Gyrophoric Acid, a Secondary Metabolite of Lichens, Exhibits Antidepressant and Anxiolytic Activity In Vivo in Wistar Rats
Abstract
1. Introduction

2. Results
2.1. Animal Behavior Analysis
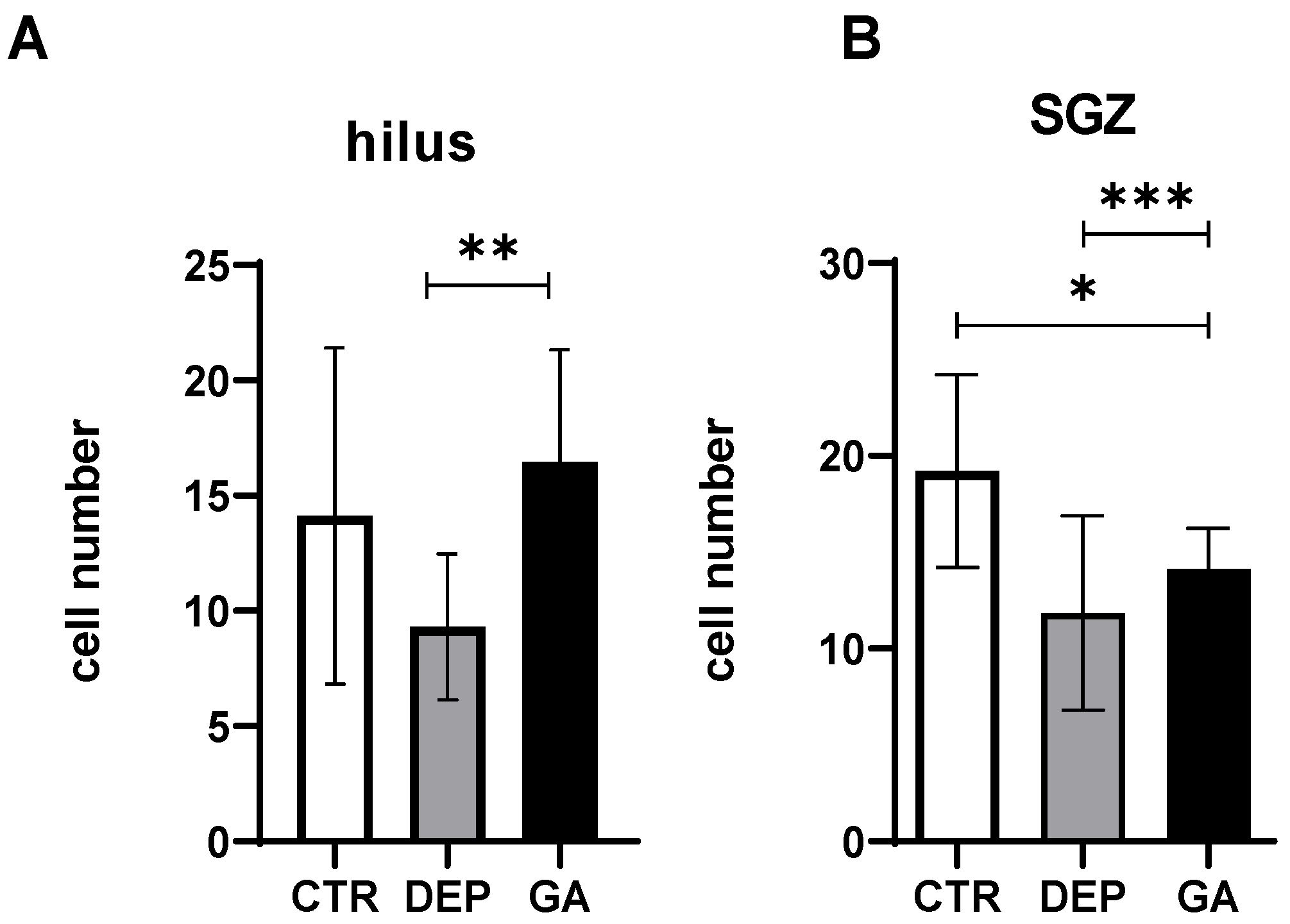
2.2. The Level of Hippocampal Neurogenesis
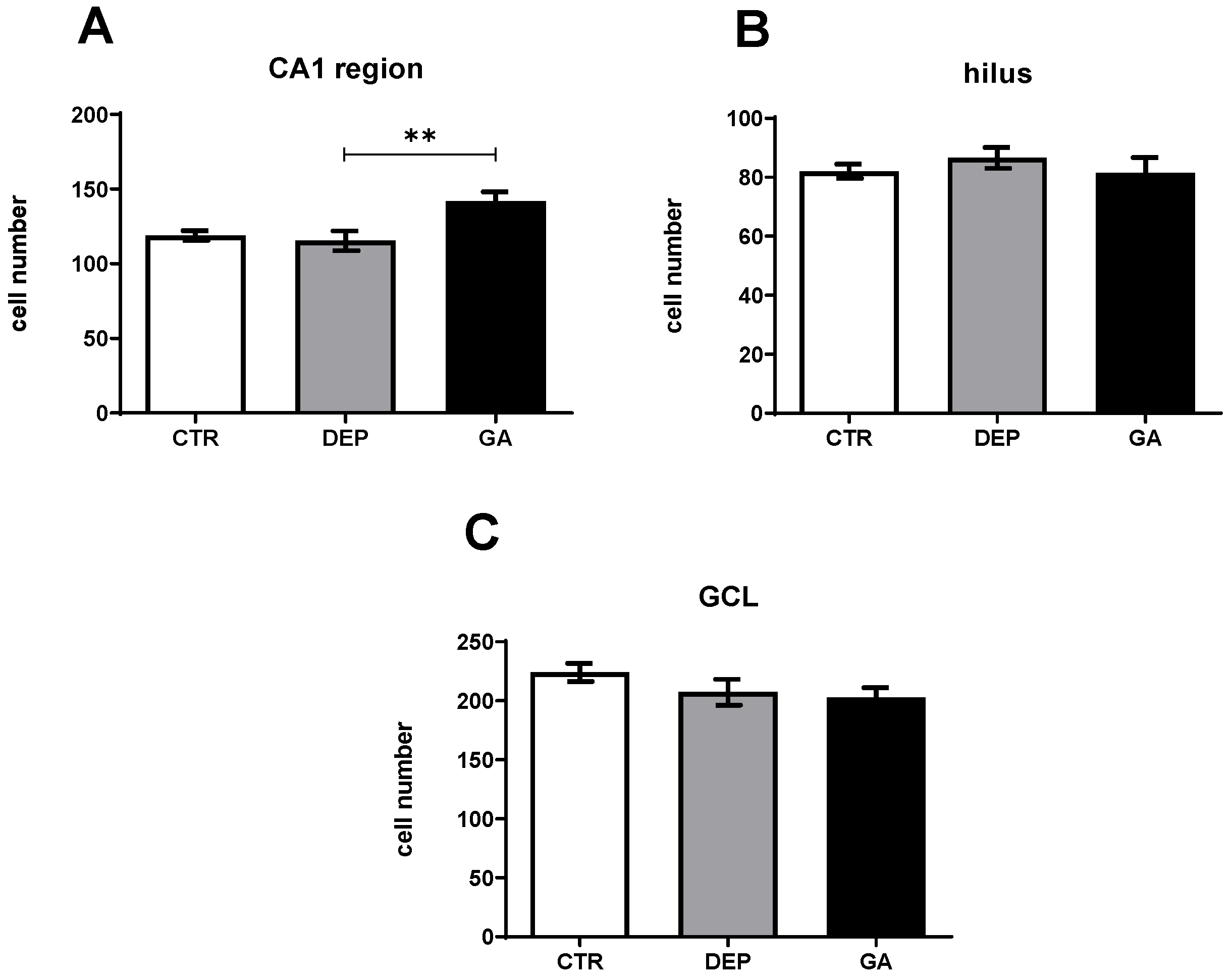
2.3. Analysis of the Number of Neun-Positive Mature Neurons
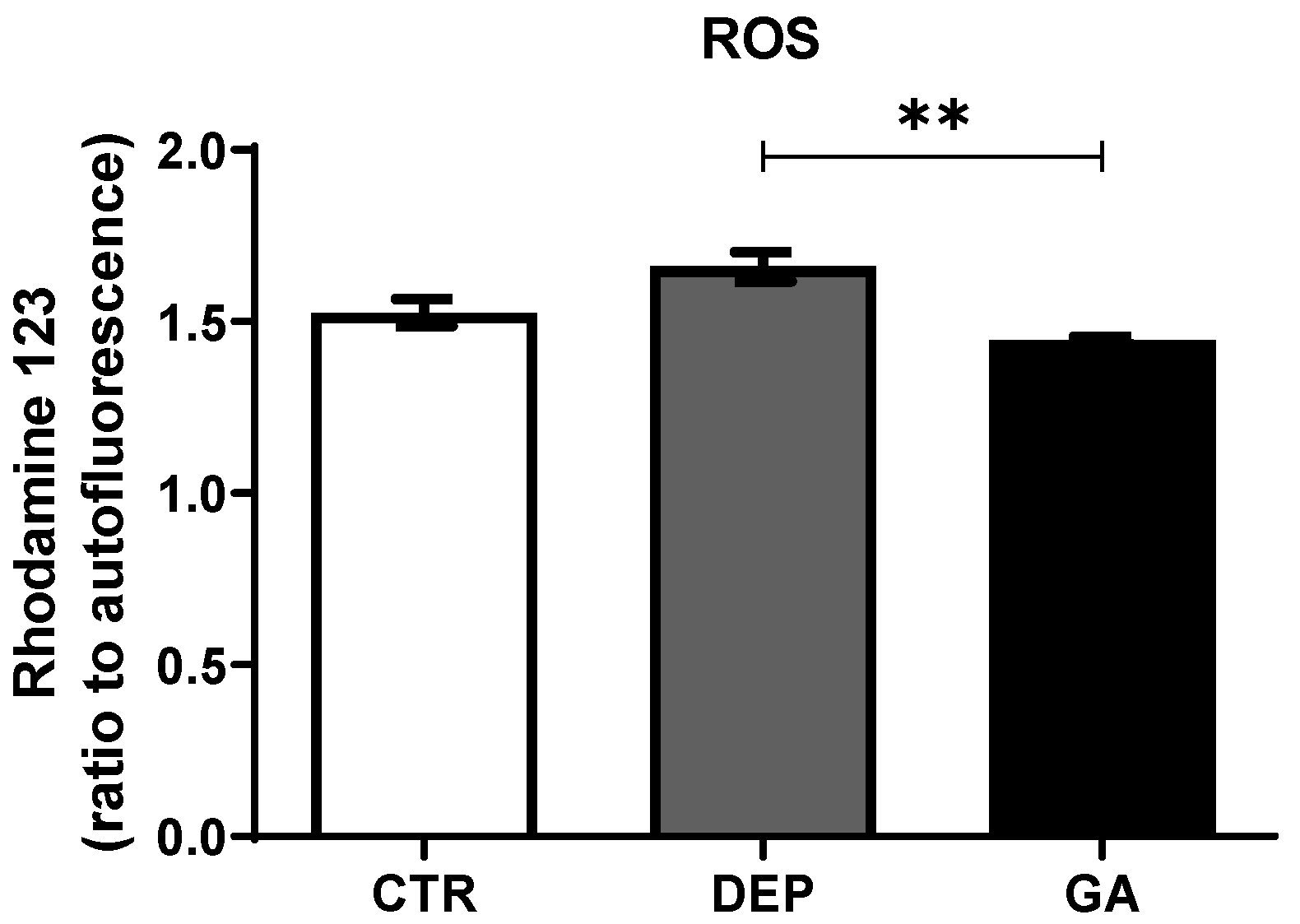
2.4. Reactive Oxygen Species Levels in Leukocytes
3. Discussion
3.1. Animal Behavior Analysis
3.2. Analysis of the Number of Proliferative Ki67 Cells and Mature Neun-Positive Neurons
3.3. Reactive Oxygen Species Levels in Leukocytes
4. Materials and Methods
4.1. Experimental Design and Laboratory Animals
4.2. Induction of Depression-like Behavior in Animals
4.3. Elevated Plus Maze Test
4.4. Collection of Blood
4.5. Assessment of Reactive Oxygen Species in Leukocytes
4.6. Immunohistochemical Staining of Ki-67-Positive Cells in the Hippocampus
4.7. Immunohistochemical Staining of Neun-Positive Mature Neurons in the Hippocampus
4.8. Assessment of Cell Number
4.9. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javaid, S.F.; Hashim, I.J.; Hashim, M.J.; Stip, E.; Samad, M.A.; Ahbabi, A.A. Epidemiology of anxiety disorders: Global burden and sociodemographic associations. Middle East Curr. Psychiatry 2023, 30, 44. [Google Scholar] [CrossRef]
- Ströhle, A.; Gensichen, J.; Domschke, K. The diagnosis and treatment of anxiety disorders. Dtsch. Ärzteblatt Int. 2018, 115, 611. [Google Scholar] [CrossRef] [PubMed]
- Tham, W.W.; Sojli, E.; Bryant, R.; McAleer, M. Common mental disorders and economic uncertainty: Evidence from the COVID-19 pandemic in the US. PLoS ONE 2021, 16, e0260726. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, H.; Sohrabi, M.; Yousefi, M.; Boustie, J. Tridepsides as potential bioactives: A review on their chemistry and the global distribution of their lichenic and non-lichenic natural sources. Front. Fungal Biol. 2023, 4, 1088966. [Google Scholar] [CrossRef] [PubMed]
- Divakar, P.K.; Crespo, A.; Kraichak, E.; Leavitt, S.D.; Singh, G.; Schmitt, I.; Lumbsch, H.T. Using a temporal phylogenetic method to harmonize family-and genus-level classification in the largest clade of lichen-forming fungi. Fungal Divers. 2017, 84, 101–117. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; González-Burgos, E.; De Vita, S.; Divakar, P.K.; Bifulco, G.; Gomez-Serranillos, M.P. Phytochemical Characterization and Pharmacological Properties of Lichen Extracts from Cetrarioid Clade by Multivariate Analysis and Molecular Docking. Evid.-Based Complement. Altern. Med. 2022, 2022, 5218248. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen extracts from Cetrarioid clade provide neuroprotection against hydrogen peroxide-induced oxidative stress. Molecules 2022, 27, 6520. [Google Scholar] [CrossRef]
- Rather, L.J.; Mir, S.S.; Ganie, S.A.; Islam, S.-U.; Li, Q. Research progress, challenges, and perspectives in microbial pigment production for industrial applications—A review. Dye. Pigment. 2022, 210, 110989. [Google Scholar] [CrossRef]
- Torres, J.M.; Torres, V.d.O.; Rodrigues, A.S.; Gianini, A.S.; Micheletti, A.C.; Honda, N.K.; Spielmann, A.A.; Lorenz, A.P. Lineages of the lichen-forming fungus Stereocaulon alpinum and their photobionts in southern South America and maritime Antarctica. Polar Biol. 2023, 46, 865–879. [Google Scholar] [CrossRef]
- Poulsen-Silva, E.; Gordillo-Fuenzalida, F.; Atala, C.; Moreno, A.A.; Otero, M.C. Bioactive lichen secondary metabolites and their presence in species from Chile. Metabolites 2023, 13, 805. [Google Scholar] [CrossRef]
- Xie, C.-M.; Wang, L.-S.; Zhao, Z.-T.; Zhang, Y.-Y.; Wang, X.-Y.; Zhang, L.-L. Revision of Immersaria and a new lecanorine genus in Lecideaceae (lichenised Ascomycota, Lecanoromycetes). MycoKeys 2022, 87, 99. [Google Scholar] [CrossRef] [PubMed]
- Lendemer, J.C. Studies in Lichens and Lichenicolous Fungi–No. 23: Notes on Appalachian taxa including newly reported disjunctions and multiple species new to North America. Opusc. Philolichenum 2023, 22, 81–97. [Google Scholar] [CrossRef]
- Printzen, C.; Holien, H.; Kantelinen, A.; Myllys, L.; Ratschow, F.; Stepanchikova, I.; Weber, L.; Timdal, E. DNA barcoding indicates the presence of unrecognized species and phylogenetic diversity within the Biatora vernalis-and B. meiocarpa-groups. Plant Fungal Syst. 2023, 68, 262–279. [Google Scholar] [CrossRef]
- Sharma, B.; Fatima, S.; Rajeshkumar, K.C.; Škaloud, P.; Divakar, P.K.; Gaikwad, S.; Ansil, P.A.; Mohan, A.S.; Sequeira, S.Y. Molecular studies of Flavopunctelia and Punctelia species and their Trebouxia photobiont from the Himalayas, India. Mycotaxon 2023, 137, 853–869. [Google Scholar] [CrossRef]
- Palice, Z.; Svoboda, S.; Vondrák, J. Hidden in the dark under umbrellas: Two new Psilolechia species (lichenized Ascomycota, Lecanorales) described from the Czech Republic. Plant Fungal Syst. 2023, 68, 285–293. [Google Scholar] [CrossRef]
- Aptroot, A.; da Silva Cáceres, M.E.; dos Santos, L.A. The taxonomy of sterile Arthoniaceae from Brazil: White crusts on overhanging tropical trees can be named. Lichenologist 2024, 56, 1–13. [Google Scholar] [CrossRef]
- Axenov-Gibanov, D.V.; Voytsekhovskaya, I.V.; Tokovenko, B.T.; Protasov, E.S.; Gamaiunov, S.V.; Rebets, Y.V.; Luzhetskyy, A.N.; Timofeyev, M.A. Actinobacteria isolated from an underground lake and moonmilk speleothem from the biggest conglomeratic karstic cave in Siberia as sources of novel biologically active compounds. PLoS ONE 2016, 11, e0149216. [Google Scholar] [CrossRef]
- Inokoshi, J.; Takagi, Y.; Uchida, R.; Masuma, R.; Ōmura, S.; Tomoda, H. Production of a new type of amidepsine with a sugar moiety by static fermentation of Humicola sp. FO-2942. J. Antibiot. 2010, 63, 9–16. [Google Scholar] [CrossRef]
- Canter, F.W.; Robertson, A.; Waters, R.B. 123. Lichen acids. Part V. A synthesis of methyl O-tetramethylgyrophorate. J. Chem. Soc. (Resumed) 1933, 493–495. [Google Scholar] [CrossRef]
- Manojlovic, N.T.; Vasiljevic, P.J.; Maskovic, P.Z.; Juskovic, M.; Bogdanovic-Dusanovic, G. Chemical composition, antioxidant, and antimicrobial activities of lichen Umbilicaria cylindrica (L.) Delise (Umbilicariaceae). Evid.-Based Complement. Altern. Med. 2012, 2012, 452431. [Google Scholar] [CrossRef]
- Singh, G.; Calchera, A.; Merges, D.; Valim, H.; Otte, J.; Schmitt, I.; Dal Grande, F. A candidate gene cluster for the bioactive natural product gyrophoric acid in lichen-forming fungi. Microbiol. Spectr. 2022, 10, e00109-22. [Google Scholar] [CrossRef] [PubMed]
- Buçukoglu, T.Z.; Albayrak, S.; Halici, M.G.; Tay, T. Antimicrobial and Antioxidant Activities of Extracts and Lichen Acids Obtained from Some U mbilicaria Species from C entral A natolia, T urkey. J. Food Process. Preserv. 2013, 37, 1103–1110. [Google Scholar] [CrossRef]
- Candan, M.; Yılmaz, M.; Tay, T.; Kıvança, M.; Türk, H. Antimicrobial activity of extracts of the lichen Xanthoparmelia pokornyi and its gyrophoric and stenosporic acid constituents. Z. Für Naturforschung C 2006, 61, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Furmanek, Ł.; Seaward, M.R. Anti-yeast potential of lichen-extracted substances—An analytical review. S. Afr. J. Bot. 2023, 161, 720–779. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Van Praag, H.; Gage, F. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol. Psychiatry 2000, 5, 262–269. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B. Antioxidant and antimicrobial properties of some lichens and their constituents. J. Med. Food 2011, 14, 1624–1630. [Google Scholar] [CrossRef]
- Madrigal, J.; Caso, J.; De Cristobal, J.; Cardenas, A.; Leza, J.; Lizasoain, I.; Lorenzo, P.; Moro, M. Effect of subacute and chronic immobilisation stress on the outcome of permanent focal cerebral ischaemia in rats. Brain Res. 2003, 979, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Ranković, B.; Mišić, M.; Sukdolak, S. The antimicrobial activity of substances derived from the lichens Physcia aipolia, Umbilicaria polyphylla, Parmelia caperata and Hypogymnia physodes. World J. Microbiol. Biotechnol. 2008, 24, 1239–1242. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.P.; Sahu, N.; Jaiswal, J.; Kumari, N.; Singh, P.R.; Sinha, R.P. Bioprospecting and Evolutionary Significance of Photoprotectors in Non-flowering Lower Plants. In Photoprotective Green Pharmacology: Challenges, Sources and Future Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 101–140. [Google Scholar]
- Lohézic-Le Dévéhat, F.; Legouin, B.; Couteau, C.; Boustie, J.; Coiffard, L. Lichenic extracts and metabolites as UV filters. J. Photochem. Photobiol. B Biol. 2013, 120, 17–28. [Google Scholar] [CrossRef]
- Russo, A.; Piovano, M.; Lombardo, L.; Garbarino, J.; Cardile, V. Lichen metabolites prevent UV light and nitric oxide-mediated plasmid DNA damage and induce apoptosis in human melanoma cells. Life Sci. 2008, 83, 468–474. [Google Scholar] [CrossRef]
- Varol, M.; Türk, A.; Candan, M.; Tay, T.; Koparal, A.T. Photoprotective activity of vulpinic and gyrophoric acids toward ultraviolet B-induced damage in human keratinocytes. Phytother. Res. 2016, 30, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.I.; Ali, M.; Wahab, A.-T.; Khan, A.; Rasheed, S.; Shyaula, S.L.; Rahman, A.-U. New antiglycation and enzyme inhibitors from Parmotrema cooperi. Sci. China Chem. 2011, 54, 1926–1931. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Karunaratne, V. Potential of lichen compounds as antidiabetic agents with antioxidative properties: A review. Oxidative Med. Cell. Longev. 2017, 2017, 2079697. [Google Scholar] [CrossRef] [PubMed]
- Bačkorová, M.; Bačkor, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. Vitr. 2011, 25, 37–44. [Google Scholar] [CrossRef]
- Bačkorová, M.; Jendželovský, R.; Kello, M.; Bačkor, M.; Mikeš, J.; Fedoročko, P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol. Vitr. 2012, 26, 462–468. [Google Scholar] [CrossRef]
- Burlando, B.; Ranzato, E.; Volante, A.; Appendino, G.; Pollastro, F.; Verotta, L. Antiproliferative effects on tumour cells and promotion of keratinocyte wound healing by different lichen compounds. Planta Medica 2009, 75, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Castañeta, G.; Villagomez, R.; Salamanca, E.; Canaviri-Paz, P.; Bravo, J.A.; Vila, J.L.; Bárcenas-Pérez, D.; Cheel, J.; Sepúlveda, B.; Giménez, A. Microwave-Assisted Semisynthesis and Leishmanicidal Activity of Some Phenolic Constituents from Lichens. Separations 2023, 10, 524. [Google Scholar] [CrossRef]
- Kumar KC, S.; Müller, K. Lichen metabolites. 2. Antiproliferative and cytotoxic activity of gyrophoric, usnic, and diffractaic acid on human keratinocyte growth. J. Nat. Prod. 1999, 62, 821–823. [Google Scholar] [CrossRef]
- Elečko, J.; Vilková, M.; Frenák, R.; Routray, D.; Ručová, D.; Bačkor, M.; Goga, M. A comparative study of isolated secondary metabolites from lichens and their antioxidative properties. Plants 2022, 11, 1077. [Google Scholar] [CrossRef]
- Goga, M.; Kello, M.; Vilkova, M.; Petrova, K.; Backor, M.; Adlassnig, W.; Lang, I. Oxidative stress mediated by gyrophoric acid from the lichen Umbilicaria hirsuta affected apoptosis and stress/survival pathways in HeLa cells. BMC Complement. Altern. Med. 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Simko, P.; Leskanicova, A.; Suvakova-Nunhart, M.; Koval, J.; Zidekova, N.; Karasova, M.; Majerova, P.; Verboova, L.; Blicharova, A.; Kertys, M.; et al. The First In Vivo Study Shows That Gyrophoric Acid Changes Behavior of Healthy Laboratory Rats. Int. J. Mol. Sci. 2024, 25, 6782. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Zambare, V.; Suntres, Z.; Christopher, L. Isolation, characterization, and breast cancer cytotoxic activity of gyrophoric acid from the lichen Umbilicaria muhlenbergii. Processes 2022, 10, 1361. [Google Scholar] [CrossRef]
- Sovrlic, M.; Manojlovic, N.; Kosanic, M.; Kocovic, A.; Tomović, J.; Vasiljević, P. Lichenochemical analysis and in vitro antioxidant activity of extracts and gyrophoric acid from lichen Umbilicaria grisea. In Proceedings of the 2nd International Conference on Chemo and BioInformatics, Kragujevac, Serbia, 28–29 September 2023. [Google Scholar]
- Simko, P.; Leskanicova, A.; Suvakova, M.; Blicharova, A.; Karasova, M.; Goga, M.; Kolesarova, M.; Bojkova, B.; Majerova, P.; Zidekova, N. Biochemical Properties of Atranorin-Induced Behavioral and Systematic Changes of Laboratory Rats. Life 2022, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, N.; Simko, P.; Leskanicova, A.; Karasova, M.; Jendzelovska, Z.; Jendzelovsky, R.; Rucova, D.; Kolesarova, M.; Goga, M.; Backor, M. Atranorin, a Secondary Metabolite of Lichens, Exhibited Anxiolytic/Antidepressant Activity in Wistar Rats. Life 2022, 12, 1850. [Google Scholar] [CrossRef] [PubMed]
- Goga, M.; Elečko, J.; Marcinčinová, M.; Ručová, D.; Bačkorová, M.; Bačkor, M. Lichen metabolites: An overview of some secondary metabolites and their biological potential. In Co-Evolution of Secondary Metabolites; Springer: Cham, Switzerland, 2020; pp. 175–209. [Google Scholar]
- Fernández-Moriano, C.; Gómez-Serranillos, M.P.; Crespo, A. Antioxidant potential of lichen species and their secondary metabolites. A systematic review. Pharm. Biol. 2016, 54, 1–17. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of lavender L). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Shtro, A.; Zarubaev, V.; Luzina, O.; Sokolov, D.; Salakhutdinov, N. Derivatives of usnic acid inhibit broad range of influenza viruses and protect mice from lethal influenza infection. Antivir. Chem. Chemother. 2015, 24, 92–98. [Google Scholar] [CrossRef]
- Sokolov, D.N.; Zarubaev, V.V.; Shtro, A.A.; Polovinka, M.P.; Luzina, O.A.; Komarova, N.I.; Salakhutdinov, N.F.; Kiselev, O.I. Anti-viral activity of (−)-and (+)-usnic acids and their derivatives against influenza virus A (H1N1) 2009. Bioorganic Med. Chem. Lett. 2012, 22, 7060–7064. [Google Scholar] [CrossRef]
- Gupta, A.; Sahu, N.; Singh, A.P.; Singh, V.K.; Singh, S.C.; Upadhye, V.J.; Mathew, A.T.; Kumar, R.; Sinha, R.P. Exploration of novel lichen compounds as inhibitors of SARS-CoV-2 Mpro: Ligand-based design, molecular dynamics, and ADMET analyses. Appl. Biochem. Biotechnol. 2022, 194, 6386–6406. [Google Scholar] [CrossRef]
- Krishnan, R.; Greeshma, G.; Lawarence, B.; Murugan, K. Insight of Bioresources from Lower Plant Groups: Reconciling the Possibilities and Responsibilities. In Conservation and Sustainable Utilization of Bioresources; Springer: Berlin/Heidelberg, Germany, 2023; pp. 59–77. [Google Scholar]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Kalin, N.H. The critical relationship between anxiety and depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.; Meerlo, P.; De Boer, S.; Strubbe, J.; Bohus, B. Social stress in rats: An animal model of depression? Acta Neuropsychiatr. 1995, 7, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.; Cao, B.-J.; Dalvi, A.; Holmes, A. Animal models of anxiety: An ethological perspective. Braz. J. Med. Biol. Res. 1997, 30, 289–304. [Google Scholar] [CrossRef]
- Smith, R.; Taylor, S.; Wilson, R.C.; Chuning, A.E.; Persich, M.R.; Wang, S.; Killgore, W.D. Lower levels of directed exploration and reflective thinking are associated with greater anxiety and depression. Front. Psychiatry 2022, 12, 782136. [Google Scholar] [CrossRef] [PubMed]
- Salgado, F.; Caballero, J.; Vargas, R.; Cornejo, A.; Areche, C. Continental and Antarctic Lichens: Isolation, identification and molecular modeling of the depside tenuiorin from the Antarctic lichen Umbilicaria antarctica as tau protein inhibitor. Nat. Prod. Res. 2020, 34, 646–650. [Google Scholar] [CrossRef]
- Cazarin, C.A.; Dalmagro, A.P.; Gonçalves, A.E.; Boeing, T.; da Silva, L.M.; Corrêa, R.; Klein-Júnior, L.C.; Pinto, B.C.; Lorenzett, T.S.; da Costa Sobrinho, T.U. Usnic acid enantiomers restore cognitive deficits and neurochemical alterations induced by Aβ1–42 in mice. Behav. Brain Res. 2021, 397, 112945. [Google Scholar] [CrossRef]
- Johnson, A.; Varberg, Z.; Benhardus, J.; Maahs, A.; Schrater, P. The hippocampus and exploration: Dynamically evolving behavior and neural representations. Front. Hum. Neurosci. 2012, 6, 216. [Google Scholar] [CrossRef]
- Pedro, A.; Monteiro, J.; Silva, A.J. Perspective Chapter: Role of the Hippocampal Formation in Navigation from a Simultaneous Location and Mapping Perspective. In Hippocampus-More Than Just Memory; IntechOpen: London, UK, 2023. [Google Scholar]
- Miller, B.R.; Hen, R. The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 2015, 30, 51–58. [Google Scholar] [CrossRef]
- Coe, C.L.; Kramer, M.; Czéh, B.; Gould, E.; Reeves, A.J.; Kirschbaum, C.; Fuchs, E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol. Psychiatry 2003, 54, 1025–1034. [Google Scholar] [CrossRef]
- Lucassen, P.; Bosch, O.; Jousma, E.; Krömer, S.; Andrew, R.; Seckl, J.; Neumann, I. Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but not low, anxiety: Possible key role of placental 11β-hydroxysteroid dehydrogenase type 2. Eur. J. Neurosci. 2009, 29, 97–103. [Google Scholar] [CrossRef]
- García-Martinez, Y.; Sánchez-Huerta, K.B.; Pacheco-Rosado, J. Quantitative characterization of proliferative cells subpopulations in the hilus of the hippocampus of adult Wistar rats: An integrative study. J. Mol. Histol. 2020, 51, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; McEwen, B.S.; Tanapat, P.; Galea, L.A.; Fuchs, E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997, 17, 2492–2498. [Google Scholar] [CrossRef]
- Czéh, B.; Welt, T.; Fischer, A.K.; Erhardt, A.; Schmitt, W.; Müller, M.B.; Toschi, N.; Fuchs, E.; Keck, M.E. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: Effects on stress hormone levels and adult hippocampal neurogenesis. Biol. Psychiatry 2002, 52, 1057–1065. [Google Scholar] [CrossRef]
- Podgorny, O.V.; Gulyaeva, N.V. Glucocorticoid-mediated mechanisms of hippocampal damage: Contribution of subgranular neurogenesis. J. Neurochem. 2021, 157, 370–392. [Google Scholar] [CrossRef] [PubMed]
- Cameron, H.A.; Woolley, C.S.; Gould, E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993, 611, 342–346. [Google Scholar] [CrossRef]
- Armanini, M.P.; Hutchins, C.; Stein, B.A.; Sapolsky, R.M. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res. 1990, 532, 7–12. [Google Scholar] [CrossRef]
- Cameron, H.A.; McEwen, B.S.; Gould, E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J. Neurosci. 1995, 15, 4687–4692. [Google Scholar] [CrossRef] [PubMed]
- Gulyaeva, N. Glucocorticoid regulation of the glutamatergic synapse: Mechanisms of stress-dependent neuroplasticity. J. Evol. Biochem. Physiol. 2021, 57, 564–576. [Google Scholar] [CrossRef]
- Bartsch, T. The hippocampus in neurological disease. In The Clinical Neurobiology of the Hippocampus: An Integrative View; Oxford University Press: Oxford, UK, 2012; p. 200. [Google Scholar]
- Seki, T.; Hori, T.; Miyata, H.; Maehara, M.; Namba, T. Analysis of proliferating neuronal progenitors and immature neurons in the human hippocampus surgically removed from control and epileptic patients. Sci. Rep. 2019, 9, 18194. [Google Scholar] [CrossRef]
- Bond, A.M.; Ming, G.-l.; Song, H. What is the relationship between hippocampal neurogenesis across different stages of the lifespan? Front. Neurosci. 2022, 16, 716. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; González-Burgos, E.; Divakar, P.K.; Gómez-Serranillos, M.P. Lichen depsides and tridepsides: Progress in pharmacological approaches. J. Fungi 2023, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.G.; Veeraval, L.; Maitra, S.; Chollet-Krugler, M.; Tomasi, S.; Lohezic-Le Devehat, F.; Boustie, J.; Chakravarty, S. Lichen-derived compounds show potential for central nervous system therapeutics. Phytomedicine 2016, 23, 1527–1534. [Google Scholar] [CrossRef]
- Porter, G.A.; O’Connor, J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. Psychiatry 2022, 12, 77. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The role of BDNF on neural plasticity in depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Matsuzaki, K.; Islam, R.; Hossain, S.; Hossain, M.E.; Katakura, M.; Arai, H.; Shido, O.; Hashimoto, M. Chronic administration of thymoquinone enhances adult hippocampal neurogenesis and improves memory in rats via regulating the BDNF signaling pathway. Neurochem. Res. 2022, 47, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Yuan, Z.; Yu, J.; Fu, X.; Deng, X.; Fu, Q.; Ma, Z.; Ma, S. Saikosaponin-d impedes hippocampal neurogenesis and causes cognitive deficits by inhibiting the survival of neural stem/progenitor cells via neurotrophin receptor signaling in mice. Clin. Transl. Med. 2020, 10, e243. [Google Scholar] [CrossRef]
- Sairanen, M.; Lucas, G.; Ernfors, P.; Castrén, M.; Castrén, E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J. Neurosci. 2005, 25, 1089–1094. [Google Scholar] [CrossRef]
- Katoh, R.S.; Asano, T.; Ueda, H.; Morishita, R.; Takeuchi, I.K.; Inaguma, Y.; Kato, K. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 2002, 16, 1328–1330. [Google Scholar] [CrossRef]
- Scharfman, H.; Goodman, J.; Macleod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef]
- Schmidt, H.D.; Duman, R.S. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology 2010, 35, 2378–2391. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, S.D.; Trentani, A.; Tiron, A.; Mao, X.; Kuhl, D.; Bramham, C.R. BDNF-induced LTP is associated with rapid Arc/Arg3. 1-dependent enhancement in adult hippocampal neurogenesis. Sci. Rep. 2016, 6, 21222. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Bramham, C.; Duarte, C. BDNF and hippocampal synaptic plasticity. Vitam. Horm. 2017, 104, 153–195. [Google Scholar]
- Cardile, V.; Graziano, A.; Avola, R.; Piovano, M.; Russo, A. Potential anticancer activity of lichen secondary metabolite physodic acid. Chem.-Biol. Interact. 2017, 263, 36–45. [Google Scholar] [CrossRef]
- Mohammadi, M.; Bagheri, L.; Badreldin, A.; Fatehi, P.; Pakzad, L.; Suntres, Z.; van Wijnen, A.J. Biological Effects of Gyrophoric Acid and Other Lichen Derived Metabolites, on Cell Proliferation, Apoptosis and Cell Signaling Pathways. Chem.-Biol. Interact. 2022, 351, 109768. [Google Scholar] [CrossRef]
- Yousuf, S.; Choudhary, M.I. Lichens: Chemistry and biological activities. Stud. Nat. Prod. Chem. 2014, 43, 223–259. [Google Scholar]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Emsen, B.; Turkez, H.; Togar, B.; Aslan, A. Evaluation of antioxidant and cytotoxic effects of olivetoric and physodic acid in cultured human amnion fibroblasts. Hum. Human. Exp. Toxicol. 2017, 36, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of lichen metabolite fumarprotocetraric acid via intracellular redox modulation. Toxicol. Appl. Pharmacol. 2017, 316, 83–94. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. Protective effects of lichen metabolites evernic and usnic acids against redox impairment-mediated cytotoxicity in central nervous system-like cells. Food Chem. Toxicol. 2017, 105, 262–277. [Google Scholar] [CrossRef]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation damage: A possible hub for major pathophysiological disorders and aging. Aging Dis. 2018, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Subbaiyan, R.; Ganesan, A.; Dhanuskodi, S.; Prakash, H.P.; Varadharajan, V. Virtual screening of the natural antifoulants: In silico approach to screen lichen metabolites against marine biofoulers. Environ. Prog. Sustain. Energy 2024, 43, e14219. [Google Scholar] [CrossRef]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of G-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef]
- Bay, M.V.; Nam, P.C.; Quang, D.T.; Mechler, A.; Hien, N.K.; Hoa, N.T.; Vo, Q.V. Theoretical study on the antioxidant activity of natural depsidones. ACS Omega 2020, 5, 7895–7902. [Google Scholar] [CrossRef]
- Kosanic, M.; Rankovic, B.; Stanojkovic, T.; Vasiljevic, P.; Manojlovic, N. Biological activities and chemical composition of lichens from Serbia. Excli J. 2014, 13, 1226. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Mohamed, H.; Ons, M.; Yosra, E.T.; Rayda, S.; Neji, G.; Moncef, N. Chemical composition and antioxidant and radical-scavenging activities of Periploca laevigata root bark extracts. J. Sci. Food Agric. 2009, 89, 897–905. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T.; Rančić, A.; Manojlović, N. Cladonia lichens and their major metabolites as possible natural antioxidant, antimicrobial and anticancer agents. LWT-Food Sci. Technol. 2014, 59, 518–525. [Google Scholar] [CrossRef]
- Melo, M.G.D.; dos Santos, J.P.A.; Serafini, M.R.; Caregnato, F.F.; de Bittencourt Pasquali, M.A.; Rabelo, T.K.; da Rocha, R.F.; Quintans, L., Jr.; de Souza Araújo, A.A.; da Silva, F.A. Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite. Toxicol. Vitr. 2011, 25, 462–468. [Google Scholar] [CrossRef]
- Siqueira, R.S.; Bonjardim, L.R.; Araújo, A.A.; Araújo, B.E.; Melo, M.G.; Oliveira, M.G.; Gelain, D.P.; Silva, F.A.; Albuquerque-Júnior, R.L.; Rocha, R.F. Antinociceptive activity of atranorin in mice orofacial nociception tests. Z. Für Naturforschung C 2010, 65, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Shamoto-Nagai, M.; Maruyama, W. Neuroprotection of multifunctional phytochemicals as novel therapeutic strategy for neurodegenerative disorders: Antiapoptotic and antiamyloidogenic activities by modulation of cellular signal pathways. Future Neurol. 2019, 14, FNL9. [Google Scholar] [CrossRef]
- Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch. Neurol. 2011, 68, 1385–1392. [Google Scholar] [CrossRef]
- Latkowska, E.; Bober, B.; Chrapusta, E.; Adamski, M.; Kaminski, A.; Bialczyk, J. Secondary metabolites of the lichen Hypogymnia physodes (L.) Nyl. and their presence in spruce (Picea abies (L.) H. Karst.) bark. Phytochemistry 2015, 118, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Waltenberger, B.; Noha, S.M.; Schuster, D.; Rollinger, J.M.; Boustie, J.; Chollet, M.; Stuppner, H.; Werz, O. Discovery of depsides and depsidones from lichen as potent inhibitors of microsomal prostaglandin E2 synthase-1 using pharmacophore models. ChemMedChem 2012, 7, 2077–2081. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Żarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia physodes Extract Component—Physodic Acid through the Blood–Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Van den Hove, D.; Leibold, N.; Strackx, E.; Martinez-Claros, M.; Lesch, K.; Steinbusch, H.; Schruers, K.; Prickaerts, J. Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. Eur. Neuropsychopharmacol. 2014, 24, 595–607. [Google Scholar] [CrossRef]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.-H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef]
- Kee, N.; Sivalingam, S.; Boonstra, R.; Wojtowicz, J. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods 2002, 115, 97–105. [Google Scholar] [CrossRef]
- Leskanicova, A.; Babincak, M.; Mochnacky, F.; Pipova Kokosova, N.; Kukelova, D.; Urbanska, N.; Kolesarova, M.; Macekova, D.; Kostolny, J.; Kiskova, T. Sex-dependent differences in stress-induced depression in Wistar rats are accompanied predominantly by changes in phosphatidylcholines and sphingomyelins. J. Physiol. Pharmacol. 2021, 72, 72. [Google Scholar]
- Pipová Kokošová, N.; Kisková, T.; Vilhanová, K.; Štafuriková, A.; Jendželovský, R.; Račeková, E.; Šmajda, B. Melatonin mitigates hippocampal and cognitive impairments caused by prenatal irradiation. Eur. J. Neurosci. 2020, 52, 3575–3594. [Google Scholar] [CrossRef] [PubMed]

| CTR | DEP | GA | |
|---|---|---|---|
| Center crossings | 3.24 ± 3.05 | 3.57 ± 3.00 | 3.32 ± 2.54 |
| Washing | 4.76 ± 2.72 | 4.75 ± 2.68 | 4.68 ± 2.83 |
| Rearing | 13.80 ± 6.30 | 14.32 ± 5.69 | 13.32 ± 6.53 |
| Defecation | 0.22 ± 0.99 | 0.20 ± 0.95 | 0.21 ± 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanska, N.; Karasova, M.; Jendzelovska, Z.; Majerník, M.; Kolesarova, M.; Kecsey, D.; Jendzelovsky, R.; Bohus, P.; Kiskova, T. Gyrophoric Acid, a Secondary Metabolite of Lichens, Exhibits Antidepressant and Anxiolytic Activity In Vivo in Wistar Rats. Int. J. Mol. Sci. 2024, 25, 11840. https://doi.org/10.3390/ijms252111840
Urbanska N, Karasova M, Jendzelovska Z, Majerník M, Kolesarova M, Kecsey D, Jendzelovsky R, Bohus P, Kiskova T. Gyrophoric Acid, a Secondary Metabolite of Lichens, Exhibits Antidepressant and Anxiolytic Activity In Vivo in Wistar Rats. International Journal of Molecular Sciences. 2024; 25(21):11840. https://doi.org/10.3390/ijms252111840
Chicago/Turabian StyleUrbanska, Nicol, Martina Karasova, Zuzana Jendzelovska, Martin Majerník, Mariana Kolesarova, Dajana Kecsey, Rastislav Jendzelovsky, Peter Bohus, and Terezia Kiskova. 2024. "Gyrophoric Acid, a Secondary Metabolite of Lichens, Exhibits Antidepressant and Anxiolytic Activity In Vivo in Wistar Rats" International Journal of Molecular Sciences 25, no. 21: 11840. https://doi.org/10.3390/ijms252111840
APA StyleUrbanska, N., Karasova, M., Jendzelovska, Z., Majerník, M., Kolesarova, M., Kecsey, D., Jendzelovsky, R., Bohus, P., & Kiskova, T. (2024). Gyrophoric Acid, a Secondary Metabolite of Lichens, Exhibits Antidepressant and Anxiolytic Activity In Vivo in Wistar Rats. International Journal of Molecular Sciences, 25(21), 11840. https://doi.org/10.3390/ijms252111840










