Cushing’s Disease Manifestation in USP8-Mutated Corticotropinoma May Be Mediated by Interactions Between WNT Signaling and SST Trafficking
Abstract
1. Introduction
2. Results
2.1. Clinical Data
2.2. USP8 Mutations Spectrum in Patients with Cushing’s Disease
2.3. Associations Between Somatic USP8 Mutation and Clinical Parameters
2.4. SST2/SST5 Expression and USP8 Mutation Status
2.5. Transcriptome Analysis of Pituitary Adenomas Harboring USP8-Mutant or USP8-WT in Patients with Cushing’s Disease
3. Discussion
4. Materials and Methods
4.1. Study Population of Patients with Cushing’s Disease
4.1.1. Hormone Measurements
4.1.2. Pituitary Magnetic Resonance Imaging
4.1.3. Immunnohistochemical (IHC) Staining
4.1.4. Statistical Analysis
4.2. DNA/RNA Purification from Tumor Samples
4.3. Sanger Sequencing of USP8
4.4. Transcriptome Sequencing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dekkers, O.M.; Biermasz, N.R.; Pereira, A.M.; Roelfsema, F.; Van Aken, M.O.; Voormolen, J.H.C.; Romijn, J.A. Mortality in Patients Treated for Cushing’s Disease Is Increased, Compared with Patients Treated for Nonfunctioning Pituitary Macroadenoma. J. Clin. Endocrinol. Metab. 2007, 92, 976–981. [Google Scholar] [CrossRef]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, J.; Juul, S.; Jørgensen, J.O.L.; Astrup, J.; Bjerre, P.; Feldt-Rasmussen, U.; Hagen, C.; Jørgensen, J.; Kosteljanetz, M.; Kristensen, L.Ø.; et al. Incidence and Late Prognosis of Cushing’s Syndrome: A Population-Based Study. J. Clin. Endocrinol. Metab. 2001, 86, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Etxabe, J.; Vazquez, J.A. Morbidity and Mortality in Cushing’s Disease: An Epidemiological Approach. Clin. Endocrinol. 1994, 40, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, O.; Olsson, D.S.; Chantzichristos, D.; Papakokkinou, E.; Dahlqvist, P.; Segerstedt, E.; Olsson, T.; Petersson, M.; Berinder, K.; Bensing, S.; et al. The Incidence of Cushing’s Disease: A Nationwide Swedish Study. Pituitary 2019, 22, 179–186. [Google Scholar] [CrossRef]
- Huang, C.; Shi, Y.; Zhao, Y. USP8 Mutation in Cushing’s Disease. Oncotarget 2015, 6, 18240. [Google Scholar] [CrossRef]
- Perez-Rivas, L.G.; Theodoropoulou, M.; Ferraù, F.; Nusser, C.; Kawaguchi, K.; Stratakis, C.A.; Rueda Faucz, F.; Wildemberg, L.E.; Assié, G.; Beschorner, R.; et al. The Gene of the Ubiquitin-Specific Protease 8 Is Frequently Mutated in Adenomas Causing Cushing’s Disease. J. Clin. Endocrinol. Metab. 2015, 100, E997–E1004. [Google Scholar] [CrossRef]
- Wanichi, I.Q.; de Paula Mariani, B.M.; Frassetto, F.P.; Siqueira, S.A.C.; de Castro Musolino, N.R.; Cunha-Neto, M.B.C.; Ochman, G.; Cescato, V.A.S.; Machado, M.C.; Trarbach, E.B.; et al. Cushing’s Disease Due to Somatic USP8 Mutations: A Systematic Review and Meta-Analysis. Pituitary 2019, 22, 435–442. [Google Scholar] [CrossRef]
- Albani, A.; Theodoropoulou, M.; Reincke, M. Genetics of Cushing’s Disease. Clin. Endocrinol. 2018, 88, 3–12. [Google Scholar] [CrossRef]
- Sesta, A.; Cassarino, M.F.; Terreni, M.; Ambrogio, A.G.; Libera, L.; Bardelli, D.; Lasio, G.; Losa, M.; Pecori Giraldi, F. Ubiquitin-Specific Protease 8 Mutant Corticotrope Adenomas Present Unique Secretory and Molecular Features and Shed Light on the Role of Ubiquitylation on ACTH Processing. Neuroendocrinology 2020, 110, 119. [Google Scholar] [CrossRef]
- Treppiedi, D.; Barbieri, A.M.; Di Muro, G.; Marra, G.; Mangili, F.; Catalano, R.; Esposito, E.; Ferrante, E.; Serban, A.L.; Locatelli, M.; et al. Genetic Profiling of a Cohort of Italian Patients with Acth-Secreting Pituitary Tumors and Characterization of a Novel Usp8 Gene Variant. Cancers 2021, 13, 4022. [Google Scholar] [CrossRef] [PubMed]
- Bujko, M.; Kober, P.; Boresowicz, J.; Rusetska, N.; Zeber-Lubecka, N.; Paziewska, A.; Pekul, M.; Zielinski, G.; Styk, A.; Kunicki, J.; et al. Differential MicroRNA Expression in USP8-Mutated and Wild-Type Corticotroph Pituitary Tumors Reflect the Difference in Protein Ubiquitination Processes. J. Clin. Med. 2021, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Reincke, M.; Sbiera, S.; Hayakawa, A.; Theodoropoulou, M.; Osswald, A.; Beuschlein, F.; Meitinger, T.; Mizuno-Yamasaki, E.; Kawaguchi, K.; Saeki, Y.; et al. Mutations in the Deubiquitinase Gene USP8 Cause Cushing’s Disease. Nat. Genet. 2015, 47, 31–38. [Google Scholar] [CrossRef]
- Hayashi, K.; Inoshita, N.; Kawaguchi, K.; Ardisasmita, A.I.; Suzuki, H.; Fukuhara, N.; Okada, M.; Nishioka, H.; Takeuchi, Y.; Komada, M.; et al. The USP8 Mutational Status May Predict Drug Susceptibility in Corticotroph Adenomas of Cushing’s Disease. Eur. J. Endocrinol. 2016, 174, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Theodoropoulou, M.; Stalla, G.K. Somatostatin Receptors: From Signaling to Clinical Practice. Front. Neuroendocrinol. 2013, 34, 228–252. [Google Scholar] [CrossRef]
- Ben-Shlomo, A.; Melmed Shlomo, S. Pituitary Somatostatin Receptor Signaling. Trends Endocrinol. Metab. 2010, 21, 123. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Fleseriu, M. Somatostatin Receptor Ligands and Resistance to Treatment in Pituitary Adenomas. J. Mol. Endocrinol. 2014, 52, R223–R240. [Google Scholar] [CrossRef]
- Treppiedi, D.; Marra, G.; Di Muro, G.; Esposito, E.; Barbieri, A.M.; Catalano, R.; Mangili, F.; Bravi, F.; Locatelli, M.; Lania, A.G.; et al. P720R USP8 Mutation Is Associated with a Better Responsiveness to Pasireotide in ACTH-Secreting PitNETs. Cancers 2022, 14, 2455. [Google Scholar] [CrossRef]
- Petukhova, N.; Poluzerova, A.; Bug, D.; Nerubenko, E.; Kostareva, A.; Tsoy, U.; Dmitrieva, R. USP8 Mutations Associated with Cushing’s Disease Alter Protein Structure Dynamics. Int. J. Mol. Sci. 2024, 25, 12697. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Song, Z.J.; Chen, J.H.; Wang, Y.F.; Li, S.Q.; Zhou, L.F.; Mao, Y.; Li, Y.M.; Hu, R.G.; Zhang, Z.Y.; et al. Recurrent Gain-of-Function USP8 Mutations in Cushing’s Disease. Cell Res. 2015, 25, 306–317. [Google Scholar] [CrossRef]
- Mizuno, E.; Iura, T.; Mukai, A.; Yoshimori, T.; Kitamura, N.; Komada, M. Regulation of Epidermal Growth Factor Receptor Down-Regulation by UBPY-Mediated Deubiquitination at Endosomes. Mol. Biol. Cell 2005, 16, 5163. [Google Scholar] [CrossRef] [PubMed]
- Albani, A.; Pérez-Rivas, L.G.; Dimopoulou, C.; Zopp, S.; Colón-Bolea, P.; Roeber, S.; Honegger, J.; Flitsch, J.; Rachinger, W.; Buchfelder, M.; et al. The USP8 Mutational Status May Predict Long-Term Remission in Patients with Cushing’s Disease. Clin. Endocrinol. 2018, 89, 454–458. [Google Scholar] [CrossRef]
- Losa, M.; Mortini, P.; Pagnano, A.; Detomas, M.; Cassarino, M.F.; Pecori Giraldi, F. Clinical Characteristics and Surgical Outcome in USP8-Mutated Human Adrenocorticotropic Hormone-Secreting Pituitary Adenomas. Endocrine 2019, 63, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Faucz, F.R.; Tirosh, A.; Tatsi, C.; Berthon, A.; Hernández-Ramírez, L.C.; Settas, N.; Angelousi, A.; Correa, R.; Papadakis, G.Z.; Chittiboina, P.; et al. Somatic USP8 Gene Mutations Are a Common Cause of Pediatric Cushing Disease. J. Clin. Endocrinol. Metab. 2017, 102, 2836–2843. [Google Scholar] [CrossRef]
- Castellnou, S.; Vasiljevic, A.; Lapras, V.; Raverot, V.; Alix, E.; Borson-Chazot, F.; Jouanneau, E.; Raverot, G.; Lasolle, H. SST5 Expression and USP8 Mutation in Functioning and Silent Corticotroph Pituitary Tumors. Endocr. Connect. 2020, 9, 243–253. [Google Scholar] [CrossRef]
- Chinezu, L.; Vasiljevic, A.; Jouanneau, E.; François, P.; Borda, A.; Trouillas, J.; Raverot, G. Expression of Somatostatin Receptors, SSTR2A and SSTR5, in 108 Endocrine Pituitary Tumors Using Immunohistochemical Detection with New Specific Monoclonal Antibodies. Hum. Pathol. 2014, 45, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.L.; Sioson, L.; Sheen, A.; Clarkson, A.; Gill, A.J. Immunohistochemical Expression of Somatostatin Receptors SSTR2A and SSTR5 in 299 Pituitary Adenomas. Pathology 2018, 50, 472–474. [Google Scholar] [CrossRef]
- Tateno, T.; Kato, M.; Tani, Y.; Oyama, K.; Yamada, S.; Hirata, Y. Differential Expression of Somatostatin and Dopamine Receptor Subtype Genes in Adrenocorticotropin (ACTH)-Secreting Pituitary Tumors and Silent Corticotroph Adenomas. Endocr. J. 2009, 56, 579–584. [Google Scholar] [CrossRef]
- van der Hoek, J.; Lamberts, S.W.J.; Hofland, L.J. The Role of Somatostatin Analogs in Cushing’s Disease. Pituitary 2004, 7, 257–264. [Google Scholar] [CrossRef]
- Hofland, L.J.; van der Hoek, J.; Feelders, R.; van Aken, M.O.; van Koetsveld, P.M.; Waaijers, M.; Sprij-Mooij, D.; Bruns, C.; Weckbecker, G.; de Herder, W.W.; et al. The Multi-Ligand Somatostatin Analogue SOM230 Inhibits ACTH Secretion by Cultured Human Corticotroph Adenomas via Somatostatin Receptor Type 5. Eur. J. Endocrinol. 2005, 152, 645–654. [Google Scholar] [CrossRef]
- Lupp, A.; Hunder, A.; Petrich, A.; Nagel, F.; Doll, C.; Schulz, S. Reassessment of Sst(5) Somatostatin Receptor Expression in Normal and Neoplastic Human Tissues Using the Novel Rabbit Monoclonal Antibody UMB-4. Neuroendocrinology 2011, 94, 255–264. [Google Scholar] [CrossRef]
- Hassaneen, W.; Cahill, D.P.; Fuller, G.N.; Levine, N.B. Immunohistochemical Detection of Somatostatin Receptor Subtype 5 (SSTR-5) in Cushing Adenoma. J. Neurooncol. 2010, 98, 151–152. [Google Scholar] [CrossRef]
- Martins, C.S.; Camargo, R.C.; Coeli-Lacchini, F.B.; Saggioro, F.P.; Moreira, A.C.; De Castro, M. USP8 Mutations and Cell Cycle Regulation in Corticotroph Adenomas. Horm. Metab. Res. 2020, 52, 117–123. [Google Scholar] [CrossRef]
- Weigand, I.; Knobloch, L.; Flitsch, J.; Saeger, W.; Monoranu, C.M.; Höfner, K.; Herterich, S.; Rotermund, R.; Ronchi, C.L.; Buchfelder, M.; et al. Impact of USP8 Gene Mutations on Protein Deregulation in Cushing Disease. J. Clin. Endocrinol. Metab. 2019, 104, 2535–2546. [Google Scholar] [CrossRef]
- Roosterman, D. Endocytosis of the Rat Somatostatin Receptors: Subtype Discrimination, Ligand Specificity, and Delineation of Carboxy-Terminal Positive and Negative Sequence Motifs. DNA Cell Biol. 1997, 16, 111–119. [Google Scholar] [CrossRef]
- Hukovic, N.; Panetta, R.; Kumar, U.; Patel, Y.C. Agonist-Dependent Regulation of Cloned Human Somatostatin Receptor Types 1-5 (HSSTR1-5): Subtype Selective Internalization or Upregulation. Endocrinology 1996, 137, 4046–4049. [Google Scholar] [CrossRef][Green Version]
- Albani, A.; Perez-Rivas, L.G.; Tang, S.; Simon, J.; Lucia, K.E.; Colón-Bolea, P.; Schopohl, J.; Roeber, S.; Buchfelder, M.; Rotermund, R.; et al. Improved Pasireotide Response in USP8 Mutant Corticotroph Tumours in Vitro. Endocr. Relat. Cancer 2022, 29, 503–511. [Google Scholar] [CrossRef]
- Carr, H.S.; Zuo, Y.; Frost, J.A. The Wnt Pathway Protein Dvl1 Targets Somatostatin Receptor 2 for Lysosome-Dependent Degradation. J. Biol. Chem. 2023, 299, 104645. [Google Scholar] [CrossRef]
- Ren, J.; Jian, F.; Jiang, H.; Sun, Y.; Pan, S.; Gu, C.; Chen, X.; Wang, W.; Ning, G.; Bian, L.; et al. Decreased Expression of SFRP2 Promotes Development of the Pituitary Corticotroph Adenoma by Upregulating Wnt Signaling. Int. J. Oncol. 2018, 52, 1934. [Google Scholar] [CrossRef]
- Liu, X.; Feng, M.; Dai, C.; Bao, X.; Deng, K.; Yao, Y.; Wang, R. Expression of EGFR in Pituitary Corticotroph Adenomas and Its Relationship with Tumor Behavior. Front. Endocrinol. 2019, 10, 482696. [Google Scholar] [CrossRef]
- Hao, H.X.; Jiang, X.; Cong, F. Control of Wnt Receptor Turnover by R-Spondin-ZNRF3/RNF43 Signaling Module and Its Dysregulation in Cancer. Cancers 2016, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Mukai, A.; Yamamoto-Hino, M.; Awano, W.; Watanabe, W.; Komada, M.; Goto, S. Balanced Ubiquitylation and Deubiquitylation of Frizzled Regulate Cellular Responsiveness to Wg/Wnt. EMBO J. 2010, 29, 2114–2125. [Google Scholar] [CrossRef] [PubMed]
- Madana, B.; Walkerb, M.P.; Young, R.; Quick, L.; Orgel, K.A.; Ryan, M.; Gupta, P.; Henrichc, I.C.; Ferrer, M.; Marine, S.; et al. USP6 Oncogene Promotes Wnt Signaling by Deubiquitylating Frizzleds. Proc. Natl. Acad. Sci. USA 2016, 113, E2945–E2954. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Maurice, M.M. The Various Roles of Ubiquitin in Wnt Pathway Regulation. Cell Cycle 2010, 9, 3700. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Srivastava, A.; Rikhari, D.; Srivastava, S. RSPO2 as Wnt Signaling Enabler: Important Roles in Cancer Development and Therapeutic Opportunities. Genes. Dis. 2024, 11, 788–806. [Google Scholar] [CrossRef]
- Yue, F.; Ku, A.T.; Stevens, P.D.; Michalski, M.N.; Jiang, W.; Tu, J.; Shi, Z.; Dou, Y.; Wang, Y.; Feng, X.-H.; et al. Loss of ZNRF3/RNF43 Unleashes EGFR in Cancer. bioRxiv 2024. [Google Scholar] [CrossRef]
- Nieman, L.K.; Biller, B.M.K.; Findling, J.W.; Newell-Price, J.; Savage, M.O.; Stewart, P.M.; Montori, V.M.; Edwards, H. The Diagnosis of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2008, 93, 1526–1540. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on Diagnosis and Management of Cushing’s Disease: A Guideline Update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef]
- Bertagna, X.; Guignat, L.; Groussin, L.; Bertherat, J. Cushing’s Disease. Best. Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 607–623. [Google Scholar] [CrossRef]
- Liu, C.; Lo, J.C.; Dowd, C.F.; Wilson, C.B.; Kunwar, S.; Aron, D.C.; Tyrrell, J.B. Cavernous and Inferior Petrosal Sinus Sampling in the Evaluation of ACTH-Dependent Cushing’s Syndrome. Clin. Endocrinol. 2004, 61, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, A.; Nemoto, S.; Takakura, K.; Sasaki, Y.; Machida, T. Selective Venous Sampling Directly from Cavernous Sinus in Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 1993, 76, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.E.; Samuels, M.H.; Nesbit, G.M.; Cook, D.M.; O’Neill, O.R.; Barnwell, S.L.; Loriaux, D.L. Cavernous Sinus Sampling Is Highly Accurate in Distinguishing Cushing’s Disease from the Ectopic Adrenocorticotropin Syndrome and in Predicting Intrapituitary Tumor Location. J. Clin. Endocrinol. Metab. 1999, 84, 1602–1610. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
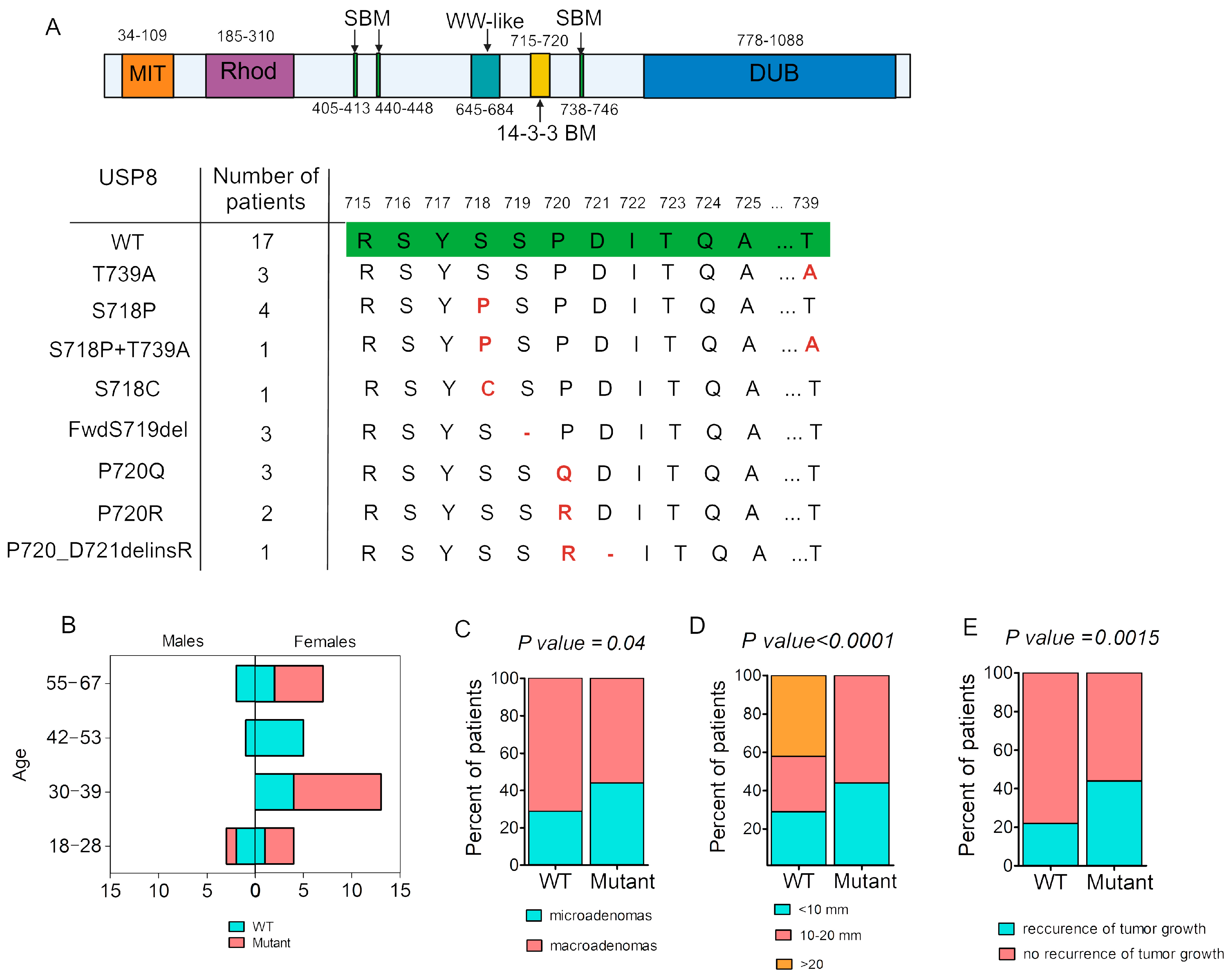
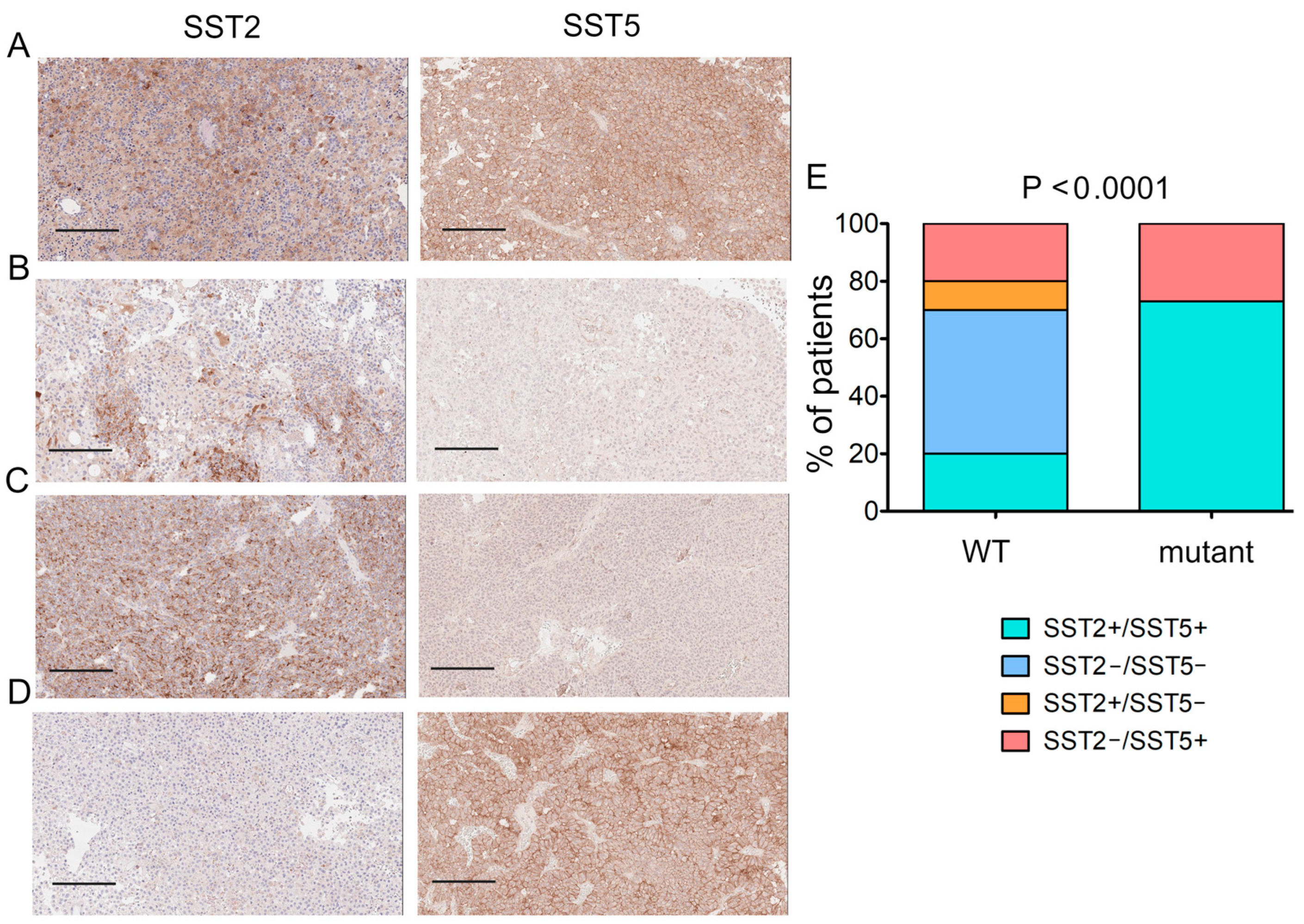
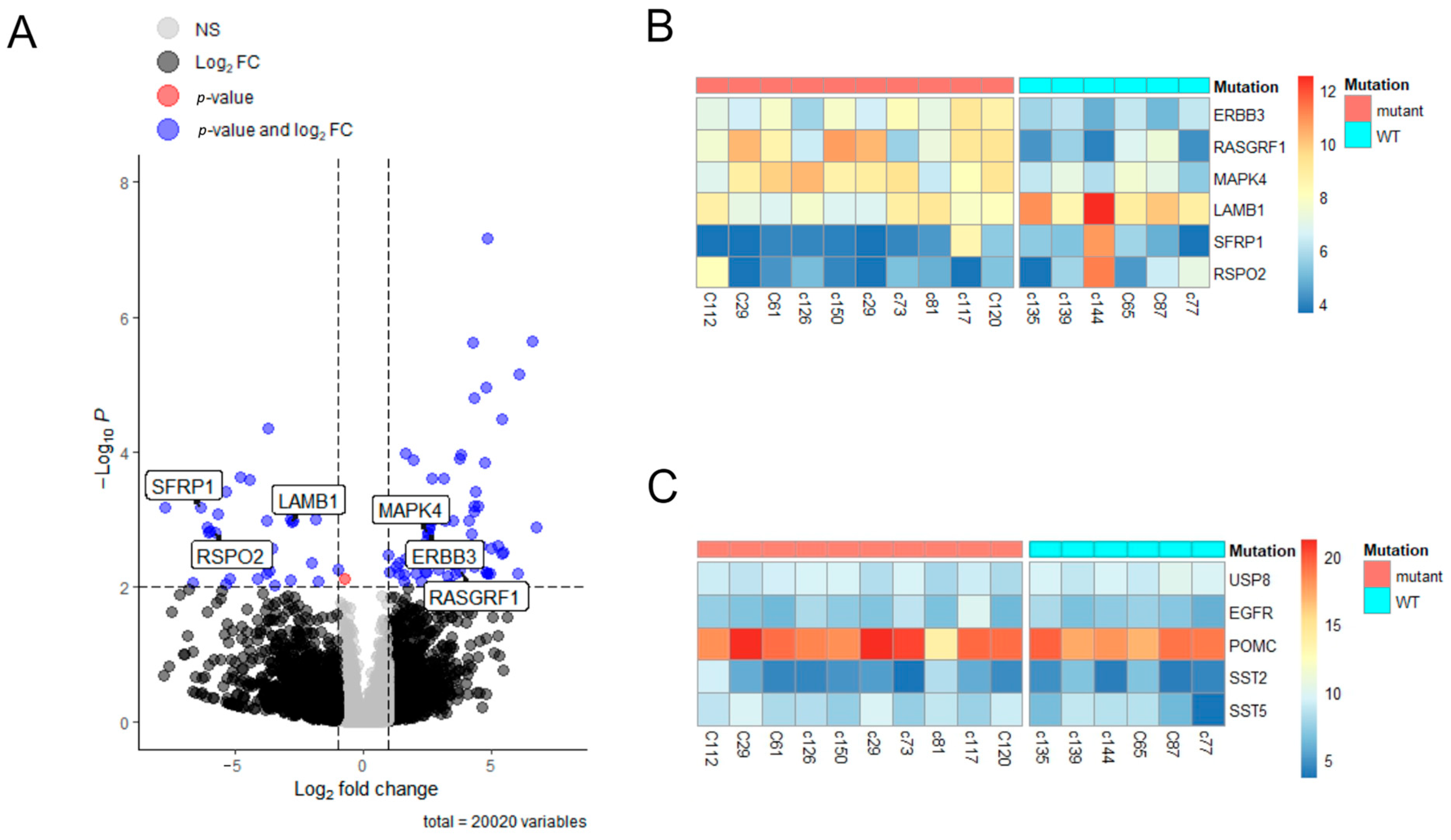
| Clinical Data | USP8-Mutant | USP8-WT | p |
|---|---|---|---|
| Sex, m/f, % | 6%/94% | 24%/76% | p = 0.001 |
| Age, years Me [25%; 75%] (min.–max.) | 36.5 [29; 56.5] (18–67) | 45 [34; 54] (21–60) | p = 0.43 |
| Microadenomas/macroadenomas, % | 44%/56% | 29%/71% | p = 0.04 |
| Maximum adenoma size, mm Me [25%; 75%] (min.–max.) | 11.25 [8; 15] (3–18) | 12 [6; 30] (4–49) | p = 0.21 |
| Adenoma volume, mL Me [25%; 75%] (min.–max.) | 0.27 [0.1; 0.43] (0.003–1.56) | 0.37 [0.043; 6.23] (0.01–42.34) | p = 0.25 |
| Morning plasma ACTH *, pg/mL Me [25%; 75%] (min.–max.) | 61.57 [50.56; 81.76] (23.54–128.3) | 82.99 [45.67; 161.2] (15.34–645.6) | p = 0.39 |
| Morning serum cortisol *, nmol/L Me [25%; 75%] (min.–max.) | 735 [588.2; 837.3] (448–1094) | 638.85 [430.1; 831.6] (261–1690) | p = 0.51 |
| 24 h UFC *, nmol/24 h Me [25%; 75%] (min.–max.) | 492.5 [271.3–696.6] (137.88–1752) | 570 [195.8; 861.8] (56.8–3392) | p = 0.93 |
| Ki67, % | 1 [0.4; 3.27] (0.1–5.3) | 1.47 [0.53; 3.64] (0.1–17.0) | p = 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nerubenko, E.; Ryazanov, P.; Kuritsyna, N.; Paltsev, A.; Ivanova, O.; Grineva, E.; Kostareva, A.; Dmitrieva, R.; Tsoy, U. Cushing’s Disease Manifestation in USP8-Mutated Corticotropinoma May Be Mediated by Interactions Between WNT Signaling and SST Trafficking. Int. J. Mol. Sci. 2024, 25, 12886. https://doi.org/10.3390/ijms252312886
Nerubenko E, Ryazanov P, Kuritsyna N, Paltsev A, Ivanova O, Grineva E, Kostareva A, Dmitrieva R, Tsoy U. Cushing’s Disease Manifestation in USP8-Mutated Corticotropinoma May Be Mediated by Interactions Between WNT Signaling and SST Trafficking. International Journal of Molecular Sciences. 2024; 25(23):12886. https://doi.org/10.3390/ijms252312886
Chicago/Turabian StyleNerubenko, Elena, Pavel Ryazanov, Natalia Kuritsyna, Artem Paltsev, Oksana Ivanova, Elena Grineva, Anna Kostareva, Renata Dmitrieva, and Uliana Tsoy. 2024. "Cushing’s Disease Manifestation in USP8-Mutated Corticotropinoma May Be Mediated by Interactions Between WNT Signaling and SST Trafficking" International Journal of Molecular Sciences 25, no. 23: 12886. https://doi.org/10.3390/ijms252312886
APA StyleNerubenko, E., Ryazanov, P., Kuritsyna, N., Paltsev, A., Ivanova, O., Grineva, E., Kostareva, A., Dmitrieva, R., & Tsoy, U. (2024). Cushing’s Disease Manifestation in USP8-Mutated Corticotropinoma May Be Mediated by Interactions Between WNT Signaling and SST Trafficking. International Journal of Molecular Sciences, 25(23), 12886. https://doi.org/10.3390/ijms252312886





