Polymorphism rs259983 of the Zinc Finger Protein 831 Gene Increases Risk of Superimposed Preeclampsia in Women with Gestational Diabetes Mellitus
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. Association between rs259983 of the ZNF831 Gene and Preeclampsia
2.3. Association between rs259983 of the ZNF831 Gene and Preeclampsia without CHTN
2.4. Association between rs259983 of the ZNF831 Gene and Superimposed Preeclampsia (SIPE)
3. Discussion
3.1. Discussion of the PE Risk Factors
3.2. Association between rs259983 of the ZNF831 with PE and SIPE
4. Materials and Methods
4.1. Ethical Statement, Exclusion/Inclusion Criteria and Study Design
4.2. Primer and Probe Design
4.3. PCR with TaqMan Probes Conditions for rs259983 of the ZNF831
4.4. Statistical and Computational Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moller, A.B.; Patten, J.H.; Hanson, C.; Morgan, A.; Say, L.; Diaz, T.; Moran, A.C. Monitoring maternal and newborn health outcomes globally: A brief history of key events and initiatives. Trop. Med. Int. Health 2019, 24, 1342–1368. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Progress in Tackling Maternal and Newborn Deaths Stalls Since 2015: Un. World Health Organization. (n.d.) Available online: https://www.who.int/news/item/09-05-2023-global-progress-in-tackling-maternal-and-newborn-deaths-stalls-since-2015--un (accessed on 5 March 2024).
- Khodzhaeva, Z.S.; Shmakov, R.G.; Savel’eva, G.M. Clinical recommendations. Preeclampsia. Eclampsia. Edema, proteinuria and hypertensive disorders during pregnancy, childbirth and the postpartum period. 2021. (In Russian). Available online: https://praesens.ru/rubricator/klinicheskie-rekomendatsii/c2175905-632f-4b64-ace4-39b4ccfa6642/ (accessed on 5 March 2024).
- Abalos, E.; Cuesta, C.; Carroli, G.; Qureshi, Z.; Widmer, M.; Vogel, J.P.; Souza, J.P. WHO Multicountry Survey on Maternal and Newborn Health Research Network. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: A secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG Int. J. Obstet. Gynecol. 2014, 121 (Suppl. S1), 14–24. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, R.N. Preeclampsia, eclampsia: Terminology and classifications. Ulyanovsk. Med. Biol. J. 2018, 2, 41–46. (In Russian) [Google Scholar]
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Danilova, L.A.; Litvinenko, L.A. Preeclampsia and newborn health. Med. Theory Pract. 2019, 4, 593–594. [Google Scholar]
- Doney, A.; Fischer, B.; Frew, D.; Cumming, A.; Flavell, D.M.; World, M.; Montgomery, H.E.; Boyle, D.; Morris, A.; Palmer, C.N. Haplotype analysis of the PPARgamma Pro12Ala and C1431T variants reveals opposing associations with body weight. BMC Genet. 2002, 3, 21. [Google Scholar] [CrossRef]
- Bilano, V.L.; Ota, E.; Ganchimeg, T.; Mori, R.; Souza, J.P. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: A WHO secondary analysis. PLoS ONE 2014, 9, e91198. [Google Scholar] [CrossRef]
- Nerenberg, K.A.; Johnson, J.A.; Leung, B.; Savu, A.; Ryan, E.A.; Chik, C.L.; Kaul, P. Risks of gestational diabetes and preeclampsia over the last decade in a cohort of Alberta women. J. Obstet. Gynaecol. Can. 2013, 35, 986–994. [Google Scholar] [CrossRef]
- HAPO Study Cooperative Research Group. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with maternal body mass index. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 575–584. [Google Scholar] [CrossRef]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco RP, V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nature reviews. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Vidaeff, A.; Pettker, C.M.; Simhan, H. ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2019, 133, 1. [Google Scholar] [CrossRef]
- Webster, K.; Fishburn, S.; Maresh, M.; Findlay, S.C.; Chappell, L.C.; Guideline Committee. Diagnosis and management of hypertension in pregnancy: Summary of updated NICE guidance. BMJ (Clin. Res. Ed.) 2019, 366, l5119. [Google Scholar] [CrossRef] [PubMed]
- Kametas, N.A.; Nzelu, D.; Nicolaides, K.H. Chronic hypertension and superimposed preeclampsia: Screening and diagnosis. Am. J. Obstet. Gynecol. 2022, 226, S1182–S1195. [Google Scholar] [CrossRef]
- Lie, R.T.; Rasmussen, S.; Brunborg, H.; Gjessing, H.K.; Lie-Nielsen, E.; Irgens, L.M. Fetal and maternal contributions to risk of pre-eclampsia: Population based study. BMJ (Clin. Res. Ed.) 1998, 316, 1343–1347. [Google Scholar] [CrossRef]
- Little, J.; Higgins, J. The HuGENet™ HuGE Review Handbook, Version 1.0. Centers for Disease Control and Prevention; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2006. [Google Scholar]
- Sollis, E.; Mosaku, A.; Abid, A.; Buniello, A.; Cerezo, M.; Gil, L.; Groza, T.; Güneş, O.; Hall, P.; Hayhurst, J.; et al. The NHGRI-EBI GWAS Catalog: Knowledgebase and deposition resource. Nucleic Acids Res. 2023, 51, D977–D985. [Google Scholar] [CrossRef]
- Steinthorsdottir, V.; McGinnis, R.; Williams, N.O.; Stefansdottir, L.; Thorleifsson, G.; Shooter, S.; Fadista, J.; Sigurdsson, J.K.; Auro, K.M.; Berezina, G.; et al. Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat. Commun. 2020, 11, 5976. [Google Scholar] [CrossRef]
- Changalidis, A.I.; Maksiutenko, E.M.; Barbitoff, Y.A.; Tkachenko, A.A.; Vashukova, E.S.; Pachuliia, O.V.; Nasykhova, Y.A.; Glotov, A.S. Aggregation of Genome-Wide Association Data from FinnGen and UK Biobank Replicates Multiple Risk Loci for Pregnancy Complications. Genes 2022, 13, 2255. [Google Scholar] [CrossRef]
- Denny, J.C.; Bastarache, L.; Ritchie, M.D.; Carroll, R.J.; Zink, R.; Mosley, J.D.; Field, J.R.; Pulley, J.M.; Ramirez, A.H.; Bowton, E.; et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat. Biotechnol. 2013, 31, 1102–1110. [Google Scholar] [CrossRef]
- Wu, Y.; Byrne, E.M.; Zheng, Z.; Kemper, K.E.; Yengo, L.; Mallett, A.J.; Yang, J.; Visscher, P.M.; Wray, N.R. Genome-wide association study of medication-use and associated disease in the UK Biobank. Nat. Commun. 2019, 10, 1891. [Google Scholar] [CrossRef]
- Elawad, T.; Scott, G.; Bone, J.N.; Elwell, H.; Lopez, C.E.; Filippi, V.; Green, M.; Khalil, A.; Kinshella, M.W.; Mistry, H.D.; et al. PRECISE Network Risk factors for pre-eclampsia in clinical practice guidelines: Comparison with the evidence. BJOG Int. J. Obstet. Gynaecol. 2024, 131, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Robillard, P.Y.; Dekker, G.; Scioscia, M.; Bonsante, F.; Iacobelli, S.; Boukerrou, M.; Hulsey, T.C. Increased BMI has a linear association with late-onset preeclampsia: A population-based study. PLoS ONE 2019, 14, e0223888. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. Pre-Pregnancy Obesity vs. Other Risk Factors in Probability Models of Preeclampsia and Gestational Hypertension. Nutrients 2020, 12, 2681. [Google Scholar] [CrossRef]
- Gong, X.; Li, J.; Jiang, Y.; Yuan, P.; Chen, L.; Yang, Y.; Li, Y.; Sun, M.; Zhao, Y.; Shi, H.; et al. Risk of preeclampsia by gestational weight gain in women with varied prepregnancy BMI: A retrospective cohort study. Front. Endocrinol. 2022, 13, 967102. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Belizán, J.M. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 75–83. [Google Scholar] [CrossRef] [PubMed]
- McCowan, L.M.; Buist, R.G.; North, R.A.; Gamble, G. Perinatal morbidity in chronic hypertension. Br. J. Obstet. Gynaecol. 1996, 103, 123–129. [Google Scholar] [CrossRef]
- Sibai, B.M.; Anderson, G.D. Pregnancy outcome of intensive therapy in severe hypertension in first trimester. Obstet. Gynecol. 1986, 67, 517–522. [Google Scholar]
- Seely, E.W.; Ecker, J. Chronic hypertension in pregnancy. Circulation 2014, 129, 1254–1261. [Google Scholar] [CrossRef]
- Garovic, V.D.; August, P. Preeclampsia and the future risk of hypertension: The pregnant evidence. Curr. Hypertens. Rep. 2013, 15, 114–121. [Google Scholar] [CrossRef]
- Chappell, L.C.; Enye, S.; Seed, P.; Briley, A.L.; Poston, L.; Shennan, A.H. Adverse perinatal outcomes and risk factors for preeclampsia in women with chronic hypertension: A prospective study. Hypertension 2008, 51, 1002–1009. [Google Scholar] [CrossRef]
- Gilbert, W.M.; Young, A.L.; Danielsen, B. Pregnancy outcomes in women with chronic hypertension: A population-based study. J. Reprod. Med. 2007, 52, 1046–1051. [Google Scholar] [PubMed]
- Zetterström, K.; Lindeberg, S.N.; Haglund, B.; Hanson, U. Maternal complications in women with chronic hypertension: A population-based cohort study. Acta Obstet. Et Gynecol. Scand. 2005, 84, 419–424. [Google Scholar] [CrossRef]
- Vanek, M.; Sheiner, E.; Levy, A.; Mazor, M. Chronic hypertension and the risk for adverse pregnancy outcome after superimposed pre-eclampsia. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2004, 86, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Tita, A.T.; Szychowski, J.M.; Boggess, K.; Dugoff, L.; Sibai, B.; Lawrence, K.; Hughes, B.L.; Bell, J.; Aagaard, K.; Edwards, R.K.; et al. Chronic Hypertension and Pregnancy (CHAP) Trial Consortium Treatment for Mild Chronic Hypertension during Pregnancy. N. Engl. J. Med. 2022, 386, 1781–1792. [Google Scholar] [CrossRef]
- Tyrmi, J.S.; Kaartokallio, T.; Lokki, A.I.; Jääskeläinen, T.; Kortelainen, E.; Ruotsalainen, S.; Karjalainen, J.; Ripatti, S.; Kivioja, A.; Laisk, T.; et al. FinnGen Project, and the Estonian Biobank Research Team Genetic Risk Factors Associated with Preeclampsia and Hypertensive Disorders of Pregnancy. JAMA Cardiol. 2023, 8, 674–683. [Google Scholar] [CrossRef]
- Dedov, I.I.; Sukhikh, G.T.; Filippov, O.S.; Arbatskaya, N.Y.; Borovik, N.V.; Burumkulova, F.F.; Galstyan, G.R.; Grigoryan, O.R.; Degtyareva, E.I.; Demidova, I.Y.; et al. Gestational diabetes mellitus: Diagnosis, treatment, postpartum care. Probl. Reprod. 2018, 24 (Suppl. S6), 115–127. (In Russian) [Google Scholar]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva Ad Hod, M.; Kitzmiler, J.L.; et al.; International Association of Diabetes and Pregnancy Study Groups Consensus Panel International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Gauderman, W.J. Sample size requirements for association studies of gene-gene interaction. Am. J. Epidemiol. 2002, 155, 478–484. [Google Scholar] [CrossRef]
- Martin, F.J.; Amode, M.R.; Aneja, A.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2023. Nucleic Acids Res. 2023, 51, D933–D941. [Google Scholar] [CrossRef]
- Kent, W.J.; Sugnet, C.W.; Furey, T.S.; Roskin, K.M.; Pringle, T.H.; Zahler, A.M.; Haussler, D. The human genome browser at, U.C.S.C. Genome Res. 2002, 12, 996–1006. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; E Bolton, E.; Brister, J.R.; Chan, J.; Comeau, D.C.; Connor, R.; DiCuccio, M.; Farrell, C.M.; Feldgarden, M.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2024, 52, D33. [Google Scholar] [CrossRef] [PubMed]
- González, J.R.; Armengol, L.; Solé, X.; Guinó, E.; Mercader, J.M.; Estivill, X.; Moreno, V. SNPassoc: An R package to perform whole genome association studies. Bioinformatics (Oxf. Engl.) 2007, 23, 644–645. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Control, n = 207 | PE, n = 216 | PE+, n = 147 | SIPE, n = 69 | |||
|---|---|---|---|---|---|---|---|
| Values | Values | p-Value 1 | Values | p-Value 1 | Values | p-Value 1 | |
| Age (mean ± SD), years | 31.30 ± 4.9 | 32.14 ± 4.8 | 0.08 | 31.6 ± 4.6 | 0.48 | 33.39 ± 4.74 | 0.002 |
| ≥35 years, n (%) | 66 (32) | 69 (32) | 0.99 | 39 (26.5) | 0.28 | 30 (43.48) | 0.08 |
| BMI (mean ± SD), kg/m2 | 26.6 ± 6.7 | 31.9 ± 8.4 | <0.00001 | 28.88 ± 6.8 | 0.0003 | 38.77 ± 7.83 | <0.00001 |
| BMI > 29.9, n (%) | 54 (26) | 120 (56) | <0.00001 | 66 (45) | 0.0002 | 54 (78) | <0.00001 |
| CHTN, n (%) | 9 (4) | 69 (32) | <0.00001 | 147 (0) | Not applicable 2 | 69 (100) | Not applicable 2 |
| IDA, n (%) | 105 (55) | 135 (63) | 0.01 | 75 (51) | 0.96 | 60 (87) | <0.00001 |
| Insulin/diet GDM treated, n (%) | 69 (33)/138 (67) | 126 (58)/90 (42) | <0.00001 | 87 (59)/60 (41) | <0.00001 | 39 (57)/30 (43) | 0.0006 |
| Gestational age at detection of GDM | 24.79 ± 7.43 | 23.37 ± 7.51 | 0.08 | 23.15 ± 8.25 | 0.17 | 23.83 ± 5.7 | 0.13 |
| Gestational age at detection of PE | Not applicable 2 | 32.83 ± 4.67 | Not applicable 2 | 33.9 ± 3.88 | Not applicable 2 | 31.83 ± 5.14 | Not applicable 2 |
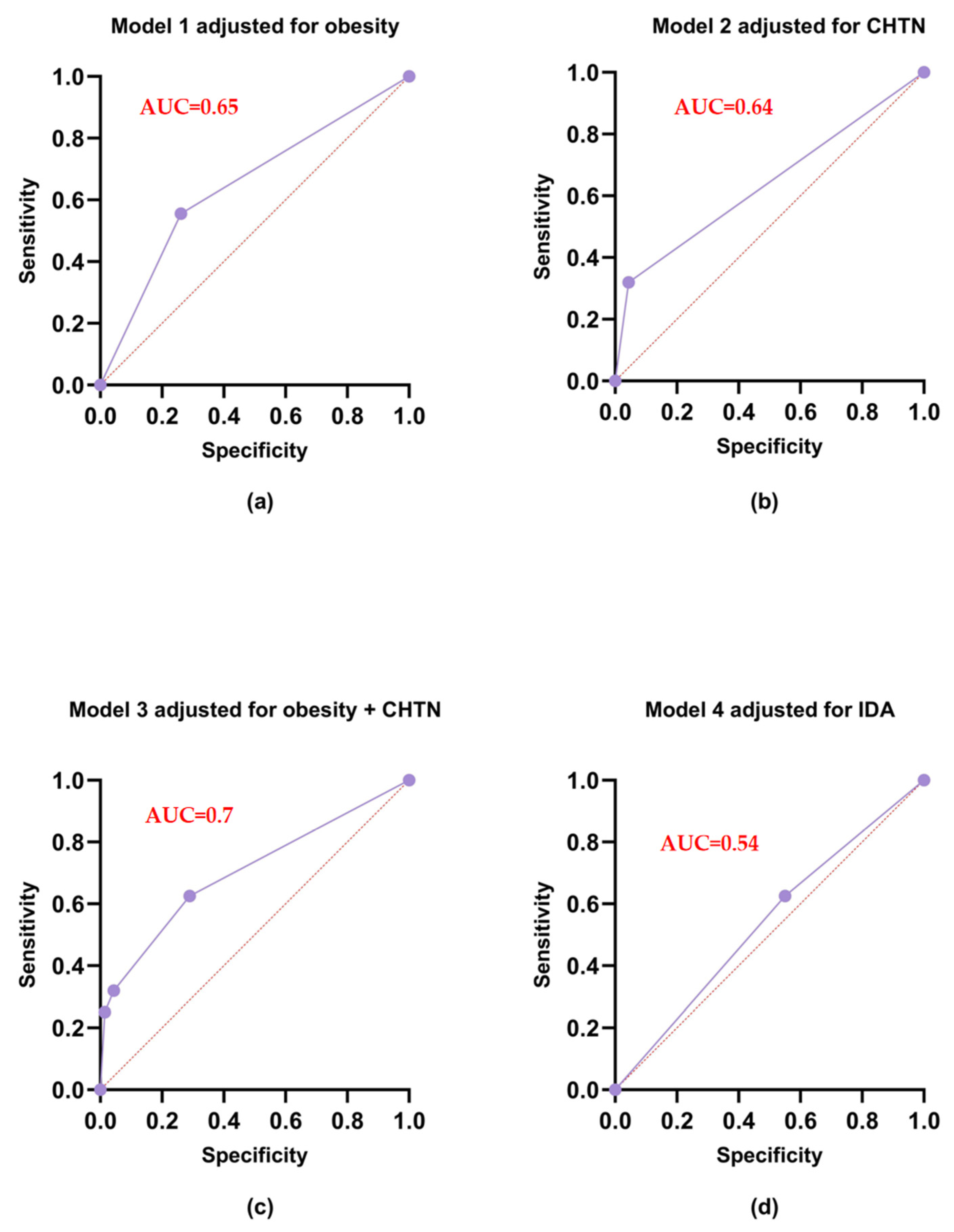
| Model | Variable | OR (95% CI) | p-Value 1 | Sensitivity | Specificity | AIC 2 |
|---|---|---|---|---|---|---|
| Model 1 adjusted for obesity | Intercept | 0.628 (0.485–0.808) | 0.0003 | 55.56 | 73.91 | 551.6 |
| Obesity | 3.542 (2.361–5.367) | <0.0001 | ||||
| Model 2 adjusted for CHTN | Intercept | 0.742 (0.599–0.918) | 0.006 | 31.94 | 95.65 | 530.5 |
| CHTN | 10.33 (5.245–22.80) | <0.0001 | ||||
| Model 3 adjusted for obesity and CHTN | Intercept | 0.531 (0.405–0.693) | <0.0001 | 62.50 | 71.01 | 513.7 |
| Obesity | 2.601 (1.687–4.034) | <0.0001 | ||||
| CHTN | 7.720 (3.847–17.226) | <0.0001 | ||||
| Model 4 adjusted for IDA | Intercept | 0.871 (0.646–1.173) | 0.36 | 62.50 | 44.93 | 530.5 |
| IDA | 1.36 (0.923–2.001) | 0.12 |
| Sample Type | Genotype Distribution, n (%) | Allele Frequency | p-Value | |||
|---|---|---|---|---|---|---|
| AA | AC | CC | A | C | ||
| Control, n = 207 | 138 (66.67) | 63 (30.43) | 6 (2.90) | 0.82 | 0.18 | 0.82 |
| PE+, n = 216 | 147 (68.06) | 57 (26.39) | 12 (5.56) | 0.81 | 0.19 | 0.07 |
| Model of Inheritance | Genotypes | PE+, n = 216 | Control, n = 207 | OR (95% of CI) | p-Value 1 | AIC |
|---|---|---|---|---|---|---|
| Codominant | AA | 147 (68.1) | 138 (66.7) | 1.00 | 0.296 | 589.8 |
| AC | 57 (26.4) | 63 (30.4) | 0.85 (0.55–1.30) | |||
| CC | 12 (5.6) | 6 (2.9) | 1.88 (0.69–5.14) | |||
| Dominant | AA | 147 (68.1) | 138 (66.7) | 1.00 | 0.761 | 590.1 |
| AC+CC | 63 (31.9) | 69 (33.3) | ||||
| Recessive | AA+AC | 204 (94.4) | 201 (97.1) | 1.00 | 0.172 | 588.4 |
| CC | 12 (5.6) | 6 (2.9) | ||||
| Overdominant | AA+CC | 159 (73.6) | 144 (69.6) | 1.00 | 0.356 | 589.4 |
| AC | 59 (26.4) | 63 (30.4) | ||||
| Log-additive | 0, 1, 2 | 216 (51.1) | 207(48.9) | 1.04 (0.74–1.46) | 0.817 | 590.2 |
| Sample Type | Genotype Distribution, n (%) | Allele Frequency | p-Value | |||
|---|---|---|---|---|---|---|
| AA | AC | CC | A | C | ||
| Control, n = 207 | 138 (66.67) | 63 (30.43) | 6 (2.90) | 0.82 | 0.18 | 0.82 |
| SIPE, n = 67 | 42 (60.9) | 18 (26) | 9 (13.1) | 0.74 | 0.26 | 0.01 |
| Model of Inheritance | Genotypes | SIPE, n = 69 | Control, n = 207 | OR (95% of CI) | p-Value 1 | AIC |
|---|---|---|---|---|---|---|
| Codominant | AA | 42 (60.9) | 138 (66.67) | 1.00 | 0.012 | 307.67 |
| AC | 18 (26.1) | 63 (30.34) | 0.94 (0.5–1.76) | |||
| CC | 9 (13.0) | 6 (2.9) | 4.93(1.66–14.65) | |||
| Dominant | AA | 42 (60.9) | 138 (66.67) | 1.00 | 0.38 | 313.7 |
| AC+CC | 27 (39.1) | 69 (33.3) | 1.29 (0.73–2.26) | |||
| Recessive | AA+AC | 60 (87) | 201 (97.1) | 1.00 | 0.003 | 305.6 |
| CC | 9 (13) | 6 (2.9) | 5.05 (1.72–14.69) | |||
| Overdominant | AA+CC | 51 (73.9) | 144 (69.6) | 1.00 | 0.49 | 313.9 |
| AC | 18 (26.1) | 63 (30.4) | 0.81 (0.44–1.49) | |||
| Log-additive | 0,1,2 | 69 (25) | 207 (75) | 1.54 (0.99–2.39) | 0.057 | 310.8 |
| Primer Type | Sequences (5’-->3’) | Tm (Salt Adjusted), °C 1 |
|---|---|---|
| Forward primer | GAGGAAGGATGTGGCGAGG | 61.6 |
| Reverse primer | GAAGCTGTGGTCAGGAGGAG | 62.5 |
| TaqMan probe for A allele | FAM-CTTGTCTCATGG A CGCTCTTGATCG-BHQ1 | 67.4 |
| TaqMan probe for C allele | HEX-CTTGTCTCATGG C CGCTCTTGATCG-BHQ1 | 69.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpova, N.; Dmitrenko, O.; Nurbekov, M. Polymorphism rs259983 of the Zinc Finger Protein 831 Gene Increases Risk of Superimposed Preeclampsia in Women with Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 11108. https://doi.org/10.3390/ijms252011108
Karpova N, Dmitrenko O, Nurbekov M. Polymorphism rs259983 of the Zinc Finger Protein 831 Gene Increases Risk of Superimposed Preeclampsia in Women with Gestational Diabetes Mellitus. International Journal of Molecular Sciences. 2024; 25(20):11108. https://doi.org/10.3390/ijms252011108
Chicago/Turabian StyleKarpova, Nataliia, Olga Dmitrenko, and Malik Nurbekov. 2024. "Polymorphism rs259983 of the Zinc Finger Protein 831 Gene Increases Risk of Superimposed Preeclampsia in Women with Gestational Diabetes Mellitus" International Journal of Molecular Sciences 25, no. 20: 11108. https://doi.org/10.3390/ijms252011108
APA StyleKarpova, N., Dmitrenko, O., & Nurbekov, M. (2024). Polymorphism rs259983 of the Zinc Finger Protein 831 Gene Increases Risk of Superimposed Preeclampsia in Women with Gestational Diabetes Mellitus. International Journal of Molecular Sciences, 25(20), 11108. https://doi.org/10.3390/ijms252011108







