Comparison of n-3 PUFA-Enriched vs. Olive-Oil-Based Lipid Emulsion on Oxidative Stress and Inflammatory Response in Critically Ill Post-Surgery Adults: Secondary Analysis of a Randomized Controlled Trial
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Effects on the Clinical Parameters and Outcomes of the Patients
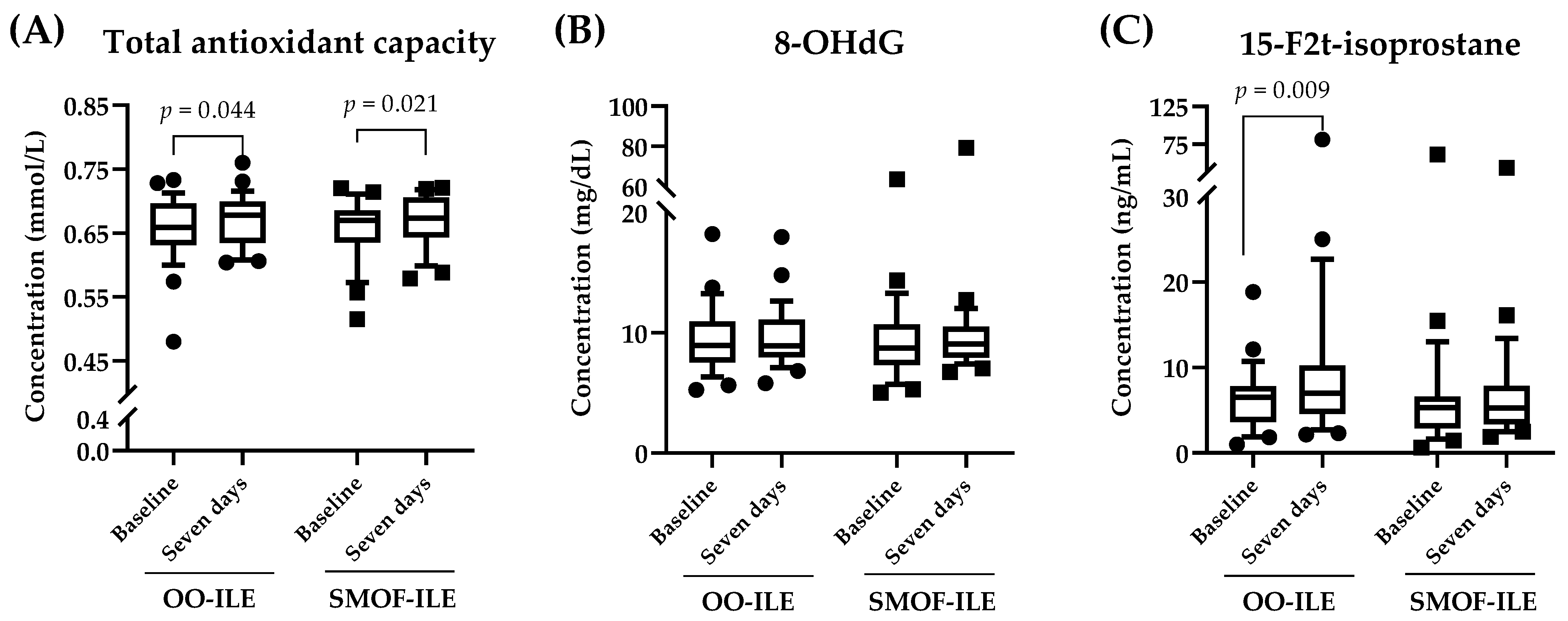
2.3. Effects of Lipid Composition on Serum Oxidative Stress Markers
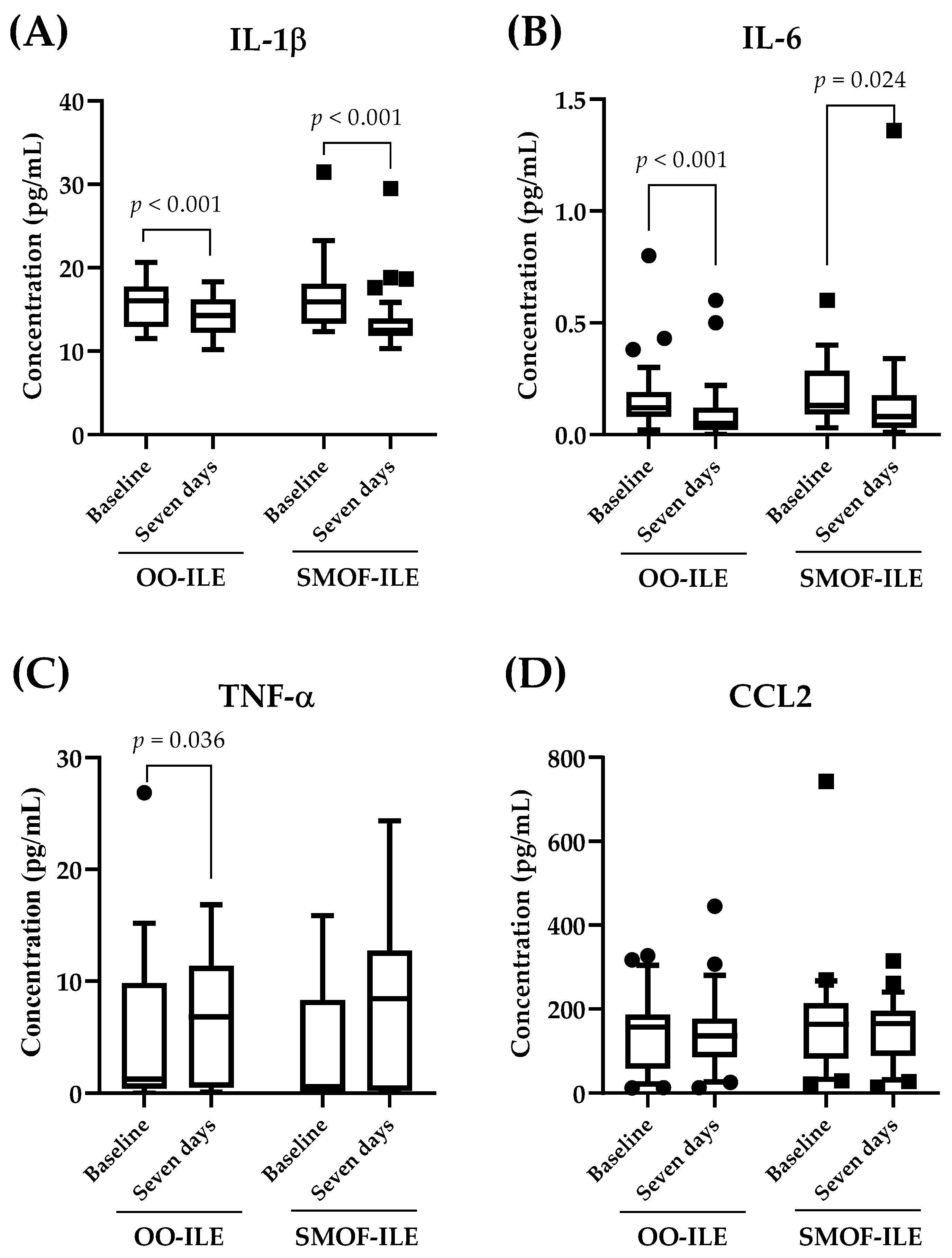
2.4. Effects of Lipid Composition on Serum Inflammatory Markers
3. Discussion
4. Materials and Methods
4.1. Study Design
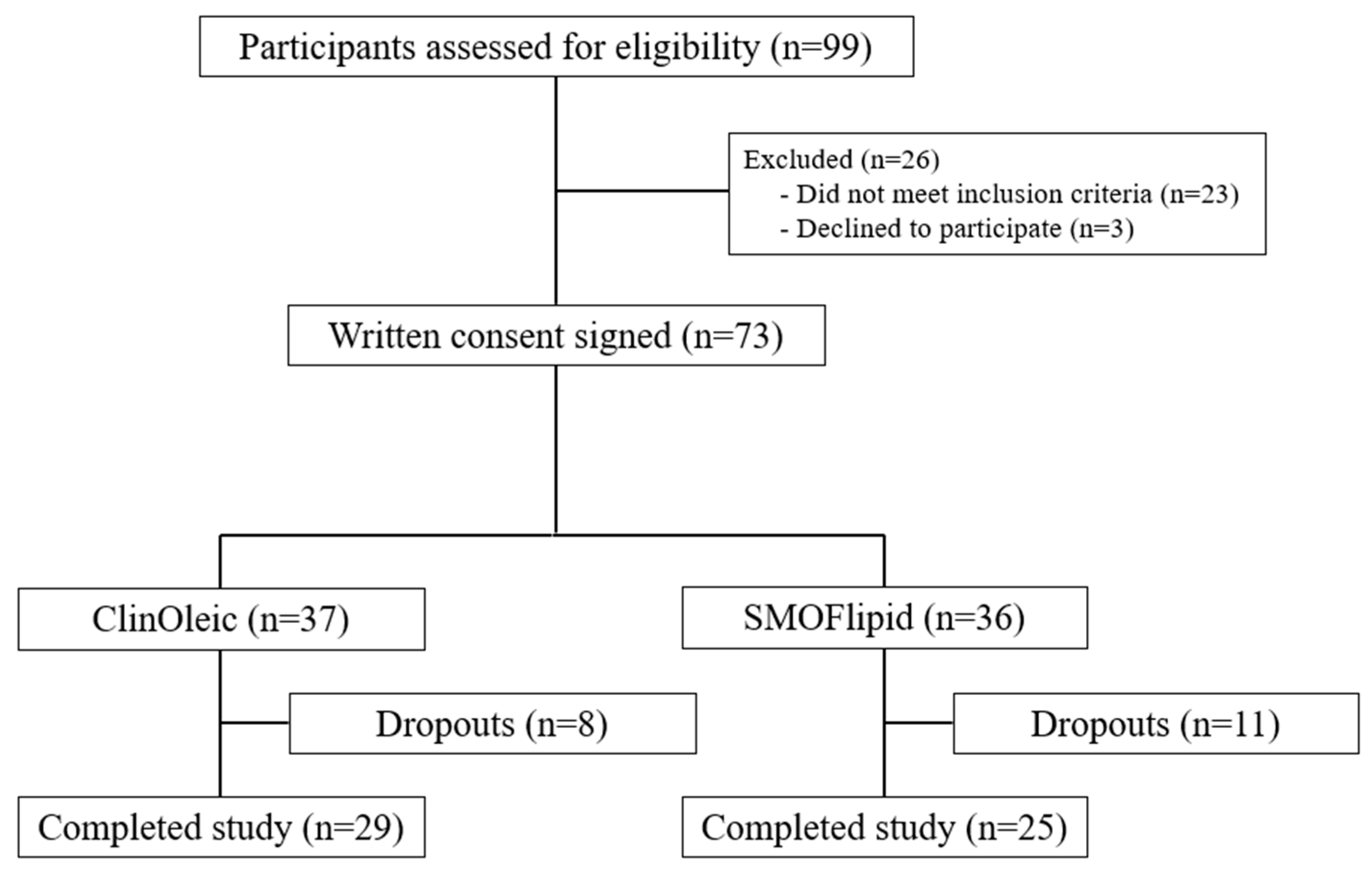
4.2. Sample Size, Recruitment, and Participants
4.3. Nutritional Intervention
4.4. Clinical and Demographic Data Collection
4.5. Biological Samples and Laboratory Analyses
4.6. Determination of Inflammatory and Oxidative Stress Biomarkers in Serum Samples
4.7. Trolox Equivalent Antioxidant Capacity Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cass, A.R.; Charlton, K.E. Prevalence of hospital-acquired malnutrition and modifiable determinants of nutritional deterioration during inpatient admissions: A systematic review of the evidence. J. Hum. Nutr. Diet. 2022, 35, 1043–1058. [Google Scholar] [CrossRef] [PubMed]
- Lew, C.C.H.; Yandell, R.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. Association between malnutrition and clinical outcome in the intensive care unit: A systematic revies. J. Parenter. Enteral Nutr. 2017, 41, 744–758. [Google Scholar] [CrossRef] [PubMed]
- Im, K.M.; Kim, E.Y. Reducing In-Hospital and 60-Day Mortality in Critically Ill Patients after Surgery with Strict Nutritional Supplementation: A Prospective, Single-Labeled, Randomized Controlled Trial. Nutrients 2023, 15, 4684. [Google Scholar] [CrossRef] [PubMed]
- Andresen, H.M.; Regueira, H.T.; Leighton, F. Oxidative stress in critically ill patients. Rev. Med. Chil. 2006, 134, 649–656. [Google Scholar] [CrossRef]
- Dresen, E.; Notz, Q.; Menger, J.; Homayr, A.L.; Lindner, M.; Radke, D.I.; Stoppe, C.; Elke, G. What the clinician needs to know about medical nutrition therapy in critically ill patients in 2023: A narrative review. Nutr. Clin. Pract. 2023, 38, 479–498. [Google Scholar] [CrossRef]
- Hamdan, M.; Puckett, Y. Total Parenteral Nutrition. In StatPearls; Ineligible Companies: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wu, G.H.; Zaniolo, O.; Schuster, H.; Schlotzer, E.; Pradelli, L. Structured triglycerides versus physical mixtures of medium- and long-chain triglycerides for parenteral nutrition in surgical or critically ill adult patients: Systematic review and meta-analysis. Clin. Nutr. 2017, 36, 150–161. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Torrinhas, R.S.; Jacintho, T.M. New parenteral lipid emulsions for clinical use. J. Parenter. Enteral Nutr. 2006, 30, 351–367. [Google Scholar] [CrossRef]
- Gupta, N.; Ali, C.; Talathi, S. SO,MCT,OO-FO-ILE is associated with better side effect profile tan SO-ILE in critically ill children receiving parenteral nutrition. J. Pediatr. Pharmacol. Ther. 2023, 28, 329–334. [Google Scholar] [CrossRef]
- Furukawa, K.; Yamamori, H.; Takagi, K.; Hayashi, N.; Suzuki, R.; Nakajima, N.; Tashiro, T. Influences of soybean oil emulsion on stress response and cell-mediated immune function in moderately or severely stressed patients. Nutrition 2002, 18, 235–240. [Google Scholar] [CrossRef]
- Haines, K.; Grisel, B.; Gorenshtein, L.; Wischmeyer, P.E. Lipid emulsions in parenteral nutrition: Does it matter? Curr. Opin. Crit. Care 2023, 29, 293–299. [Google Scholar] [CrossRef]
- Rogulska, J.; Osowska, S.; Kunecki, M.; Sobocki, J.; Ładyżyński, P.; Giebułtowicz, J. Antioxidant balance in plasma of patients on home parenteral nutrition: A pilot study comparing three different lipid emulsions. Clin. Nutr. 2021, 40, 3950–3958. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Lipids for intravenous nutrition in hospitalised adult patients: A multiple choice of options. Proc. Nutr. Soc. 2013, 72, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Al-Leswas, D.; Eltweri, A.M.; Chung, W.-Y.; Arshad, A.; Stephenson, J.A.; Al-Taan, O.; Pollard, C.; Fisk, H.L.; Calder, P.C.; Garcea, G.; et al. Intravenous omega-3 fatty acids are associated with better clinical outcome and less inflammation in patients with predicted severe acute pancreatitis: A randomised double blind controlled trial. Clin. Nutr. 2020, 39, 2711–2719. [Google Scholar] [CrossRef]
- Cai, W.; Calder, P.C.; Cury-Boaventura, M.F.; De Waele, E.; Jakubowski, J.; Zaloga, G. Biological and Clinical Aspects of an Olive Oil-Based Lipid Emulsion-A Review. Nutrients 2018, 10, 776. [Google Scholar] [CrossRef]
- Calder, P.C.; Waitzberg, D.L.; Klek, S.; Martindale, R.G. Lipids in Parenteral Nutrition: Biological Aspects. JPEN J. Parenter. Enteral Nutr. 2020, 44 (Suppl. S1), S21–S27. [Google Scholar] [CrossRef] [PubMed]
- Pradelli, L.; Mayer, K.; Klek, S.; Rosenthal, M.D.; Povero, M.; Heller, A.R.; Muscaritoli, M. Omega-3 fatty acids in parenteral nutrition—A systematic review with network meta-analysis on clinical outcomes. Clin. Nutr. 2023, 42, 590–599. [Google Scholar] [CrossRef]
- Huschak, G.; Zur Nieden, K.; Hoell, T.; Riemann, D.; Mast, H.; Stuttmann, R. Olive oil based nutrition in multiple trauma patients: A pilot study. Intensive Care Med. 2005, 31, 1202–1208. [Google Scholar] [CrossRef]
- Jia, Z.Y.; Yang, J.; Xia, Y.; Tong, D.N.; Zaloga, G.P.; Qin, H.L.; OliClinomel N4 Study Group. Safety and efficacy of an olive oil-based triple-chamber bag for parenteral nutrition: A prospective, randomized, multi-center clinical trial in China. Nutr. J. 2015, 14, 119. [Google Scholar] [CrossRef]
- Notz, Q.; Lee, Z.Y.; Menger, J.; Elke, G.; Hill, A.; Kranke, P.; Roeder, D.; Lotz, C.; Meybohm, P.; Heyland, D.K.; et al. Omega-6 sparing effects of parenteral lipid emulsions-an updated systematic review and meta-analysis on clinical outcomes in critically ill patients. Crit. Care 2022, 26, 23. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; Martindale, R.G.; Klek, S.; Calder, P.C.; Wischmeyer, P.E.; Patel, J.J. The role of lipid emulsions containing omega-3 fatty acids for medical and surgical critical care patients. Crit. Care 2024, 28, 271. [Google Scholar] [CrossRef]
- Badia-Tahull, M.B.; Llop-Talaveron, J.M.; Leiva-Badosa, E.; Biondo, S.; Farran-Teixido, L.; Ramon-Torrell, J.M.; Jodar-Masanes, R. A randomised study on the clinical progress of high-risk elective major gastrointestinal surgery patients treated with olive oil-based parenteral nutrition with or without a fish oil supplement. Br. J. Nutr. 2010, 104, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lozano Aranaga, F.; Gomez Ramos, M.J.; Sanchez Alvarez, M.D.C. Effectiveness and safety of two lipid emulsions for parenteral nutrition in postsurgical critically ill patients: Clinoleic(R) versus SMOFlipid(R). Nutr. Hosp. 2021, 38, 5–10. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enteral Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M.; Mayer, K.; Montejo-Gonzalez, J.C.; Pichard, C.; Preiser, J.C.; et al. ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef]
- Tsekos, E.; Reuter, C.; Stehle, P.; Boeden, G. Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clin. Nutr. 2004, 23, 325–330. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Hong, Y.; Zhang, H.; Hong, C.; Liu, X. Single-blinded, randomized, and controlled clinical trial evaluating the effects of Omega-3 fatty acids among septic patients with intestinal dysfunction: A pilot study. Exp. Ther. Med. 2017, 14, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, G.; Sahin, H.; Inanc, N.; Uyanik, F.; Ok, E. Impact of Omega-3 and Omega-9 fatty acids enriched total parenteral nutrition on blood chemistry and inflammatory markers in septic patients. Pak. J. Med. Sci. 2014, 30, 299–304. [Google Scholar] [CrossRef]
- Osowska, S.; Kunecki, M.; Sobocki, J.; Tokarczyk, J.; Majewska, K.; Burkacka, M.; Radkowski, M.; Makarewicz-Wujec, M.; Fisk, H.L.; Mashnafi, S.; et al. Potential for Omega-3 Fatty Acids to Protect against the Adverse Effect of Phytosterols: Comparing Laboratory Outcomes in Adult Patients on Home Parenteral Nutrition Including Different Lipid Emulsions. Biology 2022, 11, 1699. [Google Scholar] [CrossRef]
- Piper, S.N.; Schade, I.; Beschmann, R.B.; Maleck, W.H.; Boldt, J.; Rohm, K.D. Hepatocellular integrity after parenteral nutrition: Comparison of a fish-oil-containing lipid emulsion with an olive-soybean oil-based lipid emulsion. Eur. J. Anaesthesiol. 2009, 26, 1076–1082. [Google Scholar] [CrossRef]
- Crimi, E.; Sica, V.; Williams-Ignarro, S.; Zhang, H.; Slutsky, A.S.; Ignarro, L.J.; Napoli, C. The role of oxidative stress in adult critical care. Free Radic. Biol. Med. 2006, 40, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Almutairdi, A.; Mulesa, L.; Alberda, C.; Beattie, C.; Gramlich, L. Parenteral Nutrition and Lipids. Nutrients 2017, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Demirer, S.; Sapmaz, A.; Karaca, A.S.; Kepenekci, I.; Aydintug, S.; Balci, D.; Sonyurek, P.; Kose, K. Effects of postoperative parenteral nutrition with different lipid emulsions in patients undergoing major abdominal surgery. Ann. Surg. Treat. Res. 2016, 91, 309–315. [Google Scholar] [CrossRef]
- Alcazar Espin, M.N.; Macaya Redin, L.; Moreno Clari, E.; Sanchez Alvarez, C. Recommendations for specialized nutritional-metabolic management of the critical patient: Digestive tract surgery. Metabolism and Nutrition Working Group of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Med. Intensiv. Engl. Ed. 2020, 44 (Suppl. S1), 65–68. [Google Scholar] [CrossRef]
- Martindale, R.G.; Berlana, D.; Boullata, J.I.; Cai, W.; Calder, P.C.; Deshpande, G.H.; Evans, D.; Garcia-de-Lorenzo, A.; Goulet, O.J.; Li, A.; et al. Summary of Proceedings and Expert Consensus Statements from the International Summit “Lipids in Parenteral Nutrition”. JPEN J. Parenter. Enteral Nutr. 2020, 44 (Suppl. S1), S7–S20. [Google Scholar] [CrossRef]
- Kumar, N.G.; Contaifer, D.; Madurantakam, P.; Carbone, S.; Price, E.T.; Van Tassell, B.; Brophy, D.F.; Wijesinghe, D.S. Dietary bioactive fatty acids as modulators of immune function: Implications on human health. Nutrients 2019, 11, 2974. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Spiegelman, R.; Zhao, V.; Smiley, D.D.; Pinzon, I.; Griffith, D.P.; Peng, L.; Morris, T.; Luo, M.; Garcia, H.; et al. A double-blind, randomized clinical trial comparing soybean oil-based versus olive oil-based lipid emulsions in adult medical-surgical intensive care unit patients requiring parenteral nutrition. Crit. Care Med. 2013, 40, 1792–1798. [Google Scholar] [CrossRef]
- Ulusoy, H.; Kangalgil, M.; Küçük, A.O.; Özdemir, A.; Karahan, S.C.; Yaman, S.Ö.; Yavuz, H.B.; Ok, Ü. Effects of different lipid emulsions on serum adipokines, inflammatory markers and mortality in critically ill patients with sepsis: A prospective observational cohort study. Clin. Nutr. 2021, 40, 4569–4578. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; The CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef]
| Total (n = 54) | OO-ILE (n = 29) | SMOF-ILE (n = 25) | p | |
|---|---|---|---|---|
| Gender (M/F) | 36/18 | 17/12 | 19/6 | 0.249 |
| Age (years) | 68.9 ±13.5 | 69.2 ± 15.1 | 68.6 ± 11.7 | 0.864 |
| Weight (kg) | 75.0 (11.5) | 75.0 (10.0) | 75.0 (17.0) | 0.379 |
| BMI | 27.7 ± 5.3 | 27.3 ± 5.9 | 28.1 ± 4.5 | 0.570 |
| APACHE II | 15.0 (9.5) | 14.0 (8.8) | 17.0 (13.5) | 0.848 |
| Variable | OO-ILE (n = 29) | SMOF-ILE (n = 25) | Interaction Treatment × Time | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 7 Days | p | Baseline | 7 Days | p | p | |
| Albumin (g/dL) | 2.1 ± 0.5 | 2.3 ± 0.5 | 0.019 | 2.1 ± 0.4 | 2.2 ± 0.5 | 0.473 | 0.361 |
| Prealbumin (mg/dL) | 7.2 (5.9) | 14.9 (9.6) | <0.001 | 7.8 (1.7) | 14.3 (10.8) | <0.001 | 0.331 |
| Transferrin (mg/dL) | 97.7 ± 26.6 | 113.6 ± 39.1 | 0.010 | 99.3 ± 31.1 | 119.5 ± 28.9 | 0.004 | 0.262 |
| Triglycerides (mg/dL) | 94.0 (70.3) | 170.0 (90.3) | 0.001 | 104.0 (65.0) | 141.0 (79.5) | 0.041 | 0.505 |
| Cholesterol (mg/dL) | 83.5 (56.2) | 110.0 (47.0) | 0.006 | 80.0 (15.0) | 101.0 (49.0) | 0.003 | 0.330 |
| GGT (U/L) | 65.0 (57.0) | 86.0 (91.0) | 0.347 | 86.5 (68.0) | 95.0 (79.7) | 0.737 | 0.616 |
| AST(U/L) | 16.0 (13.0) | 28.0 (38.0) | 0.046 | 24.5 (42.2) | 32.0 (22.4) | 0.476 | 0.584 |
| ALT(U/L) | 22.0 (31.5) | 39.0 (42.0) | 0.078 | 22.5 (42.2) | 28.0 (28.8) | 0.382 | 0.518 |
| Bilirubin (mg/dL) | 0.33 (0.28) | 0.45 (0.65) | 0.256 | 0.52 (0.72) | 0.59 (0.68) | 0.830 | 0.400 |
| Apolipoprotein AI (mg/dL) | 58.3 ± 18.7 | 72.5 ± 18.2 | <0.001 | 50.8 ± 20.1 | 65.2 ± 18.7 | 0.002 | 0.747 |
| Apolipoprotein B (mg/dL) | 59.5 ± 26.2 | 80.1 ± 26.9 | 0.003 | 63.8 ± 27.2 | 83.4 ± 25.3 | 0.015 | 0.956 |
| SOFA score | 6.5 (5.0) | 2.0 (3.0) | <0.001 | 5.0 (5.0) | 2.0 (3.0) | <0.001 | 0.271 |
| OO-ILE (n = 29) | SMOF-ILE (n = 25) | p | |
|---|---|---|---|
| Mechanical ventilation (days) | 7.0 (8.5) | 1.0 (24.0) | 0.185 |
| CVC (days) | 16.5 (18.8) | 18.0 (16.5) | 0.908 |
| ICU length of stay (days) | 13.0 (20.2) | 17.0 (21.5) | 0.964 |
| Hospital length of stay (days) | 30.5 (22.8) | 24.0 (16.5) | 0.748 |
| Days of PN | 10.0 (6.0) | 10.0 (7.5) | 0.693 |
| VAP | 2 | 3 | 0.222 |
| CAUTI | 2 | 2 | 1 |
| CRBSI | 4 | 3 | 0.535 |
| Surgical Infection | 3 | 4 | 1 |
| Exitus | 7 | 9 | 0.446 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuartero-Corbalán, N.; Martínez-Lozano Aranaga, F.; Gómez-Ramos, M.J.; Gómez-Sánchez, M.B.; Avilés-Plaza, F.V.; Núñez-Sánchez, M.A.; Morillas-Ruiz, J.M. Comparison of n-3 PUFA-Enriched vs. Olive-Oil-Based Lipid Emulsion on Oxidative Stress and Inflammatory Response in Critically Ill Post-Surgery Adults: Secondary Analysis of a Randomized Controlled Trial. Int. J. Mol. Sci. 2024, 25, 11739. https://doi.org/10.3390/ijms252111739
Cuartero-Corbalán N, Martínez-Lozano Aranaga F, Gómez-Ramos MJ, Gómez-Sánchez MB, Avilés-Plaza FV, Núñez-Sánchez MA, Morillas-Ruiz JM. Comparison of n-3 PUFA-Enriched vs. Olive-Oil-Based Lipid Emulsion on Oxidative Stress and Inflammatory Response in Critically Ill Post-Surgery Adults: Secondary Analysis of a Randomized Controlled Trial. International Journal of Molecular Sciences. 2024; 25(21):11739. https://doi.org/10.3390/ijms252111739
Chicago/Turabian StyleCuartero-Corbalán, Nerea, Fátima Martínez-Lozano Aranaga, Maria Jesús Gómez-Ramos, María B. Gómez-Sánchez, Francisco V. Avilés-Plaza, María A. Núñez-Sánchez, and Juana M. Morillas-Ruiz. 2024. "Comparison of n-3 PUFA-Enriched vs. Olive-Oil-Based Lipid Emulsion on Oxidative Stress and Inflammatory Response in Critically Ill Post-Surgery Adults: Secondary Analysis of a Randomized Controlled Trial" International Journal of Molecular Sciences 25, no. 21: 11739. https://doi.org/10.3390/ijms252111739
APA StyleCuartero-Corbalán, N., Martínez-Lozano Aranaga, F., Gómez-Ramos, M. J., Gómez-Sánchez, M. B., Avilés-Plaza, F. V., Núñez-Sánchez, M. A., & Morillas-Ruiz, J. M. (2024). Comparison of n-3 PUFA-Enriched vs. Olive-Oil-Based Lipid Emulsion on Oxidative Stress and Inflammatory Response in Critically Ill Post-Surgery Adults: Secondary Analysis of a Randomized Controlled Trial. International Journal of Molecular Sciences, 25(21), 11739. https://doi.org/10.3390/ijms252111739






