Lifespan Extension and Motor Function Improvement Effects of Whale Meat Extract in Caenorhabditis elegans
Abstract
1. Introduction
2. Results and Discussion
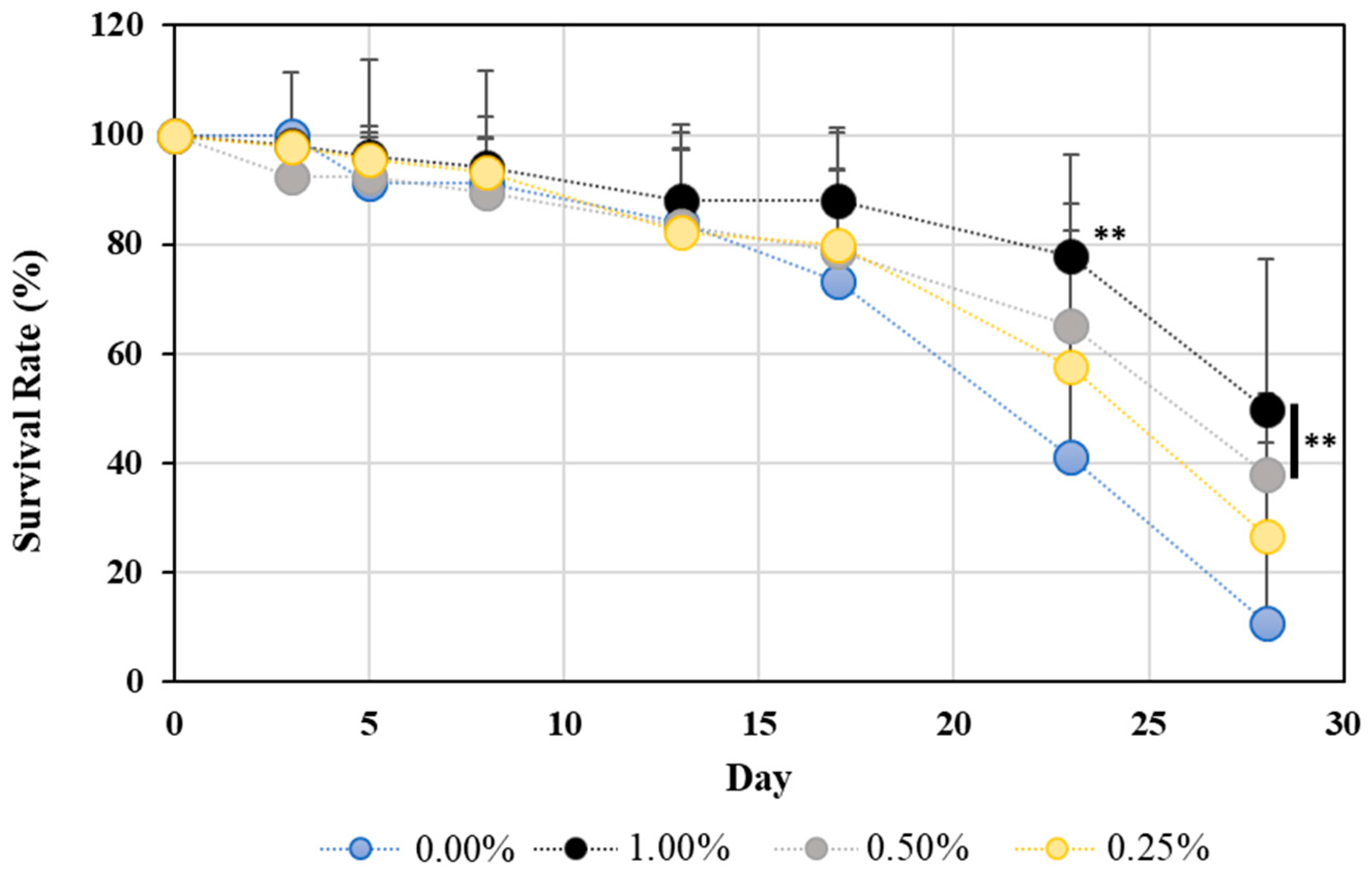
2.1. Lifespan Assay
2.2. Motor Function Assay
2.2.1. Flexion and Extension Movement Assay
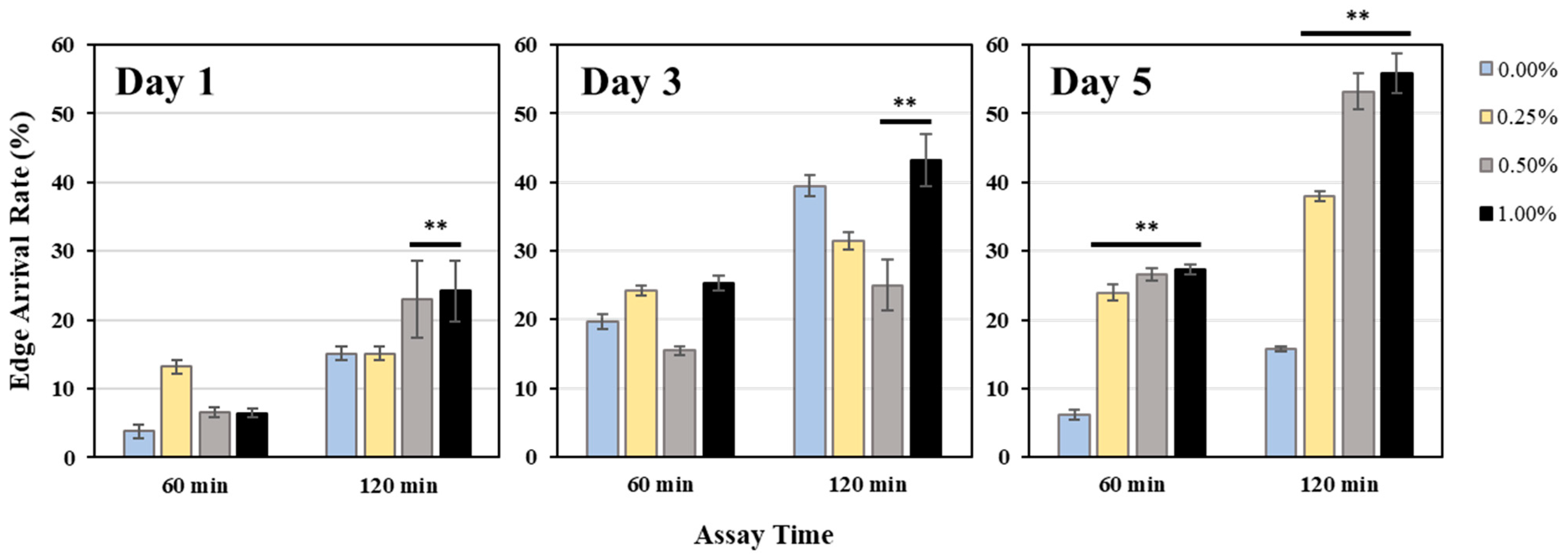
2.2.2. Edge Assay
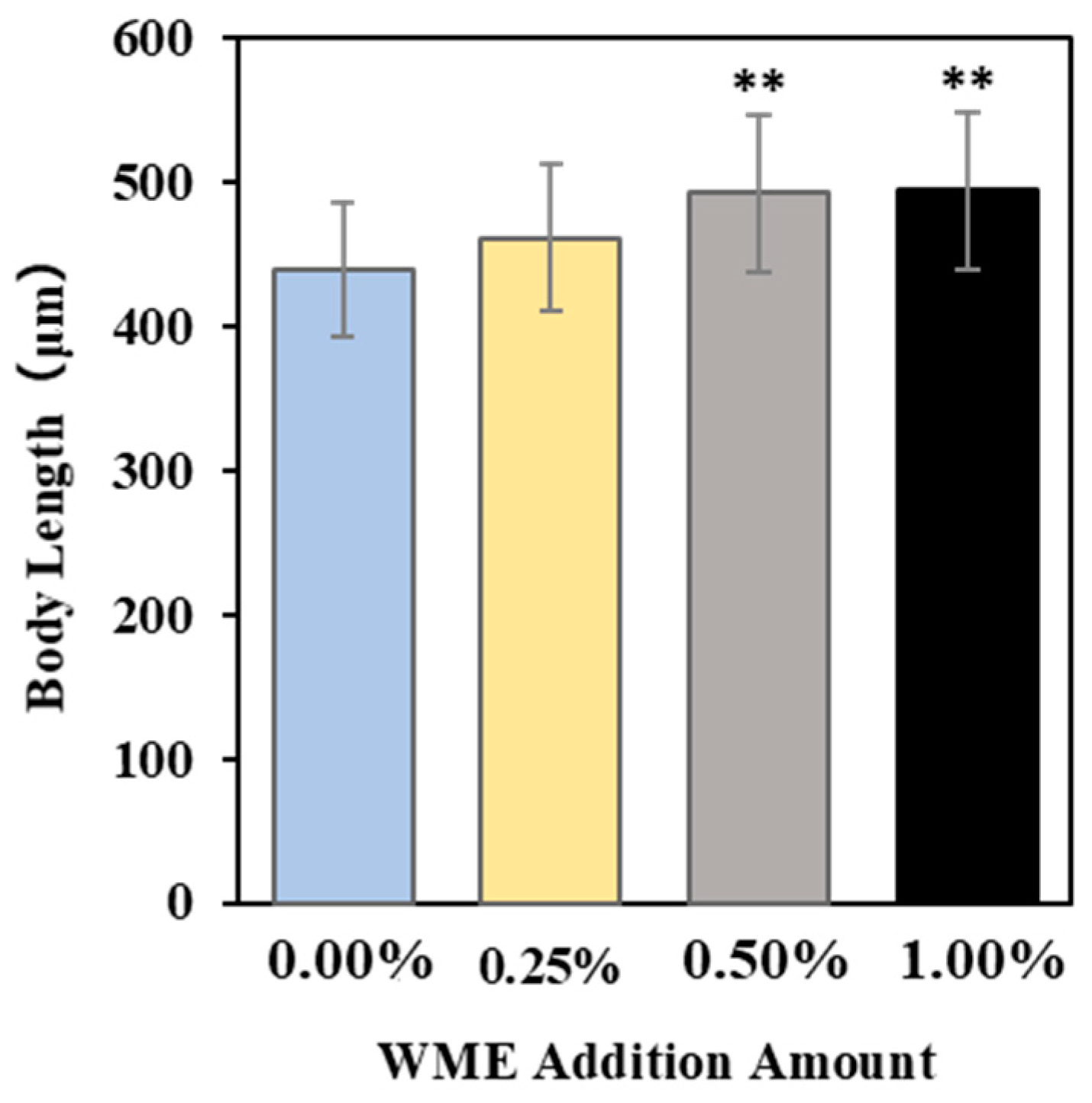
2.2.3. Body Length Measurement
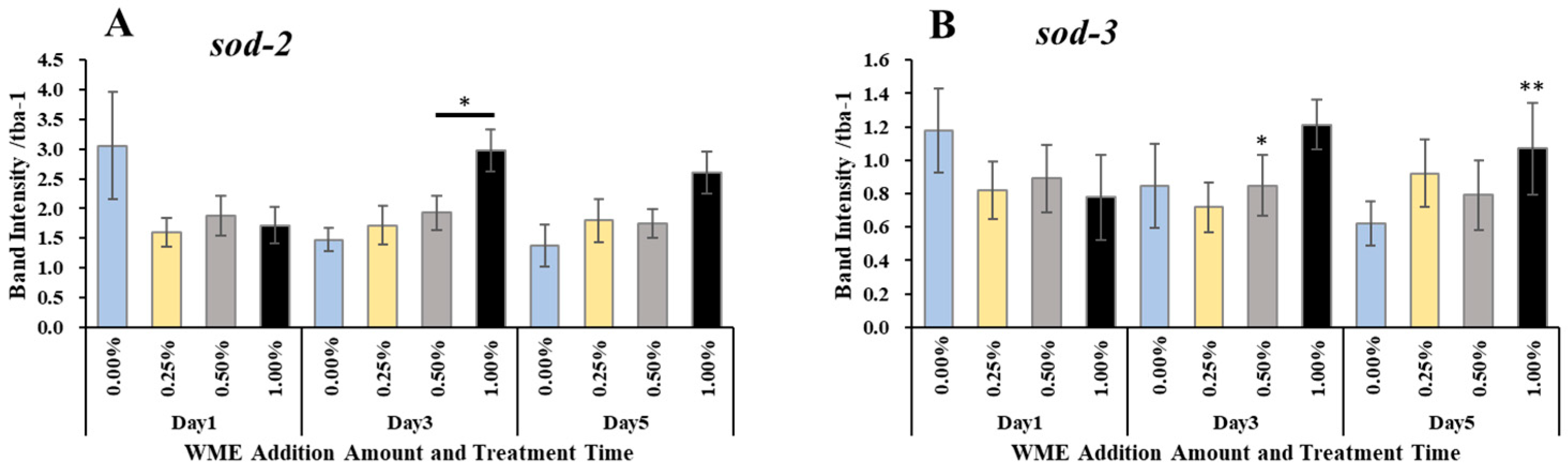
2.3. Gene Expression Analysis
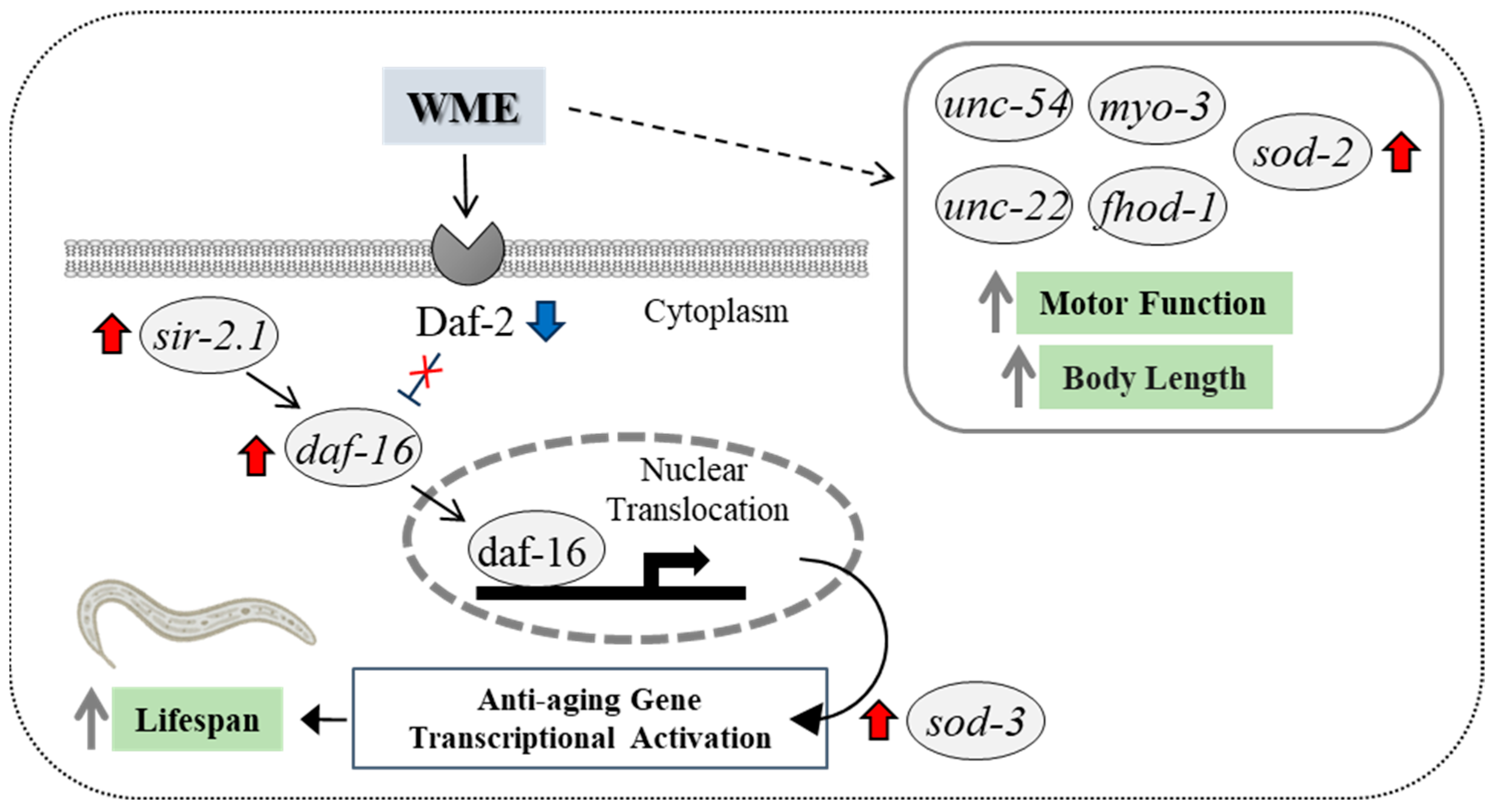
3. Discussion
4. Materials and Methods
4.1. C. elegans Maintenance Methods
4.2. C. elegans Synchronization Method
4.3. Adjustment of WME
4.4. Lifespan Assay
4.5. Motor Function Assay
4.5.1. Measurement of Bending Movement Frequency
4.5.2. Edge Assay
4.5.3. Measurement of C. elegans Body Length
4.6. RNA Extraction for Gene Expression Analysis
4.7. RT-PCR Protocol
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cocks, D.H.; Dennis, P.O.; Nelson, T.H. Isolation of 3-methyl histidine from whalemeat extract and the preparation of some derivatives. Nature 1964, 202, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.O.; Lorkin, P.A. Isolation and synthesis of balenine, a dipeptide occurring in whale-meat extract. J. Chem. Soc. 1965, 4968–4972. [Google Scholar] [CrossRef] [PubMed]
- Suyama, M.; Suzuki, T.; Yamamoto, A. Free amino acids and related compounds in whale muscle tissue. J. Tokyo Univ. Fish. 1977, 63, 189–196. [Google Scholar]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Phys. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Sale, C.; Saunders, B.; Harris, R.C. Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino Acids 2010, 39, 321–333. [Google Scholar] [CrossRef]
- Herculano, B.; Tamura, M.; Ohba, A.; Shimatani, M.; Kutsuna, N.; Hisatsune, T. β-alanyl-L-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 983–997. [Google Scholar] [CrossRef]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of anserine/carnosine supplementation on verbal episodic memory in elderly people. J. Alzheimers Dis. 2016, 50, 149–159. [Google Scholar] [CrossRef]
- Masuoka, N.; Lei, C.; Li, H.; Hisatsune, T. Influence of Imidazole-Dipeptides on Cognitive Status and Preservation in Elders: A Narrative Review. Nutrients 2021, 13, 397. [Google Scholar] [CrossRef]
- Begum, G.; Cunliffe, A.; Leveritt, M. Physiological role of carnosine in contracting muscle. Int. J. Sport Nutr. Exer. Metab. 2005, 15, 493–514. [Google Scholar] [CrossRef]
- Van der Stede, T.; Spaas, J.; de Jager, S.; De Brandt, J.; Hansen, C.; Stautemas, J.; Vercammen, B.; De Baere, S.; Croubels, S.; Van Assche, C.-H.; et al. Extensive profiling of histidine-containing dipeptides reveals species- and tissue-specific distribution and metabolism in mice, rats, and humans. Acta Physiol. 2023, 239, e14020. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Watanabe, R.; Kato, T.; Seko, T.; Matsuda, T.; Omura, Y.; Shigemura, Y.; Kawabata, Y.; Maegawa, T. Isolation of balenine from opah (Lampris megalopsis) muscle and comparison of antioxidant and iron-chelating activities with other major imidazole dipeptides. Food Chem. 2021, 364, 130343. [Google Scholar] [CrossRef] [PubMed]
- de Jager, S.; Vermeulen, A.; De Baere, S.; Van der Stede, T.; Lievens, E.; Croubels, S.; Jäger, R.; Purpura, M.; Bourgois, J.G.; Derave, W. Acute balenine supplementation in humans as a natural carnosinase-resistant alternative to carnosine. Sci. Rep. 2023, 13, 6484. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T. Anti fatigue effect of whale-meat extract (balenine), on swimming mouse model. Food Process. Ingred. 2006, 41, 62–64. (In Japanese) [Google Scholar]
- Sugino, T.; Yasunagawa, G.; Fukuda, M. Effect of whale meat extract on fatigue induced by physical load and daily activities in humans. Jpn. Pharmcol. Ther. 2013, 41, 879–893. [Google Scholar]
- Wada, N.; Yamanaka, S.; Shibato, J.; Rakwal, R.; Hirako, S.; Iizuka, Y.; Kim, H.; Matsumoto, A.; Kimura, A.; Takenoya, F.; et al. Behavioral and omics analyses study on potential involvement of dipeptide balenine through supplementation in diet of senescence-accelerated mouse prone 8. Genom. Data 2016, 10, 38–50. [Google Scholar] [CrossRef][Green Version]
- Yang, M.; Sun, L.; Kawabata, Y.; Murayama, F.; Maegawa, T.; Nikawa, T.; Hirasaka, K. Balenine, Imidazole Dipeptide Promotes Skeletal Muscle Regeneration by Regulating Phagocytosis Properties of Immune Cells. Mar. Drugs 2022, 20, 313. [Google Scholar] [CrossRef]
- Dhondt, I.; Verschuuren, C.; Zečić, A.; Loier, T.; Braeckman, B.P.; De Vos, W.H. Prediction of biological age by morphological staging of sarcopenia in C. elegans. Dis. Model Mech. 2021, 14, dmm049169. [Google Scholar] [CrossRef]
- Tissenbaum, H.A.; Guarente, L. Increased dosage of a sir-2 gene extends lifespan in C. elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in C. elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef]
- Kenyon, C. The plasticity of aging: Insights from long-lived mutants. Cell 2005, 120, 449–460. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.D.; Wang, Y.D. DAF-16/FOXO transcription factor in aging and longevity. Front. Pharmacol. 2017, 8, 548. [Google Scholar] [CrossRef]
- Lamitina, S.T.; Strange, K. Transcriptional targets of DAF-16 insulin signaling pathway protect C. elegans from extreme hypertonic stress. Am. J. Physiol. Cell Physiol. 2005, 288, 467–474. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.V. Recent Progress in Regulation of Aging by Insulin/IGF-1 Signaling in C. elegans. Mol. Cells 2022, 45, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Li, F.; Wang, T.; Luo, X.; Li, B.; You, Y.; Wu, C.; Liu, X. Jujubae Fructus extract prolongs lifespan and improves stress tolerance in C. elegans dependent on DAF-16/SOD-3. Sci. Rep. 2024, 14, 13713. [Google Scholar] [CrossRef]
- Libina, N.; Berman, J.R.; Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell 2003, 115, 489–502. [Google Scholar] [CrossRef]
- McElwee, J.; Bubb, K.; Thomas, J.H. Transcriptional outputs of the C. elegans forkhead protein DAF-16. Aging Cell 2003, 2, 111–121. [Google Scholar] [CrossRef]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.R.; Arhinger, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of C. elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef]
- Ayoub, I.M.; Eldahshan, O.A.; Roxo, M.; Zhang, S.; Wink, M.; Singab, A.N.B. Stress resistance, antiaging, and neuroprotective activities of baicalein 5,6-dimethyl ether and Alnus rugosa extract in C. elegans model. Arch. Pharm. 2024, e2400464. [Google Scholar] [CrossRef]
- Chavez, V.; Mohri-Shiomi, A.; Maadani, A.; Vega, L.A.; Garsin, D.A. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by C. elegans. Genetics 2007, 176, 1567–1577. [Google Scholar] [CrossRef]
- Jiang, S.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Rhodiola extract promotes longevity and stress resistance of C. elegans via DAF-16 and SKN-1. Food Funct. 2021, 12, 4471–4483. [Google Scholar] [CrossRef]
- Pei, H.; Lin, Z.; Yao, K.; Luo, Y.; Tong, P.; Chen, H.; Wu, Y.; Wu, Z.; Gao, J. Ovalbumin promotes innate immune response of Caenorhabditis elegans through DAF-16 and SKN-1 pathways in insulin/IGF-1 signaling. J. Physiol. Biochem. 2024, 80, 541–559. [Google Scholar] [CrossRef]
- Schaar, C.E.; Dues, D.J.; Spielbauer, K.K.; Machiela, E.; Cooper, J.F.; Senchuk, M.; Hekimi, S.; Van Raamsdonk, J.M. Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS Genet. 2015, 11, e1004972. [Google Scholar] [CrossRef]
- Xu, J.; Du, P.; Liu, X.; Xu, X.; Ge, Y.; Zhang, C. Curcumin supplementation increases longevity and antioxidant capacity in C. elegans. Front. Pharmacol. 2023, 14, 1195490. [Google Scholar] [CrossRef]
- Ma, J.; Xu, X.; Wang, R.; Yan, H.; Yao, H.; Zhang, H.; Jiang, S.; Xu, A. Lipopolysaccharide exposure induces oxidative damage in C. elegans: Protective effects of carnosine. BMC Pharmacol. Toxicol. 2020, 21, 85. [Google Scholar] [CrossRef]
- Niu, Y.; Liao, J.; Zhou, H.; Wang, C.C.; Wang, L.; Fan, Y. Flavonoids from Lycium barbarum Leaves Exhibit Anti-Aging Effects through the Redox-Modulation. Molecules 2022, 27, 4952. [Google Scholar] [CrossRef]
- Zhao, L.; Rui, Q.; Wang, D. Molecular basis for oxidative stress induced by simulated microgravity in nematode C. elegans. Sci. Total Environ. 2017, 607–608, 1381–1390. [Google Scholar] [CrossRef]
- Sakamoto, T.; Imai, H. Hydrogen peroxide produced by superoxide dismutase SOD-2 activates sperm in C. elegans. J. Biol. Chem. 2017, 292, 14804–14813. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Wu, Q.; Tang, M.; Pu, Y.; Wang, D. Chronic Al2O3-nanoparticle exposure causes neurotoxic effects on locomotion behaviors by inducing severe ROS production and disruption of ROS defense mechanisms in nematode C. elegans. J. Hazard. Mater. 2012, 219–220, 221–230. [Google Scholar] [CrossRef]
- Matheny, C.J.; Qadota, H.; Bailey, A.O.; Valdebenito-Silva, S.; Oberhauser, A.F.; Benian, G.M. The myosin chaperone UNC-45 has an important role in maintaining the structure and function of muscle sarcomeres during adult aging. Mol. Biol. Cell 2024, 35, ar98. [Google Scholar] [CrossRef]
- Adamla, F.; Ignatova, Z. Somatic expression of unc-54 and vha-6 mRNAs declines but not pan-neuronal rgef-1 and unc-119 expression in aging C. elegans. Sci. Rep. 2015, 5, 10692. [Google Scholar] [CrossRef]
- Dahl-Halvarsson, M.; Pokrzywa, M.; Rauthan, M.; Pilon, M.; Tajsharghi, H. Myosin storage myopathy in C. elegans and human cultured muscle cells. PLoS ONE 2017, 12, e0170613. [Google Scholar] [CrossRef]
- Harada, S.; Hashizume, T.; Nemoto, K.; Shao, Z.; Higashitani, N.; Etheridge, T.; Szewczyk, N.J.; Fukui, K.; Higashibata, A.; Higashitani, A. Fluid dynamics alter C. elegans body length via TGF-beta/DBL-1 neuromuscular signaling. NPJ Microgravity 2016, 2, 16006. [Google Scholar] [CrossRef]
- Sundaramurthy, S.; Votra, S.; Laszlo, A.; Davies, T.; Pruyne, D. FHOD-1 is the only formin in C. elegans that promotes striated muscle growth and Z-line organization in a cell autonomous manner. Cytoskeleton 2020, 77, 422–441. [Google Scholar] [CrossRef]



| Forward Primer | Reverse Primer | ||
|---|---|---|---|
| Accession (Gene) | Nucleotide Sequence (5′-3′) | Nucleotide Sequence (5′-3′) | Gene Name |
| NM_001269678 | agttgaatgggattggatgaag | ctatcgaggctgtgtcaatgtc | pmp-3 |
| NM_001268555 | ttgaaactggttcgtgatgttc | gtttttcaatgggatttggtgt | sir-2.1 |
| NM_065249 | atgagcatcatccacttgtctg | gtcagcgaacgtacaaactcag | daf-2 |
| AF032112 | ttcaatcgtgtggaattgtagc | tgggatgaatgtgttggaatta | daf-16 |
| NM_059889 | ctggactaatttggcaaaggac | agtagtaagcgtgctcccagac | sod-2 |
| NM_078363 | agaaccttcaaaggagctgatg | ctgcttttattgtcgagcattg | sod-3 |
| NM_073664 | ttaatgctcacgtgtctgctct | acgtctctgttctccatccaat | myo-3 |
| NM_061195 | gaatctgaattggacggagaac | ggagaaagagcatgtagggatg | unc-54 |
| NM_001313484 | agacctggtggacactgaatct | gagtgagtgaggaggaaggaga | fhod-1 |
| NM_069872 | aagctagagtgcaaggaacacc | cttaaaggtgtttccggtcttg | unc-22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibato, J.; Takenoya, F.; Kimura, A.; Yamashita, M.; Rakwal, R.; Shioda, S. Lifespan Extension and Motor Function Improvement Effects of Whale Meat Extract in Caenorhabditis elegans. Int. J. Mol. Sci. 2024, 25, 12833. https://doi.org/10.3390/ijms252312833
Shibato J, Takenoya F, Kimura A, Yamashita M, Rakwal R, Shioda S. Lifespan Extension and Motor Function Improvement Effects of Whale Meat Extract in Caenorhabditis elegans. International Journal of Molecular Sciences. 2024; 25(23):12833. https://doi.org/10.3390/ijms252312833
Chicago/Turabian StyleShibato, Junko, Fumiko Takenoya, Ai Kimura, Michio Yamashita, Randeep Rakwal, and Seiji Shioda. 2024. "Lifespan Extension and Motor Function Improvement Effects of Whale Meat Extract in Caenorhabditis elegans" International Journal of Molecular Sciences 25, no. 23: 12833. https://doi.org/10.3390/ijms252312833
APA StyleShibato, J., Takenoya, F., Kimura, A., Yamashita, M., Rakwal, R., & Shioda, S. (2024). Lifespan Extension and Motor Function Improvement Effects of Whale Meat Extract in Caenorhabditis elegans. International Journal of Molecular Sciences, 25(23), 12833. https://doi.org/10.3390/ijms252312833






