Structural Basis of Activity of HER2-Targeting Construct Composed of DARPin G3 and Albumin-Binding Domains
Abstract
1. Introduction
2. Results
2.1. Design of the Study
- To establish whether the G3 and ABD domains in the conjugates are able to associate in an aqueous solution.
- If so, what are the most likely interfaces in the resulting complexes? Can they affect the known G3 binding site for the HER2 receptor?
- How can the effect of HSA be explained on the basis of molecular structure data?
- What determines the significant difference in the action of chimeras with the “opposite orientation”—G3–ABD/ABD–G3?
2.2. NMR Spectroscopy
2.3. Molecular Modeling
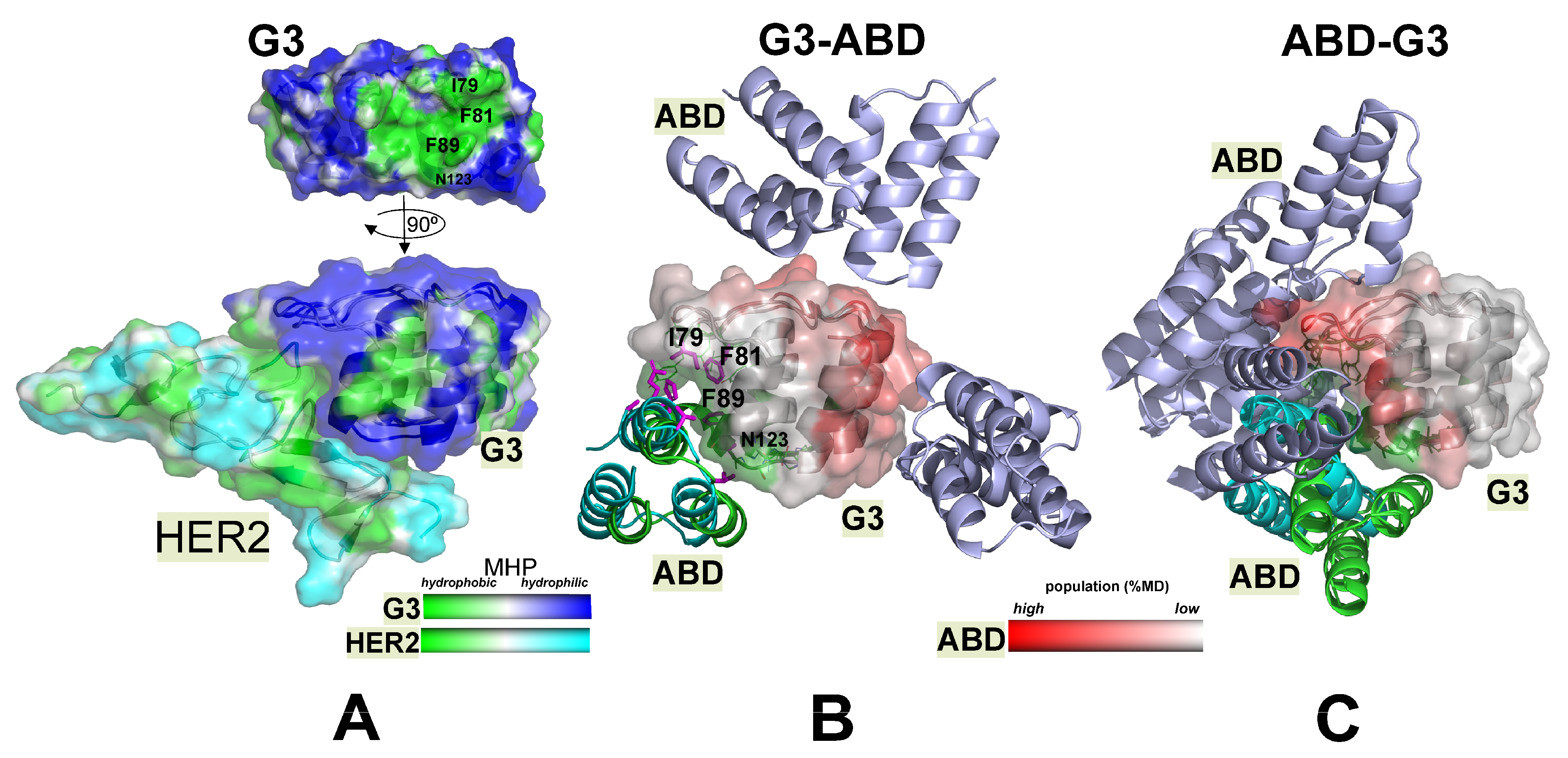
2.3.1. The HER2- and ABD-Binding Interfaces (np-Site) on the G3 Surface Are Almost Identical
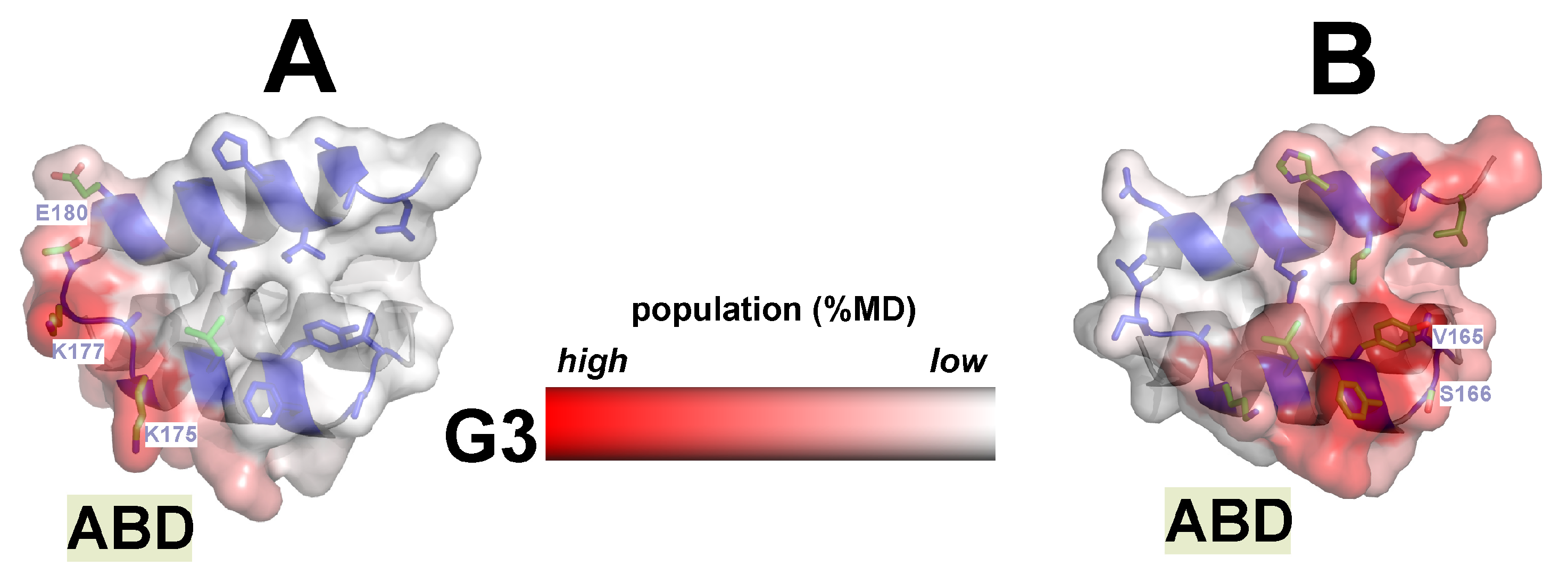
2.3.2. Alternative p-Sites of ABD Binding on G3 Surface Were Found in Both Chimeras and Practically Do Not Overlap
2.3.3. The Availability of HER2 Site Depends on the Order of the Domains in the Constructs
2.3.4. The Involvement of the HSA Motif in G3 Binding Depends on Both the Type of Construct and the Binding Interface of Its Modules
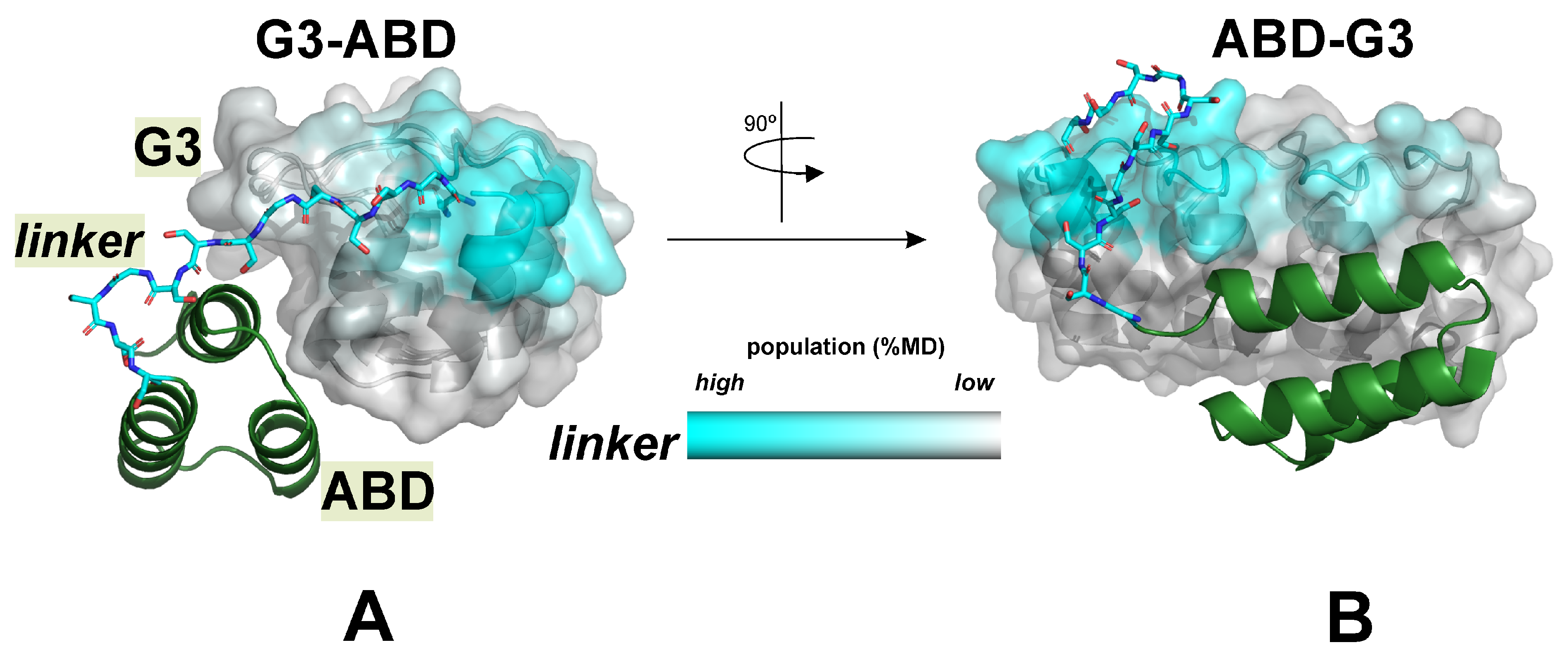
2.3.5. Role of the Domain Linker
3. Discussion
4. Materials and Methods
4.1. NMR Studies
4.2. Modeling of Interaction Between DARPin G3 and ABD
4.2.1. Building Up the Starting Spatial Models of G3–ABD and ABD–G3
- Domains are distant from each other and do not interact;
- Domains are in contact and both have a “hydrophobic” binding interface.
4.2.2. MD Simulations
4.2.3. MC Conformational Search
4.2.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mullard, A. FDA Approves 100th Monoclonal Antibody Product. Nat. Rev. Drug Discov. 2021, 20, 491–495. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Fiedler, M.; Skerra, A. Non-Antibody Scaffolds as Alternative Therapeutic Agents. In Handbook of Therapeutic Antibodies; Dübel, S., Reichert, J.M., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 435–474. ISBN 978-3-527-32937-3. [Google Scholar]
- Binz, H.K.; Stumpp, M.T.; Forrer, P.; Amstutz, P.; Plückthun, A. Designing Repeat Proteins: Well-Expressed, Soluble and Stable Proteins from Combinatorial Libraries of Consensus Ankyrin Repeat Proteins. J. Mol. Biol. 2003, 332, 489–503. [Google Scholar] [CrossRef]
- Hober, S.; Lindbo, S.; Nilvebrant, J. Bispecific Applications of Non-Immunoglobulin Scaffold Binders. Methods 2019, 154, 143–152. [Google Scholar] [CrossRef]
- Wurch, T.; Pierré, A.; Depil, S. Novel Protein Scaffolds as Emerging Therapeutic Proteins: From Discovery to Clinical Proof-of-Concept. Trends Biotechnol. 2012, 30, 575–582. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 852748. [Google Scholar] [CrossRef]
- Yan, M.; Parker, B.A.; Schwab, R.; Kurzrock, R. HER2 Aberrations in Cancer: Implications for Therapy. Cancer Treat. Rev. 2014, 40, 770–780. [Google Scholar] [CrossRef]
- Amisha, F.; Malik, P.; Saluja, P.; Gautam, N.; Patel, T.H.; Roy, A.M.; Singh, S.R.K.; Malapati, S.J. A Comprehensive Review on the Role of Human Epidermal Growth Factor Receptor 2 (HER2) as a Biomarker in Extra-Mammary and Extra-Gastric Cancers. Onco 2023, 3, 96–124. [Google Scholar] [CrossRef]
- Rubin, E.; Shan, K.; Dalal, S.; Vu, D.; Milillo-Naraine, A.; Guaqueta, D.; Ergle, A. Molecular Targeting of the Human Epidermal Growth Factor Receptor-2 (HER2) Genes across Various Cancers. Int. J. Mol. Sci. 2024, 25, 1064. [Google Scholar] [CrossRef]
- Zahnd, C.; Kawe, M.; Stumpp, M.T.; De Pasquale, C.; Tamaskovic, R.; Nagy-Davidescu, G.; Dreier, B.; Schibli, R.; Binz, H.K.; Waibel, R.; et al. Efficient Tumor Targeting with High-Affinity Designed Ankyrin Repeat Proteins: Effects of Affinity and Molecular Size. Cancer Res. 2010, 70, 1595–1605. [Google Scholar] [CrossRef]
- Bragina, O.; Chernov, V.; Schulga, A.; Konovalova, E.; Garbukov, E.; Vorobyeva, A.; Orlova, A.; Tashireva, L.; Sörensen, J.; Zelchan, R.; et al. Phase I Trial of 99m Tc-(HE) 3 -G3, a DARPin-Based Probe for Imaging of HER2 Expression in Breast Cancer. J. Nucl. Med. 2022, 63, 528–535. [Google Scholar] [CrossRef]
- Tolmachev, V.; Bodenko, V.; Oroujeni, M.; Deyev, S.; Konovalova, E.; Schulga, A.; Lindbo, S.; Hober, S.; Bragina, O.; Orlova, A.; et al. Direct In Vivo Comparison of 99mTc-Labeled Scaffold Proteins, DARPin G3 and ADAPT6, for Visualization of HER2 Expression and Monitoring of Early Response for Trastuzumab Therapy. Int. J. Mol. Sci. 2022, 23, 15181. [Google Scholar] [CrossRef]
- Deyev, S.M.; Oroujeni, M.; Garousi, J.; Gräslund, T.; Li, R.; Rosly, A.H.B.; Orlova, A.; Konovalova, E.; Schulga, A.; Vorobyeva, A.; et al. Preclinical Evaluation of HER2-Targeting DARPin G3: Impact of Albumin-Binding Domain (ABD) Fusion. Int. J. Mol. Sci. 2024, 25, 4246. [Google Scholar] [CrossRef]
- Amstutz, P.; Koch, H.; Binz, H.K.; Deuber, S.A.; Plückthun, A. Rapid Selection of Specific MAP Kinase-Binders from Designed Ankyrin Repeat Protein Libraries. Protein Eng. Des. Sel. 2006, 19, 219–229. [Google Scholar] [CrossRef][Green Version]
- Zahnd, C.; Wyler, E.; Schwenk, J.M.; Steiner, D.; Lawrence, M.C.; McKern, N.M.; Pecorari, F.; Ward, C.W.; Joos, T.O.; Plückthun, A. A Designed Ankyrin Repeat Protein Evolved to Picomolar Affinity to Her2. J. Mol. Biol. 2007, 369, 1015–1028. [Google Scholar] [CrossRef]
- Ivanova, J.R.; Benk, A.S.; Schaefer, J.V.; Dreier, B.; Hermann, L.O.; Plückthun, A.; Missirlis, D.; Spatz, J.P. Designed Ankyrin Repeat Proteins as Actin Labels of Distinct Cytoskeletal Structures in Living Cells. ACS Nano 2024, 18, 8919–8933. [Google Scholar] [CrossRef]
- Schütz, M.; Batyuk, A.; Klenk, C.; Kummer, L.; De Picciotto, S.; Gülbakan, B.; Wu, Y.; Newby, G.A.; Zosel, F.; Schöppe, J.; et al. Generation of Fluorogen-Activating Designed Ankyrin Repeat Proteins (FADAs) as Versatile Sensor Tools. J. Mol. Biol. 2016, 428, 1272–1289. [Google Scholar] [CrossRef]
- Mosavi, L.K.; Cammett, T.J.; Desrosiers, D.C.; Peng, Z. The Ankyrin Repeat as Molecular Architecture for Protein Recognition. Protein Sci. 2004, 13, 1435–1448. [Google Scholar] [CrossRef]
- Meibohm, B. Pharmacokinetics and Half-Life of Protein Therapeutics. In Therapeutic Proteins; Kontermann, R., Ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 23–38. ISBN 978-3-527-32849-9. [Google Scholar]
- Kontermann, R.E. Half-Life Extended Biotherapeutics. Expert Opin. Biol. Ther. 2016, 16, 903–915. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Ullah, A.; Shin, G.; Lim, S.I. Human Serum Albumin Binders: A Piggyback Ride for Long-Acting Therapeutics. Drug Discov. Today 2023, 28, 103738. [Google Scholar] [CrossRef]
- Andersen, J.T.; Pehrson, R.; Tolmachev, V.; Daba, M.B.; Abrahmsén, L.; Ekblad, C. Extending Half-Life by Indirect Targeting of the Neonatal Fc Receptor (FcRn) Using a Minimal Albumin Binding Domain. J. Biol. Chem. 2011, 286, 5234–5241. [Google Scholar] [CrossRef]
- Steiner, D.; Merz, F.W.; Sonderegger, I.; Gulotti-Georgieva, M.; Villemagne, D.; Phillips, D.J.; Forrer, P.; Stumpp, M.T.; Zitt, C.; Binz, H.K. Half-Life Extension Using Serum Albumin-Binding DARPin® Domains. Protein Eng. Des. Sel. 2017, 30, 583–591. [Google Scholar] [CrossRef]
- Ruiz, D.M.; Turowski, V.R.; Murakami, M.T. Effects of the Linker Region on the Structure and Function of Modular GH5 Cellulases. Sci. Rep. 2016, 6, 28504. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Protein Structure Modeling with MODELLER. In Structural Genomics; Chen, Y.W., Yiu, C.-P.B., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2199, pp. 239–255. ISBN 978-1-07-160891-3. [Google Scholar]
- Pyrkov, T.V.; Chugunov, A.O.; Krylov, N.A.; Nolde, D.E.; Efremov, R.G. PLATINUM: A Web Tool for Analysis of Hydrophobic/Hydrophilic Organization of Biomolecular Complexes. Bioinformatics 2009, 25, 1201–1202. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D.; Pastor, R.W. Update of the CHARMM All-Atom Additive Force Field for Lipids: Validation on Six Lipid Types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Potential Energy Functions for Atomic-Level Simulations of Water and Organic and Biomolecular Systems. Proc. Natl. Acad. Sci. USA 2005, 102, 6665–6670. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Efremov, R.G.; Nolde, D.E.; Vergoten, G.; Arseniev, A.S. A Solvent Model for Simulations of Peptides in Bilayers. I. Membrane-Promoting α-Helix Formation. Biophys. J. 1999, 76, 2448–2459. [Google Scholar] [CrossRef]
- Nemethy, G.; Pottle, M.S.; Scheraga, H.A. Energy Parameters in Polypeptides. 9. Updating of Geometrical Parameters, Nonbonded Interactions, and Hydrogen Bond Interactions for the Naturally Occurring Amino Acids. J. Phys. Chem. 1983, 87, 1883–1887. [Google Scholar] [CrossRef]
- Efremov, R.G.; Alix, A.J.P. Environmental Characteristics of Residues in Proteins: Three-Dimensional Molecular Hydrophobicity Potential Approach. J. Biomol. Struct. Dyn. 1993, 11, 483–507. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konshina, A.G.; Bocharov, E.V.; Konovalova, E.V.; Schulga, A.A.; Tolmachev, V.; Deyev, S.M.; Efremov, R.G. Structural Basis of Activity of HER2-Targeting Construct Composed of DARPin G3 and Albumin-Binding Domains. Int. J. Mol. Sci. 2024, 25, 11370. https://doi.org/10.3390/ijms252111370
Konshina AG, Bocharov EV, Konovalova EV, Schulga AA, Tolmachev V, Deyev SM, Efremov RG. Structural Basis of Activity of HER2-Targeting Construct Composed of DARPin G3 and Albumin-Binding Domains. International Journal of Molecular Sciences. 2024; 25(21):11370. https://doi.org/10.3390/ijms252111370
Chicago/Turabian StyleKonshina, Anastasia G., Eduard V. Bocharov, Elena V. Konovalova, Alexey A. Schulga, Vladimir Tolmachev, Sergey M. Deyev, and Roman G. Efremov. 2024. "Structural Basis of Activity of HER2-Targeting Construct Composed of DARPin G3 and Albumin-Binding Domains" International Journal of Molecular Sciences 25, no. 21: 11370. https://doi.org/10.3390/ijms252111370
APA StyleKonshina, A. G., Bocharov, E. V., Konovalova, E. V., Schulga, A. A., Tolmachev, V., Deyev, S. M., & Efremov, R. G. (2024). Structural Basis of Activity of HER2-Targeting Construct Composed of DARPin G3 and Albumin-Binding Domains. International Journal of Molecular Sciences, 25(21), 11370. https://doi.org/10.3390/ijms252111370








