Super-Resolution Microscopy as a Versatile Tool in Probing Molecular Assembly
Abstract
1. Introduction
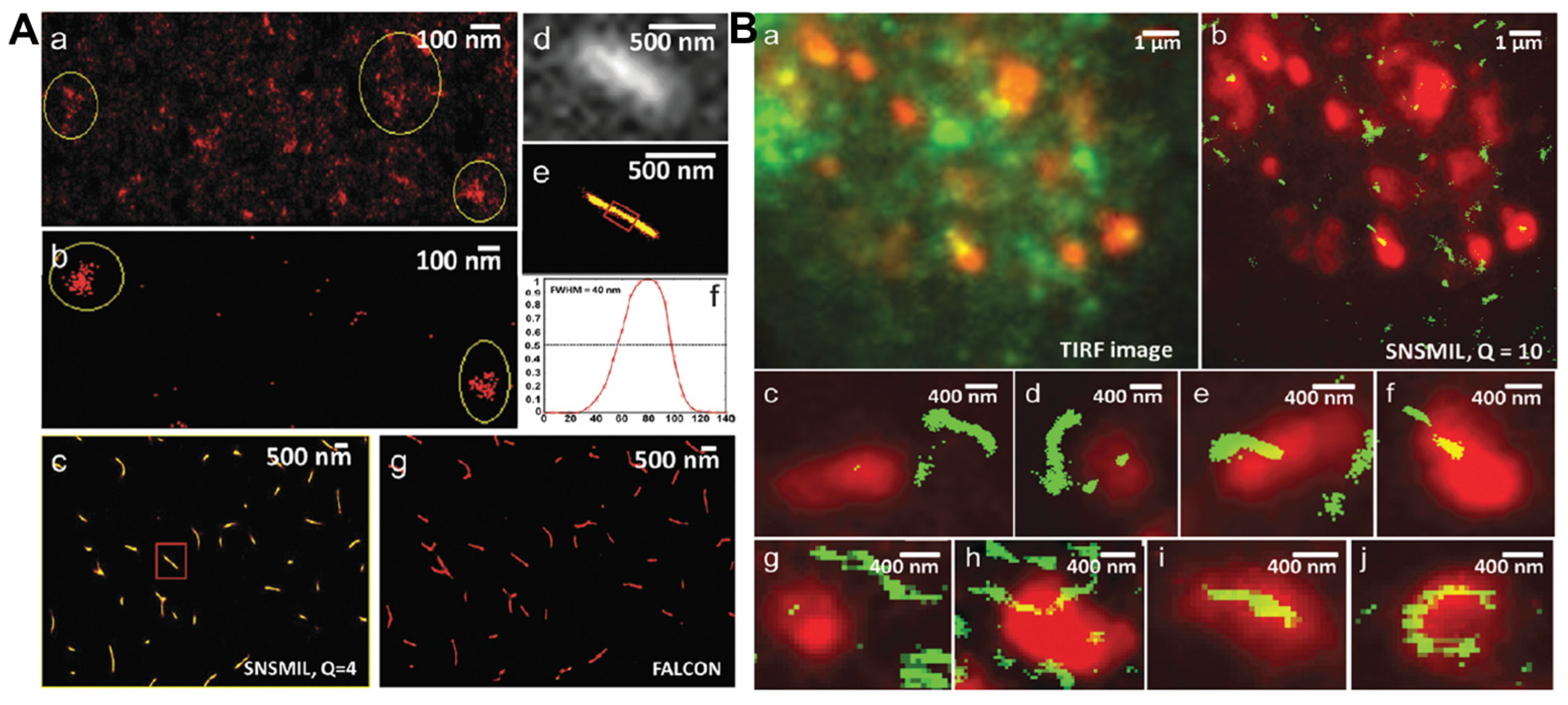
2. Classification of Super-Resolution Imaging Methods and Working Mechanisms
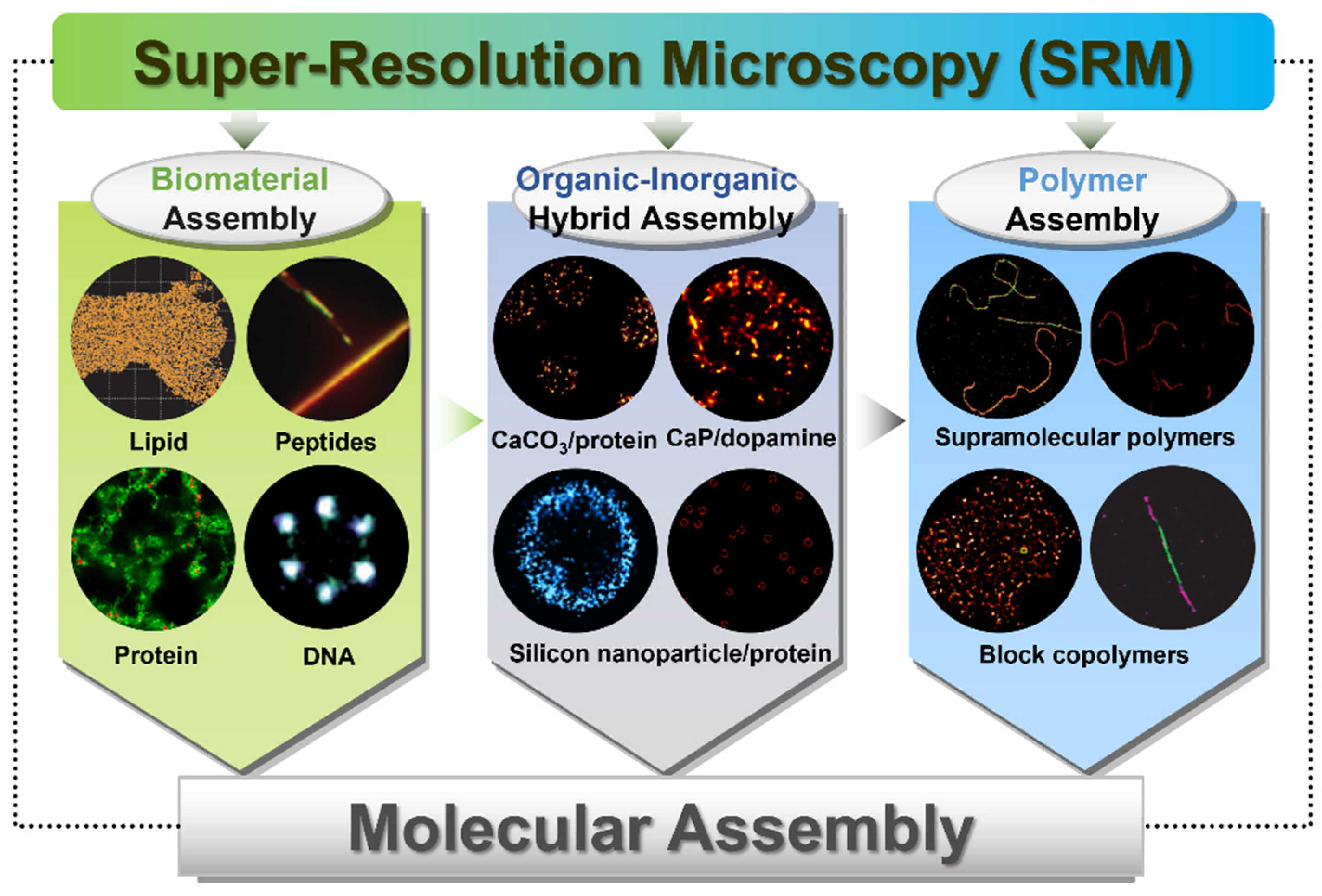
3. Molecular Assemblies Investigated by Super-Resolution Microscopy
3.1. Biomolecular Assemblies
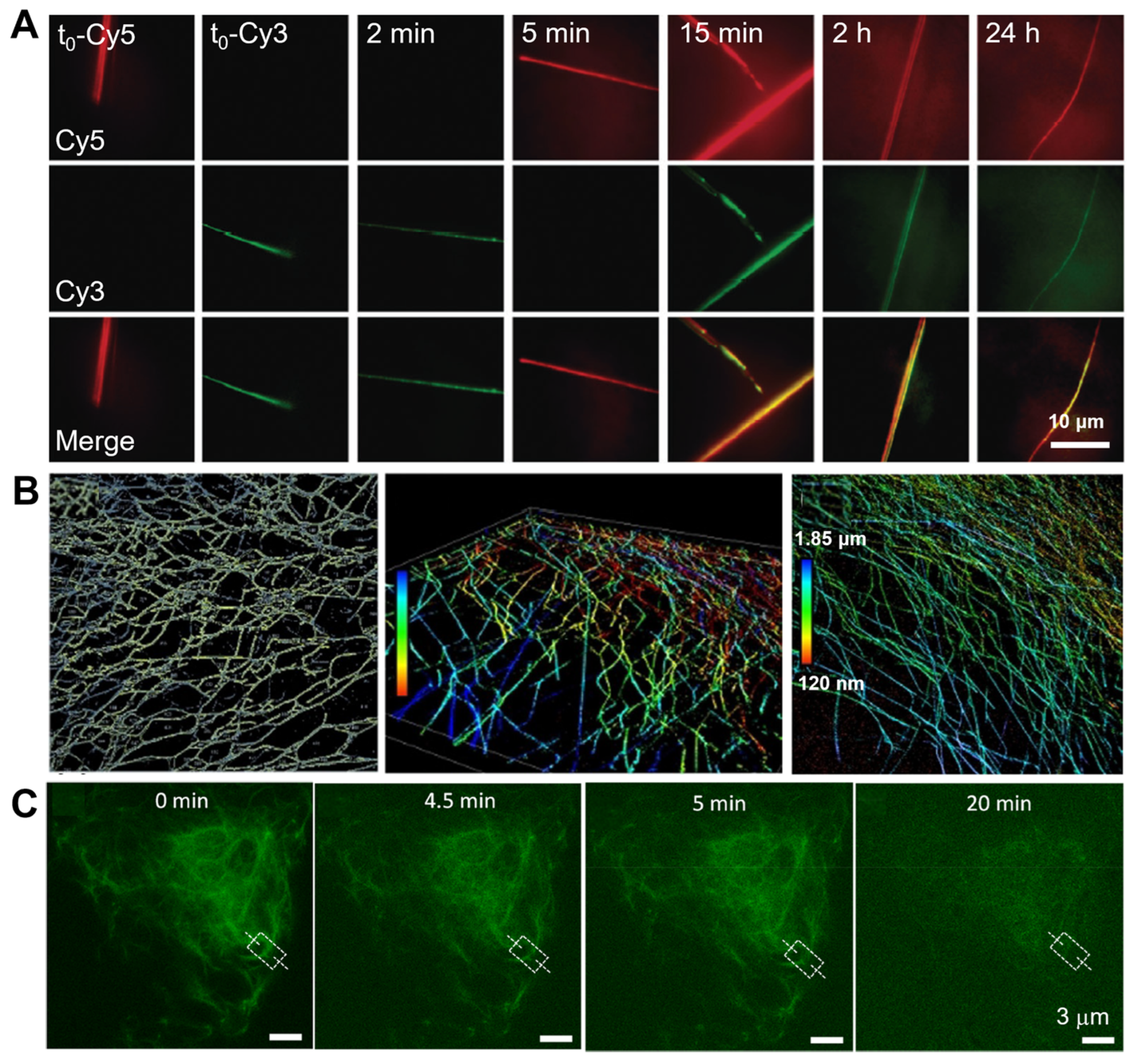
3.1.1. Lipid-Based Molecular Assemblies
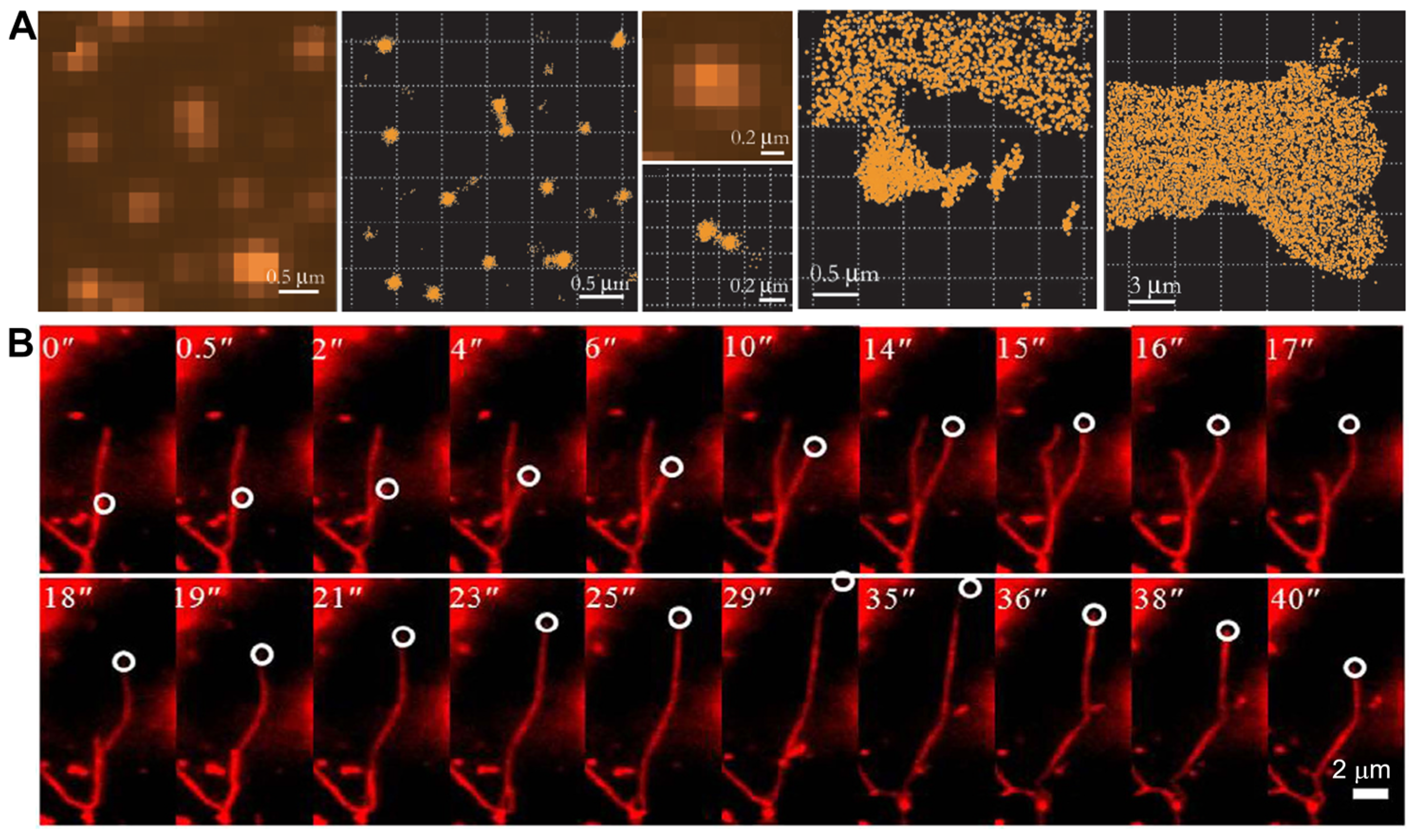
3.1.2. Peptide-Based Molecular Assemblies
3.1.3. Protein-Based Molecular Assemblies

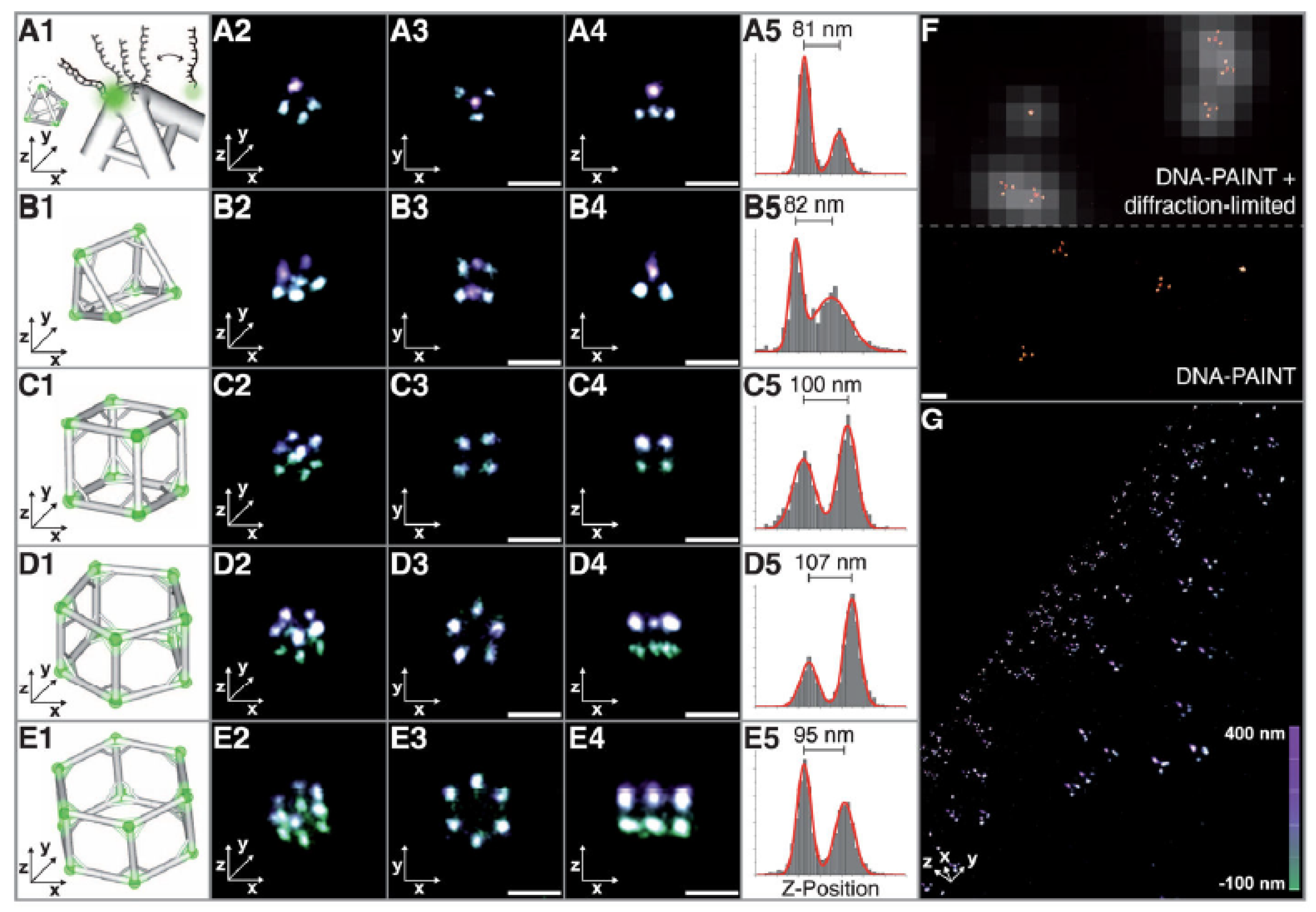
3.1.4. DNA-Based Molecular Assemblies
3.2. Organic–Inorganic Hybrid Assemblies
3.3. Polymer Assemblies
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheehan, F.; Sementa, D.; Jain, A.; Kumar, M.; Tayarani Najjaran, M.; Kroiss, D.; Ulijn, R.V. Peptide-based supramolecular systems chemistry. Chem. Rev. 2021, 121, 13869–13914. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Li, Z.; Li, Q. From single molecule to molecular aggregation science. Coord. Chem. Rev. 2023, 475, 214872. [Google Scholar] [CrossRef]
- Wang, T.; Fei, J.; Dong, Z.; Yu, F.; Li, J. Nanoarchitectonics with a membrane-embedded electron shuttle mimics the bioenergy anabolism of mitochondria. Angew. Chem. Int. Ed. 2024, 63, e202319116. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fei, J.; Yu, F.; Xu, X.; Cui, Y.; Li, J. Nanoarchitectonics of vesicle microreactors for oscillating ATP synthesis and hydrolysis. Angew. Chem. Int. Ed. 2024, e202411981. [Google Scholar]
- Chen, Q.; Bae, S.C.; Granick, S. Directed self-assembly of a colloidal kagome lattice. Nature 2011, 469, 381–384. [Google Scholar] [CrossRef]
- Sharma, A.; Czegel, D.; Lachmann, M.; Kempes, C.P.; Walker, S.I.; Cronin, L. Assembly theory explains and quantifies selection and evolution. Nature 2023, 622, 321–328. [Google Scholar] [CrossRef]
- Sun, X.; Dong, Y.; Liu, Y.; Song, N.; Li, F.; Yang, D. Self-assembly of artificial architectures in living cells-design and applications. Sci. China Chem. 2022, 65, 31–47. [Google Scholar] [CrossRef]
- Stevens, A.J.; Harris, A.R.; Gerdts, J.; Kim, K.H.; Trentesaux, C.; Ramirez, J.T.; McKeithan, W.L.; Fattahi, F.; Klein, O.D.; Fletcher, D.A.; et al. Programming multicellular assembly with synthetic cell adhesion molecules. Nature 2023, 614, 144–152. [Google Scholar] [CrossRef]
- Fu, M.; Burkart, T.; Maryshev, I.; Franquelim, H.G.; Merino-Salomon, A.; Reverte-Lopez, M.; Frey, E.; Schwille, P. Mechanochemical feedback loop drives persistent motion of liposomes. Nat. Phys. 2023, 19, 1211–1218. [Google Scholar] [CrossRef]
- Yuan, C.; Fan, W.; Zhou, P.; Xing, R.; Cao, S.; Yan, X. High-entropy non-covalent cyclic peptide glass. Nat. Nanotechnol. 2024. [Google Scholar] [CrossRef]
- Lei, Z.-C.; Wang, X.; Yang, L.; Qu, H.; Sun, Y.; Yang, Y.; Li, W.; Zhang, W.-B.; Cao, X.-Y.; Fan, C.; et al. What can molecular assembly learn from catalysed assembly in living organisms? Chem. Soc. Rev. 2024, 53, 1892–1914. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Mulvey, J.T.; Carpenter, B.P.; Talosig, R.; Patterson, J.P. A close look at molecular self-assembly with the transmission electron microscope. Chem. Rev. 2021, 121, 14232–14280. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Fei, J.; Wu, A.; Xu, X.; Li, J. Gas-induced phase transition of dipeptide supramolecular assembly. CCS Chem. 2021, 3, 8–16. [Google Scholar] [CrossRef]
- Baimanov, D.; Wu, J.; Chu, R.; Cai, R.; Wang, B.; Cao, M.; Tao, Y.; Liu, J.; Guo, M.; Wang, J.; et al. Immunological responses induced by blood protein coronas on two-dimensional mos2 nanosheets. ACS Nano 2020, 14, 5529–5542. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.W.T.; Hong, Y.; Chen, S.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. A photostable AIE luminogen for specific mitochondrial imaging and tracking. J. Am. Chem. Soc. 2013, 135, 62–65. [Google Scholar] [CrossRef]
- Jia, Y.; Xuan, M.; Feng, X.; Duan, L.; Li, J.; Li, J. Reconstitution of motor proteins through molecular assembly. Chin. J. Chem. 2020, 38, 123–129. [Google Scholar] [CrossRef]
- Kubota, R.; Tanaka, W.; Hamachi, I. Microscopic imaging techniques for molecular assemblies: Electron, atomic force, and confocal microscopies. Chem. Rev. 2021, 121, 14281–14347. [Google Scholar] [CrossRef]
- Mockl, L.; Lamb, D.C.; Brauchle, C. Super-resolved fluorescence microscopy: Nobel prize in chemistry 2014 for Eric Betzig, Stefan Hell, and William, E. Moerner. Angew. Chem. Int. Ed. 2014, 53, 13972–13977. [Google Scholar] [CrossRef]
- Sigal, Y.M.; Zhou, R.; Zhuang, X. Visualizing and discovering cellular structures with super-resolution microscopy. Science 2018, 361, 880–887. [Google Scholar] [CrossRef]
- Pujals, S.; Albertazzi, L. Super-resolution microscopy for nanomedicine research. ACS Nano 2019, 13, 9707–9712. [Google Scholar] [CrossRef]
- Komis, G.; Samajova, O.; Ovecka, M.; Samaj, J. Super-resolution microscopy in plant cell imaging. Trends Plant Sci. 2015, 20, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Andrian, T.; Gonzalez, B.S.; Tholen, M.M.E.; Wang, Y.; Albertazzi, L. Can super-resolution microscopy become a standard characterization technique for materials chemistry? Chem. Sci. 2022, 13, 2152–2166. [Google Scholar] [CrossRef] [PubMed]
- Schubert, V. Super-resolution microscopy-applications in plant cell research. Front. Plant Sci. 2017, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Gallegos Cerda, S.D.; Hernandez Varela, J.D.; Chanona Perez, J.J.; Arredondo Tamayo, B.; Mendez Mendez, J.V. Super-resolution microscopy and their applications in food materials: Beyond the resolution limits of fluorescence microscopy. Food Bioprocess Technol. 2023, 16, 268–288. [Google Scholar] [CrossRef]
- Liu, D.; Fang, G.; Wang, Y.; Meng, C.; Liu, Z.; Chen, Q.; Shao, X. Facile construction of dual-response super-resolution probes for tracking organelles dynamics. Exploration 2024, 4, 20230145. [Google Scholar] [CrossRef]
- Wang, L.; Chen, R.; Han, G.; Liu, X.; Huang, T.; Diao, J.; Sun, Y. Super-resolution analyzing spatial organization of lysosomes with an organic fluorescent probe. Exploration 2022, 2, 20210215. [Google Scholar] [CrossRef]
- Pujals, S.; Feiner Gracia, N.; Delcanale, P.; Voets, I.; Albertazzi, L. Super-resolution microscopy as a powerful tool to study complex synthetic materials. Nat. Rev. Chem. 2019, 3, 68–84. [Google Scholar] [CrossRef]
- Qiao, Q.; Liu, W.; Zhang, Y.; Chen, J.; Wang, G.; Tao, Y.; Miao, L.; Jiang, W.; An, K.; Xu, Z. In situ real-time nanoscale resolution of structural evolution and dynamics of fluorescent self-assemblies by super-resolution imaging. Angew. Chem. Int. Ed. 2022, 61, e202208678. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Xu, Z. Comparison and progress review of various super-resolution fluorescence imaging techniques. Chin. J. Chromatogr. 2021, 39, 1055–1064. [Google Scholar] [CrossRef]
- Peng, S.; Xie, Y.; Wang, L.; Liu, W.; Li, H.; Xu, Z.; Ye, M.; Liu, Z. Exploring the influence of inter- and intra-crystal diversity of surface barriers in zeolites on mass transport by using super-resolution microimaging of time-resolved guest profiles. Angew. Chem. Int. Ed. 2022, 61, e202203903. [Google Scholar] [CrossRef]
- Pohl, D.W.; Denk, W.; Lanz, M. Optical stethoscopy-image recording with resolution lambda/20. Appl. Phys. Lett. 1984, 44, 651–653. [Google Scholar] [CrossRef]
- Betzig, E.; Trautman, J.K.; Harris, T.D.; Weiner, J.S.; Kostelak, R.L. Breaking the diffraction barrier—Optical microscopy on a nanometric scale. Science 1991, 251, 1468–1470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lee, H.; Xiong, Y.; Sun, C.; Zhang, X. Far-field optical hyperlens magnifying sub-diffraction-limited objects. Science 2007, 315, 1686. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shroff, H. Faster, sharper, and deeper: Structured illumination microscopy for biological imaging. Nat. Methods 2018, 15, 1011–1019. [Google Scholar] [CrossRef]
- Heintzmann, R.; Huser, T. Super-resolution structured illumination microscopy. Chem. Rev. 2017, 117, 13890–13908. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA nanotechnology-enabled drug delivery systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef]
- Schoen, I.; Ries, J.; Klotzsch, E.; Ewers, H.; Vogel, V. Binding-activated localization microscopy of DNA structures. Nano Lett. 2011, 11, 4008–4011. [Google Scholar] [CrossRef]
- Jungmann, R.; Steinhauer, C.; Scheible, M.; Kuzyk, A.; Tinnefeld, P.; Simmel, F.C. Single-molecule kinetics and super-resolution microscopy by fluorescence imaging of transient binding on DNA origami. Nano Lett. 2010, 10, 4756–4761. [Google Scholar] [CrossRef]
- Fuerstenberg, A.; Heilemann, M. Single-molecule localization microscopy—Near-molecular spatial resolution in light microscopy with photoswitchable fluorophores. Phys. Chem. Chem. Phys. 2013, 15, 14919–14930. [Google Scholar] [CrossRef]
- Hell, S.; Stelzer, E.H.K. Fundamental improvement of resolution with a 4PI-confocal fluorescence microscope using two-photon excitation. Opt. Commun. 1992, 93, 277–282. [Google Scholar] [CrossRef]
- Gustafsson, M.G.L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 2000, 198, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, M.G.L.; Agard, D.A.; Sedat, J.W. (Im)-m-5: 3D widefield light microscopy with better than 100 nm axial resolution. J. Microsc. 1999, 195, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated-emission—Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Hell, S.W.; Kroug, M. Ground-state-depletion fluorescence microscopy—A concept for breaking the diffraction resolution limit. Appl. Phys. B-Lasers Opt. 1995, 60, 495–497. [Google Scholar] [CrossRef]
- Gustafsson, M.G.L. Nonlinear structured-illumination microscopy: Wide-field fluorescence imaging with theoretically unlimited resolution. Proc. Natl. Acad. Sci. USA 2005, 102, 13081–13086. [Google Scholar] [CrossRef]
- Heintzmann, R.; Jovin, T.M.; Cremer, C. Saturated patterned excitation microscopy—A concept for optical resolution improvement. J. Opt. Soc. Am. A 2002, 19, 1599–1609. [Google Scholar] [CrossRef]
- Bornfleth; Sätzler; Eils; Cremer. High-precision distance measurements and volume-conserving segmentation of objects near and below the resolution limit in three-dimensional confocal fluorescence microscopy. J. Microsc. 1998, 189, 118–136. [Google Scholar] [CrossRef]
- Foelling, J.; Bossi, M.; Bock, H.; Medda, R.; Wurm, C.A.; Hein, B.; Jakobs, S.; Eggeling, C.; Hell, S.W. Fluorescence nanoscopy by ground-state depletion and single-molecule return. Nat. Methods 2008, 5, 943–945. [Google Scholar] [CrossRef]
- Hess, S.T.; Girirajan, T.P.K.; Mason, M.D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 2006, 91, 4258–4272. [Google Scholar] [CrossRef]
- Rust, M.J.; Bates, M.; Zhuang, X.W. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Heilemann, M.; van de Linde, S.; Schuttpelz, M.; Kasper, R.; Seefeldt, B.; Mukherjee, A.; Tinnefeld, P.; Sauer, M. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. 2008, 47, 6172–6176. [Google Scholar] [CrossRef]
- Sharonov, A.; Hochstrasser, R.M. Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc. Natl. Acad. Sci. USA 2006, 103, 18911–18916. [Google Scholar] [CrossRef]
- Steinhauer, C.; Forthmann, C.; Vogelsang, J.; Tinnefeld, P. Superresolution microscopy on the basis of engineered dark states. J. Am. Chem. Soc. 2008, 130, 16840–16841. [Google Scholar] [CrossRef]
- Qu, X.H.; Wu, D.; Mets, L.; Scherer, N.F. Nanometer-localized multiple single-molecule fluorescence microscopy. Proc. Natl. Acad. Sci. USA 2004, 101, 11298–11303. [Google Scholar] [CrossRef]
- Dedecker, P.; Muls, B.; Deres, A.; Uji-i, H.; Hotta, J.-i.; Sliwa, M.; Soumillion, J.-P.; Muellen, K.; Enderlein, J.; Hofkens, J. Defocused wide-field imaging unravels structural and temporal heterogeneity in complex systems. Adv. Mater. 2009, 21, 1079–1090. [Google Scholar] [CrossRef]
- Kiuchi, T.; Higuchi, M.; Takamura, A.; Maruoka, M.; Watanabe, N. Multitarget super-resolution microscopy with high-density labeling by exchangeable probes. Nat. Methods 2015, 12, 743–746. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, R.; Wang, Z.; Zhou, Y.; Shen, Q.; Ji, W.; Dang, D.; Meng, L.; Tang, B.Z. Recent advances in luminescent materials for super-resolution imaging via stimulated emission depletion nanoscopy. Chem. Soc. Rev. 2021, 50, 667–690. [Google Scholar] [CrossRef]
- Mueller, T.; Schumann, C.; Kraegeloh, A. STED microscopy and its applications: New insights into cellular processes on the nanoscale. ChemPhysChem 2012, 13, 1986–2000. [Google Scholar] [CrossRef]
- Dai, M.; Jungmann, R.; Yin, P. Optical imaging of individual biomolecules in densely packed clusters. Nat. Nanotechnol. 2016, 11, 798–807. [Google Scholar] [CrossRef]
- Whelan, D.R.; Bell, T.D.M. Super-resolution single-molecule localization microscopy: Tricks of the trade. J. Phys. Chem. Lett. 2015, 6, 374–382. [Google Scholar] [CrossRef]
- Hansel, C.S.; Holme, M.N.; Gopal, S.; Stevens, M.M. Advances in high-resolution microscopy for the study of intracellular interactions with biomaterials. Biomaterials 2020, 226, 119406. [Google Scholar] [CrossRef]
- Deussner-Helfmann, N.S.; Auer, A.; Strauss, M.T.; Malkusch, S.; Dietz, M.S.; Barth, H.D.; Jungmann, R.; Heilemann, M. Correlative single-molecule FRET and DNA-PAINT imaging. Nano Lett. 2018, 18, 4626–4630. [Google Scholar] [CrossRef]
- Oleksiievets, N.; Mathew, C.; Thiele, J.C.; Gallea, J.I.; Nevskyi, O.; Gregor, I.; Weber, A.; Tsukanov, R.; Enderlein, J. Single-molecule fluorescence lifetime imaging using wide-field and confocal-laser scanning microscopy: A comparative analysis. Nano Lett. 2022, 22, 6454–6461. [Google Scholar] [CrossRef]
- Oleksiievets, N.; Sargsyan, Y.; Thiele, J.C.; Mougios, N.; Sograte-Idrissi, S.; Nevskyi, O.; Gregor, I.; Opazo, F.; Thoms, S.; Enderlein, J.; et al. Fluorescence lifetime DNA-PAINT for multiplexed super-resolution imaging of cells. Commun. Biol. 2022, 5, 38. [Google Scholar] [CrossRef]
- Ou, L.; Chen, H.; Yuan, B.; Yang, K. Membrane-specific binding of 4 nm lipid nanoparticles mediated by an entropy-driven interaction mechanism. ACS Nano 2022, 16, 18090–18100. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, H.; Li, Y.-X.; He, K.; Yang, K.; Pang, H.-B. Synergistic entry of individual nanoparticles into mammalian cells driven by free energy decline and regulated by their sizes. ACS Nano 2022, 16, 5885–5897. [Google Scholar] [CrossRef]
- Kuo, C.; Hochstrasser, R.M. Super-resolution microscopy of lipid bilayer phases. J. Am. Chem. Soc. 2011, 133, 4664–4667. [Google Scholar] [CrossRef]
- Bongiovanni, M.N.; Godet, J.; Horrocks, M.H.; Tosatto, L.; Carr, A.R.; Wirthensohn, D.C.; Ranasinghe, R.T.; Lee, J.-E.; Ponjavic, A.; Fritz, J.V.; et al. Multi-dimensional super-resolution imaging enables surface hydrophobicity mapping. Nat. Commun. 2016, 7, 13544. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Moon, S.; Kenny, S.J.; Xu, K. Spectrally resolved and functional super-resolution microscopy via ultrahigh-throughput single-molecule spectroscopy. Acc. Chem. Res. 2018, 51, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dai, L.; Wang, A.; Woelk, C.; Dobner, B.; Brezesinski, G.; Tang, Y.; Wang, X.; Li, J. The directional observation of highly dynamic membrane tubule formation induced by engulfed liposomes. Sci. Rep. 2015, 5, 16559. [Google Scholar] [CrossRef] [PubMed]
- Boreham, A.; Volz, P.; Peters, D.; Keck, C.M.; Alexiev, U. Determination of nanostructures and drug distribution in lipid nanoparticles by single molecule microscopy. Eur. J. Pharm. Biopharm. 2017, 110, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, L.; Spenkelink, L.M.; Ranson, M.; van Oijen, A.M.; Vine, K.L. Quantification of ligand density and stoichiometry on the surface of liposomes using single-molecule fluorescence imaging. J. Control. Release 2018, 278, 80–86. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef]
- Tao, K.; Makam, P.; Aizen, R.; Gazit, E. Self-assembling peptide semiconductors. Science 2017, 358, eaam9756. [Google Scholar] [CrossRef]
- Li, J.; Zhan, Z.; Du, X.; Wang, J.; Hong, B.; Xu, B. Selection of secondary structures of heterotypic supramolecular peptide assemblies by an enzymatic reaction. Angew. Chem. Int. Ed. 2018, 57, 11716–11721. [Google Scholar] [CrossRef]
- Blum, A.P.; Kammeyer, J.K.; Rush, A.M.; Callmann, C.E.; Hahn, M.E.; Gianneschi, N.C. Stimuli-responsive nanomaterials for biomedical applications. J. Am. Chem. Soc. 2015, 137, 2140–2154. [Google Scholar] [CrossRef]
- Callmann, C.E.; Thompson, M.P.; Gianneschi, N.C. Poly(peptide): Synthesis, structure, and function of peptide-polymer amphiphiles and protein-like polymers. Acc. Chem. Res. 2020, 53, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qiao, Z.; Wang, L.; Wang, H. Programmable construction of peptide-based materials in living subjects: From modular design and morphological control to theranostics. Adv. Mater. 2019, 31, 1804971. [Google Scholar] [CrossRef]
- Qi, G.; Gao, Y.; Wang, L.; Wang, H. Self-assembled peptide-based nanomaterials for biomedical imaging and therapy. Adv. Mater. 2018, 30, 1703444. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sharma, A.; Qi, J.; Peng, X.; Lee, D.Y.; Hu, R.; Lin, D.; Qu, J.; Kim, J.S. Super-resolution fluorescent materials: An insight into design and bioimaging applications. Chem. Soc. Rev. 2016, 45, 4651–4667. [Google Scholar] [CrossRef] [PubMed]
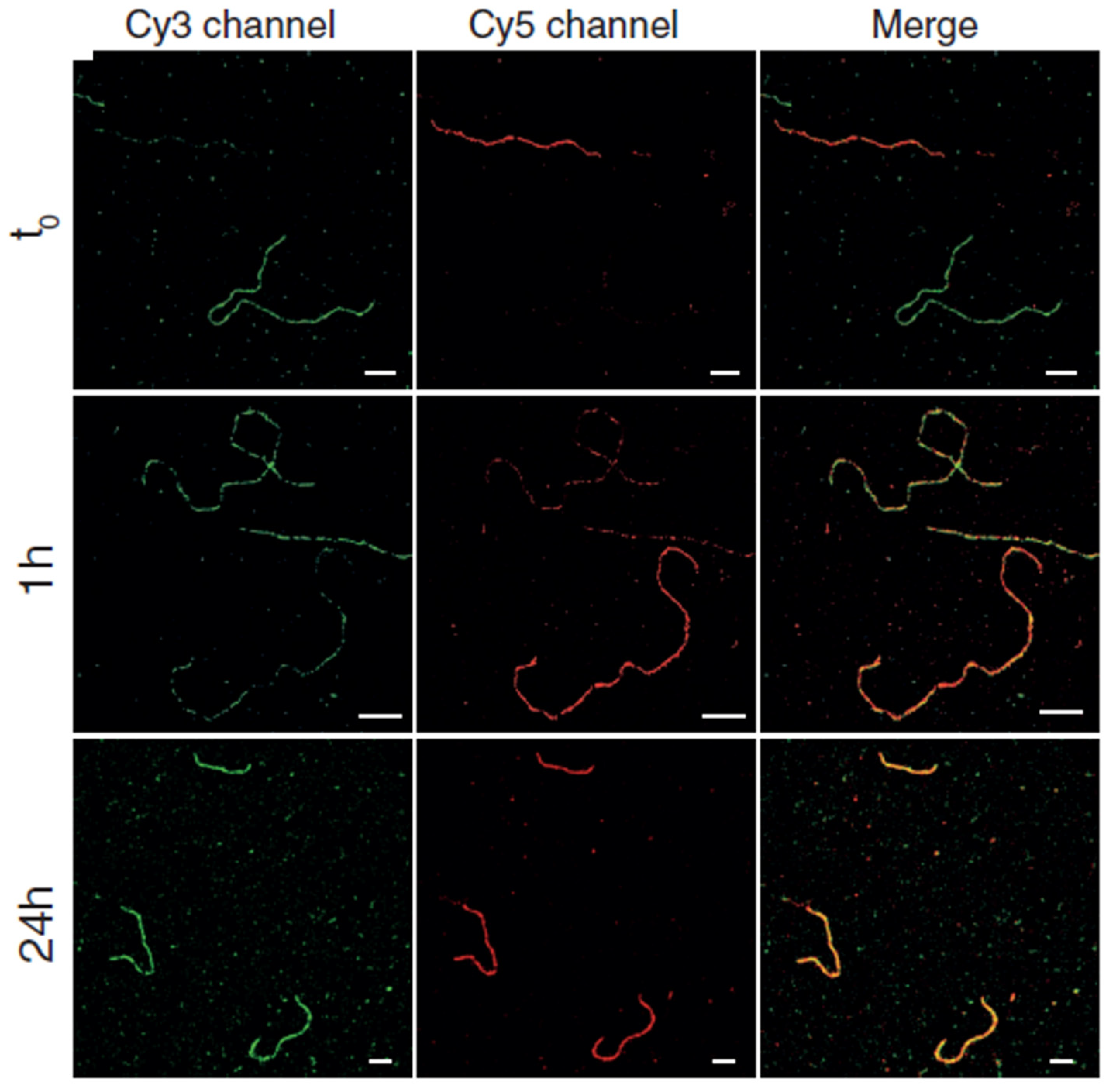
- da Silva, R.M.P.; van der Zwaag, D.; Albertazzi, L.; Lee, S.S.; Meijer, E.W.; Stupp, S.I. Super-resolution microscopy reveals structural diversity in molecular exchange among peptide amphiphile nanofibres. Nat. Commun. 2016, 7, 11561. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, C.; Fu, M.; Dai, L.; Li, J.; Gao, Y. Dynamic detection of active enzyme instructed supramolecular assemblies in situ via super-resolution microscopy. ACS Nano 2020, 14, 4882–4889. [Google Scholar] [CrossRef]
- Chien, M.; Carlini, A.S.; Hu, D.; Barback, C.V.; Rush, A.M.; Hall, D.J.; Orr, G.; Gianneschi, N.C. Enzyme-directed assembly of nanoparticles in tumors monitored by in vivo whole animal imaging and ex vivo super-resolution fluorescence imaging. J. Am. Chem. Soc. 2013, 135, 18710–18713. [Google Scholar] [CrossRef][Green Version]
- Pujals, S.; Tao, K.; Terradellas, A.; Gazit, E.; Albertazzi, L. Studying structure and dynamics of self-assembled peptide nanostructures using fluorescence and super resolution microscopy. Chem. Commun. 2017, 53, 7294–7297. [Google Scholar] [CrossRef]
- Fuentes, E.; Bohacova, K.; Fuentes Caparros, A.M.; Schweins, R.; Draper, E.R.; Adams, D.J.; Pujals, S.; Albertazzi, L. PAINT-ing fluorenylmethoxycarbonyl (Fmoc)-diphenylalanine hydrogels. Chem. Eur. J. 2020, 26, 9869–9873. [Google Scholar] [CrossRef]
- Kumar, M.; Son, J.; Huang, R.H.; Sementa, D.; Lee, M.; O’Brien, S.; Ulijn, R.V. In situ, noncovalent labeling and stimulated emission depletion-based super-resolution imaging of supramolecular peptide nanostructures. ACS Nano 2020, 14, 15056–15063. [Google Scholar] [CrossRef]
- Ye, Z.; Wei, L.; Li, Y.; Xiao, L. Efficient modulation of β-amyloid peptide fibrillation with polymer nanoparticles revealed by super-resolution optical microscopy. Anal. Chem. 2019, 91, 8582–8590. [Google Scholar] [CrossRef]
- Beun, L.H.; Albertazzi, L.; van der Zwaag, D.; de Vries, R.; Stuart, M.A.C. Unidirectional living growth of self assembled protein nanofibrils revealed by super-resolution microscopy. ACS Nano 2016, 10, 4973–4980. [Google Scholar] [CrossRef]
- Bonilla, J.C.; Clausen, M.P. Super-resolution microscopy to visualize and quantify protein microstructural organization in food materials and its relation to rheology: Egg white proteins. Food Hydrocoll. 2022, 124, 107281. [Google Scholar] [CrossRef]
- Li, R.; Ebbesen, M.F.; Glover, Z.J.; Jaeger, T.C.; Rovers, T.A.M.; Svensson, B.; Brewer, J.R.; Simonsen, A.C.; Ipsen, R.; Hougaard, A.B. Discriminating between different proteins in the microstructure of acidified milk gels by super-resolution microscopy. Food Hydrocoll. 2023, 138, 108468. [Google Scholar] [CrossRef]
- Glover, Z.J.; Bisgaard, A.H.; Andersen, U.; Povey, M.J.; Brewer, J.R.; Simonsen, A.C. Cross-correlation analysis to quantify relative spatial distributions of fat and protein in super-resolution microscopy images of dairy gels. Food Hydrocoll. 2019, 97, 105225. [Google Scholar] [CrossRef]
- Kong, G.; Xiong, M.; Liu, L.; Hu, L.; Meng, H.-M.; Ke, G.; Zhang, X.-B.; Tan, W. DNA origami-based protein networks: From basic construction to emerging applications. Chem. Soc. Rev. 2021, 50, 1846–1873. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Dong, B.; Wang, F.; Fan, C.; Zuo, X. DNA nanotechnology-empowered nanoscopic imaging of biomolecules. Chem. Soc. Rev. 2021, 50, 5650–5667. [Google Scholar] [CrossRef]
- Posnjak, G.; Yin, X.; Butler, P.; Bienek, O.; Dass, M.; Lee, S.; Sharp, I.D.; Liedl, T. Diamond-lattice photonic crystals assembled from DNA origami. Science 2024, 384, 781–785. [Google Scholar] [CrossRef]
- Rothemund, P.W.K. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef]
- LaBean, T.H.; Li, H. Constructing novel materials with DNA. Nano Today 2007, 2, 26–35. [Google Scholar] [CrossRef]
- Andersen, E.S.; Dong, M.; Nielsen, M.M.; Jahn, K.; Subramani, R.; Mamdouh, W.; Golas, M.M.; Sander, B.; Stark, H.; Oliveira, C.L.P.; et al. Self-assembly of a nanoscale DNA box with a controllable lid. Nature 2009, 459, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Jungmann, R.; Scheible, M.; Simmel, F.C. Nanoscale imaging in DNA nanotechnology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.P.; Ha, T.; Selvin, P.R. Single-molecule high-resolution imaging with photobleaching. Proc. Natl. Acad. Sci. USA 2004, 101, 6462–6465. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, C.; Jungmann, R.; Sobey, T.L.; Simmel, F.C.; Tinnefeld, P. DNA origami as a nanoscopic ruler for super-resolution microscopy. Angew. Chem. Int. Ed. 2009, 48, 8870–8873. [Google Scholar] [CrossRef] [PubMed]
- Nieves, D.J.; Gaus, K.; Baker, M.A.B. DNA-based super-resolution microscopy: DNA-PAINT. Genes 2018, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Szalai, A.M.; Siarry, B.; Lukin, J.; Giusti, S.; Unsain, N.; Caceres, A.; Steiner, F.; Tinnefeld, P.; Refojo, D.; Jovin, T.M.; et al. Super-resolution imaging of energy transfer by intensity-based STED-FRET. Nano Lett. 2021, 21, 2296–2303. [Google Scholar] [CrossRef]
- Miriklis, E.L.; Rozario, A.M.; Rothenberg, E.; Bell, T.D.M.; Whelan, D.R. Understanding DNA organization, damage, and repair with super-resolution fluorescence microscopy. Methods Appl. Fluoresc. 2021, 9, 032002. [Google Scholar] [CrossRef]
- Fu, M.; Dai, L.; Jiang, Q.; Tang, Y.; Zhang, X.; Ding, B.; Li, J. Observation of intracellular interactions between DNA origami and lysosomes by the fluorescence localization method. Chem. Commun. 2016, 52, 9240–9242. [Google Scholar] [CrossRef]
- Iinuma, R.; Ke, Y.; Jungmann, R.; Schlichthaerle, T.; Woehrstein, J.B.; Yin, P. Polyhedra self-assembled from DNA tripods and characterized with 3D DNA-PAINT. Science 2014, 344, 65–69. [Google Scholar] [CrossRef]
- Wickham, S.F.J.; Auer, A.; Min, J.; Ponnuswamy, N.; Woehrstein, J.B.; Schueder, F.; Strauss, M.T.; Schnitzbauer, J.; Nathwani, B.; Zhao, Z.; et al. Complex multicomponent patterns rendered on a 3D DNA-barrel pegboard. Nat. Commun. 2020, 11, 5768. [Google Scholar] [CrossRef]
- Tas, R.P.; Albertazzi, L.; Voets, I.K. Small peptide-protein interaction pair for genetically encoded, fixation compatible peptide-PAINT. Nano Lett. 2021, 21, 9509–9516. [Google Scholar] [CrossRef]
- Zanacchi, F.C.; Manzo, C.; Alvarez, A.S.; Derr, N.D.; Garcia-Parajo, M.F.; Lakadamyali, M. A DNA origami platform for quantifying protein copy number in super-resolution. Nat. Methods 2017, 14, 789–792. [Google Scholar] [CrossRef]
- Liu, N.; Dai, M.; Saka, S.K.; Yin, P. Super-resolution labelling with action-PAINT. Nat. Chem. 2019, 11, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yu, C.C.; Boyden, E.S.; Zhuang, X.; Kosuri, P. Tetra-gel enables superior accuracy in combined super-resolution imaging and expansion microscopy. Sci. Rep. 2021, 11, 16944. [Google Scholar] [CrossRef] [PubMed]
- Green, C.M.; Hughes, W.L.; Graugnard, E.; Kuang, W. Correlative super-resolution and atomic force microscopy of DNA nanostructures and characterization of addressable site defects. ACS Nano 2021, 15, 11597–11606. [Google Scholar] [CrossRef] [PubMed]
- Schueder, F.; Lara Gutierrez, J.; Beliveau, B.J.; Saka, S.K.; Sasaki, H.M.; Woehrstein, J.B.; Strauss, M.T.; Grabmayr, H.; Yin, P.; Jungmann, R. Multiplexed 3D super-resolution imaging of whole cells using spinning disk confocal microscopy and DNA-PAINT. Nat. Commun. 2017, 8, 2090. [Google Scholar] [CrossRef] [PubMed]
- Veis, A. A window on biomineralization. Science 2005, 307, 1419–1420. [Google Scholar] [CrossRef]
- Nudelman, F.; Sommerdijk, N. Biomineralization as an inspiration for materials chemistry. Angew. Chem. Int. Ed. 2012, 51, 6582–6596. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef]
- Crookes Goodson, W.J.; Slocik, J.M.; Naik, R.R. Bio-directed synthesis and assembly of nanomaterials. Chem. Soc. Rev. 2008, 37, 2403–2412. [Google Scholar] [CrossRef]
- Mirabello, G.; Lenders, J.J.M.; Sommerdijk, N.A.J.M. Bioinspired synthesis of magnetite nanoparticles. Chem. Soc. Rev. 2016, 45, 5085–5106. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, J.; Aymonier, C.; Fermani, S.; Kralj, D.; Falini, G.; Zhou, C. Calcium carbonate: Controlled synthesis, surface functionalization, and nanostructured materials. Chem. Soc. Rev. 2022, 51, 7883–7943. [Google Scholar] [CrossRef]
- Fu, M.; Wang, A.; Zhang, X.; Dai, L.; Li, J. Direct observation of the distribution of gelatin in calcium carbonate crystals by super-resolution fluorescence microscopy. Angew. Chem. Int. Ed. 2016, 55, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Du, J.; Xie, L.; Zhang, R. Direct observation of nacre proteins in the whole calcite by super resolution microscopy reveals diverse occlusion patterns. Cryst. Growth Des. 2017, 17, 1966–1976. [Google Scholar] [CrossRef]
- Kong, J.; Liu, C.; Yang, D.; Yan, Y.; Chen, Y.; Huang, J.; Liu, Y.; Zheng, G.; Xie, L.; Zhang, R. Alv protein plays opposite roles in the transition of amorphous calcium carbonate to calcite and aragonite during shell formation. Cryst. Growth Des. 2018, 18, 3794–3804. [Google Scholar] [CrossRef]
- Yang, X.; Yang, D.; Yan, Y.; Li, S.; Han, Z.; Ji, Y.; Zheng, G.; Xie, L.; Zhang, R. A novel matrix protein pfx regulates shell ultrastructure by binding to specific calcium carbonate crystal faces. Int. J. Biol. Macromol. 2020, 156, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Jia, Y.; Wang, C.; Xia, J.; Dai, L.; Li, J. Dopamine-mediated biomineralization of calcium phosphate as a strategy to facilely synthesize functionalized hybrids. J. Phys. Chem. Lett. 2021, 12, 10235–10241. [Google Scholar] [CrossRef] [PubMed]
- Ihli, J.; Green, D.C.; Lynch, C.; Holden, M.A.; Lee, P.A.; Zhang, S.; Robinson, I.K.; Webb, S.E.D.; Meldrum, F.C. Super-resolution microscopy reveals shape and distribution of dislocations in single-crystal nanocomposites. Angew. Chem. Int. Ed. 2019, 58, 17328–17334. [Google Scholar] [CrossRef]
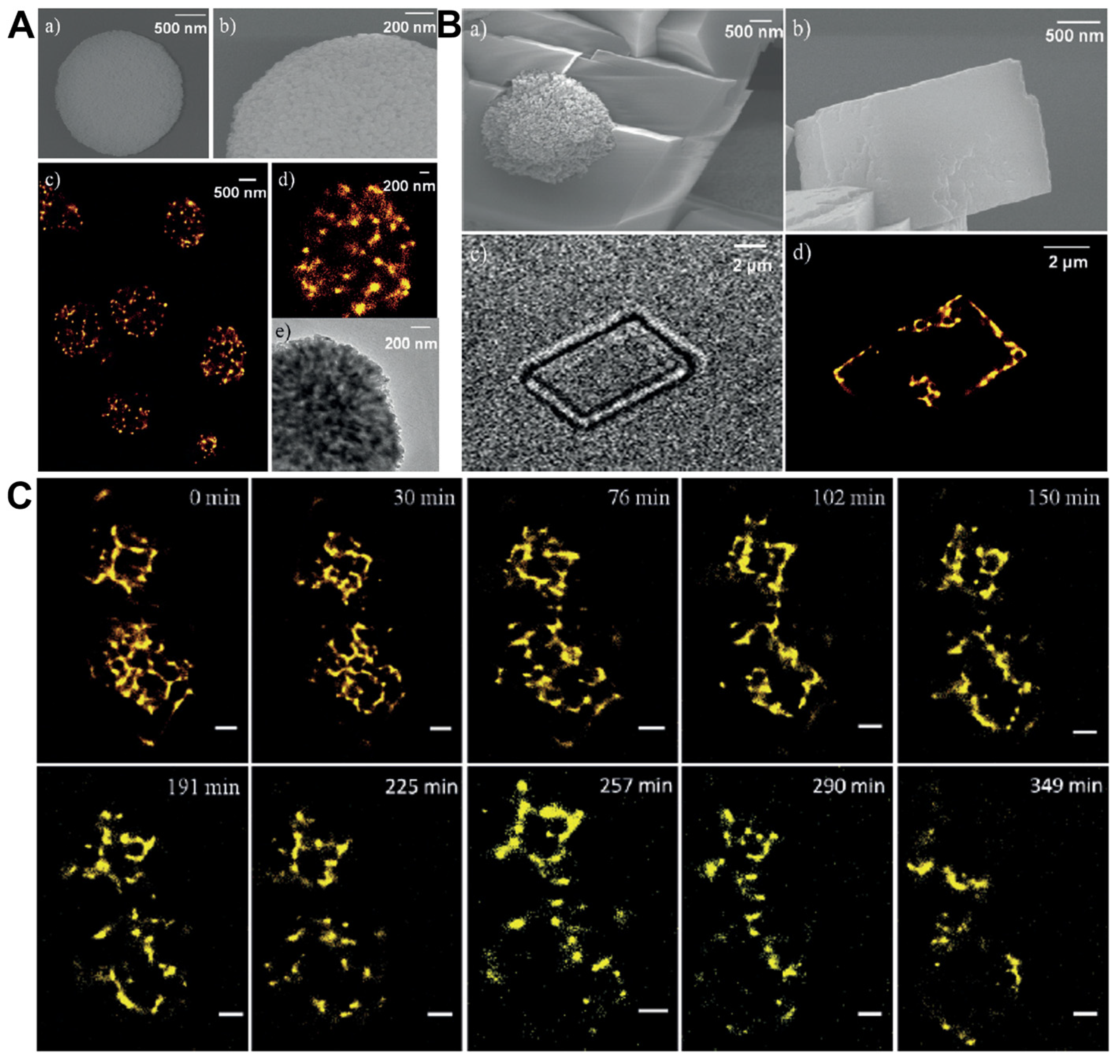
- Groger, P.; Poulsen, N.; Klemm, J.; Kroger, N.; Schlierf, M. Establishing super-resolution imaging for proteins in diatom biosilica. Sci. Rep. 2016, 6, 36824. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, S.; Ma, W.; Wu, Y.; Xin, R.; Bai, Y.; Chen, Z.; Xu, J.; Ge, J. Direct imaging of protein clusters in metal-organic frameworks. J. Am. Chem. Soc. 2024, 146, 12565–12576. [Google Scholar] [CrossRef]
- Dolci, M.; Wang, Y.; Nooteboom, S.W.; Rodriguez, P.E.D.S.; Sanchez, S.; Albertazzi, L.; Zijlstra, P. Real-time optical tracking of protein corona formation on single nanoparticles in serum. ACS Nano 2023, 17, 20167–20178. [Google Scholar] [CrossRef]
- Traldi, F.; Liu, P.; Albino, I.; Ferreira, L.; Zarbakhsh, A.; Resmini, M. Protein-nanoparticle interactions govern the interfacial behavior of polymeric nanogels: Study of protein corona formation at the air/water interface. Int. J. Mol. Sci. 2023, 24, 2810. [Google Scholar] [CrossRef]
- Nienhaus, K.; Nienhaus, G.U. Mechanistic understanding of protein corona formation around nanoparticles: Old puzzles and new insights. Small 2023, 19, 2301663. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A. The biomolecular corona of nanomedicines: Effects on nanomedicine outcomes and emerging opportunities. Curr. Opin. Biotechnol. 2024, 87, 103101. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Perez, A.; Zhang, M.; Fitzpatrick, L.W.; Izquierdo-Lozano, C.; Albertazzi, L. Advanced optical imaging for the rational design of nanomedicines. Adv. Drug Deliv. Rev. 2024, 204, 115138. [Google Scholar] [CrossRef] [PubMed]
- Baimanov, D.; Cai, R.; Chen, C. Understanding the chemical nature of nanoparticle-protein interactions. Bioconjug. Chem. 2019, 30, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, K.; Wang, H.; Nienhaus, G.U. Nanoparticles for biomedical applications: Exploring and exploiting molecular interactions at the nano-bio interface. Mater. Today Adv. 2020, 5, 100036. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Cai, R.; Ren, J.; Guo, M.; Wei, T.; Liu, Y.; Xie, C.; Zhang, P.; Guo, Z.; Chetwynd, A.J.; Ke, P.C.; et al. Dynamic intracellular exchange of nanomaterials’ protein corona perturbs proteostasis and remodels cell metabolism. Proc. Natl. Acad. Sci. USA 2022, 119, e2200363119. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Ren, J.; Andrikopoulos, N.; Velonia, K.; Tang, H.; Cai, R.; Ding, F.; Ke, P.C.; Chen, C. Chemical and biophysical signatures of the protein corona in nanomedicine. J. Am. Chem. Soc. 2022, 144, 9184–9205. [Google Scholar] [CrossRef]
- Feiner Gracia, N.; Beck, M.; Pujals, S.; Tosi, S.; Mandal, T.; Buske, C.; Linden, M.; Albertazzi, L. Super-resolution microscopy unveils dynamic heterogeneities in nanoparticle protein corona. Small 2017, 13, 1701631. [Google Scholar] [CrossRef]
- Khan, A.O.; Di Maio, A.; Guggenheim, E.J.; Chetwynd, A.J.; Pencross, D.; Tang, S.; Belinga Desaunay, M.F.A.; Thomas, S.G.; Rappoport, J.Z.; Lynch, I. Surface chemistry-dependent evolution of the nanomaterial corona on TiO2 nanomaterials following uptake and sub-cellular localization. Nanomaterials 2020, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Clemments, A.M.; Botella, P.; Landry, C.C. Spatial mapping of protein adsorption on mesoporous silica nanoparticles by stochastic optical reconstruction microscopy. J. Am. Chem. Soc. 2017, 139, 3978–3981. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Soto Rodriguez, P.E.D.; Woythe, L.; Sanchez, S.; Samitier, J.; Zijlstra, P.; Albertazzi, L. Multicolor super-resolution microscopy of protein corona on single nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 37345–37355. [Google Scholar] [CrossRef] [PubMed]
- Delcanale, P.; Miret Ontiveros, B.; Arista Romero, M.; Pujals, S.; Albertazzi, L. Nanoscale mapping functional sites on nanoparticles by points accumulation for imaging in nanoscale topography (PAINT). ACS Nano 2018, 12, 7629–7637. [Google Scholar] [CrossRef]
- Sanchez Guzman, D.; Giraudon Colas, G.; Marichal, L.; Boulard, Y.; Wien, F.; Degrouard, J.; Baeza Squiban, A.; Pin, S.; Renault, J.P.; Devineau, S. In situ analysis of weakly bound proteins reveals molecular basis of soft corona formation. ACS Nano 2020, 14, 9073–9088. [Google Scholar] [CrossRef]
- Runa, S.; Lakadamyali, M.; Kemp, M.L.; Payne, C.K. TiO2 nanoparticle-induced oxidation of the plasma membrane: Importance of the protein corona. J. Phys. Chem. B 2017, 121, 8619–8625. [Google Scholar] [CrossRef]
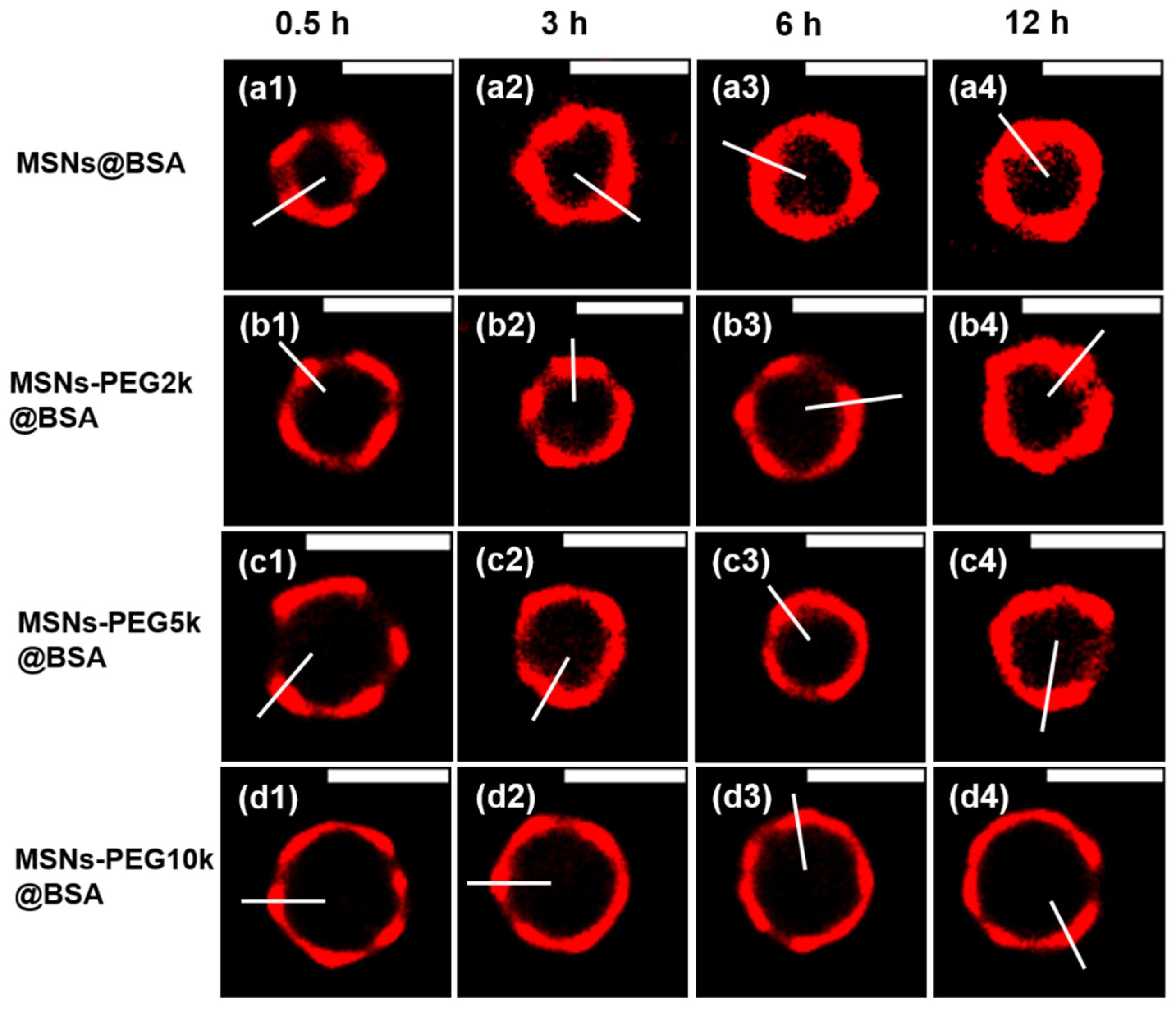
- Sun, N.; Jia, Y.; Bai, S.; Yang, Y.; Dai, L.; Li, J. Spatial mapping and quantitative evaluation of protein corona on PEGylated mesoporous silica particles by super-resolution fluorescence microscopy. J. Colloid Interface Sci. 2024, 653, 351–358. [Google Scholar] [CrossRef]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional supramolecular polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef]
- Chapman, D.V.; Du, H.; Lee, W.Y.; Wiesner, U.B. Optical super-resolution microscopy in polymer science. Prog. Polym. Sci. 2020, 111, 101312. [Google Scholar] [CrossRef]
- Xu, J.; Sun, X.; Kim, K.; Brand, R.M.; Hartman, D.; Ma, H.; Brand, R.E.; Bai, M.; Liu, Y. Ultrastructural visualization of chromatin in cancer pathogenesis using a simple small-molecule fluorescent probe. Sci. Adv. 2022, 8, eabm8293. [Google Scholar] [CrossRef]
- Jin, D.; Xi, P.; Wang, B.; Zhang, L.; Enderlein, J.; van Oijen, A.M. Nanoparticles for super-resolution microscopy and single-molecule tracking. Nat. Methods 2018, 15, 415–423. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, L.X.; Li, C.; Hu, Z.; Zhang, G.F.; Chen, Z.Q.; Chen, T.; Huang, Z.L.; Zhu, J.T.; Zhu, M.Q. Optical nanoimaging for block copolymer self-assembly. J. Am. Chem. Soc. 2015, 137, 2436–2439. [Google Scholar] [CrossRef]
- Albertazzi, L.; van der Zwaag, D.; Leenders, C.M.A.; Fitzner, R.; van der Hofstad, R.W.; Meijer, E.W. Probing exchange pathways in one-dimensional aggregates with super-resolution microscopy. Science 2014, 344, 491–495. [Google Scholar] [CrossRef]
- Baker, M.B.; Gosens, R.P.J.; Albertazzi, L.; Matsumoto, N.M.; Palmans, A.R.A.; Meijer, E.W. Exposing differences in monomer exchange rates of multicomponent supramolecular polymers in water. Chembiochem 2016, 17, 207–213. [Google Scholar] [CrossRef]
- Baker, M.B.; Albertazzi, L.; Voets, I.K.; Leenders, C.M.A.; Palmans, A.R.A.; Pavan, G.M.; Meijer, E.W. Consequences of chirality on the dynamics of a water-soluble supramolecular polymer. Nat. Commun. 2015, 6, 6234. [Google Scholar] [CrossRef]
- Aloi, A.; Jentzsch, A.V.; Vilanova, N.; Albertazzi, L.; Meijer, E.W.; Voets, I.K. Imaging nanostructures by single-molecule localization microscopy in organic solvents. J. Am. Chem. Soc. 2016, 138, 2953–2956. [Google Scholar] [CrossRef]
- Adelizzi, B.; Aloi, A.; Van Zee, N.J.; Palmans, A.R.A.; Meijer, E.W.; Voets, I.K. Painting supramolecular polymers in organic solvents by super-resolution microscopy. ACS Nano 2018, 12, 4431–4439. [Google Scholar] [CrossRef]
- Boott, C.E.; Laine, R.F.; Mahou, P.; Finnegan, J.R.; Leitao, E.M.; Webb, S.E.D.; Kaminski, C.F.; Manners, I. In situ visualization of block copolymer self-assembly in organic media by super-resolution fluorescence microscopy. Chem. Eur. J. 2015, 21, 18539–18542. [Google Scholar] [CrossRef]
- Eslami, H.; Müller-Plathe, F. Self-assembly pathways of triblock janus particles into 3D open lattices. Small 2024, 20, 2306337. [Google Scholar] [CrossRef]
- Angel Fernandez-Rodriguez, M.; Rahmani, S.; Yu, C.K.J.; Angel Rodriguez-Valverde, M.; Angel Cabrerizo-Vilchez, M.; Michel, C.A.; Lahann, J.; Hidalgo-Alvarez, R. Synthesis and interfacial activity of PMMA/PtBMA Janus and homogeneous nanoparticles at water/oil interfaces. Colloids Surf. A 2018, 536, 259–265. [Google Scholar] [CrossRef]
| Evanescent wave detection | Near-field scanning optical microscopy (NSOM) [32] | |
| Hyperlens for sub-diffraction-limited imaging [33] | ||
| High-resolution far-field optical microscopy | Enhanced resolution far-field optical microscopy | 4Pi-confocal fluoresence microscopy [41] |
| Structured illumination microscopy (SIM) [42] | ||
| (IM)-M-5 microscopy [43] | ||
| Reversible saturable optically linear fluorescence transition (RESOLFT) | Stimulated emission depletion microscopy (STED) [44] | |
| Ground-state-depletion fluorescence microscopy (GSD) [45] | ||
| Saturated structured illumination microscopy (SSIM) [46] | ||
| Saturated pattern excitation microscopy (SPEM) [47] | ||
| Single-molecule localization microscopy (SMLM) | Spectral precision distance microscopy (SPDM) [48] | |
| Ground-state-depletion microscopy followed by individual molecule return (GSDIM) [49] | ||
| Photoactivation localization microscopy (PALM) [37] | ||
| Fluorescence photoactivation localization microscopy (fPALM) [50] | ||
| Stochastic optical reconstruction microscopy (STORM) [51] | ||
| Direct stochastic optical reconstruction microscopy (dSTORM) [52] | ||
| Point accumulation for imaging in nanoscale topography (PAINT) [53] | ||
| DNA-PAINT [39] | ||
| Blink microscopy (BM) [54] | ||
| Binding-activated localization microscopy (BALM) [38] | ||
| Nanometer-localized multiple single-molecule (NALMS) [55] | ||
| Subtracting patterns in defocused imaging to enhance the resolution (SPIDER) [56] | ||
| Integrating exchangeable single-molecule localization (IRIS) [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, N.; Bai, S.; Dai, L.; Jia, Y. Super-Resolution Microscopy as a Versatile Tool in Probing Molecular Assembly. Int. J. Mol. Sci. 2024, 25, 11497. https://doi.org/10.3390/ijms252111497
Sun N, Bai S, Dai L, Jia Y. Super-Resolution Microscopy as a Versatile Tool in Probing Molecular Assembly. International Journal of Molecular Sciences. 2024; 25(21):11497. https://doi.org/10.3390/ijms252111497
Chicago/Turabian StyleSun, Nan, Shiwei Bai, Luru Dai, and Yi Jia. 2024. "Super-Resolution Microscopy as a Versatile Tool in Probing Molecular Assembly" International Journal of Molecular Sciences 25, no. 21: 11497. https://doi.org/10.3390/ijms252111497
APA StyleSun, N., Bai, S., Dai, L., & Jia, Y. (2024). Super-Resolution Microscopy as a Versatile Tool in Probing Molecular Assembly. International Journal of Molecular Sciences, 25(21), 11497. https://doi.org/10.3390/ijms252111497







