Potential Anticancer Effects of Isoflavone Prunetin and Prunetin Glycoside on Apoptosis Mechanisms
Abstract
1. Introduction
2. One Important Strategy as an Antitumor Mechanism Is Apoptosis
2.1. Major Tumor Suppression Mechanisms
2.2. Key Proteins Involved in Apoptosis
2.2.1. Cytochrome c
2.2.2. Bcl-2 Family
2.2.3. P53
2.2.4. Fas and FasL
2.2.5. Survivin
2.2.6. Caspase Family
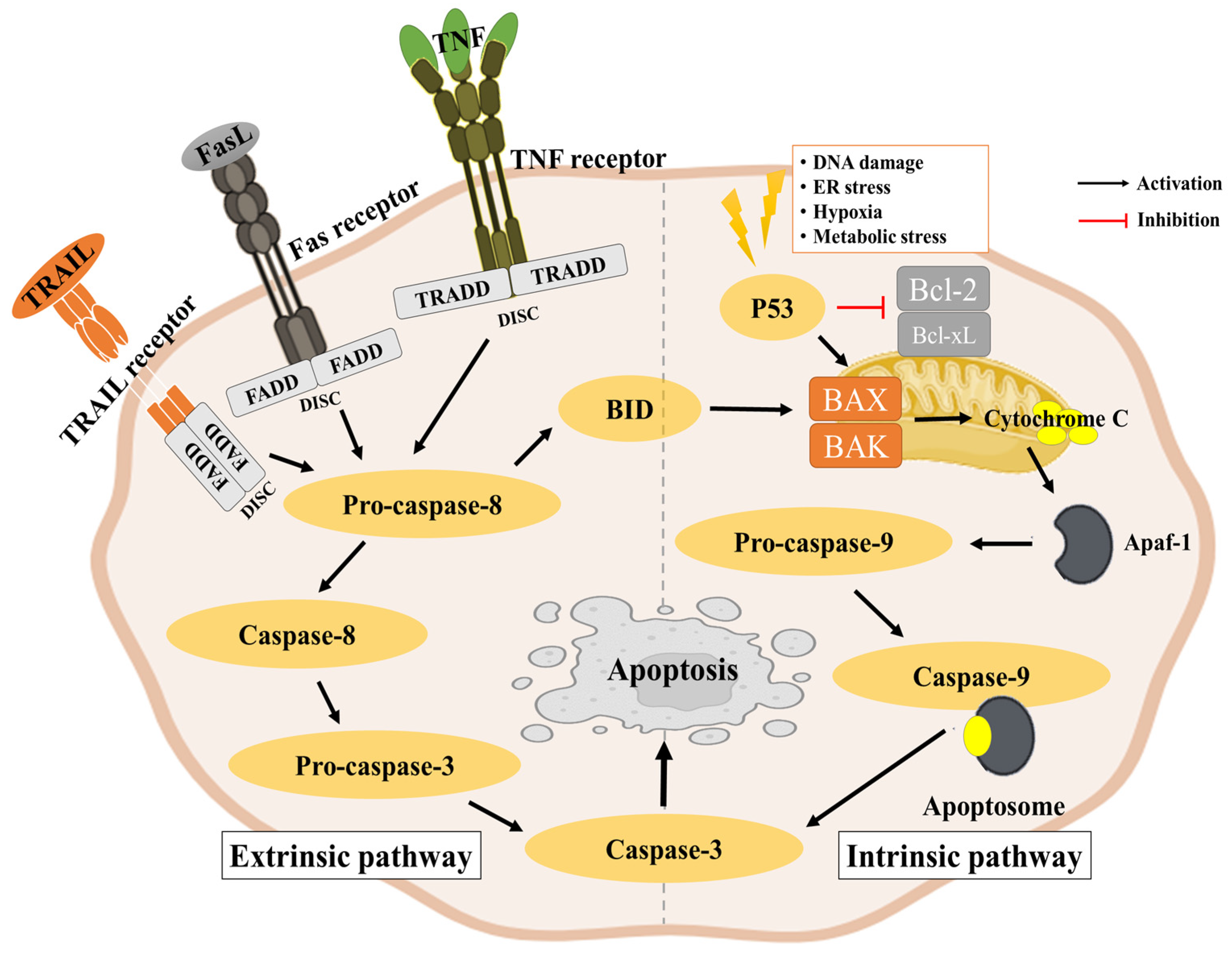
2.3. Apoptosis Pathway
2.3.1. Intrinsic Pathway
2.3.2. Extrinsic Pathway
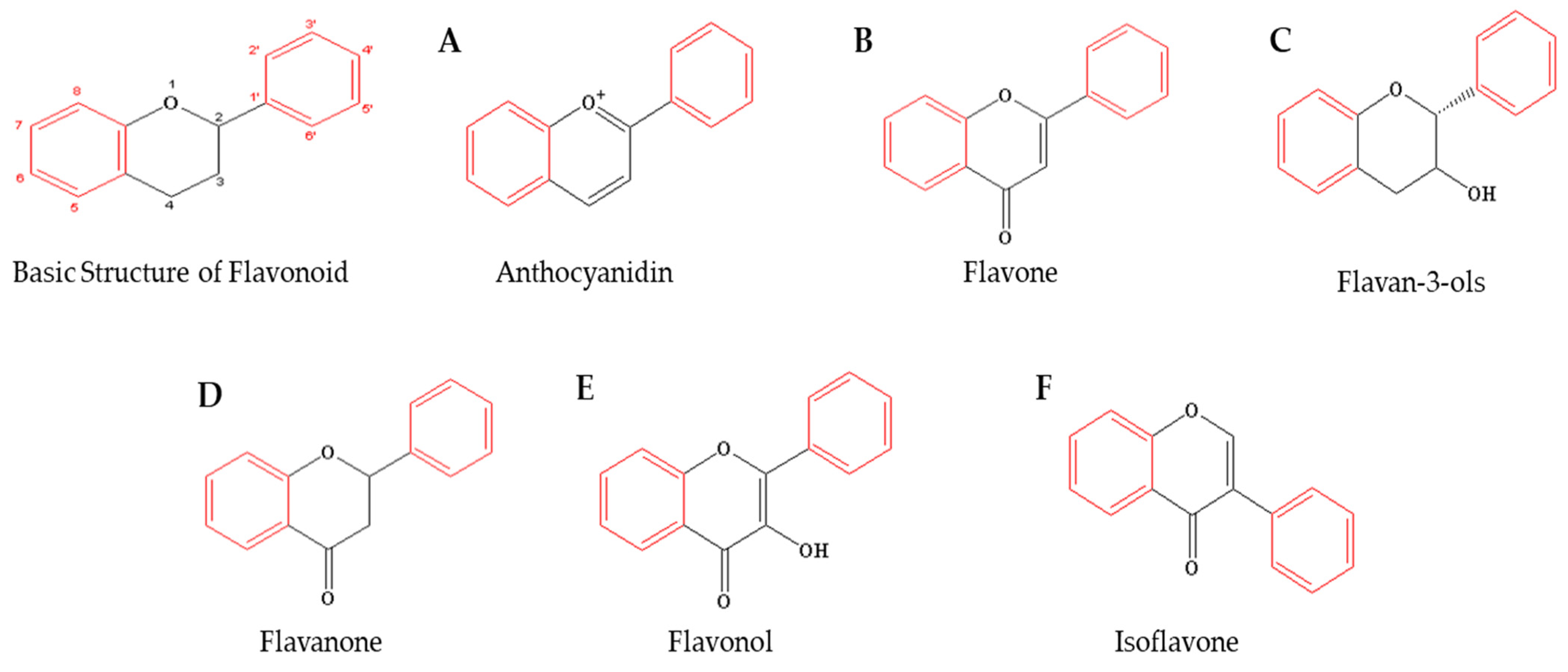
3. Flavonoids as Bioactive Compounds for Anticancer Effects
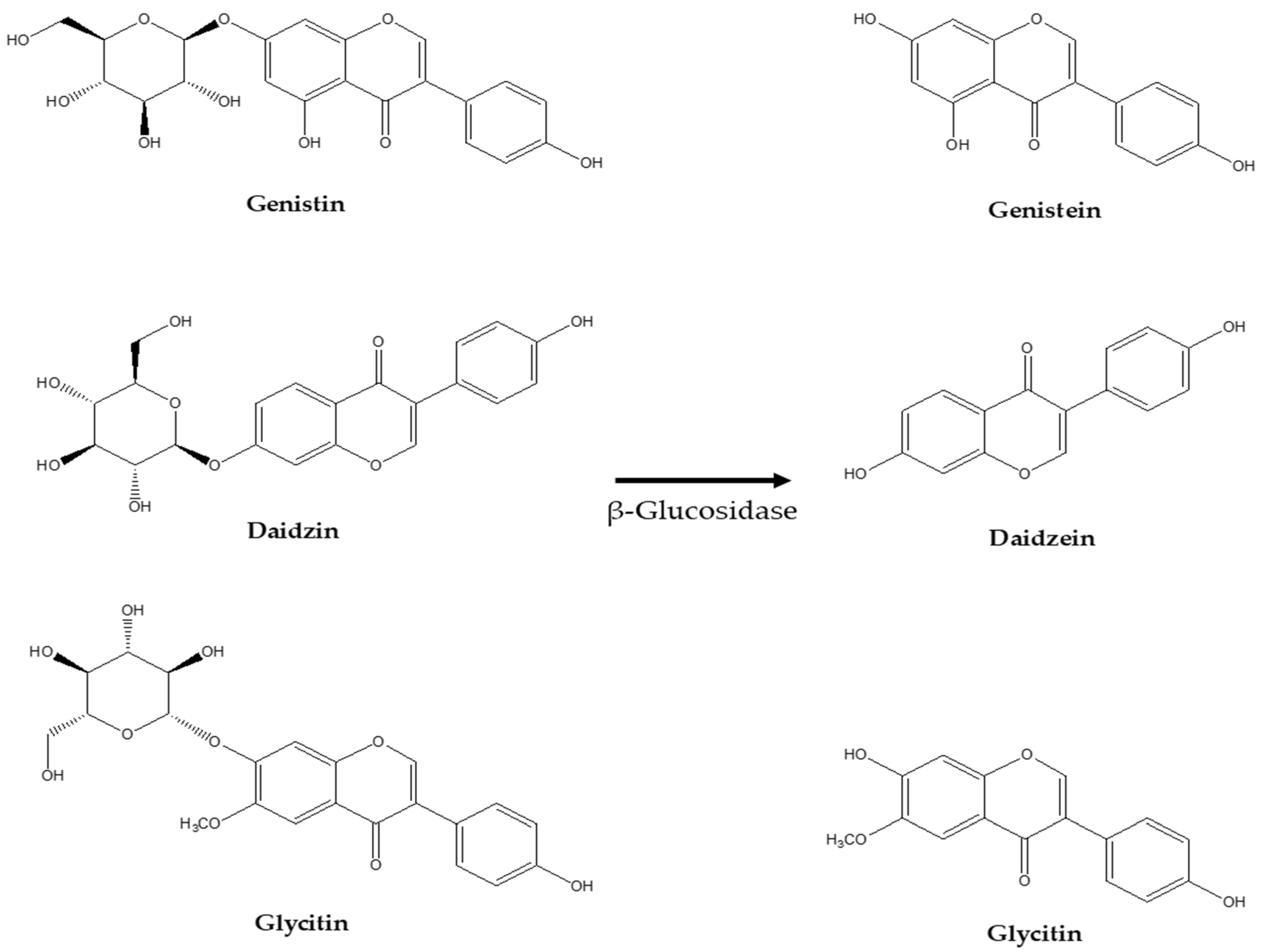
4. Potential Apoptotic Antitumor Effects of Various Isoflavone Glycosides and Aglycone Forms
| Genistin | ||||
|---|---|---|---|---|
| Cancer | Cell Line | Treatment Concentration | Apoptosis Anticancer Mechanism | Reference |
| Breast Cancer | MCF-7 | 50, 100, and 150 μM | ERα inhibition Caspase-8 and 9 activation PARP cleaved | [60] |
| Genistein | ||||
| Cancer | Cell Line | Treatment Concentration | Apoptosis Anticancer Mechanism | Reference |
| Ovarian Cancer | SK-OV-3 | 1, 5, 10, 50, and 100 μM | ● ER stress ● Caspase-mediated apoptosis ● Inhibition of angiogenesis | [63] |
| Lung Cancer | A549 | 20, 40, and 80 μM | ● Caspase-3 and 9 increased ● Overexpression of IMPDH2 and Akt inhibited | [64] |
| Leukemia | HL-60 | 20 and 50 μM | ● Bax, PARP cleavage, caspase-9 increased ● Mitochondria stress induced ● Bcl-2 and Bid decreased | [65] |
| Daidzin | ||||
| Cancer | Cell Line | Treatment Concentration | Apoptosis Anticancer Mechanism | Reference |
| Cervical Cancer | HeLa | 20 μM | ● Increases mitochondrial membrane permeability ● Caspase-8 and 9 increased | [66] |
| Daidzein | ||||
| Cancer | Cell Line | Treatment Concentration | Apoptosis Anticancer Mechanism | Reference |
| Breast Cancer | MCF-7 | 50 µM | ● Modulates estrogen signaling pathways ● Overexpression of Bax and inhibition of Bcl2 | [67] |
| Prostate Cancer | PC3 | 25 µM | ● Bax increased | [69] |
| Ovarian Cancer | SK-OV-3 | 20 µM | ● Cytochrome c released ● Caspase-3 and 9 increased ● PARP cleaved ● Raf/MEK/ERK pathway inhibited | [70] |
| Glycitin | ||||
| Cancer | Cell Line | Treatment Concentration | Apoptosis Anticancer Mechanism | Reference |
| Lung Cancer | A549 | 30, 60, 120 μM | ● Cell membrane damage ● Nuclear fragmentation | [71] |
| Glycitein | ||||
| Cancer | Cell Line | Treatment Concentration | Apoptosis Anticancer Mechanism | Reference |
| Gastric Cancer | AGS | 30 μM | ● MAPK/STAT3/NF-κB pathway and mitochondria ● Mediated apoptosis ● Cell cycle arrest | [72] |
| Breast Cancer | SKBR-3 | 20, 80, and 100 mg/mL | ● Membrane permeability increased | [73] |
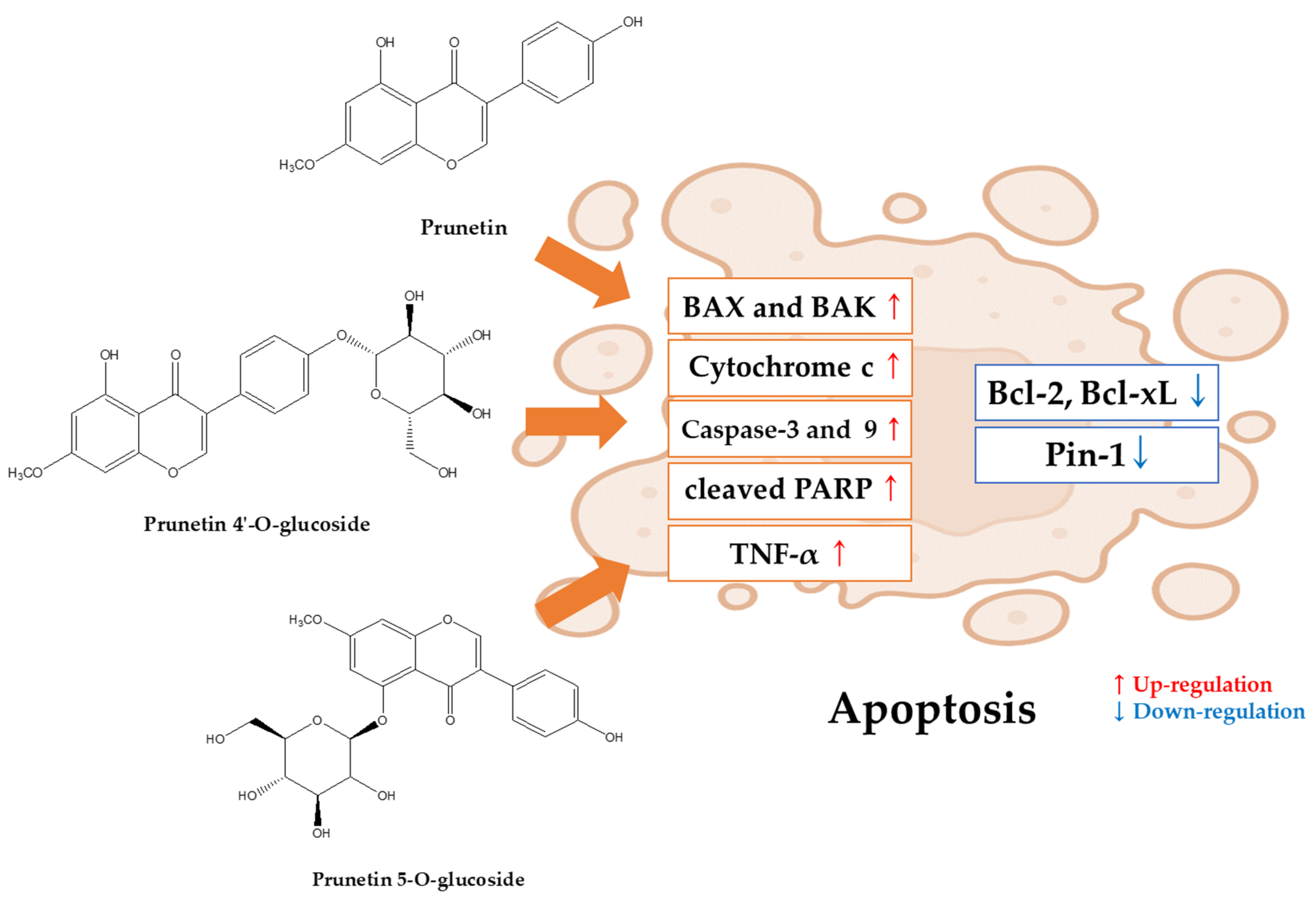
5. Potential Apoptotic Antitumor Effects of Prunetin and Prunetin Glycosides
5.1. Apoptosis and Anticancer Effects of Prunetin (In Vitro)
Apoptosis and Anticancer Effects of Prunetin (In Vivo)
5.2. Apoptosis and Anticancer Effects of Prunetin Glycosides (In Vitro)
5.2.1. Prunetin-4O-Glucoside (Prunetrin)
5.2.2. Prunetin-5O-Glucoside (Prunetinoside)
| Prunetin | ||||
|---|---|---|---|---|
| Cancer | Cell Line | Treatment Concentration | Apoptosis Anticancer Mechanism | Reference |
| Osteosarcoma | MG-63 | 20 and 25 μM | ● Bax and Caspase increased ● Bcl-2 decreased ● Pin-1 inhibited | [76] |
| Bladder Cancer | RT-4 | 21.11 and 42.22 μg/mL |
●
Caspase-3 increased ● TNF-α mediated | [77] |
| Prunetin 4′-O-glucoside | ||||
| Cancer | Cell Line | Treatment Concentration | Apoptosis Anticancer Mechanism | Reference |
| Liver Cancer | Hep3B | 10, 20 and 40 μM | ● PARP and caspase-3 activation ● Caspase-9 and Bak increased | [81] |
| HepG2 and Huh7 | 10, 15, 30 μM | ● Cytochrome c released ● Bak, Caspase-3 and 9 increased ● Bcl-xL decreased | [82] | |
| Prunetin 5-O-glucoside | ||||
| Cancer | Cell Line | Treatment Concentration | Apoptosis Anticancer Mechanism | Reference |
| Gastric Cancer | AGS | 50, 75 and 150 μM | ● PARP and caspase-3 activation | [84] |
| Prunetin | |||
|---|---|---|---|
| Cancer | Mouse Model and Dosages | Apoptosis Anticancer Mechanism | Reference |
| Lung Cancer | Swiss albino mouse, Benzo(a)pyrene, gavage, 30 mg/kg 12 and 12~18 weeks | ● Carcinoembryonic antigen (CEA) decreased | [78] |
| Liver Cancer |
Male Wistar rat, diethylnitrosamine, feed with water, 200 mg/kg |
● Cyclin-D1 downregulated ●Bcl-2, Bax, caspase-3, and caspase-9 upregulated | [79] |
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krakhmal, N.V.; Zavyalova, M.V.; Denisov, E.V.; Vtorushin, S.V.; Perelmuter, V.M. Cancer Invasion: Patterns and Mechanisms. Acta Naturae 2015, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Wild, C.P. Global cancer patterns: Causes and prevention. Lancet 2014, 383, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Busselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Ramos, S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007, 18, 427–442. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Samec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Tuli, H.S.; Garg, V.K.; Bhushan, S.; Uttam, V.; Sharma, U.; Jain, A.; Sak, K.; Yadav, V.; Lorenzo, J.M.; Dhama, K.; et al. Natural flavonoids exhibit potent anticancer activity by targeting microRNAs in cancer: A signature step hinting towards clinical perfection. Transl. Oncol. 2023, 27, 101596. [Google Scholar] [CrossRef]
- Messina, M.J.; Wood, C.E. Soy isoflavones, estrogen therapy, and breast cancer risk: Analysis and commentary. Nutr. J. 2008, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic Engineering of Isoflavones: An Updated Overview. Front. Plant Sci. 2021, 12, 670103. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Misra, P.; Khan, M.P.; Swarnkar, G.; Tewari, M.C.; Bhambhani, S.; Trivedi, R.; Chattopadhyay, N.; Trivedi, P.K. Co-expression of Arabidopsis transcription factor, AtMYB12, and soybean isoflavone synthase, GmIFS1, genes in tobacco leads to enhanced biosynthesis of isoflavones and flavonols resulting in osteoprotective activity. Plant Biotechnol. J. 2014, 12, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Whitten, P.; Kudo, S.; Okubo, K. Isoflavonoids. In Handbook of Plant and Fungal Toxicants; CRC Press: Boca Raton, FL, USA, 2020; pp. 117–137. [Google Scholar]
- Sugai, T.; Hanaya, K.; Higashibayashi, S. Semisynthesis of prunetin, a bioactive O-methylated isoflavone from naringenin, by the sequential deacetylation of chalcone intermediates and oxidative rearrangement. Biosci. Biotechnol. Biochem. 2021, 85, 143–147. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. The Potential Therapeutic Properties of Prunetin against Human Health Complications: A Review of Medicinal Importance and Pharmacological Activities. Drug Metab. Bioanal. Lett. 2022, 15, 166–177. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Abusaliya, A.; Ha, S.E.; Bhosale, P.B.; Kim, H.H.; Park, M.Y.; Vetrivel, P.; Kim, G.S. Glycosidic flavonoids and their potential applications in cancer research: A review. Mol. Cell. Toxicol. 2022, 18, 9–16. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, W.; Thompson, P.; Hannun, Y.A. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat. Commun. 2018, 9, 3490. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Arroum, T.; Luo, X.; Kang, R.; Lee, Y.J.; Tang, D.; Hüttemann, M.; Song, X. Diverse functions of cytochrome c in cell death and disease. Cell Death Differ. 2024, 31, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J. Cytochrome c in cancer therapy and prognosis. Biosci. Rep. 2022, 42, BSR20222171. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef]
- King, L.E.; Hohorst, L.; Garcia-Saez, A.J. Expanding roles of BCL-2 proteins in apoptosis execution and beyond. J. Cell Sci. 2023, 136, jcs260790. [Google Scholar] [CrossRef]
- Duffy, M.J.; Synnott, N.C.; O’Grady, S.; Crown, J. Targeting p53 for the treatment of cancer. Semin. Cancer Biol. 2022, 79, 58–67. [Google Scholar] [CrossRef]
- Zhou, S.; Chai, D.; Wang, X.; Neeli, P.; Yu, X.; Davtyan, A.; Young, K.; Li, Y. AI-powered discovery of a novel p53-Y220C reactivator. Front. Oncol. 2023, 13, 1229696. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Zhang, L.; Zhang, J. Targeting mutant p53 stabilization for cancer therapy. Front. Pharmacol. 2023, 14, 1215995. [Google Scholar] [CrossRef]
- Peter, M.E.; Hadji, A.; Murmann, A.E.; Brockway, S.; Putzbach, W.; Pattanayak, A.; Ceppi, P. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015, 22, 549–559. [Google Scholar] [CrossRef]
- Hetz, C.A.; Hunn, M.; Rojas, P.; Torres, V.; Leyton, L.; Quest, A.F. Caspase-dependent initiation of apoptosis and necrosis by the Fas receptor in lymphoid cells: Onset of necrosis is associated with delayed ceramide increase. J. Cell Sci. 2002, 115, 4671–4683. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and Tumorigenesis: Molecular Mechanisms and Therapeutic Strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Tamm, I.; Wang, Y.; Sausville, E.; Scudiero, D.A.; Vigna, N.; Oltersdorf, T.; Reed, J.C. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998, 58, 5315–5320. [Google Scholar] [PubMed]
- Huang, Y.-H.; Yeh, C.-T. Functional Compartmentalization of HSP60-Survivin Interaction between Mitochondria and Cytosol in Cancer Cells. Cells 2020, 9, 23. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef]
- Shi, Y. Caspase activation, inhibition, and reactivation: A mechanistic view. Protein Sci. 2004, 13, 1979–1987. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J. Initiator caspases in apoptosis signaling pathways. Apoptosis 2002, 7, 313–319. [Google Scholar] [CrossRef]
- Nano, M.; Mondo, J.A.; Harwood, J.; Balasanyan, V.; Montell, D.J. Cell survival following direct executioner-caspase activation. Proc. Natl. Acad. Sci. USA 2023, 120, e2216531120. [Google Scholar] [CrossRef]
- Farzaneh Behelgardi, M.; Gholami Shahvir, Z.; Asghari, S.M. Apoptosis induction in human lung and colon cancer cells via impeding VEGF signaling pathways. Mol. Biol. Rep. 2022, 49, 3637–3647. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Chaudhry, G.E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Garg, V.K.; Goel, N. Chapter Four—Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 125, pp. 73–120. [Google Scholar]
- Lossi, L. The concept of intrinsic versus extrinsic apoptosis. Biochem. J. 2022, 479, 357–384. [Google Scholar] [CrossRef] [PubMed]
- Yanumula, A.; Cusick, J.K. Biochemistry, Extrinsic Pathway of Apoptosis; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fulda, S. Targeting extrinsic apoptosis in cancer: Challenges and opportunities. Semin. Cell Dev. Biol. 2015, 39, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; Frew, A.J.; Smyth, M.J. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer 2008, 8, 782–798. [Google Scholar] [CrossRef]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- El Omari, N.; Bakrim, S.; Bakha, M.; Lorenzo, J.M.; Rebezov, M.; Shariati, M.A.; Aboulaghras, S.; Balahbib, A.; Khayrullin, M.; Bouyahya, A. Natural Bioactive Compounds Targeting Epigenetic Pathways in Cancer: A Review on Alkaloids, Terpenoids, Quinones, and Isothiocyanates. Nutrients 2021, 13, 3714. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- De Luna, F.C.F.; Ferreira, W.A.S.; Casseb, S.M.M.; de Oliveira, E.H.C. Anticancer Potential of Flavonoids: An Overview with an Emphasis on Tangeretin. Pharmaceuticals 2023, 16, 1229. [Google Scholar] [CrossRef]
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 4567. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, Y.J.; Kim, A.; Jang, D.; Kim, M.S.; Seo, D.-H.; Nam, T.G.; Rha, C.-S.; Park, C.-S.; Kim, D.-O. Enrichment of Polyglucosylated Isoflavones from Soybean Isoflavone Aglycones Using Optimized Amylosucrase Transglycosylation. Molecules 2020, 25, 181. [Google Scholar] [CrossRef] [PubMed]
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Szeja, W.; Grynkiewicz, G.; Rusin, A. Isoflavones, their Glycosides and Glycoconjugates. Synthesis and Biological Activity. Curr. Org. Chem. 2017, 21, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Abraham, K.; Appel, K.E.; Lampen, A. Risks and benefits of dietary isoflavones for cancer. Crit. Rev. Toxicol. 2011, 41, 463–506. [Google Scholar] [CrossRef]
- Hwang, S.T.; Yang, M.H.; Baek, S.H.; Um, J.-Y.; Ahn, K.S. Genistin attenuates cellular growth and promotes apoptotic cell death breast cancer cells through modulation of ERalpha signaling pathway. Life Sci. 2020, 263, 118594. [Google Scholar] [CrossRef]
- Ravindranath, M.H.; Muthugounder, S.; Presser, N.; Viswanathan, S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv. Exp. Med. Biol. 2004, 546, 121–165. [Google Scholar] [CrossRef]
- Jung, Y.; Rha, C.-S.; Baik, M.-Y.; Baek, N.-I.; Kim, D.-O. A brief history and spectroscopic analysis of soy isoflavones. Food Sci. Biotechnol. 2020, 29, 1605–1617. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.S.; Song, Y.S. Genistein as a Potential Anticancer Agent against Ovarian Cancer. J. Tradit. Complement. Med. 2012, 2, 96–104. [Google Scholar] [CrossRef]
- Xu, H.; Ma, H.; Zha, L.; Li, Q.; Pan, H.; Zhang, L. Genistein promotes apoptosis of lung cancer cells through the IMPDH2/AKT1 pathway. Am. J. Transl. Res. 2022, 14, 7040–7051. [Google Scholar] [PubMed]
- Hsiao, Y.C.; Peng, S.F.; Lai, K.C.; Liao, C.L.; Huang, Y.P.; Lin, C.C.; Lin, M.L.; Liu, K.C.; Tsai, C.C.; Ma, Y.S.; et al. Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environ. Toxicol. 2019, 34, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Xu, X.; Huang, Y. Daidzin inhibits growth and induces apoptosis through the JAK2/STAT3 in human cervical cancer HeLa cells. Saudi J. Biol. Sci. 2021, 28, 7077–7081. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chauhan, S.S. Daidzein Induces Intrinsic Pathway of Apoptosis along with ER α/β Ratio Alteration and ROS Production. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 603–610. [Google Scholar] [CrossRef]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic Potential of Isoflavones with an Emphasis on Daidzein. Oxid. Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef]
- Hsu, A.; Bray, T.M.; Helferich, W.G.; Doerge, D.R.; Ho, E. Differential effects of whole soy extract and soy isoflavones on apoptosis in prostate cancer cells. Exp. Biol. Med. 2010, 235, 90–97. [Google Scholar] [CrossRef]
- Hua, F.; Li, C.H.; Chen, X.G.; Liu, X.P. Daidzein exerts anticancer activity towards SKOV3 human ovarian cancer cells by inducing apoptosis and cell cycle arrest, and inhibiting the Raf/MEK/ERK cascade. Int. J. Mol. Med. 2018, 41, 3485–3492. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, R.; Yan, W. Glycitin exerts anticancer effect on human lung cancer cells through induction of apoptosis, cell cycle arrest, and inhibition of PI3K/AKT signal pathway. Trop. J. Pharm. Res. 2022, 21, 943–950. [Google Scholar] [CrossRef]
- Zang, Y.Q.; Feng, Y.Y.; Luo, Y.H.; Zhai, Y.Q.; Ju, X.Y.; Feng, Y.C.; Wang, J.R.; Yu, C.Q.; Jin, C.H. Glycitein induces reactive oxygen species-dependent apoptosis and G0/G1 cell cycle arrest through the MAPK/STAT3/NF-κB pathway in human gastric cancer cells. Drug Dev. Res. 2019, 80, 573–584. [Google Scholar] [CrossRef]
- Zhang, B.; Su, J.P.; Bai, Y.; Li, J.; Liu, Y.H. Inhibitory effects of O-methylated isoflavone glycitein on human breast cancer SKBR-3 cells. Int. J. Clin. Exp. Pathol. 2015, 8, 7809–7817. [Google Scholar]
- Piegholdt, S.; Rimbach, G.; Wagner, A.E.J.T.F.J. The phytoestrogen prunetin affects body composition and improves fitness and lifespan in male Drosophila melanogaster. FASEB J. 2016, 30, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Pal, S.; Yadav, M.; Maurya, R.; Trivedi, A.K.; Sanyal, S.; Chattopadhyay, N. Prunetin signals via G-protein-coupled receptor, GPR30(GPER1): Stimulation of adenylyl cyclase and cAMP-mediated activation of MAPK signaling induces Runx2 expression in osteoblasts to promote bone regeneration. J. Nutr. Biochem. 2015, 26, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, Y.; Liu, W.; Song, Z.; Wang, X.; Zhang, X. Prunetin Triggers ROS-mediated Apoptosis through the Suppression of MAPKs/STAT-3/NF-κB-p65 Signaling Pathway in Human Osteosarcoma Cells. Pharmacogn. Mag. 2024, 20, 336–346. [Google Scholar] [CrossRef]
- Köksal Karayildirim, Ç.; Nalbantsoy, A.; Karabay Yavaşoğlu, N. Prunetin inhibits nitric oxide activity and induces apoptosis in urinary bladder cancer cells via CASP3 and TNF-α genes. Mol. Biol. Rep. 2021, 48, 7251–7259. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Seshadri, V.D.; Zhang, J.J.P.M. Anticancer effect of isoflavone prunetin on benzo (a) pyrene induced lung cancer mice model. Pharmacogn. Mag. 2022, 18, 919–925. [Google Scholar]
- Li, G.; Qi, L.; Chen, H.; Tian, G.; Toxicology, M. Involvement of NF-κB/PI3K/AKT signaling pathway in the protective effect of prunetin against a diethylnitrosamine induced hepatocellular carcinogenesis in rats. J. Biochem. Mol. Toxicol. 2022, 36, e23016. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 16398538, Prunetin 4′-O-Glucoside. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Prunetin-4_-O-glucoside (accessed on 8 April 2024).
- Abusaliya, A.; Jeong, S.H.; Bhosale, P.B.; Kim, H.H.; Park, M.Y.; Kim, E.; Won, C.K.; Park, K.I.; Heo, J.D.; Kim, H.W.; et al. Mechanistic Action of Cell Cycle Arrest and Intrinsic Apoptosis via Inhibiting Akt/mTOR and Activation of p38-MAPK Signaling Pathways in Hep3B Liver Cancer Cells by Prunetrin—A Flavonoid with Therapeutic Potential. Nutrients 2023, 15, 3407. [Google Scholar] [CrossRef]
- Abusaliya, A.; Bhosale, P.B.; Kim, H.H.; Park, M.Y.; Jeong, S.H.; Lee, S.; Kim, G.S. Investigation of prunetrin induced G2/M cell cycle arrest and apoptosis via Akt/mTOR/MAPK pathways in hepatocellular carcinoma cells. Biomed. Pharmacother. 2024, 174, 116483. [Google Scholar] [CrossRef]
- Abusaliya, A.; Bhosale, P.B.; Kim, H.H.; Ha, S.E.; Park, M.Y.; Jeong, S.H.; Vetrivel, P.; Park, J.-S.; Kim, G.S. Prunetinoside Inhibits Lipopolysaccharide-Provoked Inflammatory Response via Suppressing NF-κB and Activating the JNK-Mediated Signaling Pathway in RAW264.7 Macrophage Cells. Int. J. Mol. Sci. 2022, 23, 5442. [Google Scholar] [CrossRef]
- Vetrivel, P.; Murugesan, R.; Bhosale, P.B.; Ha, S.E.; Kim, H.H.; Heo, J.D.; Kim, G.S. A Network Pharmacological Approach to Reveal the Pharmacological Targets and Its Associated Biological Mechanisms of Prunetin-5-O-Glucoside against Gastric Cancer. Cancers 2021, 13, 1918. [Google Scholar] [CrossRef]
- Dietel, M.; Lewis, M.A.; Shapiro, S. Hormone replacement therapy: Pathobiological aspects of hormone-sensitive cancers in women relevant to epidemiological studies on HRT: A mini-review. Hum. Reprod. 2005, 20, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, M.; Doseff, A.I. The Targeted Impact of Flavones on Obesity-Induced Inflammation and the Potential Synergistic Role in Cancer and the Gut Microbiota. Molecules 2020, 25, 2477. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.H.; Kim, H.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Hwang, K.H.; Moon, Y.G.; Heo, J.D.; Seong, J.K.; Ahn, M.; et al. Potential Anticancer Effects of Isoflavone Prunetin and Prunetin Glycoside on Apoptosis Mechanisms. Int. J. Mol. Sci. 2024, 25, 11713. https://doi.org/10.3390/ijms252111713
Jeong SH, Kim HH, Park MY, Bhosale PB, Abusaliya A, Hwang KH, Moon YG, Heo JD, Seong JK, Ahn M, et al. Potential Anticancer Effects of Isoflavone Prunetin and Prunetin Glycoside on Apoptosis Mechanisms. International Journal of Molecular Sciences. 2024; 25(21):11713. https://doi.org/10.3390/ijms252111713
Chicago/Turabian StyleJeong, Se Hyo, Hun Hwan Kim, Min Yeong Park, Pritam Bhangwan Bhosale, Abuyaseer Abusaliya, Kwang Hyun Hwang, Yeon Gyu Moon, Jeong Doo Heo, Je Kyung Seong, Meejung Ahn, and et al. 2024. "Potential Anticancer Effects of Isoflavone Prunetin and Prunetin Glycoside on Apoptosis Mechanisms" International Journal of Molecular Sciences 25, no. 21: 11713. https://doi.org/10.3390/ijms252111713
APA StyleJeong, S. H., Kim, H. H., Park, M. Y., Bhosale, P. B., Abusaliya, A., Hwang, K. H., Moon, Y. G., Heo, J. D., Seong, J. K., Ahn, M., Park, K. I., Won, C. K., & Kim, G. S. (2024). Potential Anticancer Effects of Isoflavone Prunetin and Prunetin Glycoside on Apoptosis Mechanisms. International Journal of Molecular Sciences, 25(21), 11713. https://doi.org/10.3390/ijms252111713









