Galangin Triggers Eryptosis and Hemolysis Through Ca2+ Nucleation and Metabolic Collapse Mediated by PKC/CK1α/COX/p38/Rac1 Signaling Axis
Abstract
1. Introduction
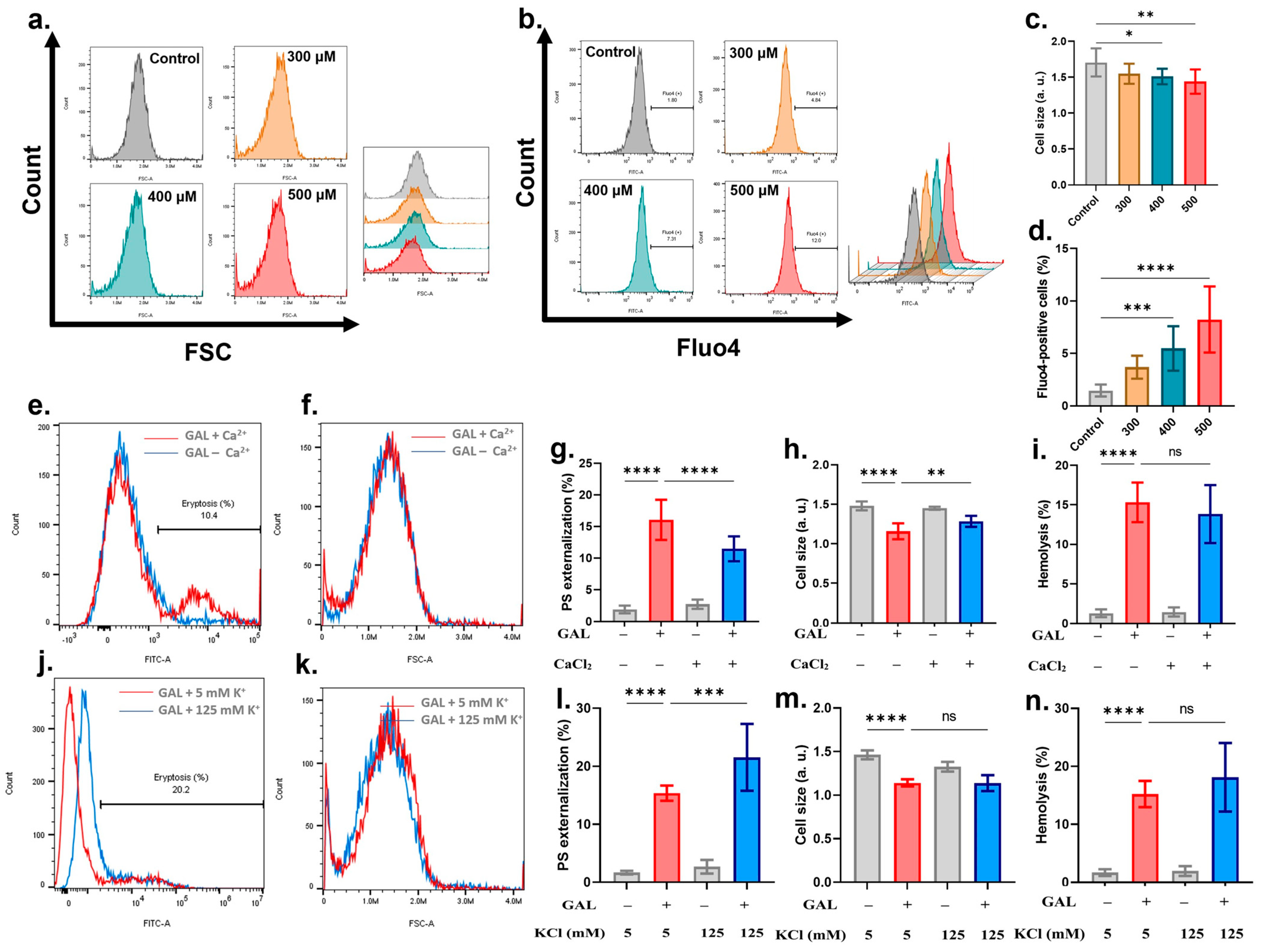
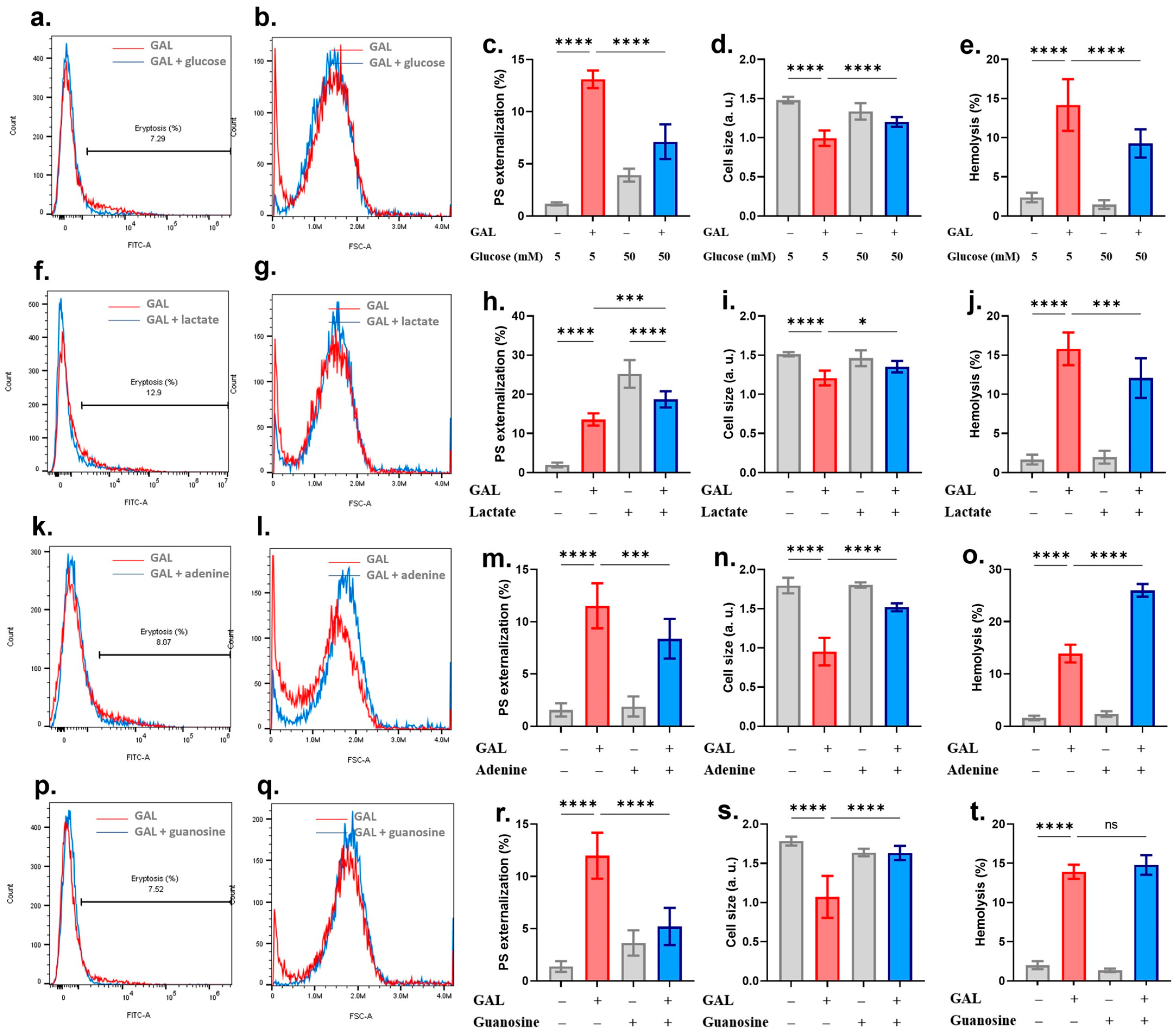
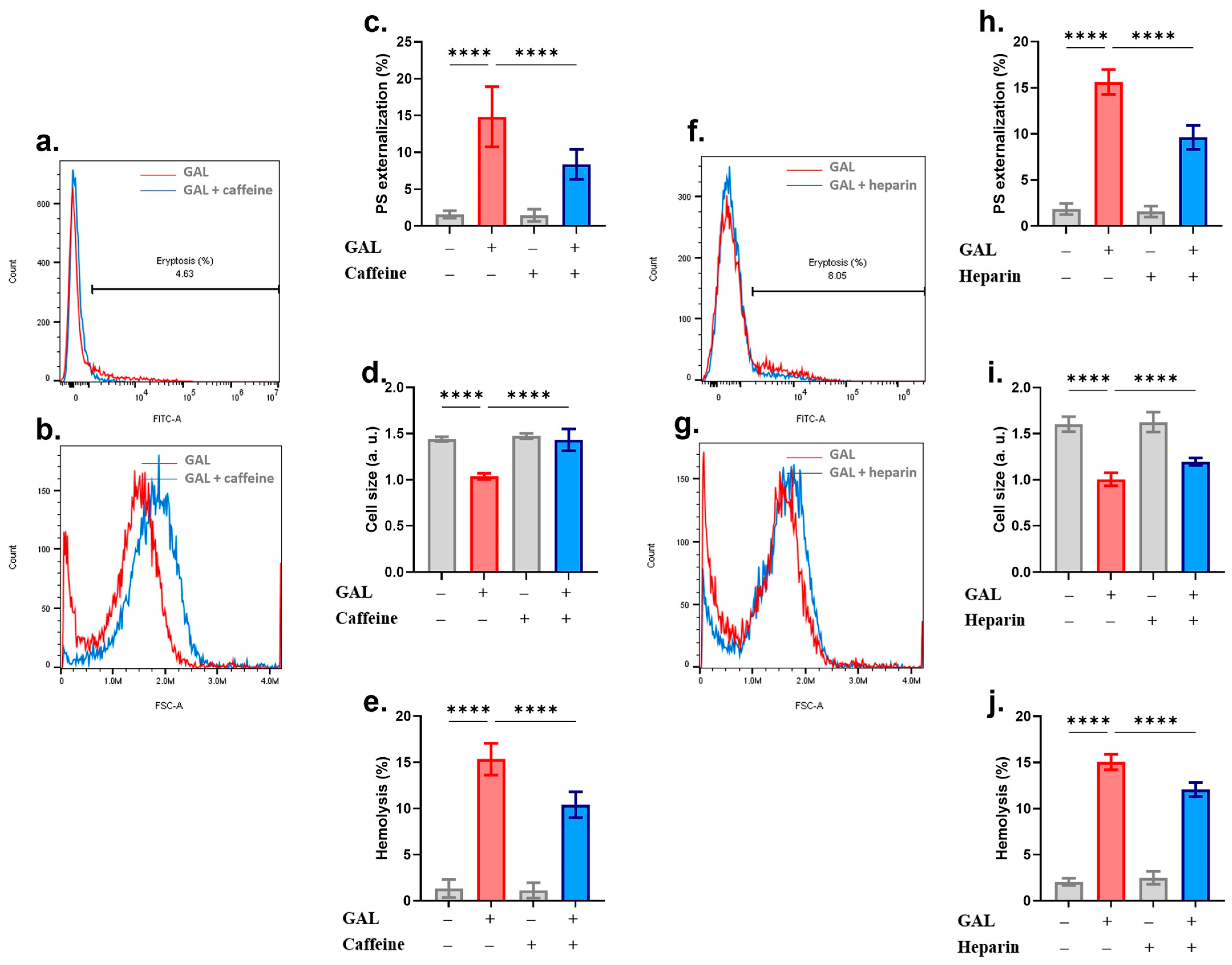
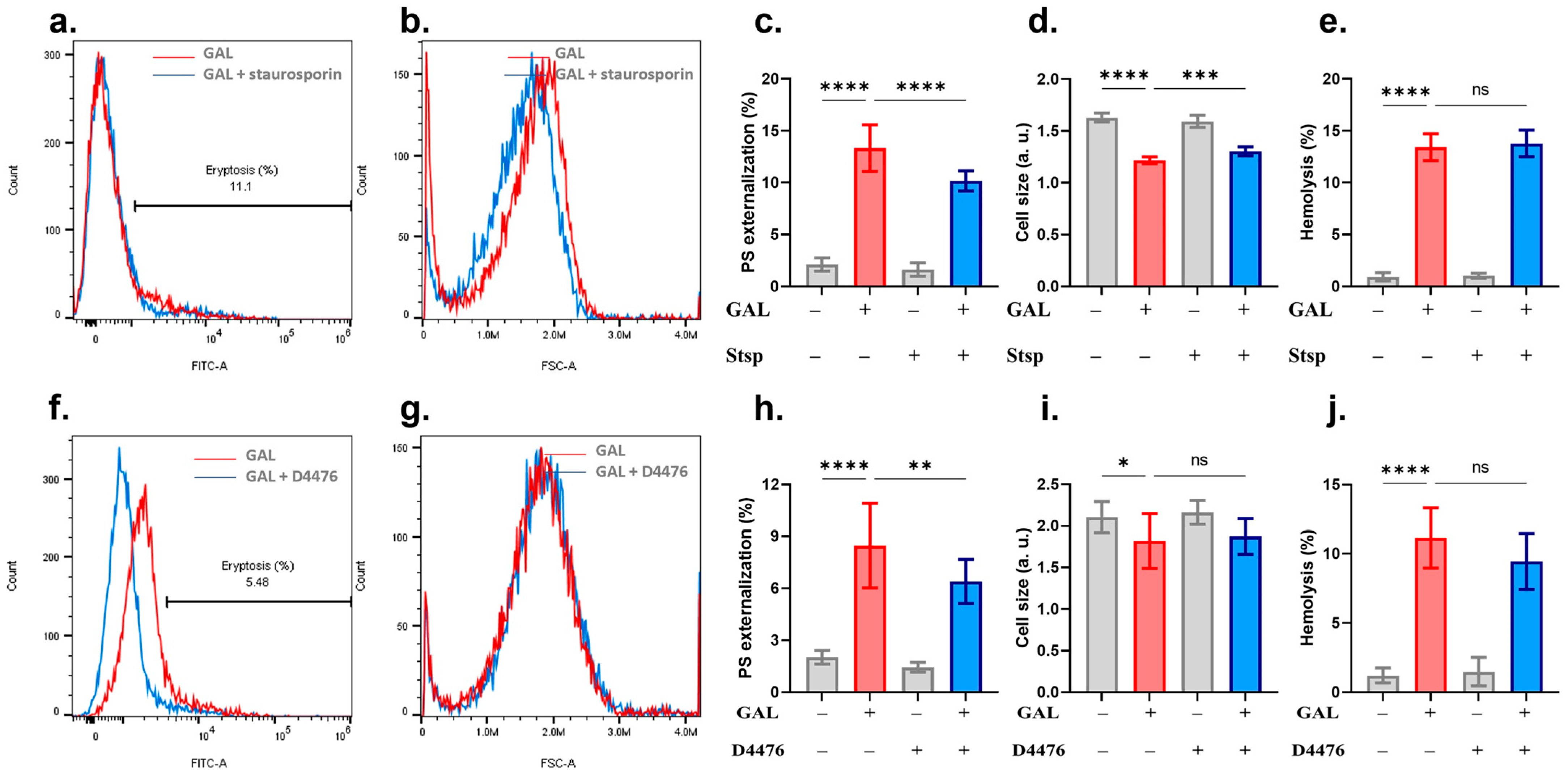
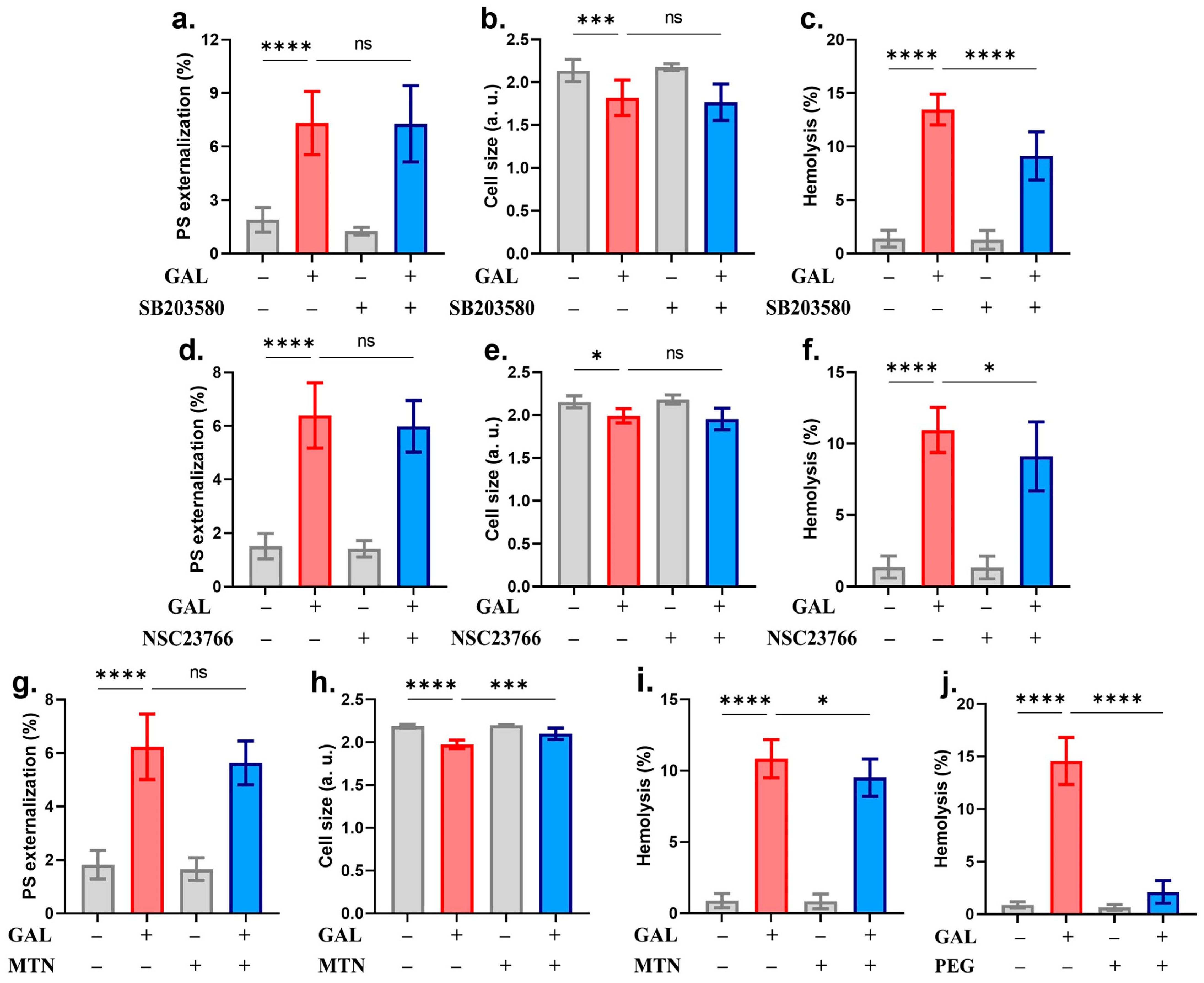
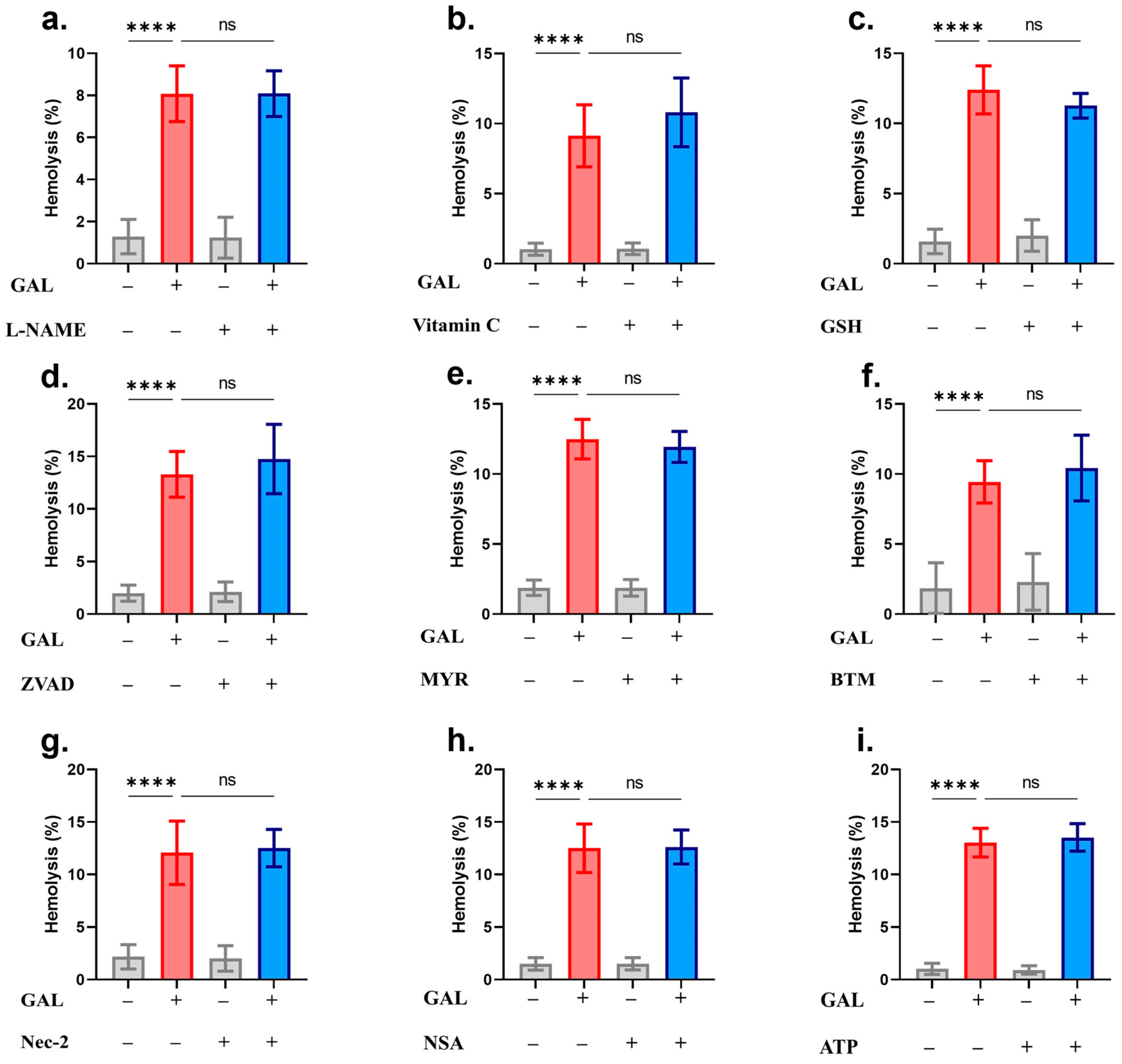
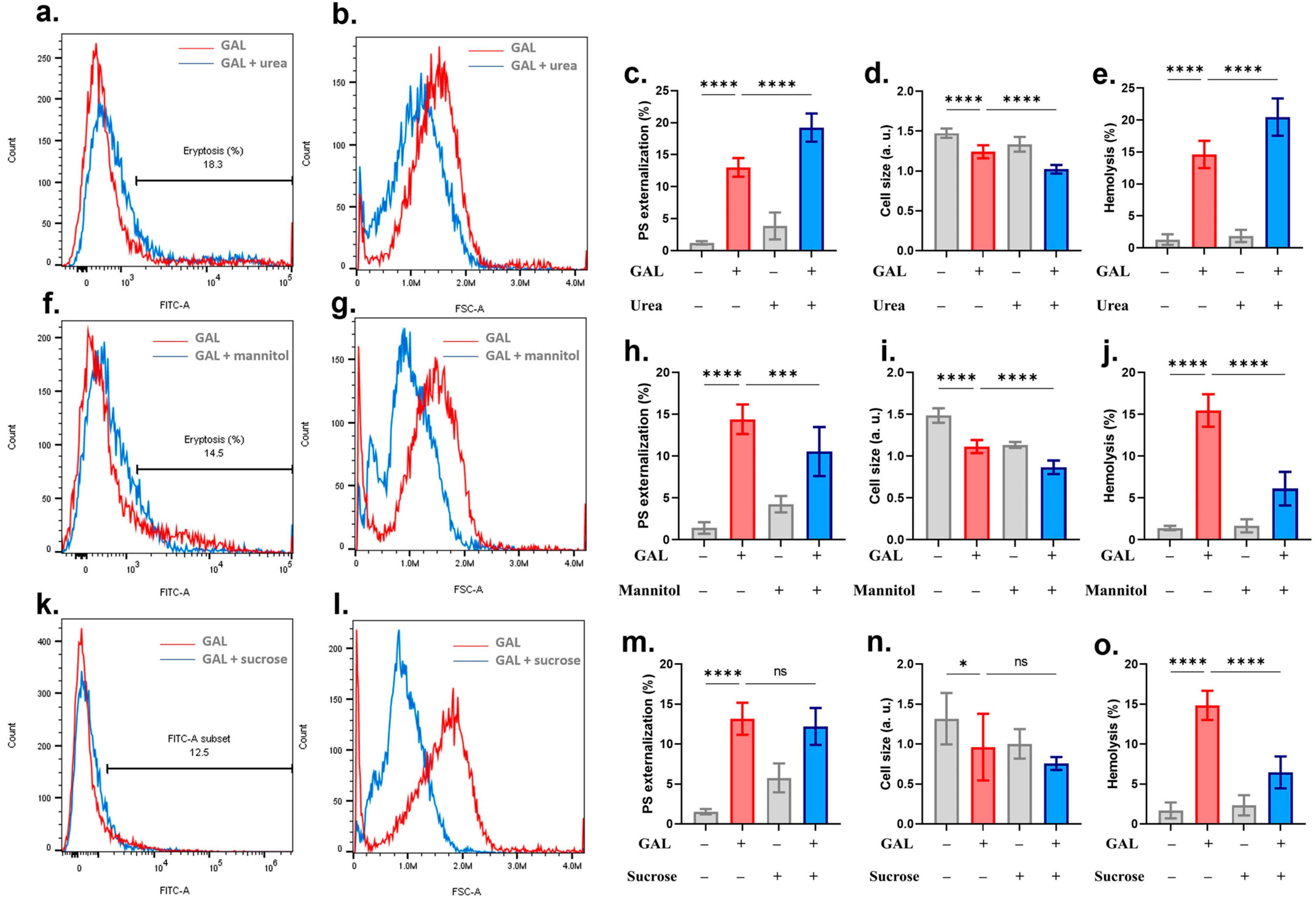
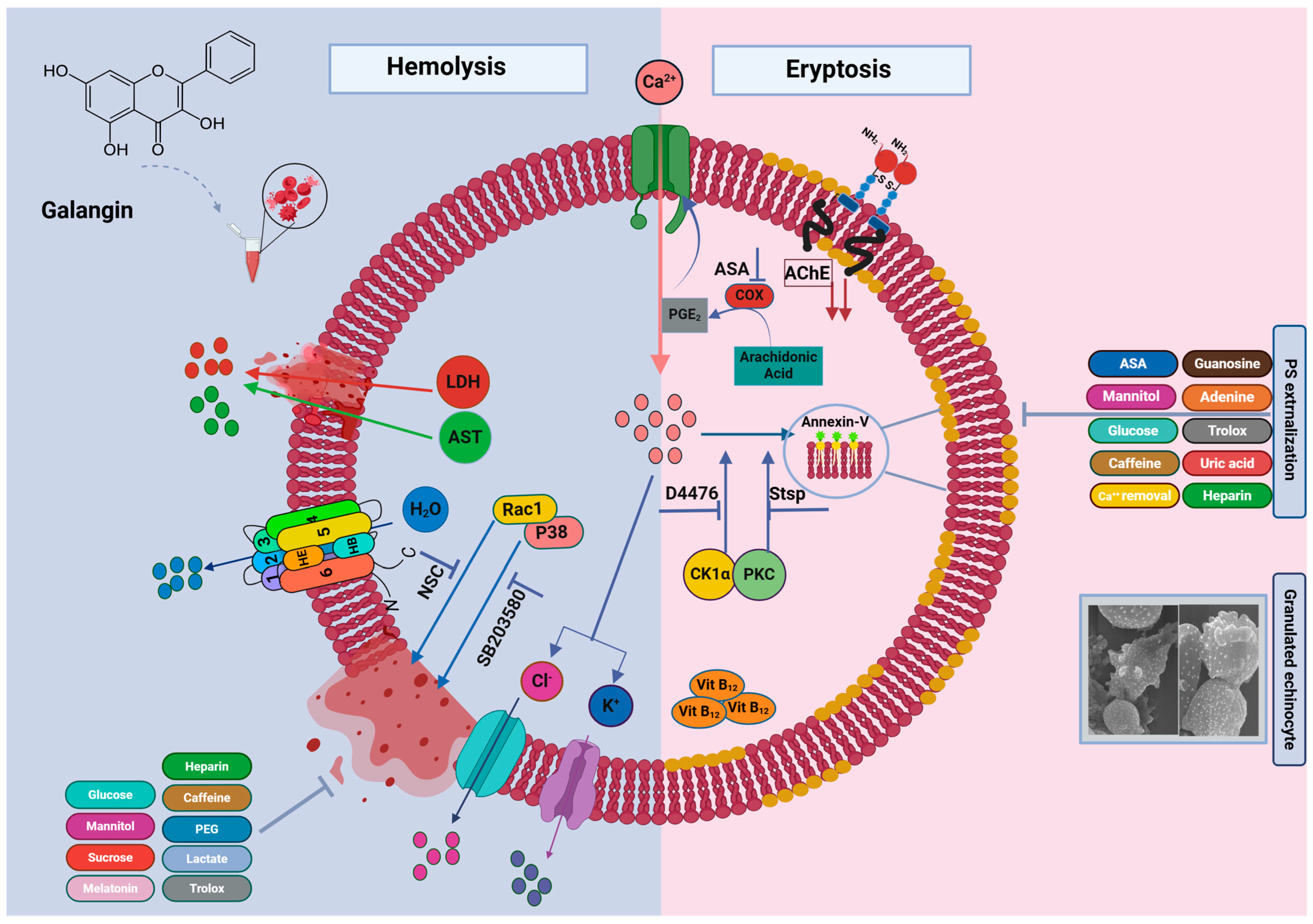
2. Results
3. Discussion
4. Materials and Methods
4.1. Blood Collection
4.2. Chemicals, Reagents, and Experimental Design
4.3. Eryptotic Markers
4.4. Hemolytic Markers
4.5. AChE Activity
4.6. RBC Aggregation
4.7. Scanning Electron Microscopy (SEM)
4.8. B12
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of phytochemicals with cyclodextrin derivatives—An insight. Biomed. Pharmacother. 2017, 88, 1122–1144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Wu, J.; Wen, M.; Su, L.J.; Luo, H. Galangin induces apoptosis in hepatocellular carcinoma cells through the caspase 8/t-Bid mitochondrial pathway. J. Asian Nat. Prod. Res. 2012, 14, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Luo, H.; Wu, J.; Lan, L.B.; Fan, D.H.; Zhu, K.D.; Chen, X.Y.; Wen, M.; Liu, H.M. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J. Gastroenterol. 2010, 16, 3377–3384. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Huang, S.; Liu, D.; Jiang, Z.; Jin, Q.; Li, C.; Da, L.; Yao, Q.; Wang, D. Galangin promotes cell apoptosis through suppression of H19 expression in hepatocellular carcinoma cells. Cancer Med. 2020, 9, 5546–5557. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.A.; Jeon, Y.K.; Nam, M.J. Galangin induces apoptosis in gastric cancer cells via regulation of ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase P. Food Chem. Toxicol. 2012, 50, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, H.; Chen, F.; Fang, J.; Xu, A.; Xi, W.; Zhang, S.; Wu, G.; Wang, Z. Galangin inhibits cell invasion by suppressing the epithelial-mesenchymal transition and inducing apoptosis in renal cell carcinoma. Mol. Med. Rep. 2016, 13, 4238–4244. [Google Scholar] [CrossRef] [PubMed]
- Han, M.A.; Lee, D.H.; Woo, S.M.; Seo, B.R.; Min, K.J.; Kim, S.; Park, J.-W.; Kim, S.H.; Choi, Y.H.; Kwon, T.K. Galangin sensitizes TRAIL-induced apoptosis through down-regulation of anti-apoptotic proteins in renal carcinoma Caki cells. Sci. Rep. 2016, 6, 18642. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; You, P.; Luo, Y.; Yang, M.; Liu, Y. Galangin Induces Apoptosis in MCF-7 Human Breast Cancer Cells Through Mitochondrial Pathway and Phosphatidylinositol 3-Kinase/Akt Inhibition. Pharmacology 2018, 102, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lin, M.L.; Meng, M.; Chen, S.S. Galangin Induces p53-independent S-phase Arrest and Apoptosis in Human Nasopharyngeal Carcinoma Cells Through Inhibiting PI3K-AKT Signaling Pathway. Anticancer Res. 2018, 38, 1377–1389. [Google Scholar] [PubMed]
- Huang, H.; Chen, A.Y.; Ye, X.; Guan, R.; Rankin, G.O.; Chen, Y.C. Galangin, a Flavonoid from Lesser Galangal, Induced Apoptosis via p53-Dependent Pathway in Ovarian Cancer Cells. Molecules 2020, 25, 1579. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Feng, Z.; Chen, A.; Qi, Q.; Han, M.; Wang, S.; Zhang, Y.; Zhang, X.; Yang, N.; Wang, J.; et al. The Natural Flavonoid Galangin Elicits Apoptosis, Pyroptosis, and Autophagy in Glioblastoma. Front. Oncol. 2019, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Chen, L.; Yang, J.; Dong, T.; Cheng, Q.; Wang, W.; Zou, Y.; Su, Y.; Dai, W.; Chen, B.; et al. Network-based Pharmacology and In vitro Validation Reveal that Galangin Induces Apoptosis in Bladder Cancer Cells by Promoting the P53 Signaling Pathway. Anticancer Agents Med. Chem. 2023, 23, 847–857. [Google Scholar] [PubMed]
- Birgegård, G.; Henry, D.; Glaspy, J.; Chopra, R.; Thomsen, L.L.; Auerbach, M. A Randomized Noninferiority Trial of Intravenous Iron Isomaltoside versus Oral Iron Sulfate in Patients with Nonmyeloid Malignancies and Anemia Receiving Chemotherapy: The PROFOUND Trial. Pharmacotherapy 2016, 36, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.; Bissinger, R.; Qadri, S.M.; Lang, F. Suicidal death of erythrocytes in cancer and its chemotherapy: A potential target in the treatment of tumor-associated anemia. Int. J. Cancer 2017, 141, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Bettiol, A.; Galora, S.; Argento, F.R.; Fini, E.; Emmi, G.; Mattioli, I.; Bagni, G.; Fiorillo, C.; Becatti, M. Erythrocyte oxidative stress and thrombosis. Expert Rev. Mol. Med. 2022, 24, e31. [Google Scholar] [CrossRef] [PubMed]
- Dreischer, P.; Duszenko, M.; Stein, J.; Wieder, T. Eryptosis: Programmed Death of Nucleus-Free, Iron-Filled Blood Cells. Cells 2022, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Floride, E.; Föller, M.; Ritter, M.; Lang, F. Caffeine inhibits suicidal erythrocyte death. Cell. Physiol. Biochem. 2008, 22, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.W.; Lau, P.M.; Tsang, H.L.; Ho, H.P.; Kwan, Y.W.; Kong, S.K. Extracellular Histones Induced Eryptotic Death in Human Erythrocytes. Cell. Physiol. Biochem. 2019, 53, 229–241. [Google Scholar] [PubMed]
- Alghareeb, S.A.; Alfhili, M.A.; Fatima, S. Molecular Mechanisms and Pathophysiological Significance of Eryptosis. Int. J. Mol. Sci. 2023, 24, 5079. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Kasahara, A.; Mizuno, S.; Uchikoga, O.; Kuroda, M.; Miyoshi, H.; Shiomi, K.; Umena, S.; Noguchi, T.; Kishimoto, N.; et al. Two cases of acute erythroid leukemia presenting with marked macrocytic anemia, reticulocytosis and hemolysis. Intern. Med. 2013, 52, 1509–1512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pretorius, E.; Du Plooy, J.N.; Bester, J. A Comprehensive Review on Eryptosis. Cell. Physiol. Biochem. 2016, 39, 1977–2000. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.A.; Kempe, D.S.; Myssina, S.; Tanneur, V.; Birka, C.; Laufer, S.; Lang, F.; Wieder, T.; Huber, S.M. PGE2 in the regulation of programmed erythrocyte death. Cell Death Differ. 2005, 12, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.K.F.; Adjobo-Hermans, M.J.W.; Brock, R.; Bosman, G.J.C.G.M. Acetylcholinesterase provides new insights into red blood cell ageing in vivo and in vitro. Blood Transfus. 2017, 15, 232–238. [Google Scholar] [PubMed]
- Abdel-Razeq, H.; Hashem, H. Recent update in the pathogenesis and treatment of chemotherapy and cancer induced anemia. Crit. Rev. Oncol. Hematol. 2020, 145, 102837. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.R.; Scully, M. How I treat microangiopathic hemolytic anemia in patients with cancer. Blood 2021, 137, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Leger, R.M.; Jain, S.; Nester, T.A.; Kaplan, H. Drug-induced immune hemolytic anemia associated with anti-carboplatin and the first example of anti-paclitaxel. Transfusion 2015, 55, 2949–2954. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M. Unveiling the Antioxidative Potential of Galangin: Complete and Detailed Mechanistic Insights through Density Functional Theory Studies. J. Org. Chem. 2024, 89, 8676–8690. [Google Scholar] [CrossRef] [PubMed]
- Viskupicova, J.; Blaskovic, D.; Galiniak, S.; Soszyński, M.; Bartosz, G.; Horakova, L.; Sadowska-Bartosz, I. Effect of high glucose concentrations on human erythrocytes in vitro. Redox Biol. 2015, 5, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Repsold, L.; Joubert, A.M. Eryptosis: An Erythrocyte’s Suicidal Type of Cell Death. Biomed Res. Int. 2018, 2018, 9405617. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, S.K.; Gu, S.; Bobbala, D.; Lang, F. Janus kinase 3 is expressed in erythrocytes, phosphorylated upon energy depletion and involved in the regulation of suicidal erythrocyte death. Cell. Physiol. Biochem. 2011, 27, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Salinas, J.V.; Muñoz-Reyes, E.G.; Guerrero-Romero, J.F.; Rodríguez-Morán, M.; Bracho-Riquelme, R.L.; Carrera-Gracia, M.A.; Quintanar-Escorza, M.A. Eryptosis and oxidative damage in type 2 diabetic mellitus patients with chronic kidney disease. Mol. Cell. Biochem. 2011, 357, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Niemoeller, O.M.; Bentzen, P.J.; Lang, E.; Lang, F. Adenosine protects against suicidal erythrocyte death. Pflugers Arch. 2007, 454, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Ammollo, C.T.; Esmon, N.L.; Esmon, C.T. Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells. J. Thromb. Haemost. 2014, 12, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Hou, G.; Xue, Z.; Zhang, L.; Zhou, Y.; Liu, C.; Liu, Y.; Li, Z. Vitamin E isomer δ-tocopherol enhances the efficiency of neural stem cell differentiation via L-type calcium channel. Neurosci. Lett. 2015, 585, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Zylinska, L.; Lisek, M.; Guo, F.; Boczek, T. Vitamin C Modes of Action in Calcium-Involved Signaling in the Brain. Antioxidants 2023, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Henning, M.; Darley, C.; Fahoum, M.; Schuler, C.B.; Frame, J. Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients 2022, 14, 3934. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, S.; Zhu, J.; Huang, S.; Zhang, A.; Jia, Z.; Ding, G.; Zhang, Y. miR-214 Protects Against Uric Acid-Induced Endothelial Cell Apoptosis. Front. Med. 2020, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Ratto, E.; Villaggio, B.; Parodi, E.L.; Pontremoli, R.; Garibotto, G.; Viazzi, F. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS ONE 2014, 9, e115210. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, J.; Jiao, H.; Oluwabiyi, C.; Li, H.; Zhao, J.; Zhou, Y.; Wang, X.; Lin, H. Enterocyte synthesizes and secrets uric acid as antioxidant to protect against oxidative stress via the involvement of Nrf pathway. Free Radic. Biol. Med. 2022, 179, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Roumeliotis, A.; Dounousi, E.; Eleftheriadis, T.; Liakopoulos, V. Dietary Antioxidant Supplements and Uric Acid in Chronic Kidney Disease: A Review. Nutrients 2019, 11, 1911. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Han, F.; Su, Y.; Sun, B.; Zhao, W.; Pan, C. High uric acid aggravates apoptosis of lung epithelial cells induced by cigarette smoke extract through downregulating PRDX2 in chronic obstructive pulmonary disease. Int. Immunopharmacol. 2023, 118, 110056. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun Bhuyan, A.; Ashiqul Haque, A.K.M.; Sahu, I.; Cao, H.; Kormann, M.S.D.; Lang, F. Inhibition of Suicidal Erythrocyte Death by Volasertib. Cell. Physiol. Biochem. 2017, 43, 1472–1486. [Google Scholar] [CrossRef] [PubMed]
- Jemaà, M.; Fezai, M.; Lang, F. Inhibition of Suicidal Erythrocyte Death by Reversine. Cell. Physiol. Biochem. 2017, 41, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.; Onishchenko, A. Casein kinase 1α mediates eryptosis: A review. Apoptosis 2023, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Klarl, B.A.; Lang, P.A.; Kempe, D.S.; Niemoeller, O.M.; Akel, A.; Sobiesiak, M.; Eisele, K.; Podolski, M.; Huber, S.M.; Wieder, T.; et al. Protein kinase C mediates erythrocyte “programmed cell death” following glucose depletion. Am. J. Physiol. Cell Physiol. 2006, 290, C244–C253. [Google Scholar] [CrossRef] [PubMed]
- Hazegh, K.; Fang, F.; Kelly, K.; Sinchar, D.; Wang, L.; Zuchelkowski, B.E.; Ufelle, A.C.; Esparza, O.; Davizon-Castillo, P.; Page, G.P.; et al. Erythrocyte mitogen-activated protein kinases mediate hemolytic events under osmotic and oxidative stress and in hemolytic diseases. Cell. Signal. 2022, 99, 110450. [Google Scholar] [CrossRef] [PubMed]
- Gatidis, S.; Zelenak, C.; Fajol, A.; Lang, E.; Jilani, K.; Michael, D.; Qadri, S.M.; Lang, F. p38 MAPK activation and function following osmotic shock of erythrocytes. Cell. Physiol. Biochem. 2011, 28, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Alsughayyir, J. Bufalin reprograms erythrocyte lifespan through p38 MAPK and Rac1 GTPase. Toxicon 2024, 240, 107636. [Google Scholar] [CrossRef] [PubMed]
- Restivo, I.; Attanzio, A.; Giardina, I.C.; Di Gaudio, F.; Tesoriere, L.; Allegra, M. Cigarette Smoke Extract Induces p38 MAPK-Initiated, Fas-Mediated Eryptosis. Int. J. Mol. Sci. 2022, 23, 14730. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, K.; Alktifan, B.; Oswald, G.; Fezai, M.; Abed, M.; Lang, F. Breakdown of phosphatidylserine asymmetry following treatment of erythrocytes with lumefantrine. Toxins 2014, 6, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Attanzio, A.; Frazzitta, A.; Cilla, A.; Livrea, M.A.; Tesoriere, L.; Allegra, M. 7-Keto-Cholesterol and Cholestan-3beta, 5alpha, 6beta-Triol Induce Eryptosis through Distinct Pathways Leading to NADPH Oxidase and Nitric Oxide Synthase Activation. Cell. Physiol. Biochem. 2019, 53, 933–947. [Google Scholar] [PubMed]
- Lang, E.; Bissinger, R.; Gulbins, E.; Lang, F. Ceramide in the regulation of eryptosis, the suicidal erythrocyte death. Apoptosis 2015, 20, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zheng, X.; Zhang, Y.; Li, X.; Chen, X.; Yin, Y.; Hu, J.; Li, J.; Guo, M.; Wang, X. What Should Be Responsible for Eryptosis in Chronic Kidney Disease? Kidney Blood Press. Res. 2022, 47, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.S.; Myssina, S.; Lang, P.A.; Tanneur, V.; Kempe, D.S.; Mack, A.F.; Huber, S.M.; Wieder, T.; Lang, F.; Duranton, C. Inhibition of erythrocyte phosphatidylserine exposure by urea and Cl−. Am. J. Physiol. Renal Physiol. 2004, 286, F1046–F1053. [Google Scholar] [CrossRef] [PubMed]
- Virzì, G.M.; Mattiotti, M.; Clementi, A.; Milan Manani, S.; Battaglia, G.G.; Ronco, C.; Zanella, M. In Vitro Induction of Eryptosis by Uremic Toxins and Inflammation Mediators in Healthy Red Blood Cells. J. Clin. Med. 2022, 11, 5329. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, R.L.; Sran, A.; Healey, G.; Veale, M.F.; Norris, P.J. In vitro measures of membrane changes reveal differences between red blood cells stored in saline-adenine-glucose-mannitol and AS-1 additive solutions: A paired study. Transfusion 2014, 54, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Lee, M.H. Flow Cytofluorometric Analysis of Molecular Mechanisms of Premature Red Blood Cell Death. Methods Mol. Biol. 2021, 2326, 155–165. [Google Scholar] [PubMed]
- Jemaà, M.; Fezai, M.; Bissinger, R.; Lang, F. Methods Employed in Cytofluorometric Assessment of Eryptosis, the Suicidal Erythrocyte Death. Cell. Physiol. Biochem. 2017, 43, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Zhbanov, A.; Yang, S. Effects of Aggregation on Blood Sedimentation and Conductivity. PLoS ONE 2015, 10, e0129337. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. 2013, e50166. [Google Scholar] [CrossRef] [PubMed]




| Inhibitor | PS Externalization | Cell Shrinkage | Hemolysis |
|---|---|---|---|
| Glucose | + | + | + |
| Trolox | + | + | + |
| Caffeine | + | + | + |
| Heparin | + | + | + |
| Ca2+ removal | + | + | − |
| Adenine | + | + | − |
| Guanosine | + | + | − |
| Uric acid | + | + | − |
| Staurosporin | + | + | − |
| Mannitol | + | − | + |
| Lactate | − | + | + |
| Melatonin | − | + | + |
| Acetylsalicylic acid | + | − | − |
| D4476 | + | − | − |
| Sucrose | − | − | + |
| Polyethylene glycol 8000 | − | − | + |
| SB203580 | − | − | + |
| NSC23766 | − | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfhili, M.A.; Alghareeb, S.A.; Alotaibi, G.A.; Alsughayyir, J. Galangin Triggers Eryptosis and Hemolysis Through Ca2+ Nucleation and Metabolic Collapse Mediated by PKC/CK1α/COX/p38/Rac1 Signaling Axis. Int. J. Mol. Sci. 2024, 25, 12267. https://doi.org/10.3390/ijms252212267
Alfhili MA, Alghareeb SA, Alotaibi GA, Alsughayyir J. Galangin Triggers Eryptosis and Hemolysis Through Ca2+ Nucleation and Metabolic Collapse Mediated by PKC/CK1α/COX/p38/Rac1 Signaling Axis. International Journal of Molecular Sciences. 2024; 25(22):12267. https://doi.org/10.3390/ijms252212267
Chicago/Turabian StyleAlfhili, Mohammad A., Sumiah A. Alghareeb, Ghada A. Alotaibi, and Jawaher Alsughayyir. 2024. "Galangin Triggers Eryptosis and Hemolysis Through Ca2+ Nucleation and Metabolic Collapse Mediated by PKC/CK1α/COX/p38/Rac1 Signaling Axis" International Journal of Molecular Sciences 25, no. 22: 12267. https://doi.org/10.3390/ijms252212267
APA StyleAlfhili, M. A., Alghareeb, S. A., Alotaibi, G. A., & Alsughayyir, J. (2024). Galangin Triggers Eryptosis and Hemolysis Through Ca2+ Nucleation and Metabolic Collapse Mediated by PKC/CK1α/COX/p38/Rac1 Signaling Axis. International Journal of Molecular Sciences, 25(22), 12267. https://doi.org/10.3390/ijms252212267







