The Absence of Thyroid-Stimulating Hormone Receptor Expression on Natural Killer T Cells: Implications for the Immune–Endocrine Interaction
Abstract
1. Introduction
2. Results
2.1. Comparison of AITD and Non-AITD Groups
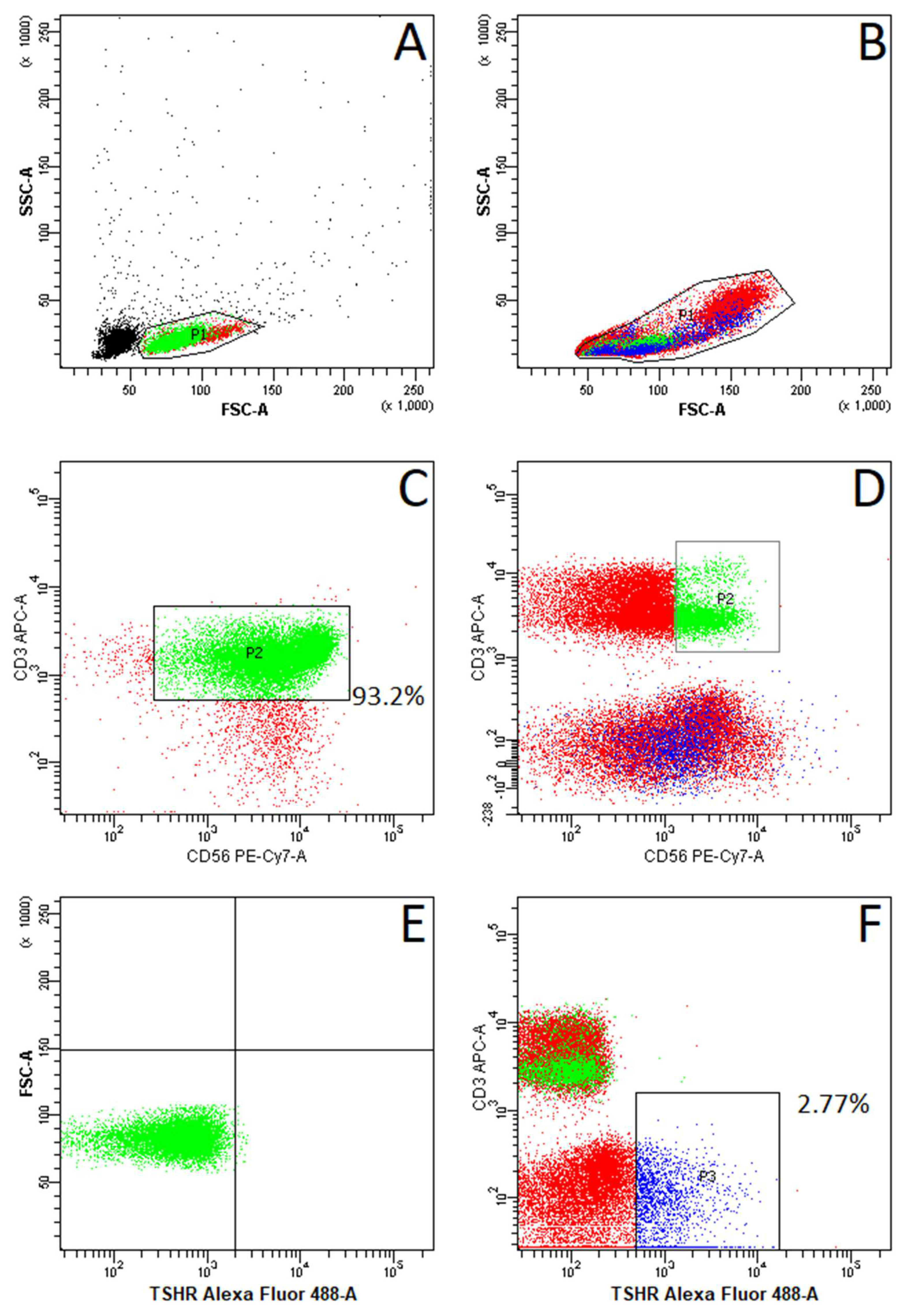
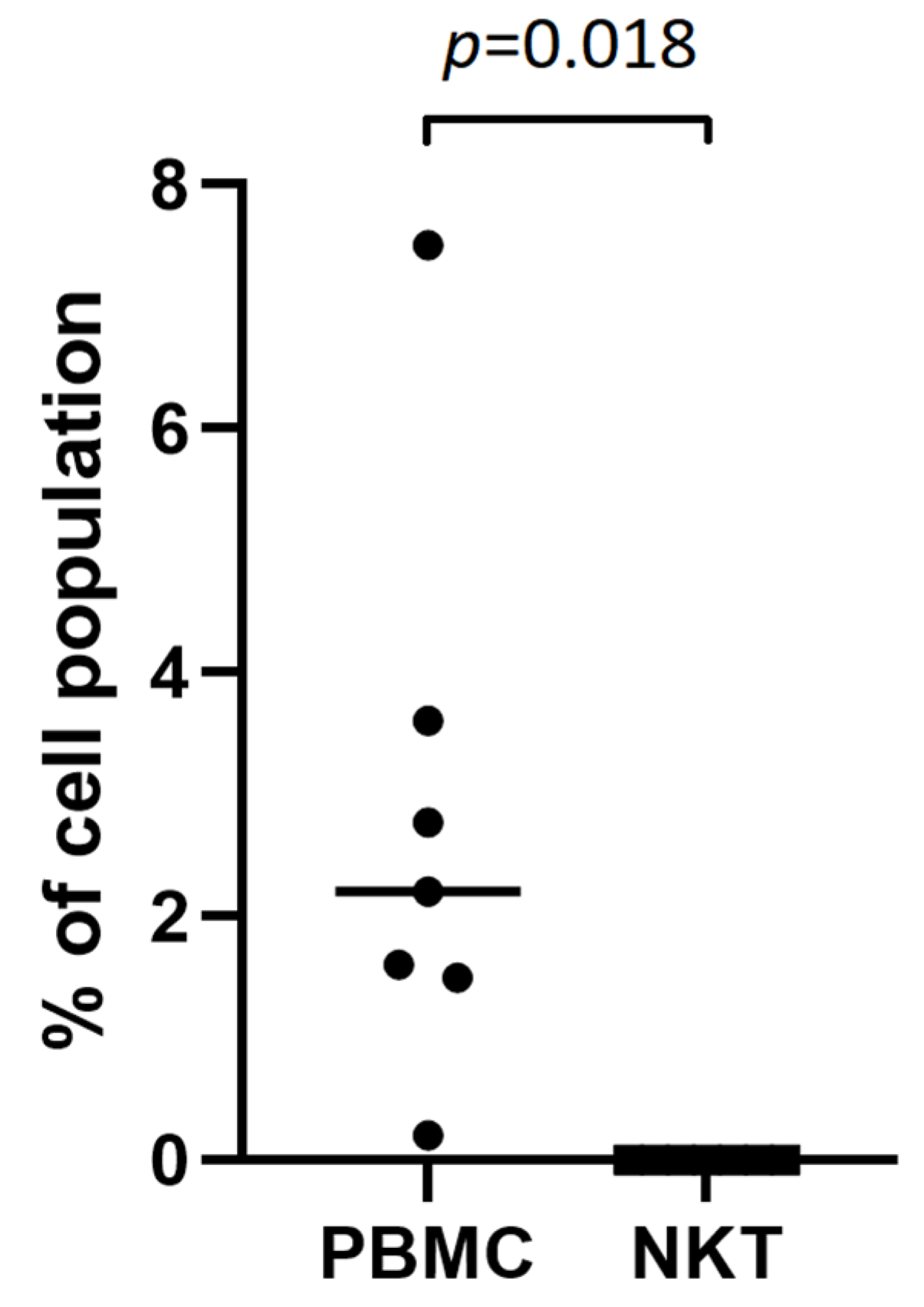
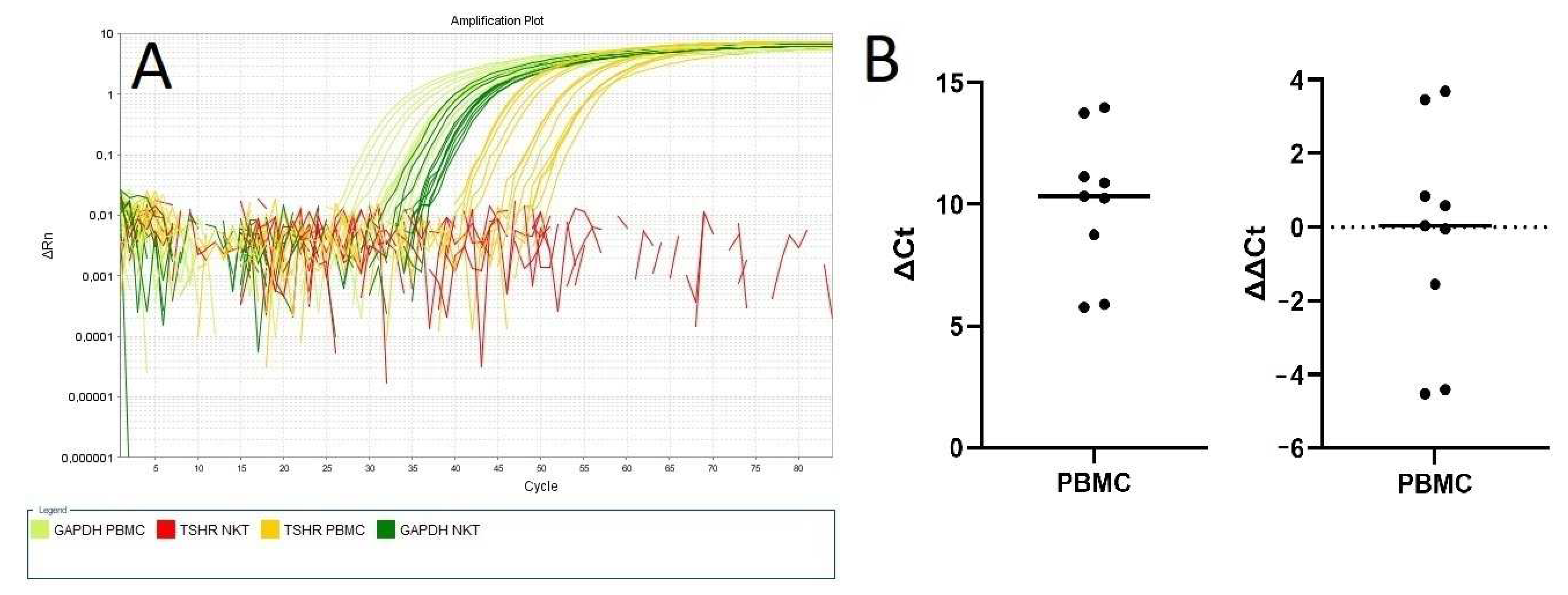
2.2. Absence of TSHR Expression on NKT Cells
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Inclusion Criteria
4.3. Biochemical Analysis
4.4. NKT Cell Isolation
4.5. Fluorescence-Activated Cell Sorting (FACS)
4.6. Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
4.7. Statistical Analysis
4.8. Ethics Procedures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AITD | autoimmune thyroid disease |
| Anti-Tg | thyroid antithyroglobulin antibodies |
| Anti-TPO | thyroid peroxidase antibodies |
| APCs | antigen-presenting cells |
| CD | cluster of differentiation |
| DCs | dendritic cells |
| pDCs | plasmocytoid dendritic cells |
| cDCs | conventional dendritic cells |
| EDTA | ethylenediamine tetraacetic acid |
| FACS | fluorescence-activated cell sorting |
| FNAB | fine-needle aspiration biopsy |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GD | Graves’s disease |
| HT | Hashimoto’s thyroiditis |
| IFN-y | interferon-gamma |
| IL-2 | interleukin-2 |
| IL-4 | interleukin-4 |
| IL-17 | interleukin-17 |
| iNKT | invariant Natural Killer T cells |
| MCH | major histocompatibility complex |
| NK | Natural Killer cells |
| NKT | Natural Killer T cells |
| NIS | sodium/iodide symporter |
| PBMCs | peripheral blood mononuclear cells |
| mRNA | messenger ribonucleic acid |
| rhTSH | recombinant human thyroid stimulating hormone |
| RNA | ribonucleic acid |
| RT-PCR | reverse-transcription polymerase chain reaction |
| SNP | single-nucleotide polymorphism |
| STAT6 | Signal Transducer and Activator of Transcription 6 |
| TCR | T-cell receptor |
| Tg | thyroglobulin |
| TPO | thyroid peroxidase |
| TRAb | thyroid-stimulating hormone receptor antibodies |
| TSH | thyroid-stimulating hormone |
| TSHR | thyroid-stimulating hormone receptor |
References
- Jara, E.L.; Muñoz-Durango, N.; Llanos, C.; Fardella, C.; González, P.A.; Bueno, S.M.; Kalergis, A.M.; Riedel, C.A. Modulating the function of the immune system by thyroid hormones and thyrotropin. Immunol. Lett. 2017, 184, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Berczi, I. Neuroendocrine Regulation of Natural Immunity. NeuroImmune Biol. 2005, 5, 215–262. [Google Scholar]
- Bağriaçik, E.U.; Klein, J.R. The thyrotropin (thyroid-stimulating hormone) receptor is expressed on murine dendritic cells and on a subset of CD45RBhigh lymph node T cells: Functional role for thyroid-stimulating hormone during immune activation. J. Immunol. 2000, 15, 6158–6165. [Google Scholar] [CrossRef] [PubMed]
- Adamczewski, Z.; Stasiołek, M.; Zygmunt, A.; Śliwka, P.W.; Wieczorek-Szukała, K.; Lewiński, A. Recombinant Human Thyroid-Stimulating Hormone Increases the Percentages of Natural Killer T Cells and B Lymphocytes in Human Peripheral Blood In Vivo. Front. Endocrinol. 2020, 11, 543845. [Google Scholar] [CrossRef]
- Miko, E.; Meggyes, M.; Doba, K.; Farkas, N.; Bogar, B.; Barakonyi, A.; Szereday, L.; Szekeres-Bartho, J.; Mezosi, E. Characteristics of peripheral blood NK and NKT-like cells in euthyroid and subclinical hypothyroid women with thyroid autoimmunity experiencing reproductive failure. J. Reprod. Immunol. 2017, 124, 62–70. [Google Scholar] [CrossRef]
- Kaszubowska, L.; Piotrowska, A.; Siedlecka-Kroplewska, K.; Kmiec, Z. NKT cells as a connecting element between innate and adaptive immunity. Postepy Biol. Komorki. 2013, 40, 697–724. [Google Scholar]
- Bojarska-Junak, A.; Tabarkiewicz, J.; Roliński, J. NKT cells: Their development, mechanisms and effects of action. Postepy Hig. Med. Dosw. 2013, 15, 65–78. [Google Scholar] [CrossRef]
- Hervier, B.; Beziat, V.; Haroche, J.; Mathian, A.; Lebon, P.; Ghillani-Dalbin, P.; Musset, L.; Debré, P.; Amoura, Z.; Vieillard, V. Phenotype and function of natural killer cells in systemic lupus erythematosus: Excess interferon-g production in patients with active disease. Arthritis Rheum. 2011, 63, 1698–1706. [Google Scholar] [CrossRef]
- Rodacki, M.; Svoren, B.; Butty, V.; Besse, W.; Laffel, L.; Benoist, C.; Mathis, D. Altered natural killer cells in type 1 diabetic patients. Diabetes 2007, 56, 177–185. [Google Scholar] [CrossRef]
- Novak, J.; Griseri, T.; Beaudoin, L.; Lehuen, A. Regulation of type 1 diabetes by NKT cells. Int. Rev. Immunol. 2007, 26, 49–72. [Google Scholar] [CrossRef]
- Zarobkiewicz, M.K.; Morawska, I.; Michalski, A.; Roliński, J.; Bojarska-Junak, A. NKT and NKT-like Cells in Autoimmune Neuroinflammatory Diseases-Multiple Sclerosis, Myasthenia Gravis and Guillain-Barre Syndrome. Int. J. Mol. Sci. 2021, 22, 9520. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, M.; Wang, J.; Li, X. Immunoregulation of NKT Cells in Systemic Lupus Erythematosus. J. Immunol. Res. 2015, 2015, 206731. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Nakamura, Y.; Matsuzuka, F.; Takamura, Y.; Miyauchi, A.; Iwatani, Y. Decrease of intrathyroidal CD161Valpha24Vbeta11 NKT cells in Graves’ disease. Endocr. J. 2008, 55, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Watanabe, K.; Niwa, M.; McLachlan, S.M.; Rapoport, B. Schistosoma mansoni and alpha-galactosylceramide: Prophylactic effect of Th1 Immune suppression in a mouse model of Graves’ hyperthyroidism. J. Immunol. 2004, 173, 2167–2173. [Google Scholar] [CrossRef]
- McLeod, D.S.; Cooper, D.S. The incidence and prevalence of thyroid autoimmunity. Endocrine 2012, 42, 252–265. [Google Scholar] [CrossRef]
- Rapoport, B.; McLachlan, S.M. The Thyrotropin Receptor in Graves’ Disease. Thyroid. Off. J. Am. Thyroid Assoc. 2007, 17, 911–922. [Google Scholar] [CrossRef]
- Vassart, G.; Dumont, J.E. The Thyrotropin Receptor and the Regulation of Thyrocyte Function and Growth. Endocr. Rev. 1992, 13, 596–611. [Google Scholar] [CrossRef]
- Chu, Y.-D.; Yeh, C.-T. The Molecular Function and Clinical Role of Thyroid Stimulating Hormone Receptor in Cancer Cells. Cells 2020, 9, 1730. [Google Scholar] [CrossRef]
- Williams, G.R. Extrathyroidal Expression of TSH Receptor. Ann. Endocrinol. 2011, 72, 68–73. [Google Scholar] [CrossRef]
- Postiglione, M.P.; Parlato, R.; Rodriguez-Mallon, A.; Rosica, A.; Mithbaokar, P.; Maresca, M.; Marians, R.C.; Davies, T.F.; Zannini, M.S.; De Felice, M.; et al. Role of the Thyroid-Stimulating Hormone Receptor Signaling in Development and Differentiation of the Thyroid Gland. Proc. Natl. Acad. Sci. USA 2002, 99, 15462–15467. [Google Scholar] [CrossRef]
- Wenzek, C.; Boelen, A.; Westendorf, A.M.; Engel, D.R.; Moeller, L.C.; Führer, D. The Interplay of Thyroid Hormones and the Immune System—Where We Stand and Why We Need to Know About It. Eur. J. Endocrinol. 2022, 186, R65–R77. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Takeshita, A.; Luo, W.; Ashizawa, K.; Yokoyama, N.; Nagataki, S. High affinity binding of thyrotropin (TSH) and thyroid-stimulating autoantibody for the TSH receptor extracellular domain. Thyroid 1994, 4, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Cuddihy, R.M.; Dutton, C.M.; Bahn, R.S. A polymorphism in the extracellular domain of the thyrotropin receptor is highly associated with autoimmune thyroid disease in females. Thyroid 1995, 5, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Xu, K.; Jia, W.; Lan, L.; Zheng, X.; Yang, X.; Cui, D. Association between TSHR gene polymorphism and the risk of Graves’ disease: A meta-analysis. J. Biomed. Res. 2016, 30, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Jiang, S.J.; Wang, D.K.; Dong, H.; Chen, G.; Fang, K.; Cui, J.R.; Lu, F.E. Association of polymorphisms of rs179247 and rs12101255 in thyroid stimulating hormone receptor intron 1 with an increased risk of Graves’ disease: A meta-analysis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 473–479. [Google Scholar] [CrossRef]
- Xiong, H.; Wu, M.; Yi, H.; Wang, X.; Wang, Q.; Nadirshina, S.; Zhou, X.; Liu, X. Genetic associations of the thyroid stimulating hormone receptor gene with Graves diseases and Graves ophthalmopathy: A meta-analysis. Sci. Rep. 2016, 6, 30356. [Google Scholar] [CrossRef]
- Burek, C.L.; Sharma, R.B.; Rose, N.R. NKT cell regulation of autoimmune thyroiditis. Autoimmunity 2003, 36, 405–408. [Google Scholar] [CrossRef]
- Sharma, R.B.; Fan, X.; Caturegli, P.; Rose, N.R.; Burek, C.L. Invariant NKT Cell Lines Derived from the NOD.H2 Mouse Enhance Autoimmune Thyroiditis. J. Thyroid Res. 2011, 2011, 895923. [Google Scholar]
- Guo, H.; Xu, B.; Yang, X.; Wang, Y.; Liu, X.; Cui, C.; Jiang, Y. A high frequency of peripheral blood NKG2D+NK and NKT cells in euthyroid patients with new onset hashimoto’s thyroiditis—A pilot study. Immunol. Investig. 2014, 43, 312–323. [Google Scholar] [CrossRef]
- Savina, A.; Amigorena, S. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 2007, 219, 143–156. [Google Scholar] [CrossRef]
- Bendelac, A.; Savage, P.B.; Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.S.; Karadimitris, A. Role and regulation of CD1d in normal and pathological B cells. J. Immunol. 2014, 193, 4761–4768. [Google Scholar] [CrossRef] [PubMed]
- Brigl, M.; Brenner, M.B. CD1: Antigen presentation and T cell function. Annu. Rev. Immunol. 2004, 22, 817–890. [Google Scholar] [CrossRef] [PubMed]
- Shortman, K.; Liu, Y.J. Mouse and Human Dendritic Cell Subtypes. Nat. Rev. 2002, 21, 151–161. [Google Scholar] [CrossRef]
- Reizis, B. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity 2019, 50, 37–50. [Google Scholar] [CrossRef]
- Turley, S.J. Dendritic cells: Inciting and inhibiting autoimmunity. Curr. Opin. Immunol. 2002, 14, 765–770. [Google Scholar] [CrossRef]
- Leskela, S.; Rodríguez-Munoz, A.; de la Fuente, H.; Figueroa-Vega, N.; Bonay, P.; Martín, P.; Serrano, A.; Sánchez-Madrid, F.; González-Amaro, R.; Marazuela, M. Plasmacytoid dendritic cells in patients with autoimmune thyroid disease. J. Clin. Endocrin. Metab. 2013, 98, 2822–2833. [Google Scholar] [CrossRef][Green Version]
- Stasiołek, M.; . Śliwka, P.W.; Stasiak, M.; Krawczyk-Rusiecka, K.; Skowrońska-Jóźwiak, E.; Adamczewski, Z.; Lewiński, A. Differences of the Structure of Immune Regulatory Cell Populations between Cellular Material from Sonographically Detected Focal Thyroid Lesions and Peripheral Blood in Humans. Int. J. Mol. Sci. 2019, 20, 918. [Google Scholar] [CrossRef]
- Quadbeck, B.; Eckstein, A.K.; Tews, S.; Walz, M.; Hoermann, R.; Mann, K.; Gieseler, R. Maturation of thyroidal dendritic cells in Graves’ disease. Scand. J. Immunol. 2002, 55, 612–620. [Google Scholar] [CrossRef]
- Hammerstad, S.S.; Jahnsen, F.L.; Tauriainen, S.; Hyöty, H.; Paulsen, T.; Norheim, I.; Dahl-Jørgensen, K. Inflammation and increased myxovirus resistance protein A expression in thyroid tissue in the early stages of Hashimoto’s thyroiditis. Thyroid 2013, 23, 334–341. [Google Scholar] [CrossRef]
- Hammerstad, S.S.; Jahnsen, F.; Tauriainen, S.; Hyöty, H.; Paulsen, T.; Norheim, I.; Dahl-Jørgensen, K. Immunological Changes and Increased Expression of Myxovirus Resistance Protein A in Thyroid Tissue of Patients with Recent Onset and Untreated Graves’ Disease. Thyroid 2014, 24, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Bessoles, S.; Fouret, F.; Dudal, S.; Besra, G.S.; Sanchez, F.; Lafont, V. IL-2 triggers specific signaling pathways in human NKT cells leading to the production of pro- and anti-inflammatory cytokines. J. Leukoc. Biol. 2008, 84, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, J. Increased interleukin-2 level in patients with primary hypothyroidism. Clin. Immunol. Immunopathol. 1992, 63, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, J.; Zylińska, K.; Pawlikowski, M.; Stepień, H. Stimulatory effect of thyrotropin (TSH) on interleukin-2 (IL-2) release from human peripheral blood lymphocytes. A dose-response study in vitro. Horm. Metab. Res. 1993, 25, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Douglas, R.S.; Afifiyan, N.F.; Hwang, C.J.; Chong, K.; Haider, U.; Richards, P.; Gianoukakis, A.G.; Smith, T.J. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J. Clin. Endocrinol. Metab. 2010, 95, 430–438. [Google Scholar] [CrossRef]
- Fernando, R.; Placzek, E.; Reese, E.A.; Placzek, A.T.; Schwartz, S.; Trierweiler, A.; Niziol, L.M.; Raychaudhuri, N.; Atkins, S.; Scanlan, T.S.; et al. Elevated Serum Tetrac in Graves’ Disease: Potential Pathogenic Role in Thyroid-Associated Ophthalmopathy. J. Clin. Endocrinol. Metab. 2017, 102, 776–785. [Google Scholar] [CrossRef]
- Gillespie, E.F.; Papageorgiou, K.I.; Fernando, R.; Raychaudhuri, N.; Cockerham, K.P.; Charara, L.K.; Goncalves, A.C.; Zhao, S.X.; Ginter, A.; Lu, Y.; et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J. Clin. Endocrinol. Metab. 2012, 97, E740–E746. [Google Scholar] [CrossRef]
- Ma, R.; Gan, L.; Guo, J.; Peng, Z.; Wu, J.; Harrison, A.R.; Qian, J. Insights into Ferroptosis: Targeting Glycolysis to Treat Graves’ Orbitopathy. J. Clin. Endocrinol. Metab. 2022, 16, 1994–2003. [Google Scholar] [CrossRef]
- Zulkarnain, Z.; Ulhaq, Z.S.; Sujuti, H.; Soeatmadji, D.W.; Zufry, H.; Wuragil, D.K.; Marhendra, A.P.W.; Riawan, W.; Kurniawati, S.; Oktanella, Y.; et al. Comparative performance of ELISA and dot blot assay for TSH-receptor antibody detection in Graves’ disease. J. Clin. Lab. Anal. 2022, 36, e24288. [Google Scholar] [CrossRef]
- Wu, Z.; Xi, Z.; Xiao, Y.; Zhao, X.; Li, J.; Feng, N.; Hu, L.; Zheng, R.; Zhang, N.; Wang, S.; et al. TSH-TSHR axis promotes tumor immune evasion. J. Immunother. Cancer 2022, 10, e004049. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, D.K.; Seo, E.J.; Choi, E.J.; Kwon, Y.W.; Jang, I.H.; Lee, J.C.; Kim, H.Y.; Shong, M.; Kim, J.H.; et al. Role of Krüppel-Like Factor 4 in the Maintenance of Chemoresistance of Anaplastic Thyroid Cancer. Thyroid 2017, 27, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Oh, S.W.; Youn, H.; Na, J.; Kang, K.W.; Park, D.J.; Park, Y.J.; Jang, J.J.; Lee, K.E.; Jung, K.C.; et al. Thyroid-Related Protein Expression in the Human Thymus. Int. J. Endocrinol. 2017, 2017, 8159892. [Google Scholar] [CrossRef]
- Gao, H.; Lu, X.; Huang, H.; Ji, H.; Zhang, L.; Su, Z. Thyroid-stimulating hormone level is negatively associated with fertilization rate in patients with polycystic ovary syndrome undergoing in vitro fertilization. Int. J. Gynaecol. Obstet. 2021, 155, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Muller, I.; Moran, C.; Lecumberri, B.; Decallonne, B.; Robertson, N.; Jones, J.; Dayan, C.M. 2019 European Thyroid Association Guidelines on the Management of Thyroid Dysfunction following Immune Reconstitution Therapy. Eur. Thyroid J. 2019, 8, 173–185. [Google Scholar] [CrossRef] [PubMed]

| Patients with AITD Mean ± SD (n = 28) | Patients without AITD Mean ± SD (n = 58) | p Value (AITD Versus Non-AITD Group) | |
|---|---|---|---|
| Age | 55.54 ± 11.4 | 61.72 ± 14.43 | p = 0.05 |
| Female/Male (n) | 23/5 | 45/13 | p = 0.63 |
| fT4 (0.93–1.7 ng/dL) | 1.25 ± 0.27 | 1.27 ± 0.34 | p = 0.9 |
| fT3 (2–4.4 pg/mL) | 2.83 ± 1.01 | 2.94 ± 0.69 | p = 0.56 |
| TSH (0.27–4.2 uIU/mL) | 1.6 ± 2.39 | 1.3 ± 1.1 | p = 0.42 |
| anti-TPO (<34 IU/mL) | 157.28 ± 149.04 | 11.39 ± 4.9 | p < 0.05 * |
| anti-Tg (<115 IU/mL) | 187.37 ± 193.65 | 15.5 ± 8.12 | p < 0.05 * |
| TRAb (0.8–1.75 IU/L) | 2.63 ± 5.17 | 0.99 ± 0.26 | p = 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamska-Fita, E.; Śliwka, P.W.; Karbownik-Lewińska, M.; Lewiński, A.; Stasiak, M. The Absence of Thyroid-Stimulating Hormone Receptor Expression on Natural Killer T Cells: Implications for the Immune–Endocrine Interaction. Int. J. Mol. Sci. 2024, 25, 11434. https://doi.org/10.3390/ijms252111434
Adamska-Fita E, Śliwka PW, Karbownik-Lewińska M, Lewiński A, Stasiak M. The Absence of Thyroid-Stimulating Hormone Receptor Expression on Natural Killer T Cells: Implications for the Immune–Endocrine Interaction. International Journal of Molecular Sciences. 2024; 25(21):11434. https://doi.org/10.3390/ijms252111434
Chicago/Turabian StyleAdamska-Fita, Emilia, Przemysław Wiktor Śliwka, Małgorzata Karbownik-Lewińska, Andrzej Lewiński, and Magdalena Stasiak. 2024. "The Absence of Thyroid-Stimulating Hormone Receptor Expression on Natural Killer T Cells: Implications for the Immune–Endocrine Interaction" International Journal of Molecular Sciences 25, no. 21: 11434. https://doi.org/10.3390/ijms252111434
APA StyleAdamska-Fita, E., Śliwka, P. W., Karbownik-Lewińska, M., Lewiński, A., & Stasiak, M. (2024). The Absence of Thyroid-Stimulating Hormone Receptor Expression on Natural Killer T Cells: Implications for the Immune–Endocrine Interaction. International Journal of Molecular Sciences, 25(21), 11434. https://doi.org/10.3390/ijms252111434









