basile, M.S.; Libra, M.; Falzone, L. Functional Roles of Matrix Metalloproteinases and Their Inhibitors in Melanoma. Cells 2020, 9, 1151. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, R.; Kheirouri, S.; Ghodsi, R.; Ojaghi, H. The effects of zinc treatment on matrix metalloproteinases: A systematic review. J. Trace Elements Med. Biol. 2019, 56, 107–115. [Google Scholar] [CrossRef]
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol. 2019, 56, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Tallant, C.; Marrero, A.; Gomis-Rüth, F. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. et Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1803, 20–28. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.S.; Overall, C.M. Updated Biological Roles for Matrix Metalloproteinases and New “Intracellular” Substrates Revealed by Degradomics. Biochemistry 2009, 48, 10830–10845. [Google Scholar] [CrossRef]
- Abdool, A.Y.; Abbas, L.; Sebaei, T.; Schmitt, E.; Sikora, A. How Can We Design an Inhibitor with an Enhanced Binding Affinity That Is Selective for MMP12? Protein Modeling Reports 4. 2021. Available online: https://nsuworks.nova.edu/protein_modeling_reports/4 (accessed on 10 December 2024).
- Wilson, C.L.; Ouellette, A.J.; Satchell, D.P.; Ayabe, T.; López-Boado, Y.S.; Stratman, J.L.; Hultgren, S.J.; Matrisian, L.M.; Parks, W.C. Regulation of Intestinal α-Defensin Activation by the Metalloproteinase Matrilysin in Innate Host Defense. Science 1999, 286, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Lemaître, V.; D’Armiento, J. Matrix metalloproteinases in development and disease. Birth Defects Res. Part C Embryo Today Rev. 2006, 78, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Lenci, E.; Cosottini, L.; Trabocchi, A. Novel matrix metalloproteinase inhibitors: An updated patent review (2014–2020). Expert Opin. Ther. Patents 2021, 31, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ma, X.; Fang, G.; Tian, X.; Ge, C. Matrix metalloproteinase inhibitors (MMPIs) as attractive therapeutic targets: Recent progress and current challenges. NanoImpact 2021, 21, 100293. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2010, 278, 28–45. [Google Scholar] [CrossRef]
- Jackson, H.W.; Defamie, V.; Waterhouse, P.; Khokha, R. TIMPs: Versatile extracellular regulators in cancer. Nat. Rev. Cancer 2016, 17, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef]
- Srivastava, P.; Lone, T.A.; Kapoor, R.; Mittal, R.D. Association of Promoter Polymorphisms in MMP2 and TIMP2 with Prostate Cancer Susceptibility in North India. Arch. Med. Res. 2012, 43, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta BBA Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Rivera, S.; Khrestchatisky, M.; Kaczmarek, L.; Rosenberg, G.A.; Jaworski, D.M. Metzincin proteases and their inhibitors: Foes or friends in nervous system physiology? J. Neurosci. 2010, 30, 15337–15357. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.X.; Rapti, M.; Tsigkou, A.; Lee, M.H. Expanding the Activity of Tissue Inhibitors of Metalloproteinase (TIMP)-1 against Surface-Anchored Metalloproteinases by the Replacement of Its C-Terminal Domain: Implications for Anti-Cancer Effects. PLoS ONE 2015, 10, e0136384. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wu, S. Tissue inhibitor of metalloproteinase 2 inhibits activation of the β-catenin signaling in melanoma cells. Cell Cycle 2015, 14, 1666–1674. [Google Scholar] [CrossRef]
- Jia, J.; Li, F.; Tang, X.S.; Xu, S.; Gao, Y.; Shi, Q.; Guo, W.; Wang, X.; He, D.; Guo, P. Long noncoding RNA DANCR promotes invasion of prostate cancer through epigenetically silencing expression of TIMP2/3. Oncotarget 2016, 7, 37868–37881. [Google Scholar] [CrossRef]
- Chang, R.M.; Fu, Y.; Zeng, J.; Zhu, X.Y.; Gao, Y. Cancer-derived exosomal miR-197-3p confers angiogenesis via targeting TIMP2/3 in lung adenocarcinoma metastasis. Cell Death Dis. 2022, 13, 1032. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; García-Hernández, A.A.; Ramos, C. Matrix Metalloproteinases’ Role in Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1245, 97–131. [Google Scholar]
- Hey, S.; Linder, S. Matrix metalloproteinases at a glance. J. Cell Sci. 2024, 137, jcs261898. [Google Scholar] [CrossRef]
- Song, K.; Yu, Z.; Zu, X.; Li, G.; Hu, Z.; Xue, Y. Collagen Remodeling along Cancer Progression Providing a Novel Opportunity for Cancer Diagnosis and Treatment. Int. J. Mol. Sci. 2022, 23, 10509. [Google Scholar] [CrossRef]
- Afshar, K.; Sanaei, M.; Ravari, M.S.; Pourbagheri-Sigaroodi, A.; Bashash, D. An overview of extracellular matrix and its remodeling in the development of cancer and metastasis with a glance at therapeutic approaches. Cell Biochem. Funct. 2023, 41, 930–952. [Google Scholar] [CrossRef] [PubMed]
- Erler, J.T.; Bennewith, K.L.; Cox, T.R.; Lang, G.; Bird, D.; Koong, A.; Le, Q.-T.; Giaccia, A.J. Hypoxia-Induced Lysyl Oxidase Is a Critical Mediator of Bone Marrow Cell Recruitment to Form the Premetastatic Niche. Cancer Cell 2009, 15, 35–44. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Eck, S.M.; Hoopes, P.J.; Petrella, B.L.; Coon, C.I.; Brinckerhoff, C.E. Matrix metalloproteinase-1 promotes breast cancer angiogenesis and osteolysis in a novel in vivo model. Breast Cancer Res. Treat. 2008, 116, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.; Nicolae, I.; Ene, C.D. Angiogenic systemic response to the hypoxic microenvironment in prostate tumorigenesis: A pilot study. Exp. Ther. Med. 2023, 26, 483. [Google Scholar]
- Schmalfeldt, B.; Prechtel, D.; Härting, K.; Späthe, K.; Rutke, S.; Konik, E.; Fridman, R.; Berger, U.; Schmitt, M.; Kuhn, W.; et al. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin. Cancer Res. 2001, 7, 2396–2404. [Google Scholar]
- Brinzea, A.; Nedelcu, R.I.; Ion, D.A.; Turcu, G.; Antohe, M.; Hodorogea, A.; Calinescu, A.; Pirici, D.; Popescu, R.; Popescu, C.M.; et al. Matrix metalloproteinases expression in lentigo maligna/lentigo maligna melanoma—A review of the literature and personal experience. Rom. J. Morphol. Embryol. 2019, 60, 1091–1095. [Google Scholar]
- Manolescu, B.S.M.; Lazar, A.M.; Ţiplica, G.S.; Zurac, S.A.; Reboşapcă, A.; Andreescu, B.; Popp, C.G. MMP1, MMP9, MMP11 and MMP13 in melanoma and its metastasis—Key points in understanding the mechanisms and celerity of tumor dissemination. Romanian J. Morphol. Embryol. 2024, 65, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Redondo, P.; Lloret, P.; Idoate, M.; Inoges, S. Expression and serum levels of MMP-2 and MMP-9 during human melanoma progression. Clin. Exp. Dermatol. 2005, 30, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Q.; Hu, G.; Van Poznak, C.; Fleisher, M.; Reiss, M.; Massagué, J.; Kang, Y. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009, 23, 1882–1894. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.; Bai, J.; Xue, Y.; Peng, Q. MMP1 and MMP9 are potential prognostic biomarkers and targets for uveal melanoma. BMC Cancer 2021, 21, 1068. [Google Scholar] [CrossRef]
- Blackburn, J.S.; Liu, I.; Coon, C.I.; Brinckerhoff, C.E. A matrix metalloproteinase-1/protease activated receptor-1 signaling axis promotes melanoma invasion and metastasis. Oncogene 2009, 28, 4237–4248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, B.; Li, Y.; Liu, Y.; Zhang, D.; Wang, X.; Gu, Q.; Zhao, J.; Dong, X.; Liu, Z.; et al. Dual effects of collagenase-3 on melanoma: Metastasis promotion and disruption of vasculogenic mimicry. Oncotarget 2015, 6, 8890–8899. [Google Scholar] [CrossRef]
- Zigrino, P.; Kuhn, I.; Bäuerle, T.; Zamek, J.; Fox, J.W.; Neumann, S.; Licht, A.; Schorpp-Kistner, M.; Angel, P.; Mauch, C. Stromal Expression of MMP-13 Is Required for Melanoma Invasion and Metastasis. J. Investig. Dermatol. 2009, 129, 2686–2693. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Suhaimi, S.A.; Chan, S.C.; Rosli, R. Matrix Metallopeptidase 3 Polymorphisms: Emerging genetic Markers in Human Breast Cancer Metastasis. J. Breast Cancer 2020, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, E.; Braeuer, R.R.; Kamiya, T.; Mobley, A.K.; Huang, L.; Vasquez, M.E.; Velazquez-Torres, G.; Chakravarti, N.; Ivan, C.; Prieto, V.; et al. NFAT1 Directly Regulates IL8 and MMP3 to Promote Melanoma Tumor Growth and Metastasis. Cancer Res. 2016, 76, 3145–3155. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Bongers, L.R.; McClain, C.B.; Saxena, M.; Bongers, G.; Merad, M.; Bhardwaj, N. MMP2 and TLRs modulate immune responses in the tumor microenvironment. J. Clin. Investig. 2021, 6, e144913. [Google Scholar] [CrossRef]
- Dąbrowska, E.; Przylipiak, A.; Zajkowska, M.; Piskor, B.M.; Sidorkiewicz, I.; Szmitkowski, M.; Lawicki, S. Possible Diagnostic Application of CXCL12 and CXCR4 as Tumor Markers in Breast Cancer Patients. Anticancer. Res. 2020, 40, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, N.K.; Muhammadnejad, A.; Rahmati, M.; Ghanadan, A.; Razavirad, A. Diagnostic Values of Ki67, Cox2, CD31, E-cadherin, VEGF, MMP2, and MMP9 Protein Expression in Human Melanoma. Asian Pac. J. Cancer Biol. 2024, 9, 329–335. [Google Scholar] [CrossRef]
- Rotte, A.; Martinka, M.; Li, G. MMP2 expression is a prognostic marker for primary melanoma patients. Cell. Oncol. 2012, 35, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Desch, A.; Strozyk, E.A.; Bauer, A.T.; Huck, V.; Niemeyer, V.; Wieland, T.; Schneider, S.W. Highly invasive melanoma cells activate the vascular endothelium via an MMP-2/integrin αvβ5-induced secretion of VEGF-A. Am. J. Pathol. 2012, 181, 693–705. [Google Scholar] [CrossRef]
- Niland, S.; Eble, J.A. Hold on or Cut? Integrin- and MMP-Mediated Cell–Matrix Interactions in the Tumor Microenvironment. Int. J. Mol. Sci. 2020, 22, 238. [Google Scholar] [CrossRef] [PubMed]
- Bastian, A.; Nichita, L.; Zurac, S. Matrix Metalloproteinases in Melanoma With and Without Regression. In The Role of Matrix Metalloproteinase in Human Body Pathologies; Tavascio, F., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Thakur, V.; Bedogni, B. The membrane tethered matrix metalloproteinase MT1-MMP at the forefront of melanoma cell invasion and metastasis. Pharmacol. Res. 2016, 111, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Moro, N.; Mauch, C.; Zigrino, P. Metalloproteinases in melanoma. Eur. J. Cell Biol. 2014, 93, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Mao, R.; Shen, K.; Zheng, Y.; Li, Y.; Liu, J.; Ni, L. Atractylenolide I-mediated Notch pathway inhibition attenuates gastric cancer stem cell traits. Biochem. Biophys. Res. Commun. 2014, 450, 353–359. [Google Scholar] [CrossRef]
- Kümper, M.; Hessenthaler, S.; Zamek, J.; Niland, S.; Pach, E.; Mauch, C.; Zigrino, P. Loss of Endothelial Cell Matrix Metalloproteinase 14 Reduces Melanoma Growth and Metastasis by Increasing Tumor Vessel Stability. J. Investig. Dermatol. 2021, 142, 1923–1933.e5. [Google Scholar] [CrossRef] [PubMed]
- Pach, E.; Kümper, M.; Fromme, J.E.; Zamek, J.; Metzen, F.; Koch, M.; Mauch, C.; Zigrino, P. Extracellular Matrix Remodeling by Fibroblast-MMP14 Regulates Melanoma Growth. Int. J. Mol. Sci. 2021, 22, 12276. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Jang, N.H.; Lee, H.J. Natural Products as Regulators against Matrix Metalloproteinases for the Treatment of Cancer. Biomedicines 2024, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.G.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 714–770. [Google Scholar] [CrossRef]
- Parsons, S.L.; Watson, S.A.; Steele, R.J. Phase I/II trial of batimastat, a matrix metalloproteinase inhibitor, in patients with malignant ascites. Eur. J. Surg. Oncol. 1997, 23, 526–531. [Google Scholar] [CrossRef]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
- Nabipoorashrafi, S.A.; Shomali, N.; Sadat-Hatamnezhad, L.; Mahami-Oskouei, M.; Mahmoudi, J.; Shotorbani, B.S.; Akbari, M.; Xu, H.; Shotorbani, S.S. miR-143 acts as an inhibitor of migration and proliferation as well as an inducer of apoptosis in melanoma cancer cells in vitro. IUBMB Life 2020, 72, 2034–2044. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Fingleton, B. MMPs as therapeutic targets--still a viable option? Semin. Cell Dev. Biol. 2008, 19, 61–68. [Google Scholar] [CrossRef]
- Conlon, G.A.; Murray, G.I. Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2018, 247, 629–640. [Google Scholar] [CrossRef]
- Remacle, A.G.; Golubkov, V.S.; Shiryaev, S.A.; Dahl, R.; Stebbins, J.L.; Chernov, A.V.; Cheltsov, A.V.; Pellecchia, M.; Strongin, A.Y. Novel MT1-MMP small-molecule inhibitors based on insights into hemopexin domain function in tumor growth. Cancer Res. 2012, 72, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.; Barralet, J.; Harvey, E.J.; Merle, G. Detecting the PEX Like Domain of Matrix Metalloproteinase-14 (MMP-14) with Therapeutic Conjugated CNTs. Biosensors 2022, 12, 884. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, K.; Dufour, A.; Li, J.; Kuscu, C.; Pulkoski-Gross, A.; Zhi, J.; Hu, Y.; Sampson, N.S.; Zucker, S.; Cao, J. Inhibition of Matrix Metalloproteinase 14 (MMP-14)-mediated Cancer Cell Migration. J. Biol. Chem. 2011, 286, 33167–33177. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Peggs, K.S.; A Quezada, S.; Korman, A.J.; Allison, J.P. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr. Opin. Immunol. 2006, 18, 206–213. [Google Scholar] [CrossRef]
- Tarhini, A.; Lo, E.; Minor, D.R. Releasing the Brake on the Immune System: Ipilimumab in Melanoma and Other Tumors. Cancer Biotherapy Radiopharm. 2010, 25, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Letendre, P.; Monga, V.; Milhem, M.; Zakharia, Y. Ipilimumab: From Preclinical Development to Future Clinical Perspectives in Melanoma. Futur. Oncol. 2016, 13, 625–636. [Google Scholar] [CrossRef]
- Monteiro, F.S.M.; Soares, A.; Debiasi, M.; Schutz, F.A.; Maluf, F.C.; Bastos, D.A.; Sasse, A.; Cauduro, C.G.; Mendes, G.O.; Ziegelmann, P.K.; et al. First-line Treatment of Metastatic Renal Cell Carcinoma in the Immuno-oncology Era: Systematic Review and Network Meta-analysis. Clin. Genitourin. Cancer 2020, 18, 244–251.e4. [Google Scholar] [CrossRef]
- Thomas, J.; Leal, A.; Overman, M.J. Clinical Development of Immunotherapy for Deficient Mismatch Repair Colorectal Cancer. Clin. Color. Cancer 2020, 19, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Saji, S.; Sato, F.; Noda, M.; Toi, M. Potential Clinical Applications of Matrix Metalloproteinase Inhibitors and their Future Prospects. Int. J. Biol. Markers 2013, 28, 117–130. [Google Scholar] [CrossRef]
- Li, N.-G.; Tang, Y.-P.; Duan, J.-A.; Shi, Z.-H. Matrix metalloproteinase inhibitors: A patent review (2011–2013). Expert Opin. Ther. Patents 2014, 24, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Madsen, D.H.; Hansen, M.; Schmidt, H.; Svane, I.M.; Karsdal, M.A.; Willumsen, N. Non-invasive biomarkers derived from the extracellular matrix associate with response to immune checkpoint blockade (anti-CTLA-4) in metastatic melanoma patients. J. Immunother. Cancer 2018, 6, 152. [Google Scholar] [CrossRef]
- Bhandaru, M.; Rotte, A. Monoclonal Antibodies for the Treatment of Melanoma: Present and Future Strategies. Methods Mol. Biol. 2018, 1904, 83–108. [Google Scholar] [CrossRef]
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Moraes, F.Y.; Zadeh, G. Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 2177–2178. [Google Scholar] [PubMed]
- Tawbi, H.A.; Forsyth, P.A.; Algazi, A.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Regan, M.M.; Werner, L.; Rao, S.; Gupte-Singh, K.; Hodi, F.S.; Kirkwood, J.M.; Kluger, H.M.; Larkin, J.; Postow, M.A.; Ritchings, C.; et al. Treatment-Free Survival: A Novel Outcome Measure of the Effects of Immune Checkpoint Inhibition—A Pooled Analysis of Patients With Advanced Melanoma. J. Clin. Oncol. 2019, 37, 3350–3358. [Google Scholar] [CrossRef]
- Tarhini, A.; McDermott, D.; Ambavane, A.; Gupte-Singh, K.; Aponte-Ribero, V.; Ritchings, C.; Benedict, A.; Rao, S.; Regan, M.M.; Atkins, M. Clinical and Economic Outcomes Associated with Treatment Sequences In Patients With BRAF -Mutant Advanced Melanoma. Immunotherapy 2018, 11, 283–295. [Google Scholar] [CrossRef]
- Wei, M.; Liu, X.; Cao, C.; Yang, J.; Lv, Y.; Huang, J.; Wang, Y.; Qin, Y. An engineered PD-1-based and MMP-2/9-oriented fusion protein exerts potent antitumor effects against melanoma. BMB Rep. 2018, 51, 572–577. [Google Scholar] [CrossRef]
- Beckman, R.A.; Weiner, L.M.; Davis, H.M. Antibody constructs in cancer therapy: Protein engineering strategies to improve exposure in solid tumors. Cancer 2007, 109, 170–179. [Google Scholar] [CrossRef]
- Zhong, G.; Zhang, S.; Li, Y.; Liu, X.; Gao, R.; Miao, Q.; Zhen, Y. A tandem scFv-based fusion protein and its enediyne-energized analogue show intensified therapeutic efficacy against lung carcinoma xenograft in athymic mice. Cancer Lett. 2010, 295, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S.; Cao, J.; Chen, W.-T. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 2000, 19, 6642–6650. [Google Scholar] [CrossRef]
- Dezutter-Dambuyant, C.; Durand, I.; Alberti, L.; Bendriss-Vermare, N.; Valladeau-Guilemond, J.; Duc, A.; Magron, A.; Morel, A.-P.; Sisirak, V.; Rodriguez, C.; et al. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. OncoImmunology 2015, 5, e1091146. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Evans, K.S.; Xiao, C.; DeVito, N.; Theivanthiran, B.; Holtzhausen, A.; Siska, P.J.; Blobe, G.C.; Hanks, B.A. Stromal Fibroblasts Mediate Anti–PD-1 Resistance via MMP-9 and Dictate TGFβ Inhibitor Sequencing in Melanoma. Cancer Immunol. Res. 2018, 6, 1459–1471. [Google Scholar] [CrossRef]

| Subgroup | MMP | Main Function | Role in Carcinogenesis | Expression in Melanoma |
|---|---|---|---|---|
| Collagenases | MMP-1 | Collagen type I and III degradation | Facilitate tumor invasion in melanoma | ↑ |
| MMP-13 | Collagen type I, II, III, and IV degradation | Involved in invasive phenotype of melanoma | ↑ | |
| Gelatinases | MMP-2 | Collagen type IV degradation | Metastasis in melanoma | ↑ |
| MMP-9 | Degrade collage type IV | Tumor angiogenesis in melanoma | ↑ | |
| Stromelysins | MMP-3 | Collagen type I degradation Activate MMP-1, MMP-9 | Activate pro-MMPs in melanoma | ↑ |
| Membrane type MMPs | MMP-14 | Collagen type I, II, and III degradation | Tumor invasion Activation of MMP-2 Tumor angiogenesis in melanoma | ↑ |
| MMPI | Activity | Source |
|---|---|---|
| Batimastat | Binds to and inhibits the catalytic zinc ion of various MMPs | [76] |
| Marimastat | Binds to and inhibits the catalytic zinc ion of various MMPs | [75] |
| Prinomastat | Selectively inhibits MMP-2, MMP-3, MMP-9, MMP-13, and MMP-14 | [77] |
| Tanomastat | Binds to zinc and selectively inhibits MMP-2, MMP-3, MMP-9, MMP-13, and MMP-14 | [77] |
| Andekaliksymab | Monoclonal antibodies against MMP-1, MMP-2, MMP-9, MMP-3, and MMP-14 | [77] |
| miR-143 | Decrease melanoma cell metastasis by targeting MMP-9 | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczygielski, O.; Dąbrowska, E.; Niemyjska, S.; Przylipiak, A.; Zajkowska, M. Targeting Matrix Metalloproteinases and Their Inhibitors in Melanoma. Int. J. Mol. Sci. 2024, 25, 13558. https://doi.org/10.3390/ijms252413558
Szczygielski O, Dąbrowska E, Niemyjska S, Przylipiak A, Zajkowska M. Targeting Matrix Metalloproteinases and Their Inhibitors in Melanoma. International Journal of Molecular Sciences. 2024; 25(24):13558. https://doi.org/10.3390/ijms252413558
Chicago/Turabian StyleSzczygielski, Orest, Emilia Dąbrowska, Sylwia Niemyjska, Andrzej Przylipiak, and Monika Zajkowska. 2024. "Targeting Matrix Metalloproteinases and Their Inhibitors in Melanoma" International Journal of Molecular Sciences 25, no. 24: 13558. https://doi.org/10.3390/ijms252413558
APA StyleSzczygielski, O., Dąbrowska, E., Niemyjska, S., Przylipiak, A., & Zajkowska, M. (2024). Targeting Matrix Metalloproteinases and Their Inhibitors in Melanoma. International Journal of Molecular Sciences, 25(24), 13558. https://doi.org/10.3390/ijms252413558







