Proinflammatory Cytokines Enhance the Mineralization, Proliferation, and Metabolic Activity of Primary Human Osteoblast-like Cells
Abstract
1. Introduction
2. Results
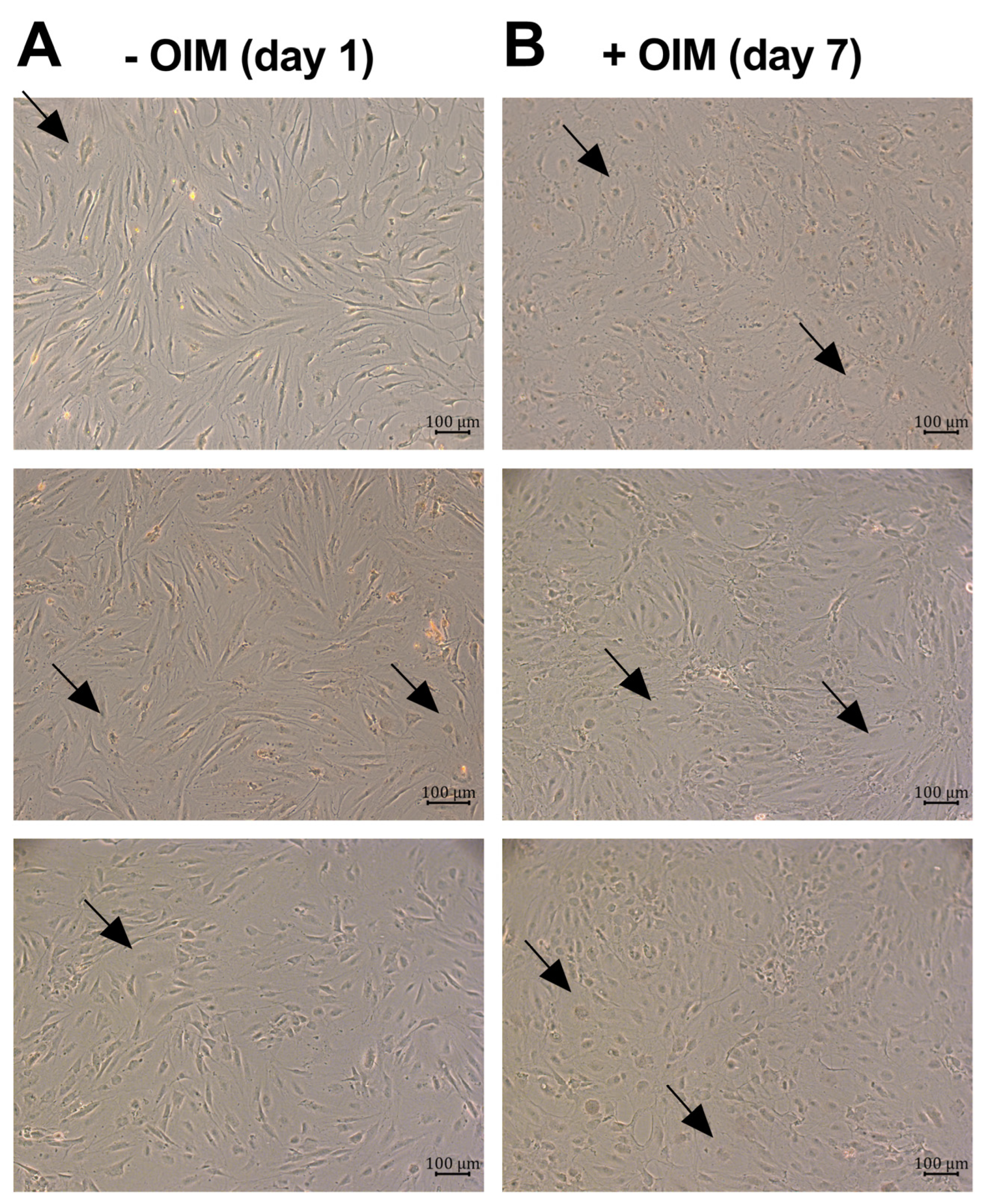
2.1. OBs Secreted the Proinflammatory Cytokines IL-6 and IL-8 During Osteogenesis
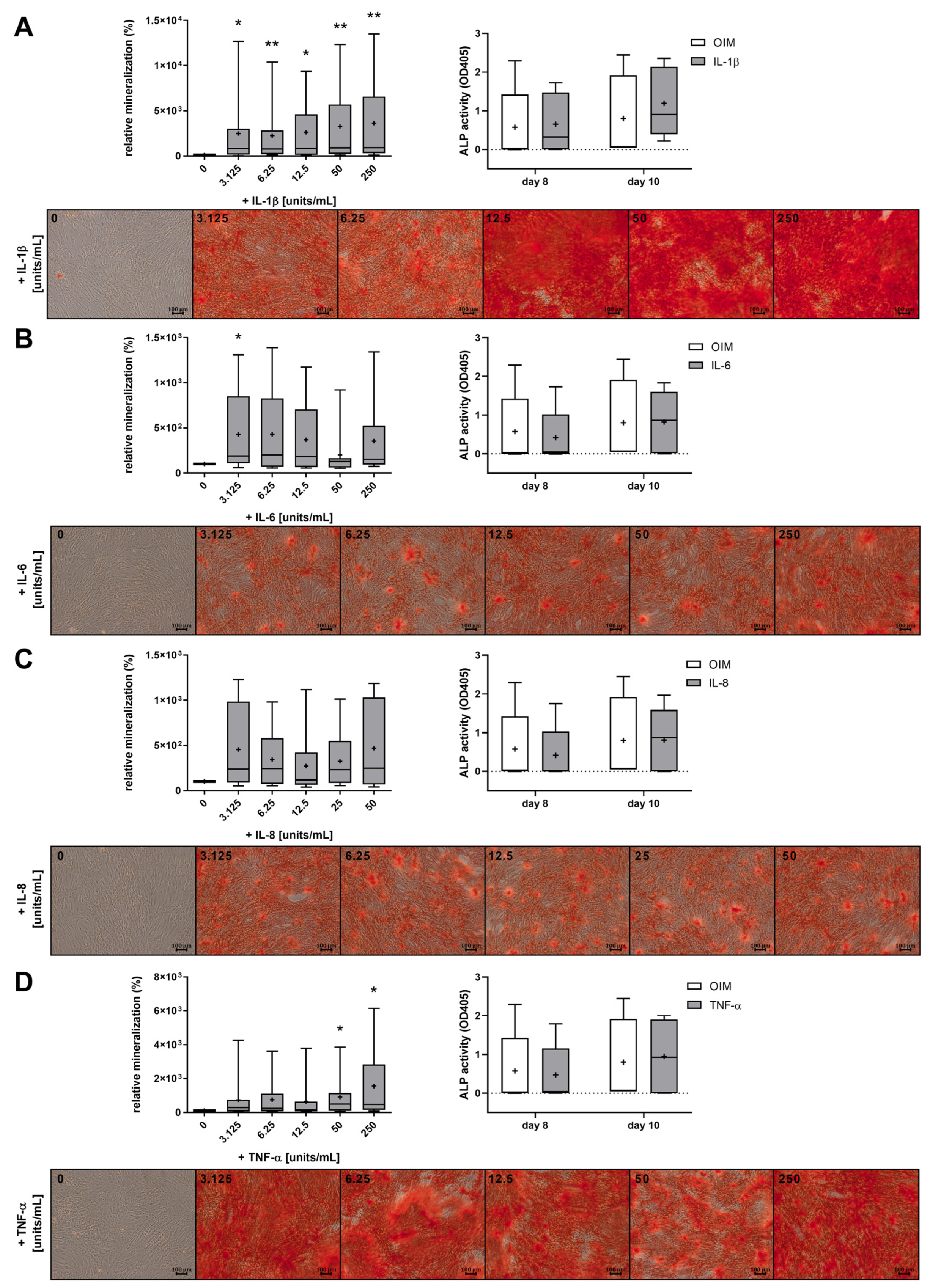
2.2. The Proinflammatory Cytokines IL-1β, IL-6, IL-8, and TNF-α Increased the Mineralization of OBs
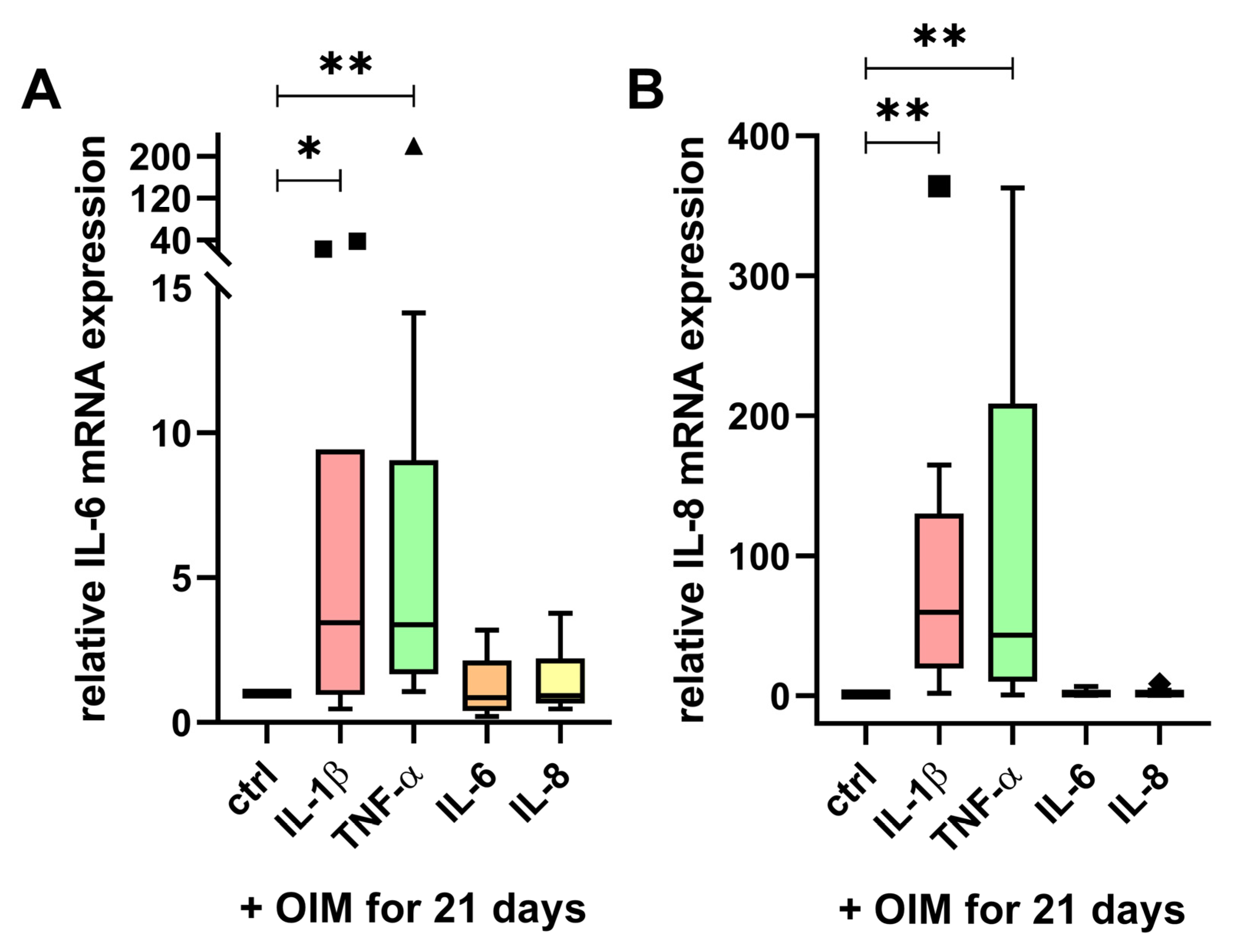
2.3. IL-6 and IL-8 mRNA Expression Were Enhanced by IL-1β and TNF-α
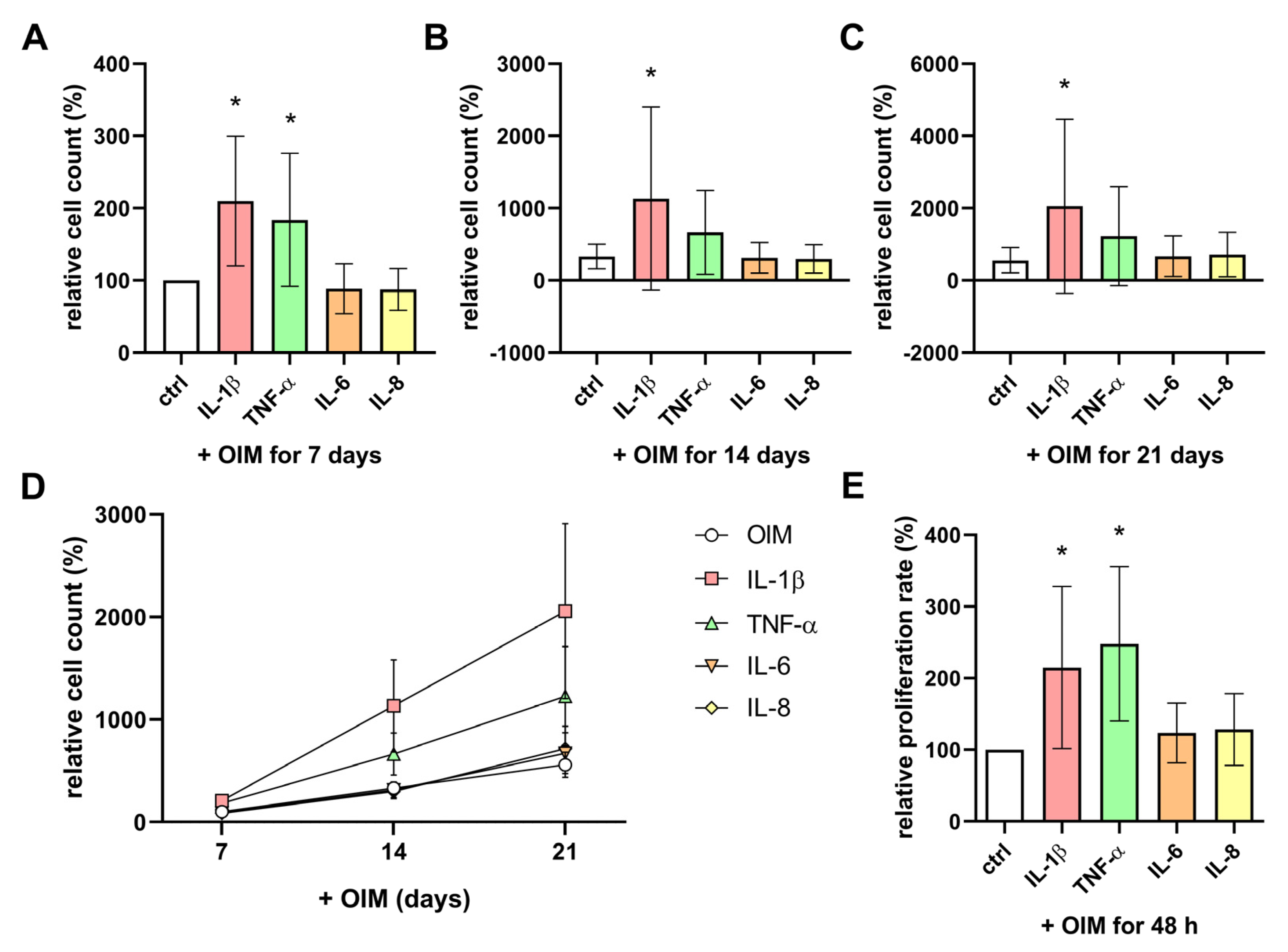
2.4. IL-1β and TNF-α Enhanced the Cell Count and Proliferation Rate of OBs During Osteogenesis
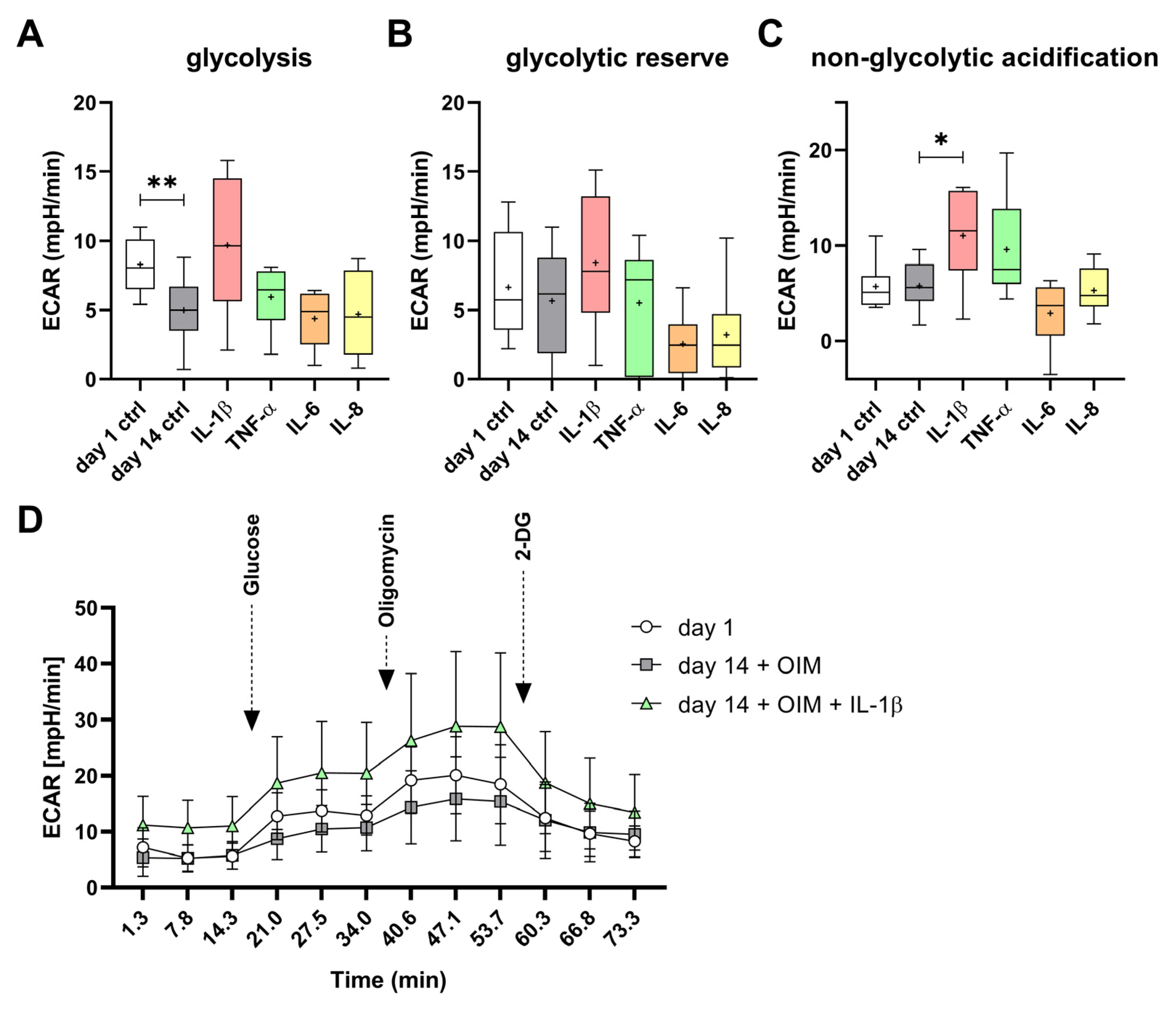
2.5. IL-1β and TNF-α Had Effects on Mitochondrial and Glycolytic Metabolism of the OBs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bone Material, Ethics Approval, and Patient Information
4.3. Isolation of Human Osteoblast-like Cells
4.4. Cell Culture
4.5. Alizarin Red S Staining
4.6. Extracellular ALP Activity
4.7. Quantification of Cytokines in Cell Supernatants by Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Real-Time qPCR
4.9. MTT Assay for Cell Count Quantification
4.10. BrdU Assay for Cell Proliferation Rate
4.11. Seahorse Assay: Evaluation of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR)
4.12. Microscopic Images
4.13. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livshits, G.; Kalinkovich, A. Targeting chronic inflammation as a potential adjuvant therapy for osteoporosis. Life Sci. 2022, 306, 120847. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Nagpal, M.; Singh, M. Osteoblast-n-Osteoclast: Making Headway to Osteoporosis Treatment. Curr. Drug Targets 2020, 21, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Ashai, S.; Harvey, N.C. Rheumatoid arthritis and bone health. Clin. Med. 2020, 20, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Magrey, M.; Khan, M.A. Osteoporosis in Ankylosing Spondylitis. Curr. Rheumatol. Rep. 2010, 12, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Okamoto, K.; Takayanagi, H. Osteoimmunology. Cold Spring Harb. Perspect. Med. 2019, 9, a031245. [Google Scholar] [CrossRef] [PubMed]
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The Effect of Inflammation on Bone. Front. Physiol. 2021, 11, 511799. [Google Scholar] [CrossRef]
- Kushioka, J.; Chow, S.K.-H.; Toya, M.; Tsubosaka, M.; Shen, H.; Gao, Q.; Li, X.; Zhang, N.; Goodman, S.B. Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy. Inflamm. Regen. 2023, 43, 29. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Hojo, H.; Ohba, S.; Chung, U.-I. Signaling pathways regulating the specification and differentiation of the osteoblast lineage. Regen. Ther. 2015, 1, 57–62. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of Osteoblast Differentiation by Cytokine Networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef]
- Sonomoto, K.; Yamaoka, K.; Oshita, K.; Fukuyo, S.; Zhang, X.; Nakano, K.; Okada, Y.; Tanaka, Y. Interleukin-1β induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase–like orphan receptor 2 pathway. Arthritis Rheum. 2012, 64, 3355–3363. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L. IL-1β inhibits osteogenesis of human bone marrow-derived mesenchymal stem cells by activating FoxD3/microRNA-496 to repress wnt signaling. Genesis 2017, 55, e23040. [Google Scholar] [CrossRef] [PubMed]
- Osta, B.; Benedetti, G.; Miossec, P. Classical and Paradoxical Effects of TNF-α on Bone Homeostasis. Front. Immunol. 2014, 5, 48. [Google Scholar] [CrossRef]
- Yang, A.; Lu, Y.; Xing, J.; Li, Z.; Yin, X.; Dou, C.; Dong, S.; Luo, F.; Xie, Z.; Hou, T.; et al. IL-8 Enhances Therapeutic Effects of BMSCs on Bone Regeneration via CXCR2-Mediated PI3k/Akt Signaling Pathway. Cell. Physiol. Biochem. Int. J. Exp. Cell Physiol. Biochem. Pharmacol. 2018, 48, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies, Inc. Seahorse XF Glycolysis Stress Test Kit User Guide. Published online October 2019. Available online: https://www.agilent.com/cs/library/usermanuals/public/XF_Glycolysis_Stress_Test_Kit_User_Guide.pdf (accessed on 4 April 2024).
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Ye, F.; Li, J.; Xu, P.; Xie, Z.; Zheng, G.; Liu, W.; Ye, G.; Yu, W.; Lin, J.; Su, Z.; et al. Osteogenic differentiation of mesenchymal stem cells promotes c-Jun-dependent secretion of interleukin 8 and mediates the migration and differentiation of CD4+ T cells. Stem Cell Res. Ther. 2022, 13, 58. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Lan, T.; Liang, P.; Tao, Q. Upregulation of interleukin-8 and activin A induces osteoclastogenesis in ameloblastoma. Int. J. Mol. Med. 2019, 43, 2329–2340. [Google Scholar] [CrossRef]
- Mukaida, N.; Mahe, Y.; Matsushima, K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 1990, 265, 21128–21133. [Google Scholar] [CrossRef]
- Choi, H.M.; Oh, D.H.; Bang, J.S.; Yang, H.-I.; Yoo, M.C.; Kim, K.S. Differential effect of IL-1β and TNF-α on the production of IL-6, IL-8 and PGE2 in fibroblast-like synoviocytes and THP-1 macrophages. Rheumatol. Int. 2010, 30, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.; Porter, R.M.; Wehling, N.; O’Sullivan, R.P.; Liu, F.; Boskey, A.; Estok, D.M.; Harris, M.B.; Vrahas, M.S.; Evans, C.H.; et al. Inflammatory Cytokines Induce a Unique Mineralizing Phenotype in Mesenchymal Stem Cells Derived from Human Bone Marrow. J. Biol. Chem. 2013, 288, 29494–29505. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.K.; Raggatt, L.-J.; Alexander, K.A.; Kuliwaba, J.S.; Fazzalari, N.L.; Schroder, K.; Maylin, E.R.; Ripoll, V.M.; Hume, D.A.; Pettit, A.R. Osteal Tissue Macrophages Are Intercalated throughout Human and Mouse Bone Lining Tissues and Regulate Osteoblast Function In Vitro and In Vivo. J. Immunol. 2008, 181, 1232–1244. [Google Scholar] [CrossRef]
- Meniailo, M.E.; Malashchenko, V.V.; Shmarov, V.A.; Gazatova, N.D.; Melashchenko, O.B.; Goncharov, A.G.; Seledtsova, G.V.; Seledtsov, V.I. Interleukin-8 favors pro-inflammatory activity of human monocytes/macrophages. Int. Immunopharmacol. 2018, 56, 217–221. [Google Scholar] [CrossRef]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co–culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef]
- Frost, A.; Jonsson, K.B.; Nilsson, O.; Ljunggren, Ö. Inflammatory cytokines regulate proliferation of cultured human osteoblasts. Acta Orthop. Scand. 1997, 68, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ghali, O.; Chauveau, C.; Hardouin, P.; Broux, O.; Devedjian, J.-C. TNF-α’s effects on proliferation and apoptosis in human mesenchymal stem cells depend on RUNX2 expression. J. Bone Miner. Res. 2010, 25, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Sapozhnikova, A.; Lu, C.; Hu, D.; Li, X.; Miclau, T.; Marcucio, R.S. Action of IL-1β during fracture healing. J. Orthop. Res. 2010, 28, 778–784. [Google Scholar] [CrossRef]
- Shen, L.; Hu, G.; Karner, C.M. Bioenergetic Metabolism in Osteoblast Differentiation. Curr. Osteoporos. Rep. 2022, 20, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Shum, L.C.; White, N.S.; Mills, B.N.; de Mesy Bentley, K.L.; Eliseev, R.A. Energy Metabolism in Mesenchymal Stem Cells During Osteogenic Differentiation. Stem Cells Dev. 2016, 25, 114–122. [Google Scholar] [CrossRef]
- Chen, C.-T.; Shih, Y.-R.V.; Kuo, T.K.; Lee, O.K.; Wei, Y.-H. Coordinated Changes of Mitochondrial Biogenesis and Antioxidant Enzymes During Osteogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Guntur, A.R.; Le, P.T.; Farber, C.R.; Rosen, C.J. Bioenergetics During Calvarial Osteoblast Differentiation Reflect Strain Differences in Bone Mass. Endocrinology 2014, 155, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Guntur, A.R.; Gerencser, A.A.; Le, P.T.; DeMambro, V.E.; Bornstein, S.A.; Mookerjee, S.A.; Maridas, D.E.; Clemmons, D.E.; Brand, M.D.; Rosen, C.J. Osteoblast-like MC3T3-E1 Cells Prefer Glycolysis for ATP Production but Adipocyte-like 3T3-L1 Cells Prefer Oxidative Phosphorylation. J. Bone Miner. Res. 2018, 33, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Pike Winer, L.S.; Wu, M. Rapid Analysis of Glycolytic and Oxidative Substrate Flux of Cancer Cells in a Microplate. PLoS ONE 2014, 9, e109916. [Google Scholar] [CrossRef]
- Kumar, S.; Votta, B.J.; Rieman, D.J.; Badger, A.M.; Gowen, M.; Lee, J.C. IL-1- and TNF-induced bone resorption is mediated by p38 mitogen activated protein kinase. J. Cell. Physiol. 2001, 187, 294–303. [Google Scholar] [CrossRef]
- Nishimoto, N.; Hashimoto, J.; Miyasaka, N.; Yamamoto, K.; Kawai, S.; Takeuchi, T.; Murata, N.; van der Heijde, D.; Kishimoto, T. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): Evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann. Rheum. Dis. 2007, 66, 1162–1167. [Google Scholar] [CrossRef]
- Vis, M.M.; Havaardsholm, A.E.; Haugeberg, G.; Uhlig, T.; Voskuyl, A.E.; Van De Stadt, R.J.; Dijkmans, B.A.C.; Woolf, A.; Kvien, T.K.; Lems, W.F. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NF B ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 1495–1499. [Google Scholar] [CrossRef]
- Musacchio, E.; Valvason, C.; Botsios, C.; Ostuni, F.; Furlan, A.; Ramonda, R.; Modesti, V.; Sartori, L.; Punzi, L. The Tumor Necrosis Factor-α-blocking Agent Infliximab Inhibits Interleukin 1ß (IL-1ß) and IL-6 Gene Expression in Human Osteoblastic Cells. J. Rheumatol. 2009, 36, 1575–1579. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2013, 64, 1697–1707. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the Inflammatory Response for Enhanced Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Torzilli, P.A.; Bhargava, M.; Park, S.; Chen, C.T.C. Mechanical load inhibits IL-1 induced matrix degradation in articular car-tilage. Osteoarthritis Cartilage. 2010, 18, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Thompson, C.L.; Ali, A.; Wang, W.; Chapple, J.P.; Mitchison, H.M.; Beales, P.L.; Wann, A.K.T.; Knight, M.M. Mechanical loading inhibits cartilage inflammatory signalling via an HDAC6 and IFT-dependent mechanism regulating primary cilia elongation. Osteoarthritis Cartilage. 2019, 27, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. Diagnosis of osteoporosis and assessment of fracture risk. Lancet Lond. Engl. 2002, 359, 1929–1936. [Google Scholar] [CrossRef]
- Neidlinger-Wilke, C.; Stalla, I.; Claes, L.; Brand, R.; Hoellen, I.; Rübenacker, S.; Arand, M.; Kinzl, L. Human osteoblasts from younger normal and osteoporotic donors show differences in proliferation and TGFβ-release in response to cyclic strain. J. Biomech. 1995, 28, 1411–1418. [Google Scholar] [CrossRef]
- Robey, P.G.; Termine, J.D. Human bone cells in vitro. Calcif. Tissue Int. 1985, 37, 453–460. [Google Scholar] [CrossRef]
- Jaiswal, N.; Haynesworth, S.E.; Caplan, A.I.; Bruder, S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell. Biochem. 1997, 64, 295–312. [Google Scholar] [CrossRef]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red s stains for calcium. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1969, 17, 110–124. [Google Scholar] [CrossRef]
- Sabokbar, A.; Millett, P.J.; Myer, B.; Rushton, N. A rapid, quantitative assay for measuring alkaline phosphatase activity in osteoblastic cells in vitro. Bone Miner. 1994, 27, 57–67. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Van Merloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]


| Target Gene | Forward Primer (5′->3′) | Reverse Primer (5′->3′) | Product Length |
|---|---|---|---|
| IL-6 | TCCAGTTCCTGCAGAAAAAGGCAA | TGGTTCTGTGCCTGCAGCTT | 100 |
| IL-8 | CCACCGGAAGGAACCATCTCAC | CCTTGGCAAAACTGCACCTTCAC | 114 |
| TFRC | TTCAGGTCAAAGACAGCGCTCA | CTATACGCCACATAACCCCCAGG | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bousch, J.F.; Beyersdorf, C.; Schultz, K.; Windolf, J.; Suschek, C.V.; Maus, U. Proinflammatory Cytokines Enhance the Mineralization, Proliferation, and Metabolic Activity of Primary Human Osteoblast-like Cells. Int. J. Mol. Sci. 2024, 25, 12358. https://doi.org/10.3390/ijms252212358
Bousch JF, Beyersdorf C, Schultz K, Windolf J, Suschek CV, Maus U. Proinflammatory Cytokines Enhance the Mineralization, Proliferation, and Metabolic Activity of Primary Human Osteoblast-like Cells. International Journal of Molecular Sciences. 2024; 25(22):12358. https://doi.org/10.3390/ijms252212358
Chicago/Turabian StyleBousch, Juliana Franziska, Christoph Beyersdorf, Katharina Schultz, Joachim Windolf, Christoph Viktor Suschek, and Uwe Maus. 2024. "Proinflammatory Cytokines Enhance the Mineralization, Proliferation, and Metabolic Activity of Primary Human Osteoblast-like Cells" International Journal of Molecular Sciences 25, no. 22: 12358. https://doi.org/10.3390/ijms252212358
APA StyleBousch, J. F., Beyersdorf, C., Schultz, K., Windolf, J., Suschek, C. V., & Maus, U. (2024). Proinflammatory Cytokines Enhance the Mineralization, Proliferation, and Metabolic Activity of Primary Human Osteoblast-like Cells. International Journal of Molecular Sciences, 25(22), 12358. https://doi.org/10.3390/ijms252212358







