Thymol Impacts the Progression of Endometriosis by Disrupting Estrogen Signaling Pathways and Inflammatory Responses
Abstract
1. Introduction
2. Results
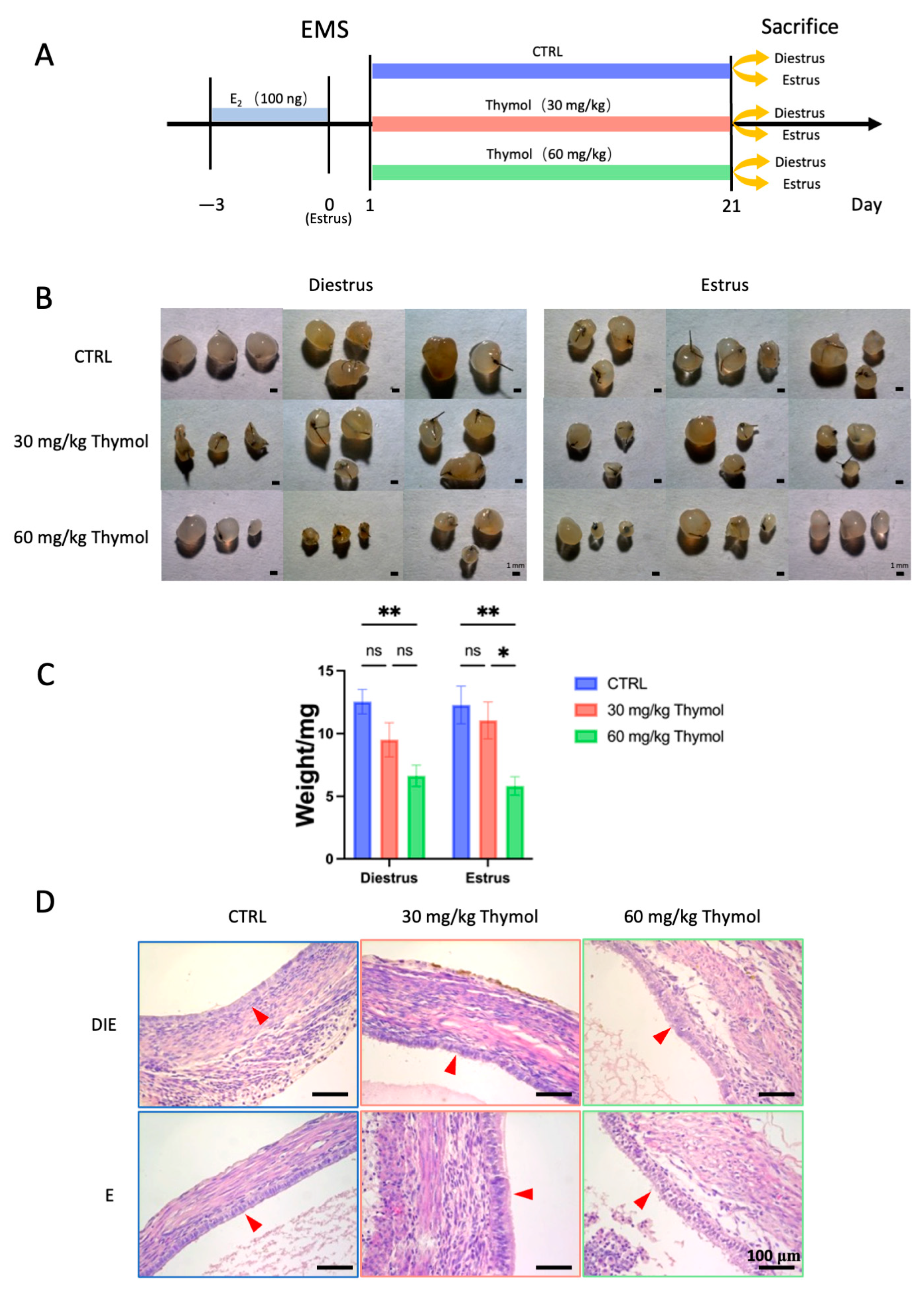
2.1. Thymol Impedes Endometriotic Lesion Growth by Affecting Cell Proliferation and Apoptosis
2.2. Thymol Mitigates the Inflammatory Reaction in Endometriotic Lesions
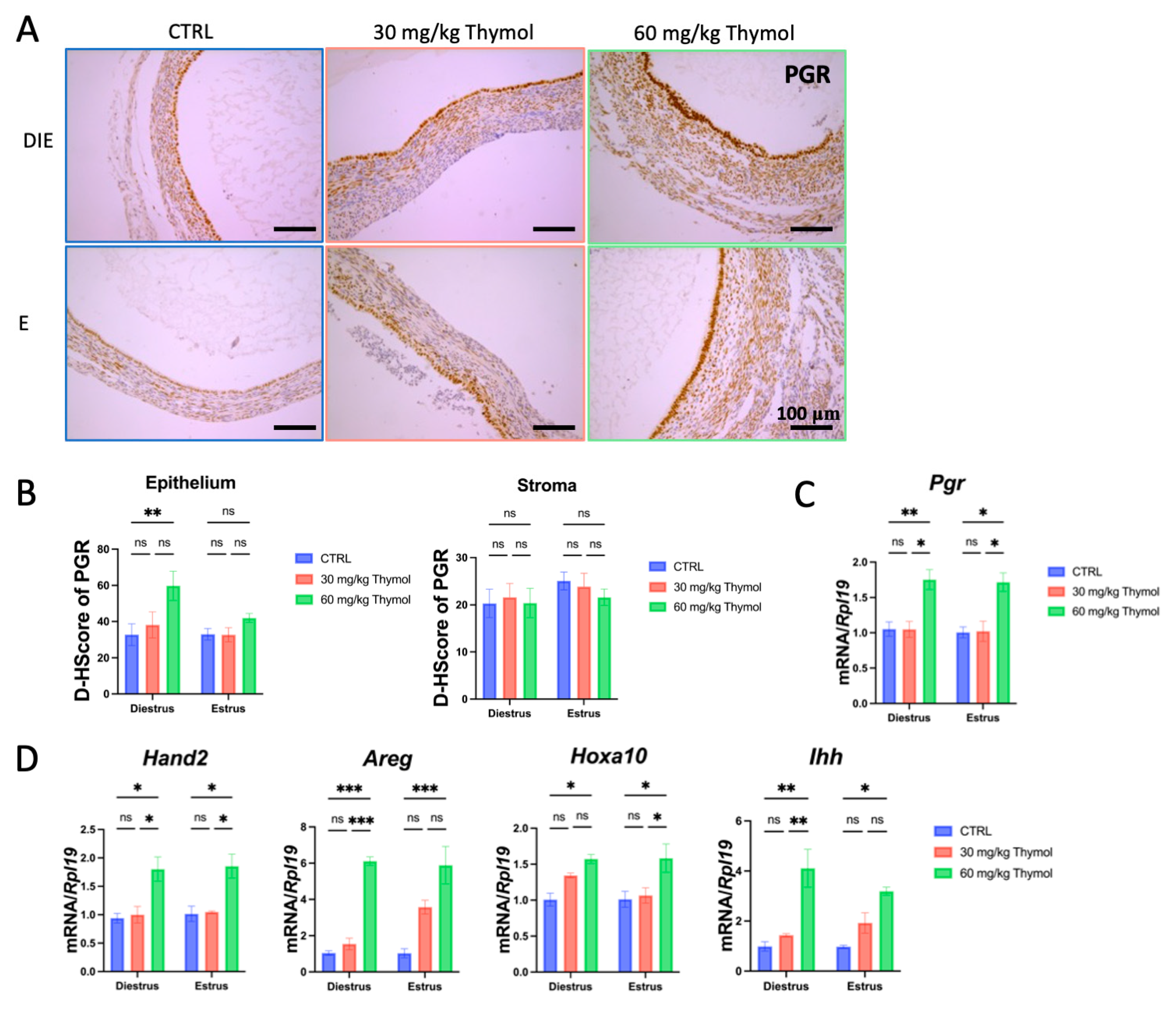
2.3. Thymol Rebalances Estrogen–Progestogen Signaling in Ectopic Tissue
2.4. Thymol Acts as an Antagonist of Estrogen
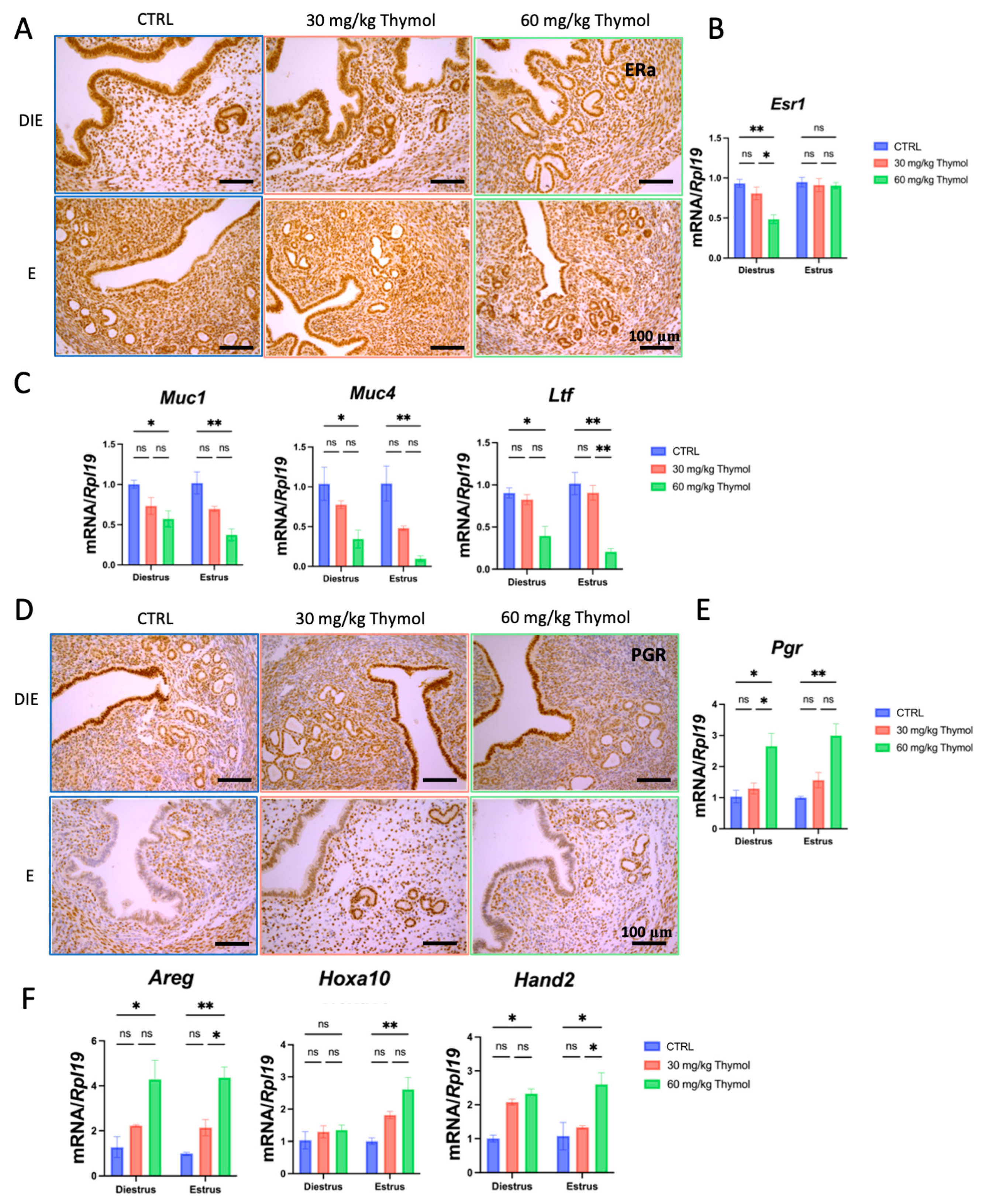
2.5. Thymol Affects Estrogen and Progesterone Signaling in Eutopic Endometrium Without Impacting the Establishment of Pregnancy
3. Discussion
4. Materials and Methods
4.1. Animal and Ethics Approval
4.2. Establishment of Murine Endometriotic Model
4.3. Induction of Pregnancy and Treatment with Thymol
4.4. Histological Staining
4.5. Cell Culture and Treatments
4.6. Cell Viability Assay
4.7. qPCR Assay
4.8. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCs | Mesothelial cells |
| EDCs | Endocrine-disrupting chemicals |
| ESR1 | Estrogen receptor 1 |
| PGR | Progesterone receptor |
| BPA | Bisphenol A |
| MUC1 | Recombinant Mucin 1 |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| DES | Diethylstilbestrol |
| IFN-γ | Interferon-γ |
| TNF-α | Tumor Necrosis Factor-α |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| Ptgs2 | Prostaglandin-Endoperoxide Synthase 2 |
| COX2 | Cyclooxygenase-2 |
| CTRL | Control group |
| Ihh | Indian hedgehog |
| HAND2 | Heart and neural crest derivatives-expressed transcript 2 |
| HOXA10 | Homeobox gene A10 |
| AREG | Amphiregulin |
| MUC | Mucin |
| LFT | Lactotransferrin |
| F4/80 | Mouse EGF-like module-containing mucin-like hormone receptor-like 1 |
| Ly6G | Lymphocyte antigen 6complex, locus g |
References
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Stern, R.C.; Dash, R.; Bentley, R.C.; Snyder, M.J.; Haney, A.F.; Robboy, S.J. Malignancy in endometriosis: Frequency and comparison of ovarian and extraovarian types. Int. J. Gynecol. Pathol. 2001, 20, 133–139. [Google Scholar] [CrossRef]
- Schindler, A.E. Dienogest in long-term treatment of endometriosis. Int. J. Women’s Health 2011, 3, 175–184. [Google Scholar] [CrossRef]
- Holoch, K.J.; Lessey, B.A. Endometriosis and infertility. Clin. Obstet. Gynecol. 2010, 53, 429–438. [Google Scholar] [CrossRef]
- Sepulcri Rde, P.; do Amaral, V.F. Depressive symptoms, anxiety, and quality of life in women with pelvic endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 142, 53–56. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef]
- Polak, G.; Banaszewska, B.; Filip, M.; Radwan, M.; Wdowiak, A. Environmental Factors and Endometriosis. Int. J. Environ. Res. Public Health 2021, 18, 11025. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.G.; Rudnicki, M.; Yu, J.; Shu, Y.; Taylor, R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 2017, 96, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.C.; Winuthayanon, W.; Korach, K.S. What’s new in estrogen receptor action in the female reproductive tract. J. Mol. Endocrinol. 2016, 56, R55–R71. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Imir, G.; Utsunomiya, H.; Thung, S.; Gurates, B.; Tamura, M.; Lin, Z. Aromatase in endometriosis and uterine leiomyomata. J. Steroid. Biochem. Mol. Biol. 2005, 95, 57–62. [Google Scholar] [CrossRef]
- Al-Sabbagh, M.; Lam, E.W.; Brosens, J.J. Mechanisms of endometrial progesterone resistance. Mol. Cell. Endocrinol. 2012, 358, 208–215. [Google Scholar] [CrossRef]
- Xue, Q.; Lin, Z.; Cheng, Y.H.; Huang, C.C.; Marsh, E.; Yin, P.; Milad, M.P.; Confino, E.; Reierstad, S.; Innes, J.; et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol. Reprod. 2007, 77, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jung, S.Y.; Wu, S.P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.J.; et al. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef]
- Ramírez-Pavez, T.N.; Martínez-Esparza, M.; Ruiz-Alcaraz, A.J.; Marín-Sánchez, P.; Machado-Linde, F.; García-Peñarrubia, P. The Role of Peritoneal Macrophages in Endometriosis. Int. J. Mol. Sci. 2021, 22, 10792. [Google Scholar] [CrossRef]
- Akoum, A.; Kong, J.; Metz, C.; Beaumont, M.C. Spontaneous and stimulated secretion of monocyte chemotactic protein-1 and macrophage migration inhibitory factor by peritoneal macrophages in women with and without endometriosis. Fertil. Steril. 2002, 77, 989–994. [Google Scholar] [CrossRef]
- Xie, Q.; He, H.; Wu, Y.H.; Zou, L.J.; She, X.L.; Xia, X.M.; Wu, X.Q. Eutopic endometrium from patients with endometriosis modulates the expression of CD36 and SIRP-α in peritoneal macrophages. J. Obstet. Gynaecol. Res. 2019, 45, 1045–1057. [Google Scholar] [CrossRef]
- Jantz, M.A.; Antony, V.B. Pathophysiology of the pleura. Respiration 2008, 75, 121–133. [Google Scholar] [CrossRef]
- Jonjić, N.; Peri, G.; Bernasconi, S.; Sciacca, F.L.; Colotta, F.; Pelicci, P.; Lanfrancone, L.; Mantovani, A. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J. Exp. Med. 1992, 176, 1165–1174. [Google Scholar] [CrossRef]
- Becker, C.M.; Gattrell, W.T.; Gude, K.; Singh, S.S. Reevaluating response and failure of medical treatment of endometriosis: A systematic review. Fertil. Steril. 2017, 108, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Sachedina, A.; Todd, N. Dysmenorrhea, Endometriosis and Chronic Pelvic Pain in Adolescents. J. Clin. Res. Pediatr. Endocrinol. 2020, 12 (Suppl. 1), 7–17. [Google Scholar] [CrossRef] [PubMed]
- Schjerning, A.M.; McGettigan, P.; Gislason, G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat. Rev. Cardiol. 2020, 17, 574–584. [Google Scholar] [CrossRef]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. Sci. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Bina, F.; Soleymani, S.; Toliat, T.; Hajimahmoodi, M.; Tabarrai, M.; Abdollahi, M.; Rahimi, R. Plant-derived medicines for treatment of endometriosis: A comprehensive review of molecular mechanisms. Pharmacol. Res. 2019, 139, 76–90. [Google Scholar] [CrossRef]
- Park, S.; Song, G.; Lim, W. Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J. Nutr. Biochem. 2020, 78, 108328. [Google Scholar] [CrossRef]
- Kolahdouz Mohammadi, R.; Arablou, T. Resveratrol and endometriosis: In vitro and animal studies and underlying mechanisms (Review). Biomed. Pharmacother. 2017, 91, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.D.M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef]
- Amiri, H. Essential oils composition and antioxidant properties of three thymus species. Evid. Based Complement. Alternat. Med. 2012, 2012, 728065. [Google Scholar] [CrossRef]
- Guo, C.; Zheng, L.; Chen, S.; Liang, X.; Song, X.; Wang, Y.; Hua, B.; Qiu, L. Thymol ameliorates ethanol-induced hepatotoxicity via regulating metabolism and autophagy. Chem.-Biol. Interact. 2023, 370, 110308. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Luna, A.; Lema-Alba, R.C.; Dambolena, J.S.; Zygadlo, J.A.; Labaque, M.C.; Marin, R.H. Thymol as natural antioxidant additive for poultry feed: Oxidative stability improvement. Poult. Sci. 2017, 96, 3214–3220. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, D.L.; Xie, L.N.; Ma, Y.R.; Wu, P.P.; Li, C.; Liu, W.F.; Zhang, K.; Zhou, R.P.; Xu, X.T.; et al. Synergistic anti-inflammatory effects of silibinin and thymol combination on LPS-induced RAW264.7 cells by inhibition of NF-κB and MAPK activation. Phytomedicine 2020, 78, 153309. [Google Scholar] [CrossRef]
- Gabbai-Armelin, P.R.; Sales, L.S.; Ferrisse, T.M.; De Oliveira, A.B.; De Oliveira, J.R.; Giro, E.M.A.; Brighenti, F.L. A systematic review and meta-analysis of the effect of thymol as an anti-inflammatory and wound healing agent: A review of thymol effect on inflammation and wound healing: A review of thymol effect on inflammation and wound healing. Phytother. Res. 2022, 36, 3415–3443. [Google Scholar] [CrossRef]
- Dou, X.; Yan, D.; Liu, S.; Gao, L.; Shan, A. Thymol Alleviates LPS-Induced Liver Inflammation and Apoptosis by Inhibiting NLRP3 Inflammasome Activation and the AMPK-mTOR-Autophagy Pathway. Nutrients 2022, 14, 2809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Fu, Y.; Wei, Z.; Yu, Y.; Zhang, X.; Yang, Z. Thymol attenuates allergic airway inflammation in ovalbumin (OVA)-induced mouse asthma. Fitoterapia 2014, 96, 131–137. [Google Scholar] [CrossRef]
- Marquardt, R.M.; Kim, T.H.; Shin, J.H.; Jeong, J.W. Progesterone and Estrogen Signaling in the Endometrium: What Goes Wrong in Endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef]
- Kitawaki, J.; Kado, N.; Ishihara, H.; Koshiba, H.; Kitaoka, Y.; Honjo, H. Endometriosis: The pathophysiology as an estrogen-dependent disease. J. Steroid. Biochem. Mol. Biol. 2002, 83, 149–155. [Google Scholar] [CrossRef]
- Agic, A.; Djalali, S.; Diedrich, K.; Hornung, D. Apoptosis in endometriosis. Gynecol. Obstet. Investig. 2009, 68, 217–223. [Google Scholar] [CrossRef]
- Burns, K.A.; Pearson, A.M.; Slack, J.L.; Por, E.D.; Scribner, A.N.; Eti, N.A.; Burney, R.O. Endometriosis in the Mouse: Challenges and Progress Toward a ‘Best Fit’ Murine Model. Front. Physiol. 2021, 12, 806574. [Google Scholar] [CrossRef] [PubMed]
- Pelch, K.E.; Sharpe-Timms, K.L.; Nagel, S.C. Mouse model of surgically-induced endometriosis by auto-transplantation of uterine tissue. J. Vis. Exp. JoVE 2012, 59, e3396. [Google Scholar] [CrossRef]
- Liu, Y.N.; Kang, J.W.; Zhang, Y.; Song, S.S.; Xu, Q.X.; Zhang, H.; Lu, L.; Wei, S.W.; Liang, C.; Su, R.W. Vanillin prevents the growth of endometriotic lesions through anti-inflammatory and antioxidant pathways in a mouse model. Food Funct. 2023, 14, 6730–6744. [Google Scholar] [CrossRef]
- Castro, J.; Maddern, J.; Grundy, L.; Manavis, J.; Harrington, A.M.; Schober, G.; Brierley, S.M. A mouse model of endometriosis that displays vaginal, colon, cutaneous, and bladder sensory comorbidities. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21430. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Quattrone, F.; Pannese, M.; Ulisse, A.; Candiani, M.; Diaz-Alonso, J.; Velasco, G.; Panina-Bordignon, P. The cannabinoid receptor CB1 contributes to the development of ectopic lesions in a mouse model of endometriosis. Hum. Reprod. 2017, 32, 175–184. [Google Scholar]
- Lousse, J.C.; Van Langendonckt, A.; Defrere, S.; Ramos, R.G.; Colette, S.; Donnez, J. Peritoneal endometriosis is an inflammatory disease. Front. Biosci. (Elite Ed.) 2012, 4, 23–40. [Google Scholar] [CrossRef]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Sekine, I.; Ishimaru, T.; Masuzaki, H. Immunopathogenesis of pelvic endometriosis: Role of hepatocyte growth factor, macrophages and ovarian steroids. Am. J. Reprod. Immunol. 2008, 60, 383–404. [Google Scholar] [CrossRef]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Update 2019, 25, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Bergqvist, A.; Bruse, C.; Carlberg, M.; Carlström, K. Interleukin 1beta, interleukin-6, and tumor necrosis factor-alpha in endometriotic tissue and in endometrium. Fertil. Steril. 2001, 75, 489–495. [Google Scholar] [CrossRef]
- Carmona, F.; Chapron, C.; Martínez-Zamora, M.; Santulli, P.; Rabanal, A.; Martínez-Florensa, M.; Lozano, F.; Balasch, J. Ovarian endometrioma but not deep infiltrating endometriosis is associated with increased serum levels of interleukin-8 and interleukin-6. J. Reprod. Immunol. 2012, 95, 80–86. [Google Scholar] [CrossRef]
- Volpato, L.K.; Horewicz, V.V.; Bobinski, F.; Martins, D.F.; Piovezan, A.P. Annexin A1, FPR2/ALX, and inflammatory cytokine expression in peritoneal endometriosis. J. Reprod. Immunol. 2018, 129, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Jaeger-Lansky, A.; Schmidthaler, K.; Kuessel, L.; Gstöttner, M.; Waidhofer-Söllner, P.; Zlabinger, G.J.; Wenzl, R.; Eiwegger, T. Local and systemic levels of cytokines and danger signals in endometriosis-affected women. J. Reprod. Immunol. 2018, 130, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.C.; Gong, J.; Nyachoti, M.; Yang, C. Thymol Improves Barrier Function and Attenuates Inflammatory Responses in Porcine Intestinal Epithelial Cells during Lipopolysaccharide (LPS)-Induced Inflammation. J. Agric. Food Chem. 2019, 67, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Yang, H.; Yan, H.J.; Ge, R.F.; Wang, Y.X.; Xue, S.S.; Li, L.; Lyu, L.Y.; Che, C.Y. Thymol Protects against Aspergillus Fumigatus Keratitis by Inhibiting the LOX-1/IL-1β Signaling Pathway. Curr. Med. Sci. 2022, 42, 620–628. [Google Scholar] [CrossRef]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef]
- Zakhari, A.; Delpero, E.; McKeown, S.; Tomlinson, G.; Bougie, O.; Murji, A. Endometriosis recurrence following post-operative hormonal suppression: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef]
- Bull, J.R.; Rowland, S.P.; Scherwitzl, E.B.; Scherwitzl, R.; Danielsson, K.G.; Harper, J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit. Med. 2019, 2, 83. [Google Scholar] [CrossRef]
- Kuan, K.K.W.; Gibson, D.A.; Whitaker, L.H.R.; Horne, A.W. Menstruation Dysregulation and Endometriosis Development. Front. Reprod. Health 2021, 3, 756704. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Ogaly, H.A.; Abdel-Rahman, R.F.; Mohamed, M.A.E.; Ahmed-Farid, O.A.; Khattab, M.S.; Abd-Elsalam, R.M. Thymol ameliorated neurotoxicity and cognitive deterioration in a thioacetamide-induced hepatic encephalopathy rat model; involvement of the BDNF/CREB signaling pathway. Food Funct. 2022, 13, 6180–6194. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-M.; Zhou, C.-Y.; Meng, X.-L.; Wang, P.; Li, W. Thymol exerts anti-inflammatory effect in dextran sulfate sodium-induced experimental murine colitis. Trop. J. Pharm. Res. 2018, 17, 1803–1810. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shaukat, A.; Zhang, H.; Yang, Y.-F.; Li, H.-X.; Li, G.-Y.; Liu, Y.-N.; Liang, C.; Kang, J.-W.; Li, S.-C.; et al. Thymol Impacts the Progression of Endometriosis by Disrupting Estrogen Signaling Pathways and Inflammatory Responses. Int. J. Mol. Sci. 2024, 25, 13150. https://doi.org/10.3390/ijms252313150
Zhang Y, Shaukat A, Zhang H, Yang Y-F, Li H-X, Li G-Y, Liu Y-N, Liang C, Kang J-W, Li S-C, et al. Thymol Impacts the Progression of Endometriosis by Disrupting Estrogen Signaling Pathways and Inflammatory Responses. International Journal of Molecular Sciences. 2024; 25(23):13150. https://doi.org/10.3390/ijms252313150
Chicago/Turabian StyleZhang, Yu, Aftab Shaukat, Han Zhang, Yao-Feng Yang, Hui-Xia Li, Guang-Ya Li, Ying-Nan Liu, Chen Liang, Jin-Wen Kang, Shao-Chuan Li, and et al. 2024. "Thymol Impacts the Progression of Endometriosis by Disrupting Estrogen Signaling Pathways and Inflammatory Responses" International Journal of Molecular Sciences 25, no. 23: 13150. https://doi.org/10.3390/ijms252313150
APA StyleZhang, Y., Shaukat, A., Zhang, H., Yang, Y.-F., Li, H.-X., Li, G.-Y., Liu, Y.-N., Liang, C., Kang, J.-W., Li, S.-C., & Su, R.-W. (2024). Thymol Impacts the Progression of Endometriosis by Disrupting Estrogen Signaling Pathways and Inflammatory Responses. International Journal of Molecular Sciences, 25(23), 13150. https://doi.org/10.3390/ijms252313150





