Gliotic Response and Reprogramming Potential of Human Müller Cell Line MIO-M1 Exposed to High Glucose and Glucose Fluctuations †
Abstract
1. Introduction
2. Results
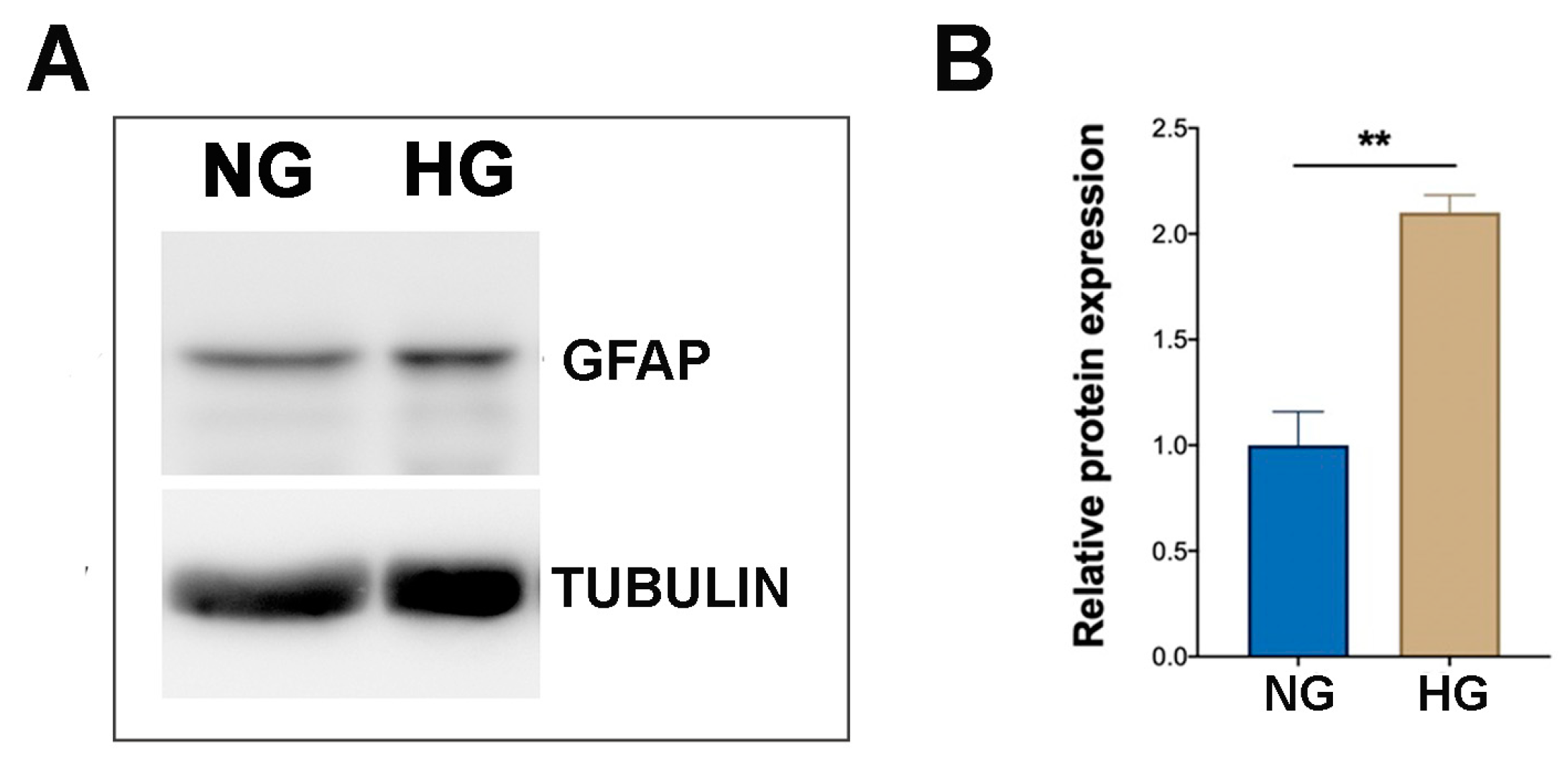
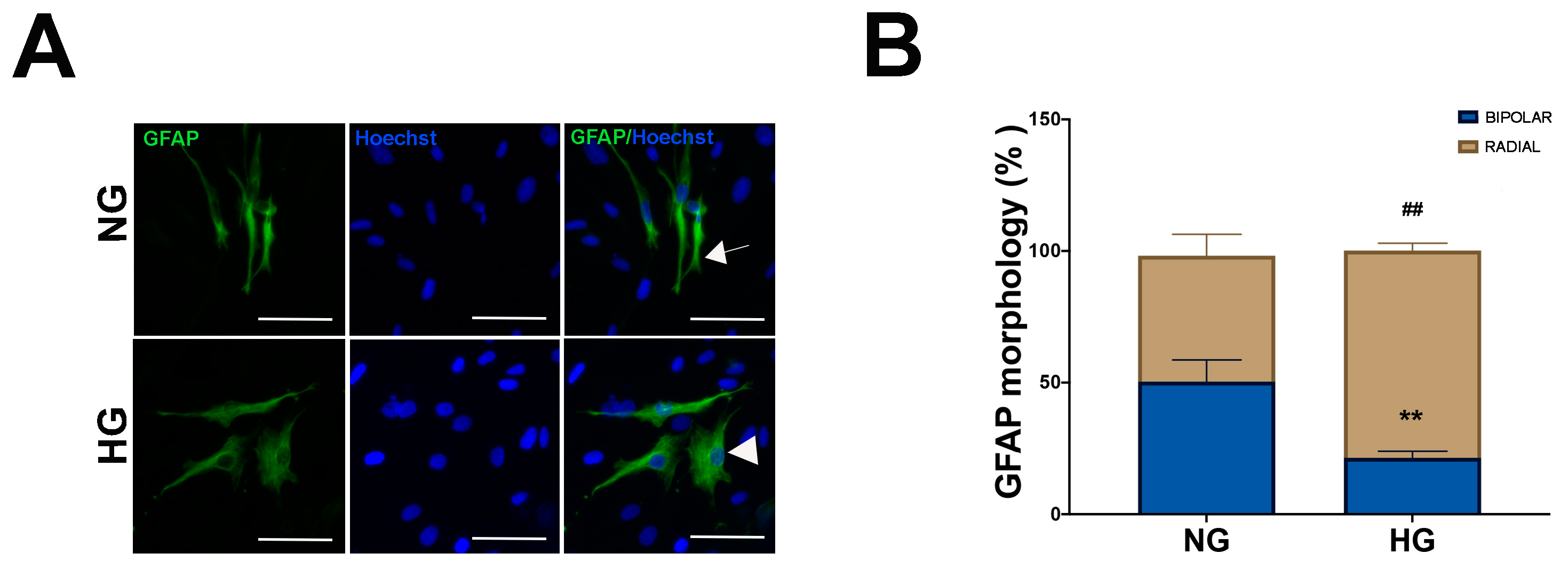
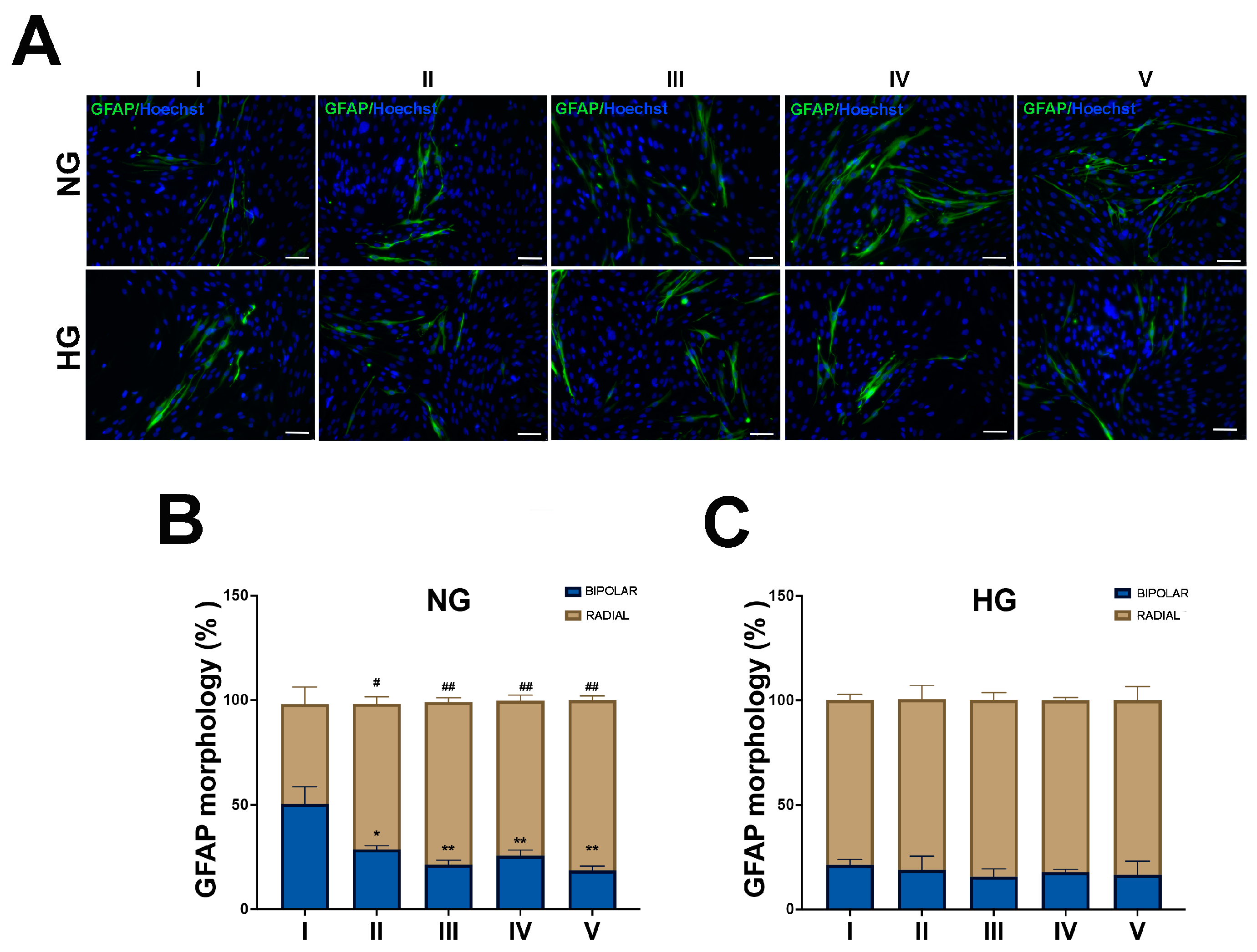
2.1. HG Condition and Treatments with Sustained High-Glucose and GFs Induce Reactive Gliosis in MIO-M1 Cells
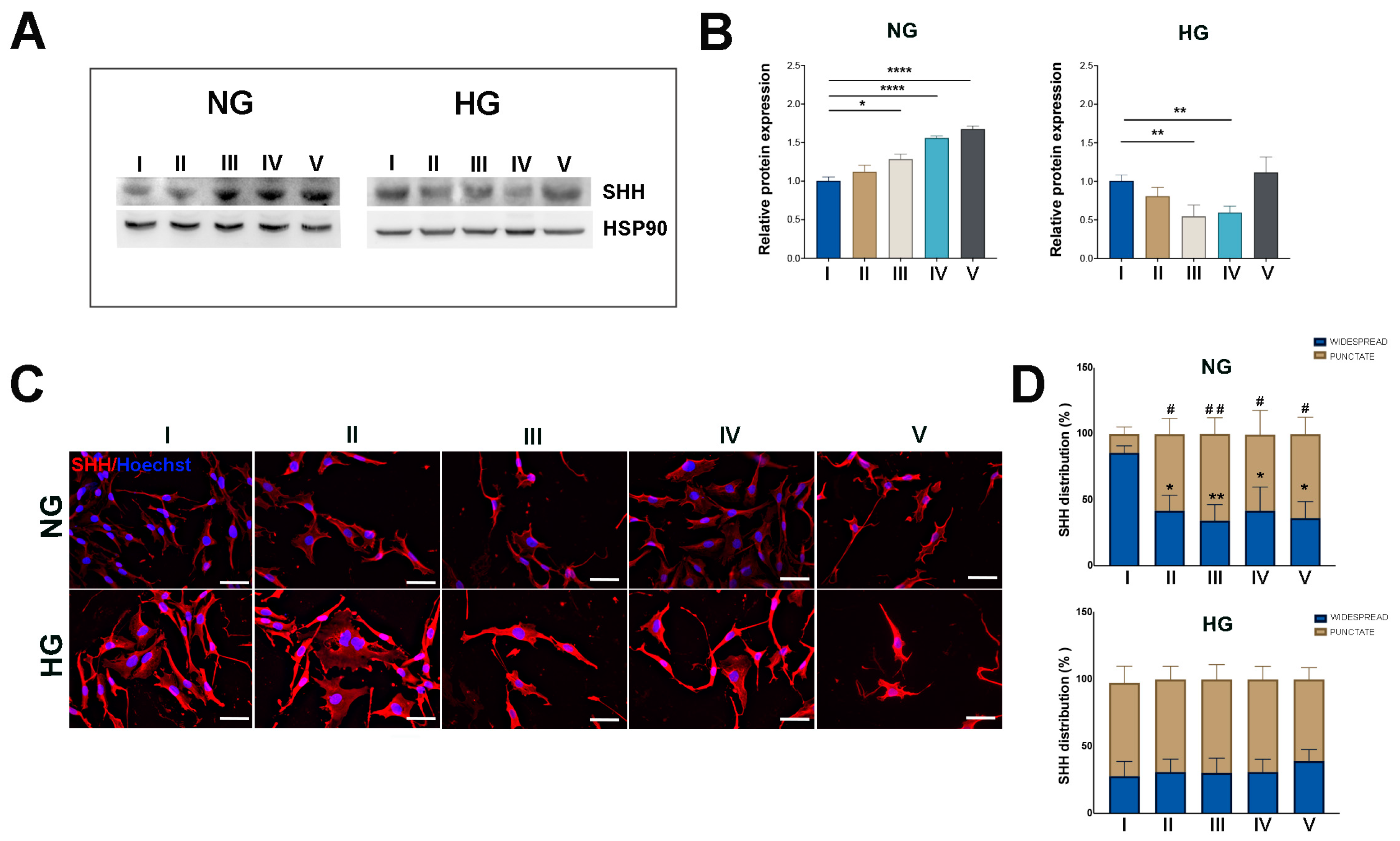
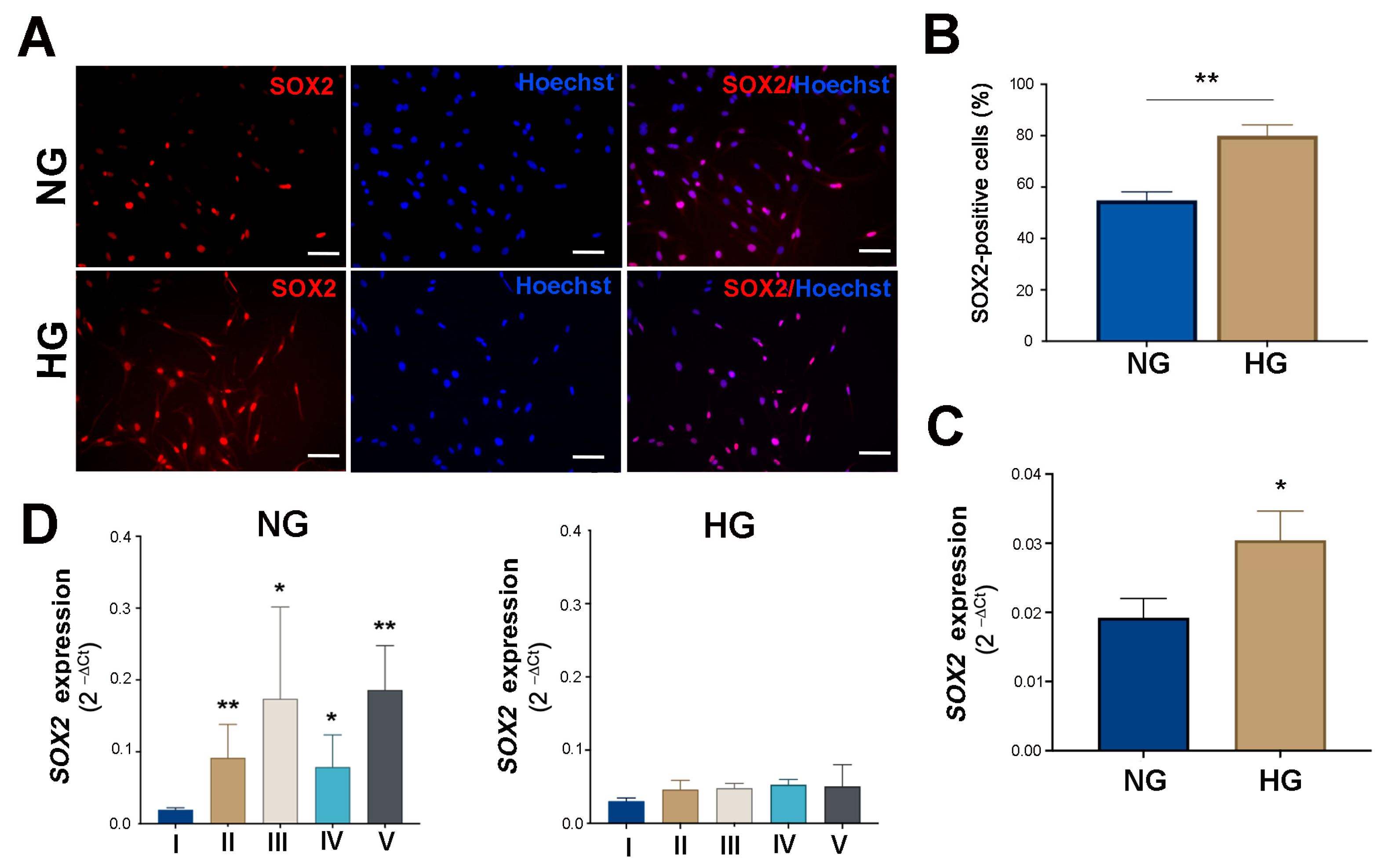
2.2. HG Condition and Treatments with Sustained High-Glucose and GFs Induce Dedifferentiation in MIO-M1 Cells
3. Discussion
4. Materials and Methods
4.1. MIO-M1 Cell Cultures Treatment
4.2. Western Blot (WB)
4.3. Immunofluorescence (IF)
4.4. RNA Extraction and qRT-PCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisma, J.H.; Dulle, J.E.; Fort, P.E. Current knowledge on diabetic retinopathy from human donor tissues. World J. Diabetes 2015, 6, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C.; European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR). Neurodegeneration in the diabetic eye: New insights and therapeutic perspectives. Trends Endocrinol. Metab. TEM 2014, 25, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Hernández, C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog. Retin. Eye Res. 2015, 48, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J. Clin. Investig. 1998, 102, 783–791. [Google Scholar] [CrossRef]
- Lieth, E.; Gardner, T.W.; Barber, A.J.; Antonetti, D.A.; Penn State Retina Research Group. Retinal neurodegeneration: Early pathology in diabetes. Clin. Experiment. Ophthalmol. 2000, 28, 3–8. [Google Scholar] [CrossRef]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Müller cells and diabetic retinopathy. Vision Res. 2017, 139, 93–100. [Google Scholar] [CrossRef]
- Bringmann, A.; Iandiev, I.; Pannicke, T.; Wurm, A.; Hollborn, M.; Wiedemann, P.; Osborne, N.N.; Reichenbach, A. Cellular signaling and factors involved in Müller cell gliosis: Neuroprotective and detrimental effects. Prog. Retin. Eye Res. 2009, 28, 423–451. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, A.; Gaddini, L.; Villa, M.; Varano, M.; Parravano, M.; Monteleone, V.; Cavallo, F.; Leo, L.; Mallozzi, C.; Malchiodi-Albedi, F.; et al. Neuroprotection by rat Müller glia against high glucose-induced neurodegeneration through a mechanism involving ERK1/2 activation. Exp. Eye Res. 2014, 125, 20–29. [Google Scholar] [CrossRef]
- Picconi, F.; Parravano, M.; Ylli, D.; Pasqualetti, P.; Coluzzi, S.; Giordani, I.; Malandrucco, I.; Lauro, D.; Scarinci, F.; Giorno, P.; et al. Retinal neurodegeneration in patients with type 1 diabetes mellitus: The role of glycemic variability. Acta Diabetol. 2017, 54, 489–497. [Google Scholar] [CrossRef]
- Vujosevic, S.; Midena, E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J. Diabetes Res. 2013, 2013, 905058. [Google Scholar] [CrossRef]
- Li, Q.; Puro, D.G. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3109–3116. [Google Scholar]
- Reichenbach, A.; Bringmann, A. New functions of Müller cells. Glia 2013, 61, 651–678. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qi, S.; Wang, C. The role of retinal Müller cells in diabetic retinopathy and related therapeutic advances. Front. Cell Dev. Biol. 2022, 10, 1047487. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D. Müller glia cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef]
- Sharma, P.; Ramachandran, R. Retina regeneration: Lessons from vertebrates. Oxf. Open Neurosci. 2022, 1, kvac012. [Google Scholar] [CrossRef]
- Fischer, A.J.; Reh, T.A. Müller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 2001, 4, 247–252. [Google Scholar] [CrossRef]
- Gu, D.; Wang, S.; Zhang, S.; Zhang, P.; Zhou, G. Directed transdifferentiation of Müller glial cells to photoreceptors using the sonic hedgehog signaling pathway agonist purmorphamine. Mol. Med. Rep. 2017, 16, 7993–8002. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zheng, H.; Xiao, H.-L.; She, Z.-J.; Zhou, G.-M. Sonic hedgehog promotes stem-cell potential of Müller glia in the mammalian retina. Biochem. Biophys. Res. Commun. 2007, 363, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, H.-C.; Ma, J.; Yu, X.-L. Effect of Sonic hedgehog gene-modified bone marrow mesenchymal stem cells on graft-induced retinal gliosis and retinal ganglion cells survival in diabetic mice. Int. J. Ophthalmol. 2024, 17, 34–41. [Google Scholar] [CrossRef]
- Jorstad, N.L.; Wilken, M.S.; Grimes, W.N.; Wohl, S.G.; VandenBosch, L.S.; Yoshimatsu, T.; Wong, R.O.; Rieke, F.; Reh, T.A. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature 2017, 548, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Pollak, J.; Wilken, M.S.; Ueki, Y.; Cox, K.E.; Sullivan, J.M.; Taylor, R.J.; Levine, E.M.; Reh, T.A. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development 2013, 140, 2619–2631. [Google Scholar] [CrossRef]
- Ueki, Y.; Wilken, M.S.; Cox, K.E.; Chipman, L.; Jorstad, N.; Sternhagen, K.; Simic, M.; Ullom, K.; Nakafuku, M.; Reh, T.A. Transgenic expression of the proneural transcription factor Ascl1 in Müller glia stimulates retinal regeneration in young mice. Proc. Natl. Acad. Sci. USA 2015, 112, 13717–13722. [Google Scholar] [CrossRef]
- Picconi, F.; Parravano, M.; Sciarretta, F.; Fulci, C.; Nali, M.; Frontoni, S.; Varano, M.; Caccuri, A.M. Activation of retinal Müller cells in response to glucose variability. Endocrine 2019, 65, 542–549. [Google Scholar] [CrossRef]
- Belecky-Adams, T.L.; Chernoff, E.C.; Wilson, J.M.; Dharmarajan, S. Reactive Muller Glia as Potential Retinal Progenitors. In Neural Stem Cells—New Perspectives; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef][Green Version]
- Limb, G.A.; E Salt, T.; Munro, P.M.G.; E Moss, S.; Khaw, P.T. In vitro characterization of a spontaneously immortalized human Müller cell line (MIO-M1). Investig. Ophthalmol. Vis. Sci. 2002, 43, 864–869. [Google Scholar]
- Lawrence, J.M.; Singhal, S.; Bhatia, B.; Keegan, D.J.; Reh, T.A.; Luthert, P.J.; Khaw, P.T.; Limb, G.A. MIO-M1 Cells and Similar Müller Glial Cell Lines Derived from Adult Human Retina Exhibit Neural Stem Cell Characteristics. Stem Cells 2007, 25, 2033–2043. [Google Scholar] [CrossRef]
- Bringmann, A.; Wiedemann, P. Müller glial cells in retinal disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef]
- Jian, Q.; Tao, Z.; Li, Y.; Yin, Z.Q. Acute retinal injury and the relationship between nerve growth factor, Notch1 transcription and short-lived dedifferentiation transient changes of mammalian Müller cells. Vision Res. 2015, 110 Pt A, 107–117. [Google Scholar] [CrossRef][Green Version]
- Todd, L.; Hooper, M.J.; Haugan, A.K.; Finkbeiner, C.; Jorstad, N.; Radulovich, N.; Wong, C.K.; Donaldson, P.C.; Jenkins, W.; Chen, Q.; et al. Efficient stimulation of retinal regeneration from Müller glia in adult mice using combinations of proneural bHLH transcription factors. Cell Rep. 2021, 37, 109857. [Google Scholar] [CrossRef] [PubMed]
- Tail, M.; Zhang, H.; Zheng, G.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K.; Younsi, A. The Sonic Hedgehog Pathway Modulates Survival, Proliferation, and Differentiation of Neural Progenitor Cells under Inflammatory Stress In Vitro. Cells 2022, 11, 736. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Reh, T.A. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev. Biol. 2000, 220, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J. Neurosci. 2007, 27, 7028–7040. [Google Scholar] [CrossRef]
- Fausett, B.V.; Goldman, D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J. Neurosci. 2006, 26, 6303–6313. [Google Scholar] [CrossRef]
- Ooto, S.; Akagi, T.; Kageyama, R.; Akita, J.; Mandai, M.; Honda, Y.; Takahashi, M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc. Natl. Acad. Sci. USA 2004, 101, 13654–13659. [Google Scholar] [CrossRef]
- Gallina, D.; Todd, L.; Fischer, A.J. A comparative analysis of Müller glia-mediated regeneration in the vertebrate retina. Exp. Eye Res. 2014, 123, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Bongini, R. Turning Müller glia into neural progenitors in the retina. Mol. Neurobiol. 2010, 42, 199–209. [Google Scholar] [CrossRef]
- Hu, D.; Helms, J.A. The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development 1999, 126, 4873–4884. [Google Scholar] [CrossRef]
- Shkumatava, A.; Fischer, S.; Müller, F.; Strahle, U.; Neumann, C.J. Sonic hedgehog, secreted by amacrine cells, acts as a short-range signal to direct differentiation and lamination in the zebrafish retina. Development 2004, 131, 3849–3858. [Google Scholar] [CrossRef]
- Shkumatava, A.; Neumann, C.J. Shh directs cell-cycle exit by activating p57Kip2 in the zebrafish retina. EMBO Rep. 2005, 6, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Dakubo, G.D.; Wang, Y.P.; Mazerolle, C.; Campsall, K.; McMahon, A.P.; Wallace, V.A. Retinal ganglion cell-derived sonic hedgehog signaling is required for optic disc and stalk neuroepithelial cell development. Development 2003, 130, 2967–2980. [Google Scholar] [CrossRef]
- Levine, E.M.; Roelink, H.; Turner, J.; Reh, T.A. Sonic hedgehog promotes rod photoreceptor differentiation in mammalian retinal cells in vitro. J. Neurosci. 1997, 17, 6277–6288. [Google Scholar] [CrossRef]
- Wang, Y.P.; Dakubo, G.; Howley, P.; Campsall, K.D.; Mazarolle, C.J.; Shiga, S.A.; Lewis, P.M.; McMahon, A.P.; Wallace, V.A. Development of normal retinal organization depends on Sonic hedgehog signaling from ganglion cells. Nat. Neurosci. 2002, 5, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Yang, X.-J. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev. Biol. 2001, 233, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Todd, L.; Fischer, A.J. Hedgehog signaling stimulates the formation of proliferating Müller glia-derived progenitor cells in the chick retina. Development 2015, 142, 2610–2622. [Google Scholar]
- Zhao, X.; Li, Y.; Lin, S.; Cai, Y.; Zhang, J.; Yu, X.; Yang, H.; Yang, L.; Chen, X.; Luo, Y.; et al. The Effects of Sonic Hedgehog on Retinal Muller Cells Under High-Glucose Stress. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2773–2782. [Google Scholar] [CrossRef]
- Allahyari, R.V.; Clark, K.L.; Shepard, K.A.; Garcia, A.D.R. Sonic hedgehog signaling is negatively regulated in reactive astrocytes after forebrain stab injury. Sci. Rep. 2019, 9, 565. [Google Scholar] [CrossRef]
- Salas, L.F.S.; García-Venzor, A.; Beltramone, N.; Capurro, C.; Toiber, D.; Silberman, D.M. Metabolic Imbalance Effect on Retinal Müller Glial Cells Reprogramming Capacity: Involvement of Histone Deacetylase SIRT6. Front. Genet. 2021, 12, 769723. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, X.; Qiu, M.; Chen, P.; Tang, H.; Hu, Y.; Huang, Y. Prediabetes and the risk of cancer: A meta-analysis. Diabetologia 2014, 57, 2261–2269. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, B.; D’Addato, G.; Salvatore, G.; Menduni, M.; Frontoni, S.; Carbone, L.; Camaioni, A.; Klinger, F.G.; De Felici, M.; Picconi, F.; et al. Gliotic Response and Reprogramming Potential of Human Müller Cell Line MIO-M1 Exposed to High Glucose and Glucose Fluctuations. Int. J. Mol. Sci. 2024, 25, 12877. https://doi.org/10.3390/ijms252312877
Russo B, D’Addato G, Salvatore G, Menduni M, Frontoni S, Carbone L, Camaioni A, Klinger FG, De Felici M, Picconi F, et al. Gliotic Response and Reprogramming Potential of Human Müller Cell Line MIO-M1 Exposed to High Glucose and Glucose Fluctuations. International Journal of Molecular Sciences. 2024; 25(23):12877. https://doi.org/10.3390/ijms252312877
Chicago/Turabian StyleRusso, Benedetta, Giorgia D’Addato, Giulia Salvatore, Marika Menduni, Simona Frontoni, Luigi Carbone, Antonella Camaioni, Francesca Gioia Klinger, Massimo De Felici, Fabiana Picconi, and et al. 2024. "Gliotic Response and Reprogramming Potential of Human Müller Cell Line MIO-M1 Exposed to High Glucose and Glucose Fluctuations" International Journal of Molecular Sciences 25, no. 23: 12877. https://doi.org/10.3390/ijms252312877
APA StyleRusso, B., D’Addato, G., Salvatore, G., Menduni, M., Frontoni, S., Carbone, L., Camaioni, A., Klinger, F. G., De Felici, M., Picconi, F., & La Sala, G. (2024). Gliotic Response and Reprogramming Potential of Human Müller Cell Line MIO-M1 Exposed to High Glucose and Glucose Fluctuations. International Journal of Molecular Sciences, 25(23), 12877. https://doi.org/10.3390/ijms252312877







