Advanced PROTAC and Quantitative Proteomics Strategy Reveals Bax Inhibitor-1 as a Critical Target of Icaritin in Burkitt Lymphoma
Abstract
1. Introduction
2. Results
2.1. Design and Synthesis of ICT-PROTACs
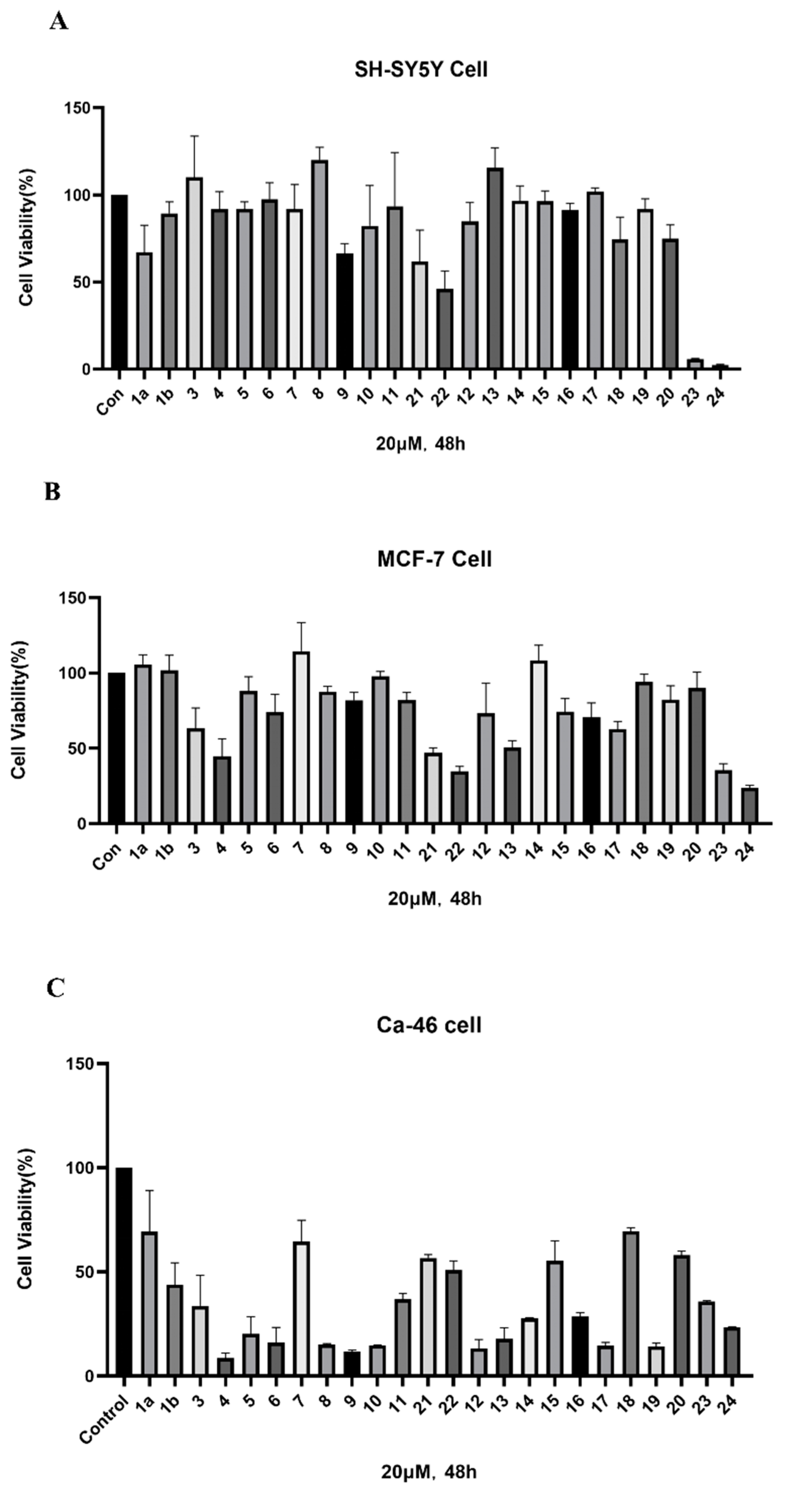
2.2. ICT-PROTACs Inhibited the Tumor Cells’ Proliferation
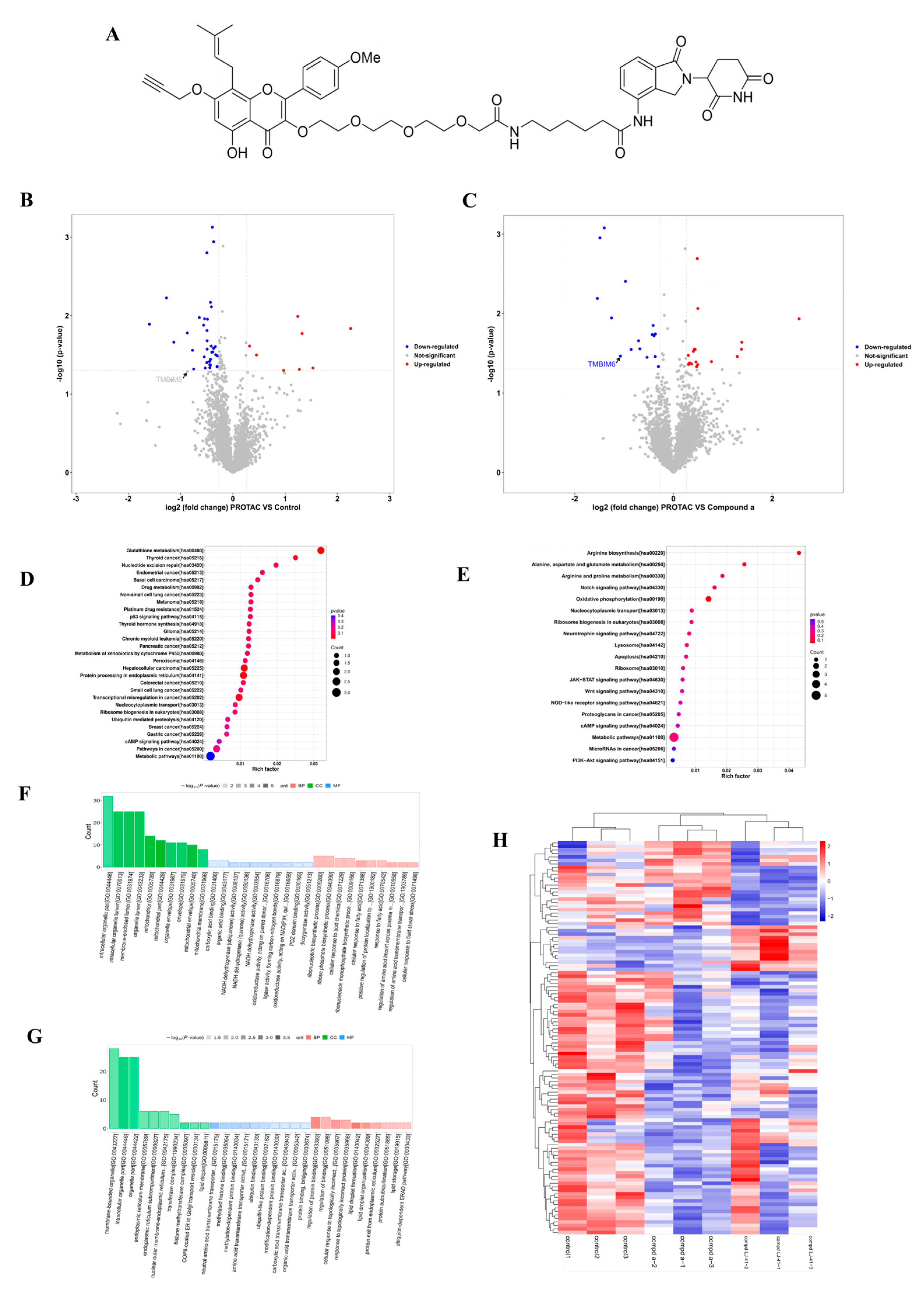
2.3. Proteomic Profiling Identifies BI-1 as a Potential Target of LJ-41
2.4. LJ-41 Induces BI-1 Degradation Via the Ubiquitin-Proteasome System
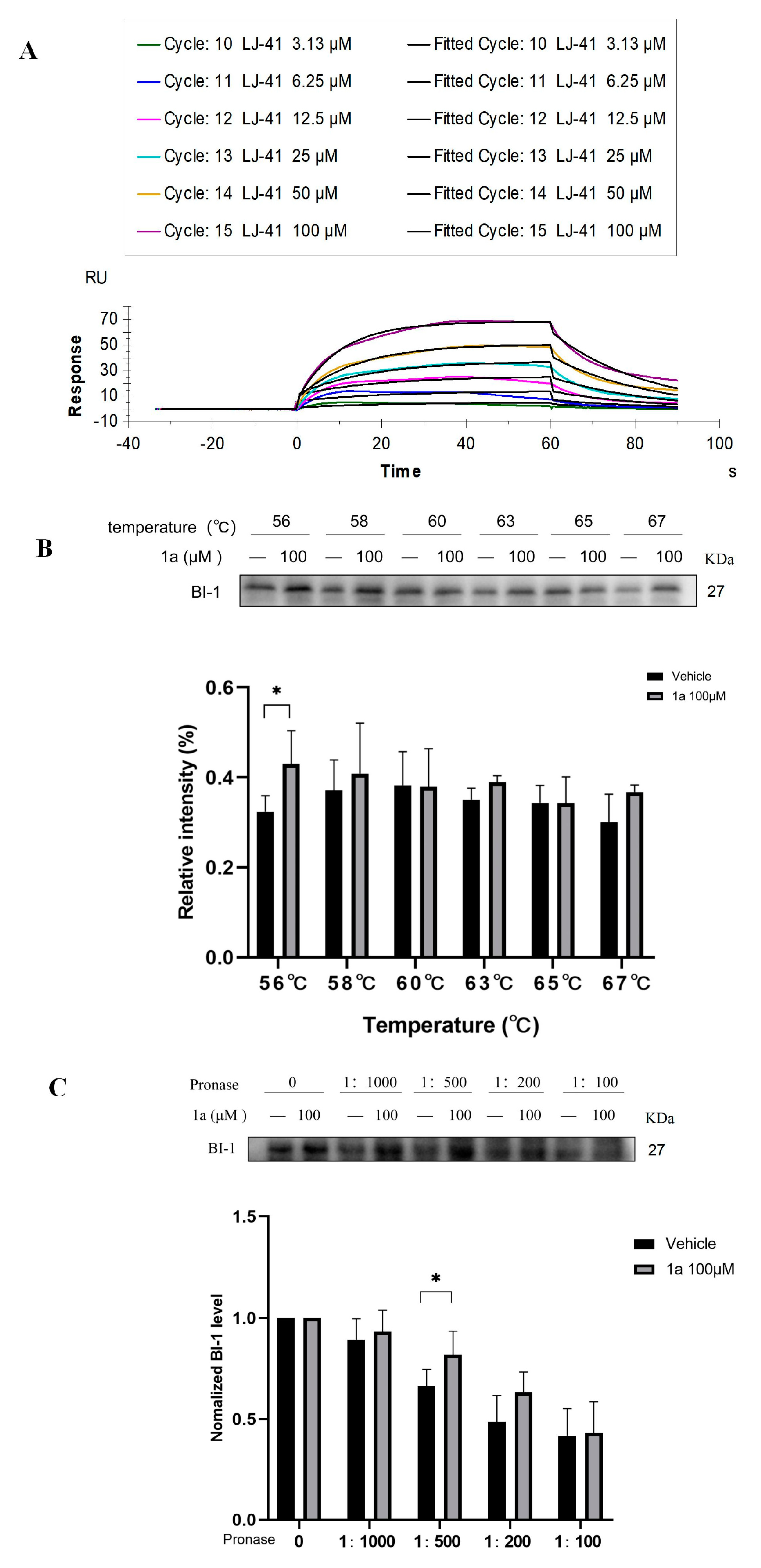
2.5. Direct Binding of LJ-41 to BI-1
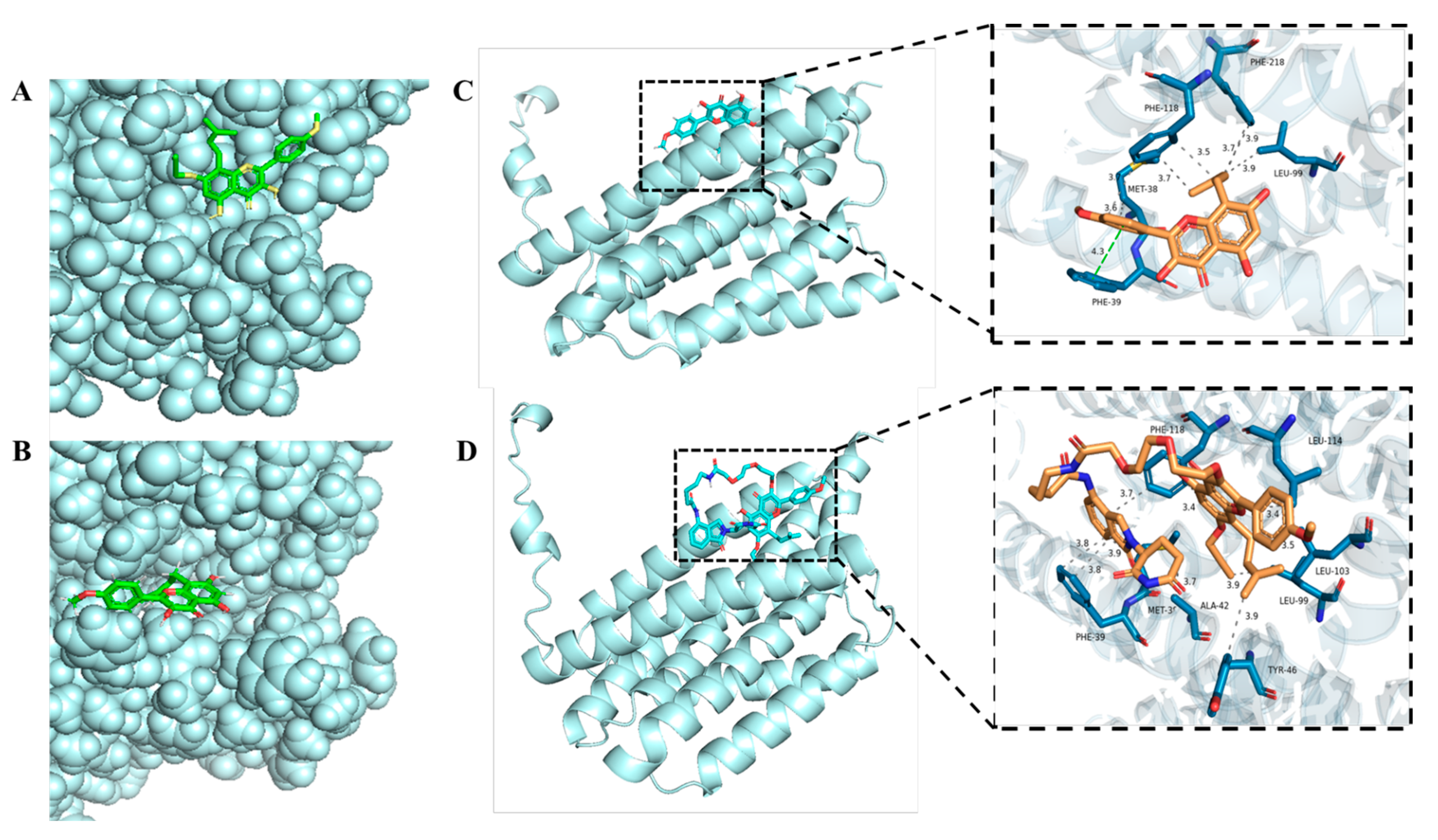
2.6. Molecular Docking Analysis
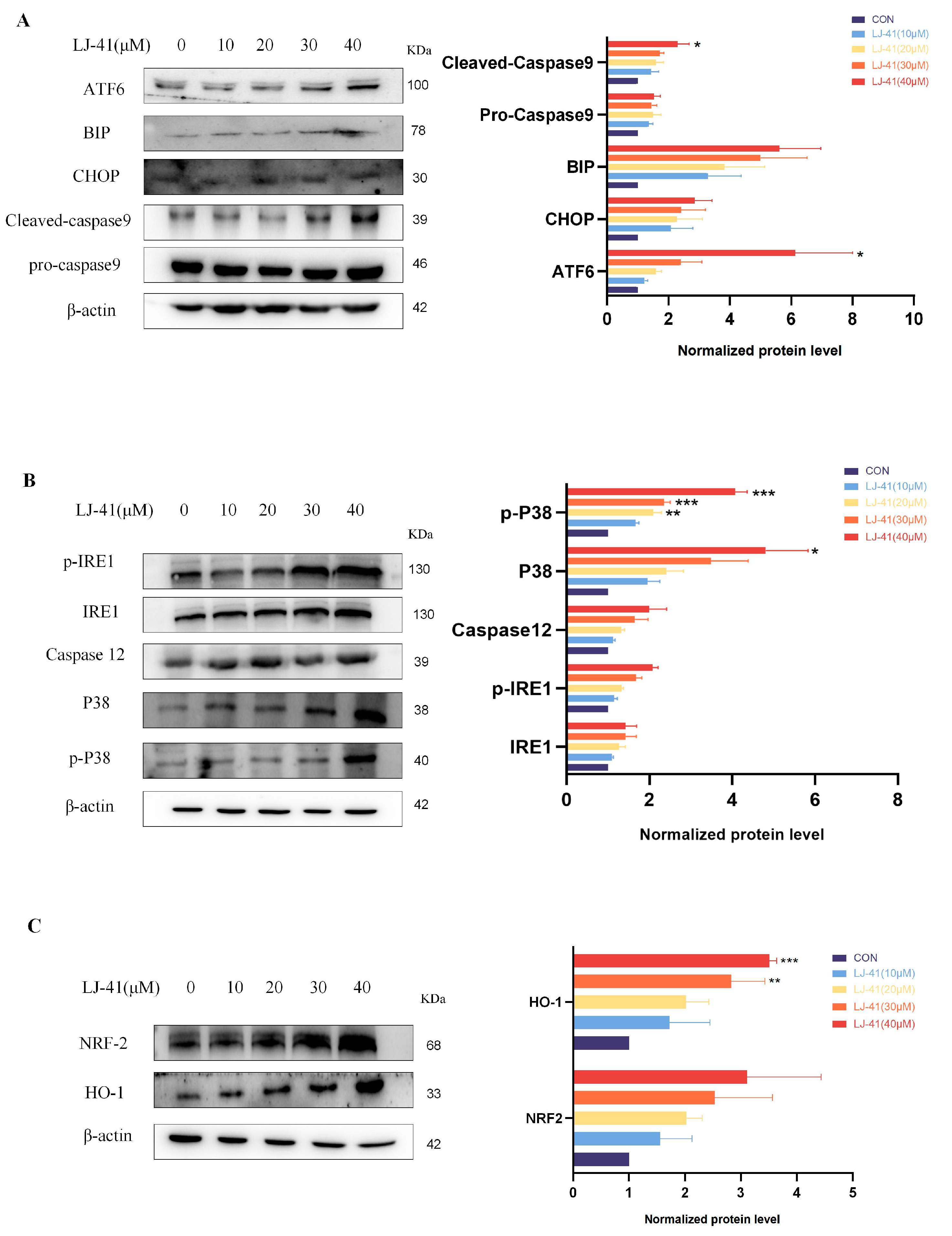
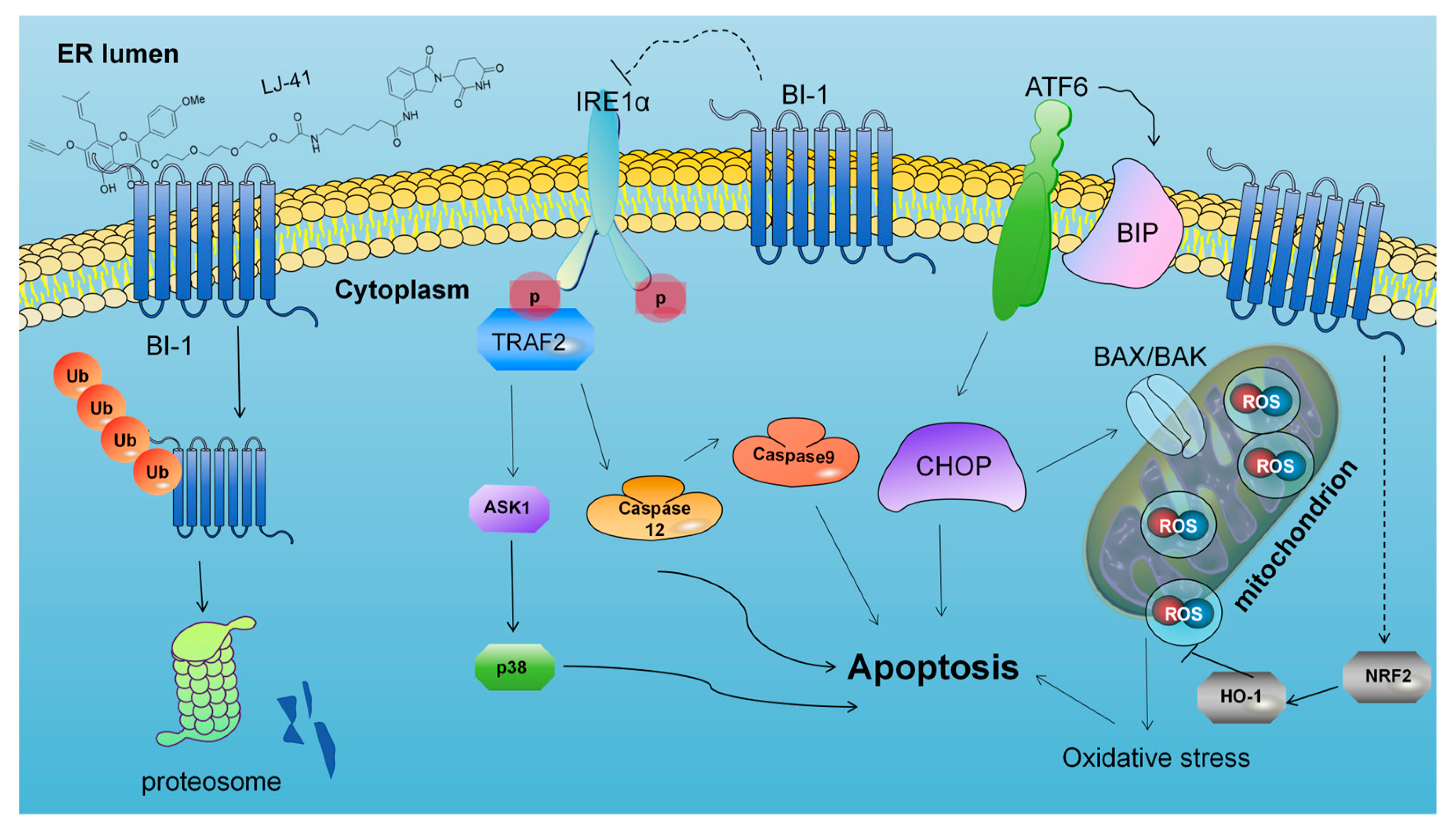
2.7. LJ-41-Induced Apoptosis Via ERS Pathways
3. Discussion
3.1. Enhanced PROTACs for Improved Anti-Tumor Efficacy
3.2. Methodological Advancements in Target Identification
3.3. BI-1 as a Novel Target for Burkitt Lymphoma
3.4. Mechanistic Insights into ER Stress-Induced Apoptosis
4. Materials and Methods
4.1. Cell Culture and Chemicals
4.2. CCK-8 Assay
4.3. Mass Spectrometry for Proteomics
4.4. Protein Extraction and Western Blotting
4.5. Cellular Thermal Shift Assay (CETSA)
4.6. Drug Affinity Responsive Target Stability (DARTS) Assay
4.7. Surface Plasmon Resonance (SPR) Assay
4.8. Molecular Docking Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, S.; Guo, X.; Han, F.; He, Z.; Wang, Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 1163–1185. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B. Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T.; Int Nat Prod Sci, T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Y.; Wang, W.; Sun, D.; Liang, J.; Zhu, M.; Li, H.; Chen, L. PROTAC technology as a novel tool to identify the target of lathyrane diterpenoids. Acta Pharm. Sin. B 2022, 12, 4262–4265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, Z.W.; Chen, D.; Gu, Z.C.; Yan, W.L.; Yue, L.Y.; Zhu, R.G.; Zhao, Y.L.; Chen, L.; Zhao, Q.J.; et al. Facilitated Drug Repurposing with Artemisinin-Derived PROTACs: Unveiling PCLAF as a Therapeutic Target. J. Med. Chem. 2023, 66, 11335–11350. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.W.; Cui, G.; Leung, G.P.H.; Luk, S.C.W.; Hoi, M.P.M.; Wang, L.; Mahady, G.B.; Lee, S.M.Y. Icaritin protects against oxidative stress-induced injury in cardiac H9c2 cells via Akt/Nrf2/HO-1 and calcium signalling pathways. J. Funct. Foods 2015, 18, 213–223. [Google Scholar] [CrossRef]
- Zhang, W.; Xing, B.; Yang, L.; Shi, J.; Zhou, X. Icaritin Attenuates Myocardial Ischemia and Reperfusion Injury Via An-ti-Inflammatory and Anti-Oxidative Stress Effects in Rats. Am. J. Chin. Med. 2015, 43, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Xie, X.-H.; Wang, X.-L.; Wan, C.; Lee, Y.-W.; Chen, S.-H.; Pei, D.-Q.; Wang, Y.-X.; Li, G.; Qin, L. Icaritin, an Exogenous Phytomolecule, Enhances Osteogenesis but Not Angiogenesis-An In Vitro Efficacy Study. PLoS ONE 2012, 7, e41264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, T.; Shu, T.; Kang, L.; Wu, J.; Xing, J.; Lu, Z.; Chen, S.; Lv, J. Icaritin, a novel plant-derived osteoinductive agent, enhances the osteogenic differentiation of human bone marrow- and human adipose tissue-derived mesenchymal stem cells. Int. J. Mol. Med. 2017, 39, 984–992. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Huang, N.-Q.; Feng, F.; Li, Y.; Luo, X.-M.; Tu, L.; Qu, J.-Q.; Xie, Y.-M.; Luo, Y. Icaritin Improves Memory and Learning Ability by Decreasing BACE-1 Expression and the Bax/Bcl-2 Ratio in Senescence-Accelerated Mouse Prone 8 (SAMP8) Mice. Evid. Based Complement. Altern. Med. 2020, 2020, 8963845. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, X.; Gao, Z.-Y.; Lin, M.; Zhao, X.; Sun, Y.; Pu, X.-P. Icaritin Provides Neuroprotection in Parkinson’s Disease by Attenuating Neuroinflammation, Oxidative Stress, and Energy Deficiency. Antioxidants 2021, 10, 529. [Google Scholar] [CrossRef]
- Zhu, S.; Xing, C.; Zhang, G.; Peng, H.; Wang, Z. Icaritin induces cellular senescence by accumulating the ROS production and regulation of the Jak2/Stat3/p21 pathway in imatinib-resistant, chronic myeloid leukemia cells. Am. J. Transl. Res. 2021, 13, 8860–8872. [Google Scholar] [PubMed]
- Yang, X.-J.; Xi, Y.-M.; Li, Z.-J. Icaritin: A Novel Natural Candidate for Hematological Malignancies Therapy. BioMed Res. Int. 2019, 2019, 4860268. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Guo, J.; Hu, M.; Gao, Y.; Huang, L. Icaritin Exacerbates Mitophagy and Synergizes with Doxorubicin to Induce Immunogenic Cell Death in Hepatocellular Carcinoma. Acs Nano 2020, 14, 4816–4828. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Jiang, W.; Xue, J.; Cheng, Y.; Wang, J.; Yang, R.; Zhang, X. Icaritin promotes apoptosis and inhibits proliferation by down-regulating AFP gene expression in hepatocellular carcinoma. Bmc Cancer 2021, 21, 318. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, N.; Dong, J.; Wang, X.; Liu, L.; Huang, J. Estrogen receptor-alpha 36 is involved in icaritin induced growth inhibition of triple-negative breast cancer cells. J. Steroid Biochem. Mol. Biol. 2017, 171, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Qi, X.-W.; Liu, X.-Z.; Yang, Z.-Y.; Cai, R.-L.; Cui, H.-J.; Chen, L.; Yu, S.-C. Icaritin enhances the efficacy of cetuximab against triple-negative breast cancer cells. Oncol. Lett. 2020, 19, 3950–3958. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Long, X.; Yang, C.; Chen, H.; Sharkey, C.; Rashid, K.; Hu, M.; Liu, Y.; Huang, Q.; Chen, Q.; et al. Icaritin reduces prostate cancer progression via inhibiting high-fat diet-induced serum adipokine in TRAMP mice model. J. Cancer 2020, 11, 6556–6564. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, W.; Liang, L.; Duan, X.; Deng, J.; Zhou, Y. Natural product-derived icaritin exerts anti-glioblastoma effects by positively modulating estrogen receptor beta. Exp. Ther. Med. 2020, 19, 2841–2850. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Duan, F.; Jiang, H.; Chen, M.-F.; Tang, S.-Y. Icaritin Induces Apoptosis of HepG2 Cells via the JNK1 Signaling Pathway Independent of the Estrogen Receptor. Planta Medica 2010, 76, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Liu, M.; Wang, Y.; Luo, S.; Xu, Y.; Ye, B.; Zheng, L.; Meng, K.; Li, L. Icaritin Induces Anti-tumor Immune Responses in Hepatocellular Carcinoma by Inhibiting Splenic Myeloid-Derived Suppressor Cell Generation. Front. Immunol. 2021, 12, 609295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.; Zhang, Y.; Zhou, K. Bax inhibitor-1 mediates apoptosis-resistance in human nasopharyngeal carcinoma cells. Mol. Cell. Biochem. 2010, 333, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Lai, Y.-K.; Zhang, J.-F.; Luo, H.-Q.; Zhang, M.-H.; Zhou, K.-Y.; Kung, H.-F. Lentivirus-Mediated RNA Interference Targeting Bax Inhibitor-1 Suppresses Ex Vivo Cell Proliferation and In Vivo Tumor Growth of Nasopharyngeal Carcinoma. Hum. Gene Ther. 2011, 22, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Grzmil, M.; Thelen, P.; Hemmerlein, B.; Schweyer, S.; Voigt, S.; Mury, D.; Burfeind, P. Bax inhibitor-1 is overexpressed in prostate cancer and its specific down-regulation by RNA interference leads to cell death in human prostate carcinoma cells. Am. J. Pathol. 2003, 163, 543–552. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. Embo Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Chen, H.; Gao, J.; Liu, Y.; Li, J.; Wang, J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J. Mol. Cell. Cardiol. 2019, 136, 27–41. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Z.; Chen, P.; Zhang, J.; Wang, T.; Zhou, J.; Zhang, H. Discovery of a new class of PROTAC BRD4 degraders based on a dihydroquinazolinone derivative and lenalidomide/pomalidomide. Bioorganic Med. Chem. 2020, 28, 115228. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Hu, B.; Wang, M.; Xu, F.; Miao, B.; Yang, C.-Y.; Wang, M.; Liu, Z.; Hayes, D.F.; Chinnaswamy, K.; et al. Discovery of ERD-308 as a Highly Potent Proteolysis Targeting Chimera (PROTAC) Degrader of Estrogen Receptor (ER). J. Med. Chem. 2019, 62, 1420–1442. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ran, T.; Chen, H. 3D based generative PROTAC linker design with reinforcement learning. Brief. Bioinform. 2023, 24, bbad323. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, C.; Zhao, L.; Yuan, Z.; Chen, Y.; Jiang, Y. Phthalimide conjugations for the degradation of oncogenic PI3K. Eur. J. Med. Chem. 2018, 151, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Peng, Y.; Zhao, Q.; Chen, Y. Small molecule PROTACs: An emerging technology for targeted therapy in drug discovery. Rsc Adv. 2019, 9, 16967–16976. [Google Scholar] [CrossRef]
- Bondeson, D.P.; Smith, B.E.; Burslem, G.M.; Buhimschi, A.D.; Hines, J.; Jaime-Figueroa, S.; Wang, J.; Hamman, B.D.; Ishchenko, A.; Crews, C.M. Lessons in PROTAC Design from Selective Degradation with a Promiscuous Warhead. Cell Chem. Biol. 2018, 25, 78–87.e5. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-T.; Dobrovolsky, D.; Paulk, J.; Yang, G.; Weisberg, E.L.; Doctor, Z.M.; Buckley, D.L.; Cho, J.-H.; Ko, E.; Jang, J.; et al. A Chemoproteomic Approach to Query the Degradable Kinome Using a Multi-kinase Degrader. Cell Chem. Biol. 2018, 25, 88–99.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiao, Y. Target Identification of Bioactive Natural Products. Acta Chim. Sin. 2018, 76, 177–189. [Google Scholar] [CrossRef]
- Li, B.; Qi, Z.-P.; He, D.-L.; Chen, Z.-H.; Liu, J.-Y.; Wong, M.-W.; Zhang, J.-W.; Xu, E.-P.; Shi, Q.; Cai, S.-L.; et al. NLRP7 deubiquitination by USP10 promotes tumor progression and tumor-associated macrophage polarization in colorectal cancer. J. Exp. Clin. Cancer Res. 2021, 40, 126. [Google Scholar] [CrossRef] [PubMed]
- Ripka, S.; Riedel, J.; Neesse, A.; Griesmann, H.; Buchholz, M.; Ellenrieder, V.; Moeller, F.; Barth, P.; Gress, T.M.; Michl, P. Glutamate Receptor GRIA3-Target of CUX1 and Mediator of Tumor Progression in Pancreatic Cancer. Neoplasia 2010, 12, 659–667. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhang, Y.H.; Qin, X.J.; Wang, Y.X.; Fu, J. Circular RNA circDENND4C facilitates proliferation, migration and glycolysis of colorectal cancer cells through miR-760/GLUT1 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2387–2400. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Ishiyama, T.; Uchihara, T.; Inadome, Y.; Iijima, T.; Morishita, Y.; Kano, J.; Goya, T.; Noguchi, M. Expression of the Bax inhibitor-1 gene in pulmonary adenocarcinoma. Cancer 2006, 106, 648–653. [Google Scholar] [CrossRef]
- Lu, B.; Li, Y.; Li, H.; Zhang, Y.; Xu, J.; Ren, L.; Fu, S.; Zhou, Y. Bax inhibitor-1 is overexpressed in non-small cell lung cancer and promotes its progression and metastasis. Int. J. Clin. Exp. Pathol. 2015, 8, 1411–1418. [Google Scholar] [PubMed]
- Grzmil, M.; Kaulfuss, S.; Thelen, P.; Hemmerlein, B.; Schweyer, S.; Obenauer, S.; Kang, T.W.; Burfeind, P. Expression and functional analysis of Bax inhibitor-I in human breast cancer cells. J. Pathol. 2006, 208, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-F.; Sui, C.-L.; Wu, W.-C.; Wang, J.-J.; Yang, D.H.A.; Chen, Y.-C.; Yu, W.C.Y.; Chang, H.-S. Klf10 induces cell apoptosis through modulation of BI-1 expression and Ca2+ homeostasis in estrogen-responding adenocarcinoma cells. Int. J. Biochem. Cell Biol. 2011, 43, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Simpson, L.M.; Glennie, L.; Brewer, A.; Zhao, J.-F.; Crooks, J.; Shpiro, N.; Sapkota, G.P. Target protein localization and its impact on PROTAC-mediated degradation. Cell Chem. Biol. 2022, 29, 1482–1504.e7. [Google Scholar] [CrossRef] [PubMed]
- Almen, M.S.; Jacobsson, J.A.; Shaik, J.H.A.; Olszewski, P.K.; Cedernaes, J.; Alsio, J.; Sreedharan, S.; Levine, A.S.; Fredriksson, R.; Marcus, C.; et al. The obesity gene, TMEM18, is of ancient origin, found in majority of neuronal cells in all major brain regions and associated with obesity in severely obese children. Bmc Med. Genet. 2010, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Lisbona, F.; Rojas-Rivera, D.; Thielen, P.; Zamorano, S.; Todd, D.; Martinon, F.; Glavic, A.; Kress, C.; Lin, J.H.; Walter, P.; et al. BAX Inhibitor-1 Is a Negative Regulator of the ER Stress Sensor IRE1 alpha. Mol. Cell 2009, 33, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Bailly-Maitre, B.; Belgardt, B.F.; Jordan, S.D.; Coornaert, B.; von Freyend, M.J.; Kleinridders, A.; Mauer, J.; Cuddy, M.; Kress, C.L.; Willmes, D.; et al. Hepatic Bax Inhibitor-1 Inhibits IRE1 alpha and Protects from Obesity-associated Insulin Resistance and Glucose Intolerance. J. Biol. Chem. 2010, 285, 6198–6207. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, W.; Palmer, A.E.; Reed, J.C. BI-1 regulates endoplasmic reticulum Ca2+ Homeostasis downstream of bcl-2 family proteins. J. Biol. Chem. 2008, 283, 11477–11484. [Google Scholar] [CrossRef]
- Kim, H.-R.; Lee, G.-H.; Cho, E.Y.; Chae, S.-W.; Ahn, T.; Chae, H.-J. Bax inhibitor 1 regulates ER-stress-induced ROS accumulation through the regulation of cytochrome P450 2E1. J. Cell Sci. 2009, 122, 1126–1133. [Google Scholar] [CrossRef]
- Lee, G.-H.; Oh, K.-J.; Kim, H.-R.; Han, H.-S.; Lee, H.-Y.; Park, K.-G.; Nam, K.-H.; Koo, S.-H.; Chae, H.-J. Effect of BI-1 on insulin resistance through regulation of CYP2E1. Sci. Rep. 2016, 6, 32229. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Kim, H.-R.; Chae, H.-J. Bax inhibitor-1 regulates the expression of P450 2E1 through enhanced lysosome activity. Int. J. Biochem. Cell Biol. 2012, 44, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Kim, H.-K.; Chae, S.-W.; Kim, D.-S.; Ha, K.-C.; Cuddy, M.; Kress, C.; Reed, J.C.; Kim, H.-R.; Chae, H.-J. Bax inhibitor-1 regulates endoplasmic reticulum stress-associated reactive oxygen species and heme oxygenase-1 expression. J. Biol. Chem. 2007, 282, 21618–21628. [Google Scholar] [CrossRef]
- Cencini, E.; Calomino, N.; Sicuranza, A.; Gozzetti, A.; Fabbri, A.; Bocchia, M. Are small lymphocytic lymphoma and chronic lymphocytic leukemia the same disease? The unsolved dilemma. J. Chemother. 2024, 36, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Cencini, E.; Sicuranza, A.; Fabbri, A.; Ferrigno, I.; Rigacci, L.; Cox, M.C.; Raspadori, D.; Bocchia, M. Study of gene polymorphisms as predictors of treatment efficacy and toxicity in patients with indolent non-hodgkin lymphomas and mantle cell lymphoma receiving bendamustine and rituximab. Br. J. Haematol. 2019, 184, 223–231. [Google Scholar] [CrossRef] [PubMed]
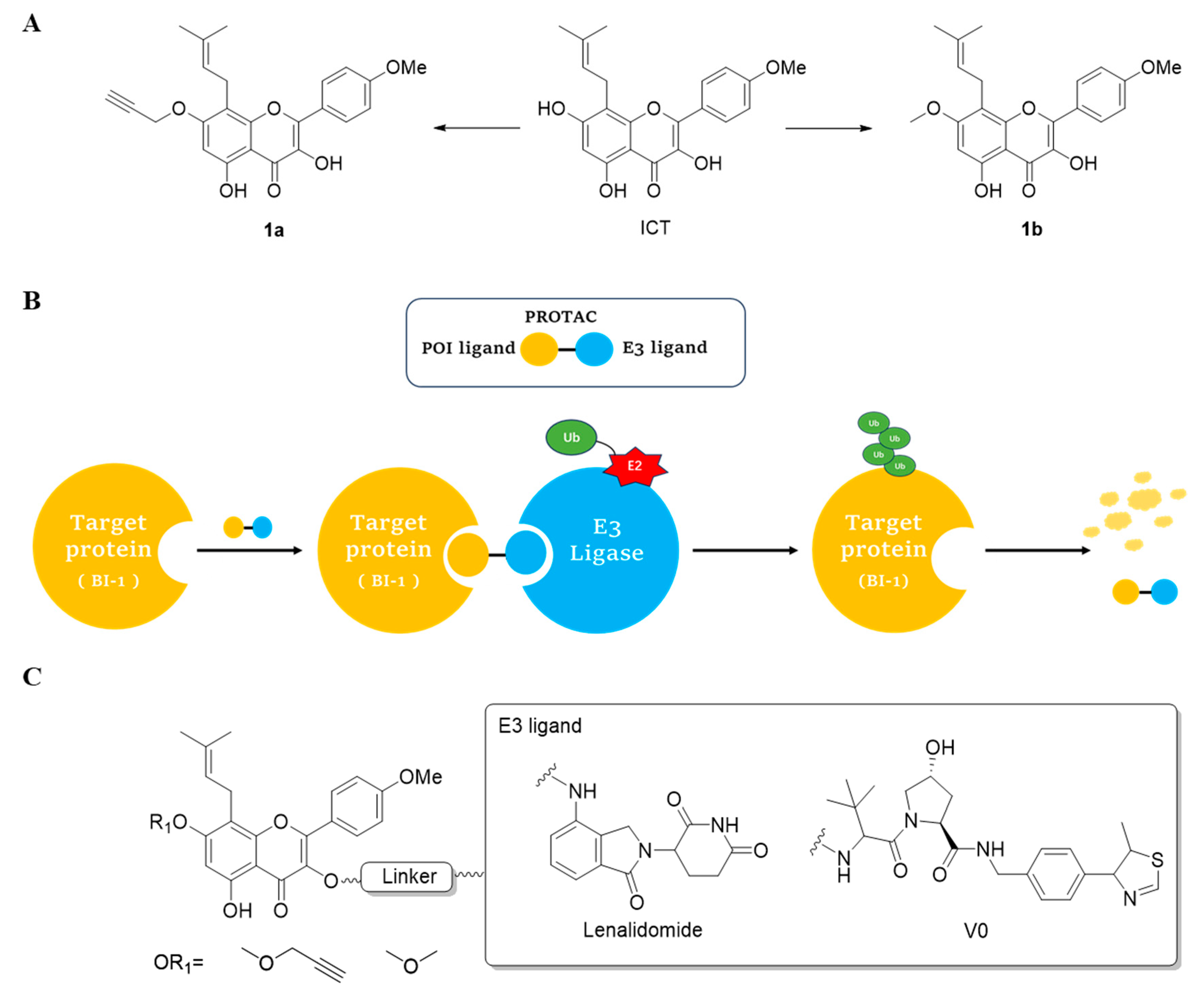

| Compounds | POI Ligand | Linker | E3 Ligand |
|---|---|---|---|
| 3 |  |  | Ledonamide |
| 4 | 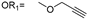 |  | Ledonamide |
| 5 | 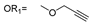 | 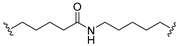 | Ledonamide |
| 6 | 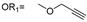 |  | Ledonamide |
| 7 |  | 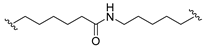 | Ledonamide |
| 8 |  | 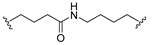 | Ledonamide |
| 9 |  |  | Ledonamide |
| 10 | 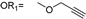 | 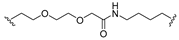 | Ledonamide |
| 11 | 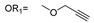 | 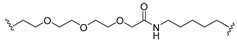 | Ledonamide |
| 12 |  |  | Ledonamide |
| 13 | 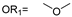 |  | Ledonamide |
| 14 | 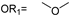 |  | Ledonamide |
| 15 |  | 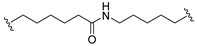 | Ledonamide |
| 16 |  |  | Ledonamide |
| 17 |  | 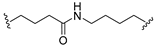 | Ledonamide |
| 18 | 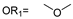 | 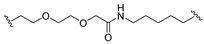 | Ledonamide |
| 19 |  | 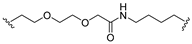 | Ledonamide |
| 20 |  |  | Ledonamide |
| 21 | 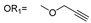 | 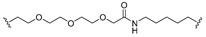 | V0 |
| 22 |  |  | V0 |
| 23 |  | 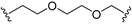 | V0 |
| 24 | 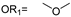 | 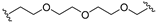 | V0 |
| Compounds | IC50 (μM) a | Compounds | IC50 (μM) a | Compounds | IC50 (μM) a | Compounds | IC50 (μM) a |
|---|---|---|---|---|---|---|---|
| ICT | 66.91 ± 0.81 | 1a | 48.19 ± 2.31 | 1b | 50.68 ± 0.57 | Celastrol b | 0.88 ± 0.14 |
| 3 | 5.79 ± 0.38 | 4 | 6.69 ± 0.34 | 5 | 7.48 ± 0.26 | 6 | 13.14 ± 1.61 |
| 7 | >20 | 8 | 4.60 ± 0.26 | 9 | 5.81 ± 0.72 | 10 | 5.52 ± 0.59 |
| 11 | 8.71 ± 1.26 | 12 | 6.92 ± 1.79 | 13 | 7.46 ± 0.56 | 14 | >20 |
| 15 | >20 | 16 | 9.14 ± 2.53 | 17 | 5.41 ± 2.11 | 18 | >20 |
| 19 | 5.82 ± 0.92 | 20 | >20 | 21 | >20 | 22 | >20 |
| 23 | 6.09 ± 0.15 |
| Gene Name | Accession | Description | Localization | Log_Fold Change | p-Value |
|---|---|---|---|---|---|
| NLRP13 | Q86W25 | NACHT, LRR, and PYD domains-containing protein 13 | cytosol | −1.54 | p < 0.05 |
| CXXC1 | Q9P0U4 | CXXC-type zinc finger protein 1 | cytosol | −1.48 | p < 0.05 |
| TECPR2 | O15040 | Tectonin beta-propeller repeat-containing protein 2 | nucleus | −1.40 | p < 0.05 |
| GRIA3 | P42263 | Glutamate receptor 3 | plasma membrane | −1.25 | p < 0.05 |
| TMBIM6 | P55061 | Bax inhibitor 1 | endoplasmic reticulum | −1.07 | p < 0.05 |
| DENND4C | Q5VZ89 | DENN domain-containing protein 4C | cytosol | −0.97 | p < 0.05 |
| FSD1 | Q9BTV5 | Fibronectin type III and SPRY domain-containing protein 1 | nucleus | −0.86 | p < 0.05 |
| TMEM18 | Q96B42 | Transmembrane protein 18 | nucleus | −0.71 | p < 0.05 |
| NMD3 | Q96D46 | 60S ribosomal export protein NMD3 | nucleus | −0.68 | p < 0.05 |
| Gene Name | Accession | Description | Localization | Log_Fold Change | p-Value |
|---|---|---|---|---|---|
| NLRP13 | Q86W25 | NACHT, LRR, and PYD domains-containing protein 13 | cytosol | −1.60 | p < 0.05 |
| TECPR2 | O15040 | Tectonin beta-propeller repeat-containing protein 2 | nucleus | −1.27 | p < 0.05 |
| GRIA3 | P42263 | Glutamate receptor 3 | plasma membrane | −1.13 | p < 0.05 |
| NMD3 | Q96D46 | 60S ribosomal export protein NMD3 | nucleus | −0.87 | p < 0.05 |
| TMBIM6 | P55061 | Bax inhibitor 1 | endoplasmic reticulum | −0.87 | p = 0.051 |
| NAB2 | Q15742 | NGFI-A-binding protein 2 | cytosol | −0.77 | p < 0.05 |
| UPRT | Q96BW1 | Uracil phosphoribosyltransferase homolog | nucleus | −0.75 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Zhang, Z.; Li, J.; Xu, M.; Lu, W.; Chen, M.; Shi, J.; Wang, Q.; Zhang, H.; Huang, S.; et al. Advanced PROTAC and Quantitative Proteomics Strategy Reveals Bax Inhibitor-1 as a Critical Target of Icaritin in Burkitt Lymphoma. Int. J. Mol. Sci. 2024, 25, 12944. https://doi.org/10.3390/ijms252312944
Zhang P, Zhang Z, Li J, Xu M, Lu W, Chen M, Shi J, Wang Q, Zhang H, Huang S, et al. Advanced PROTAC and Quantitative Proteomics Strategy Reveals Bax Inhibitor-1 as a Critical Target of Icaritin in Burkitt Lymphoma. International Journal of Molecular Sciences. 2024; 25(23):12944. https://doi.org/10.3390/ijms252312944
Chicago/Turabian StyleZhang, Peixi, Ziqing Zhang, Jie Li, Meng Xu, Weiming Lu, Ming Chen, Jiaqi Shi, Qiaolai Wang, Hengyuan Zhang, Shi Huang, and et al. 2024. "Advanced PROTAC and Quantitative Proteomics Strategy Reveals Bax Inhibitor-1 as a Critical Target of Icaritin in Burkitt Lymphoma" International Journal of Molecular Sciences 25, no. 23: 12944. https://doi.org/10.3390/ijms252312944
APA StyleZhang, P., Zhang, Z., Li, J., Xu, M., Lu, W., Chen, M., Shi, J., Wang, Q., Zhang, H., Huang, S., Lian, C., Liu, J., Ma, J., & Liu, J. (2024). Advanced PROTAC and Quantitative Proteomics Strategy Reveals Bax Inhibitor-1 as a Critical Target of Icaritin in Burkitt Lymphoma. International Journal of Molecular Sciences, 25(23), 12944. https://doi.org/10.3390/ijms252312944






