In Vitro Evaluation of New 5-Nitroindazolin-3-one Derivatives as Promising Agents against Trypanosoma cruzi
Abstract
1. Introduction

2. Results and Discussion
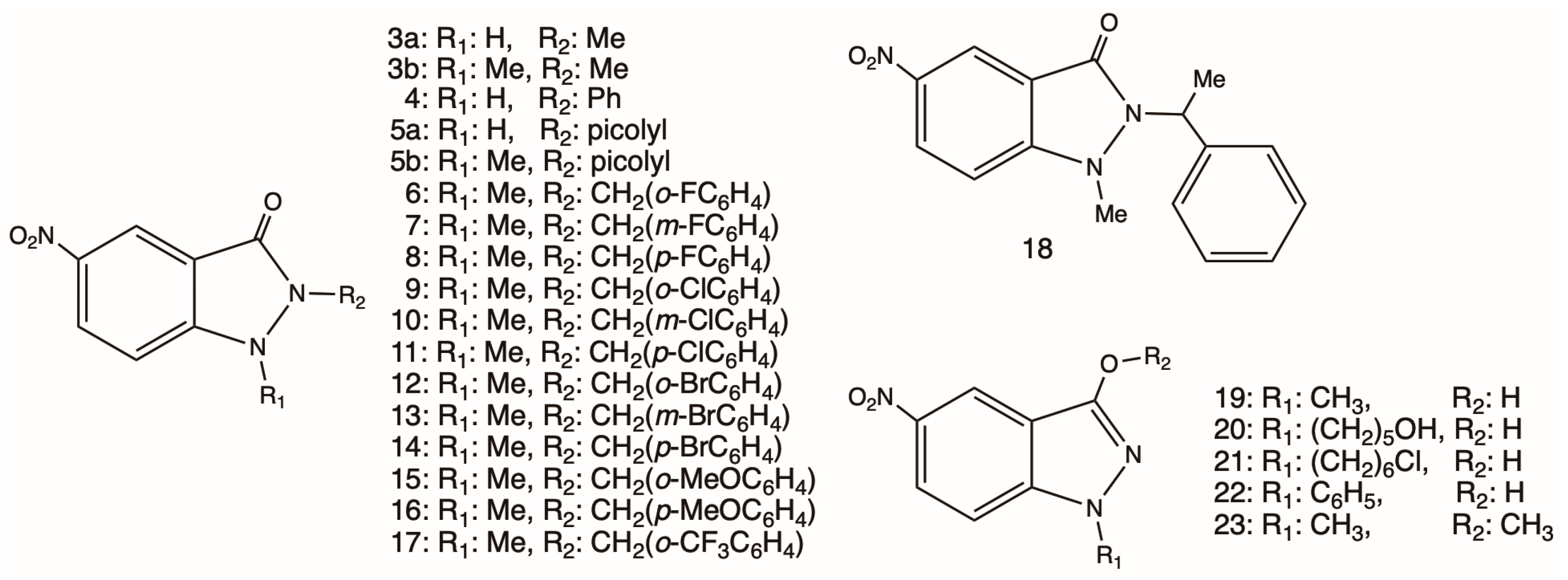
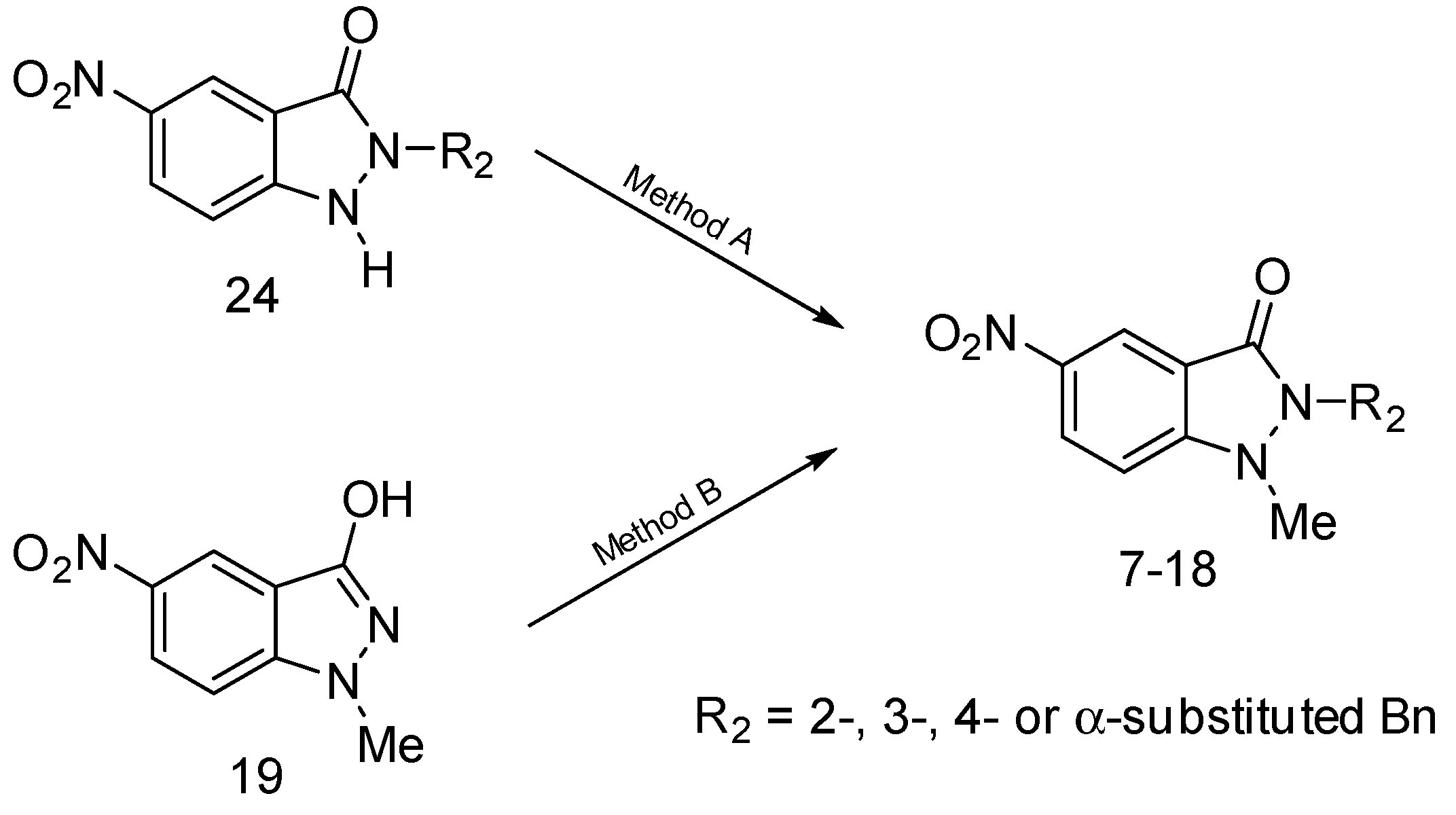
2.1. Synthesis of 1,2-Disubstituted 5-nitroindazolin-3-ones
2.2. Cyclic Voltammetry
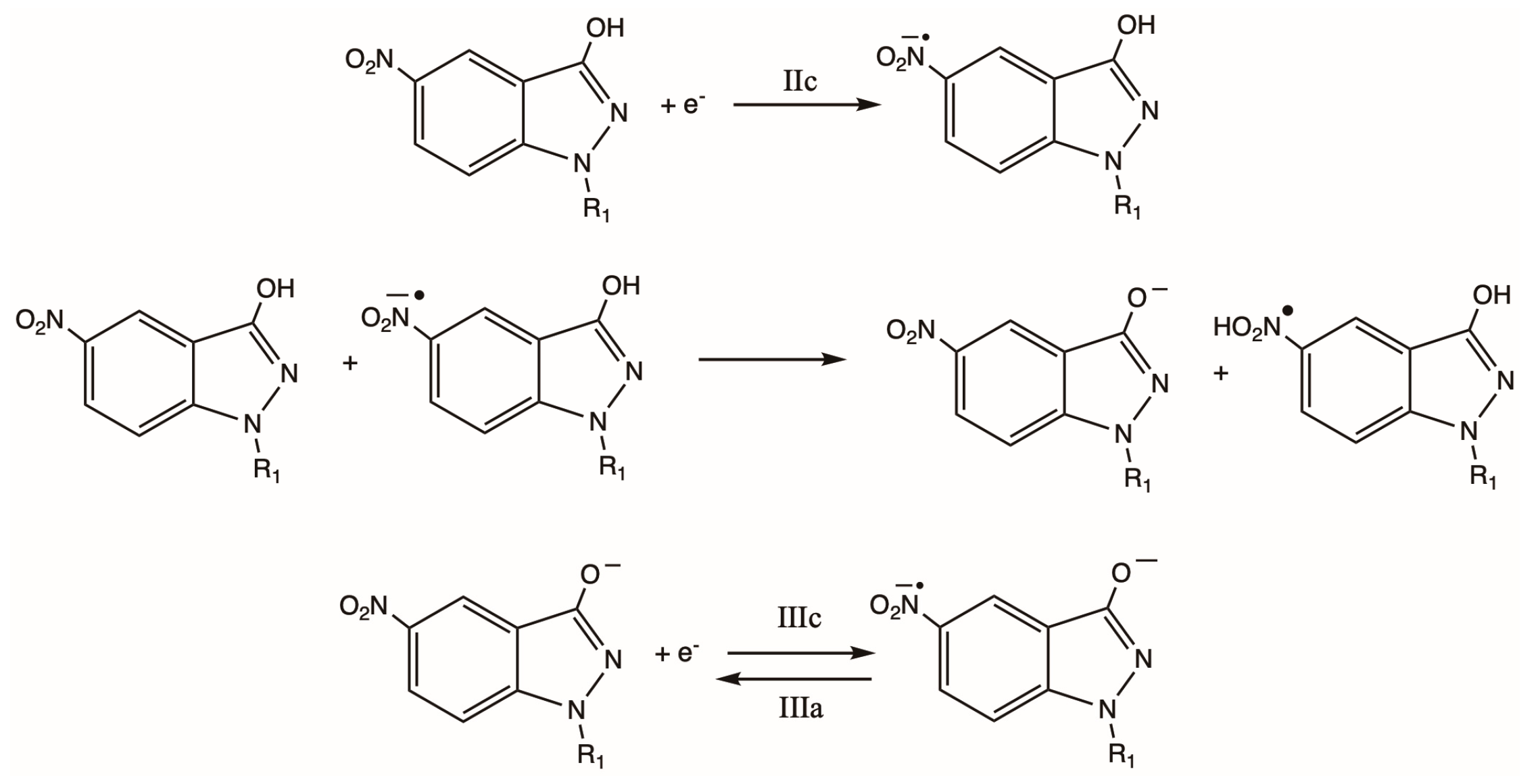
Characterization of Radicals by ESR
2.3. Determination of Cytotoxic Activity against T. cruzi (Dm28c) Epimastigotes and Trypomastigote Forms
2.4. Action Mechanism
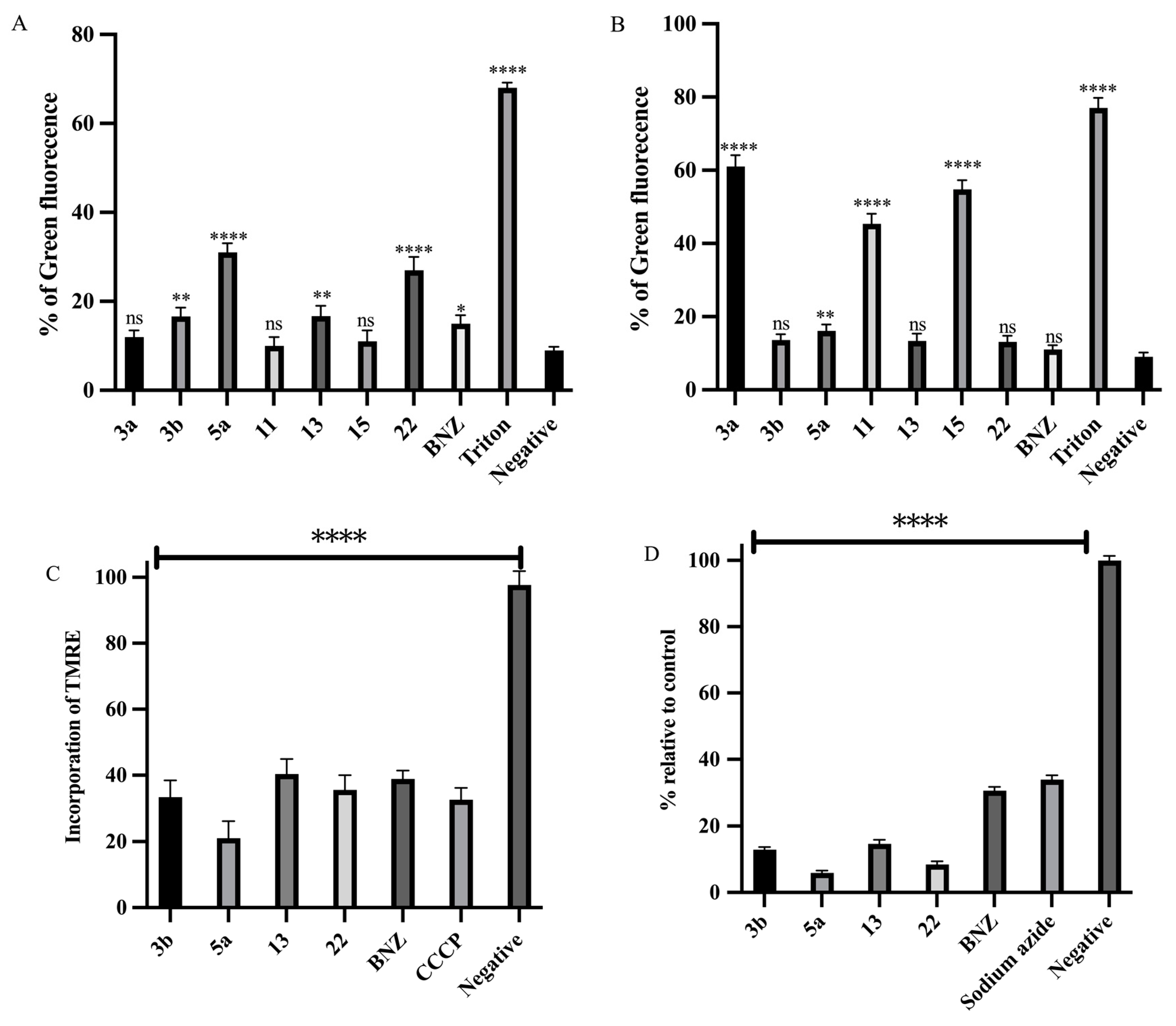
2.4.1. Plasma Membrane Permeability
2.4.2. Mitochondrial Membrane Potential (ψm)
2.4.3. Analysis of ATP Levels
2.4.4. Intracellular ROS Generation
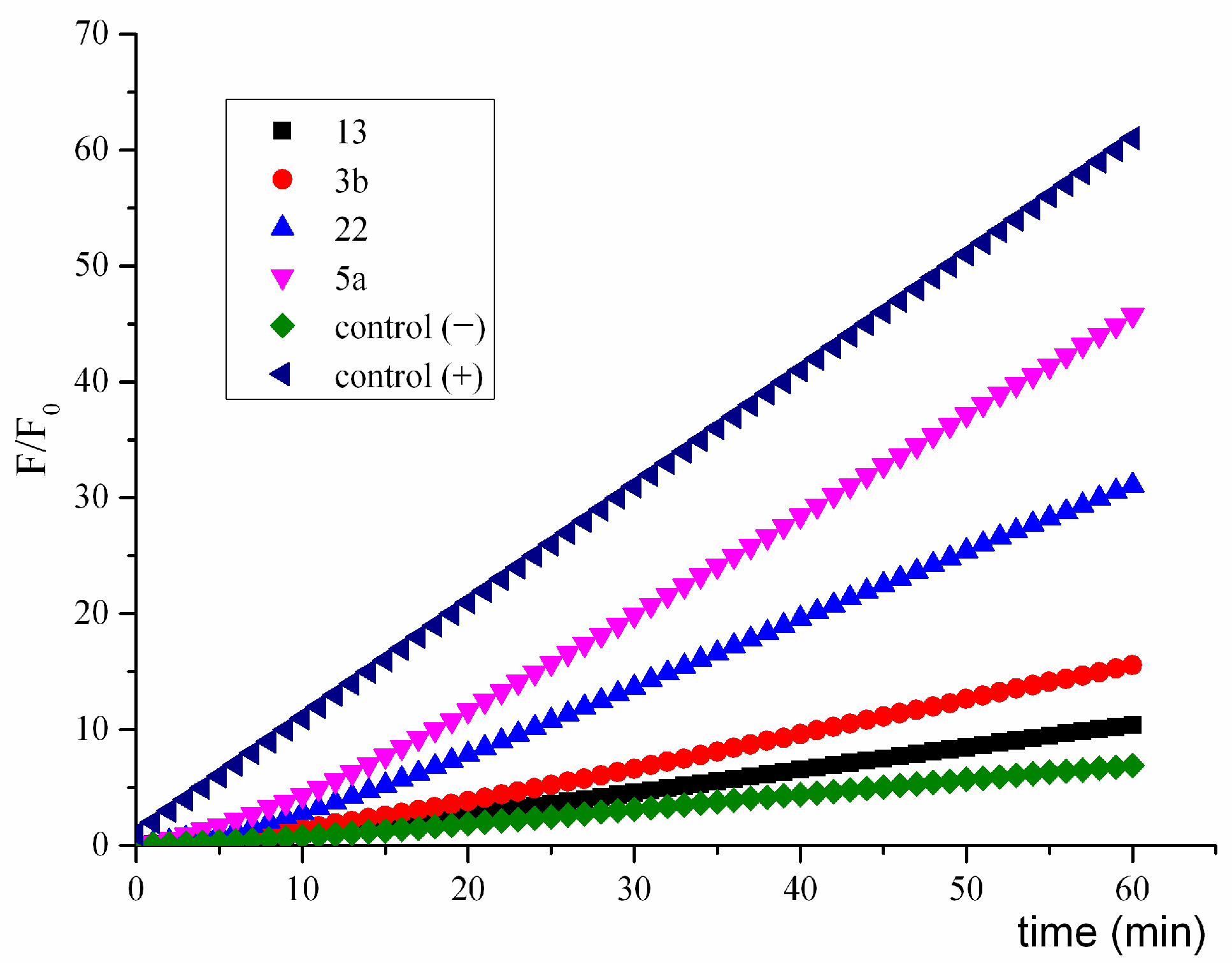
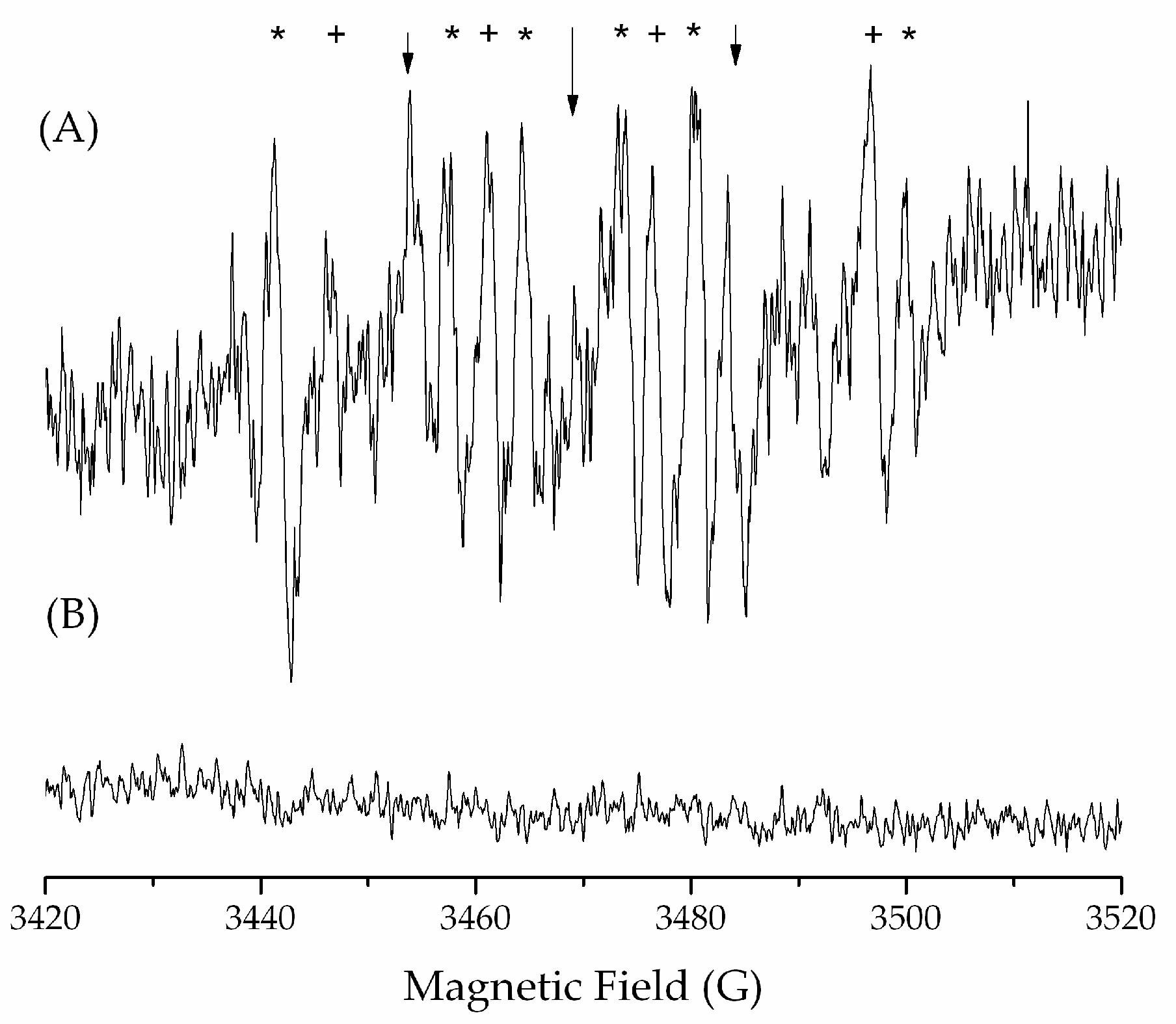
2.4.5. Study of the Generation of Radical Species by Spin Trapping
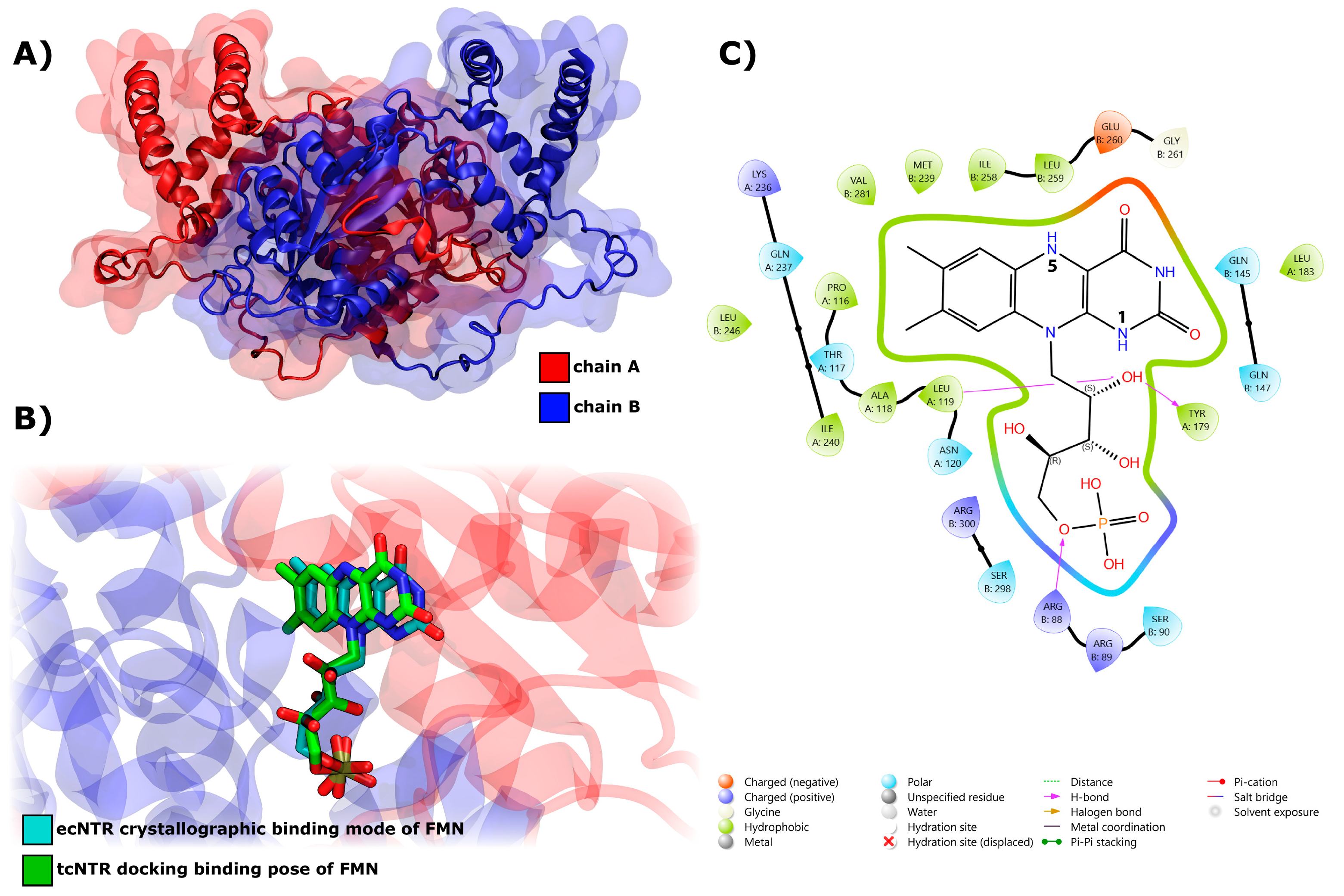
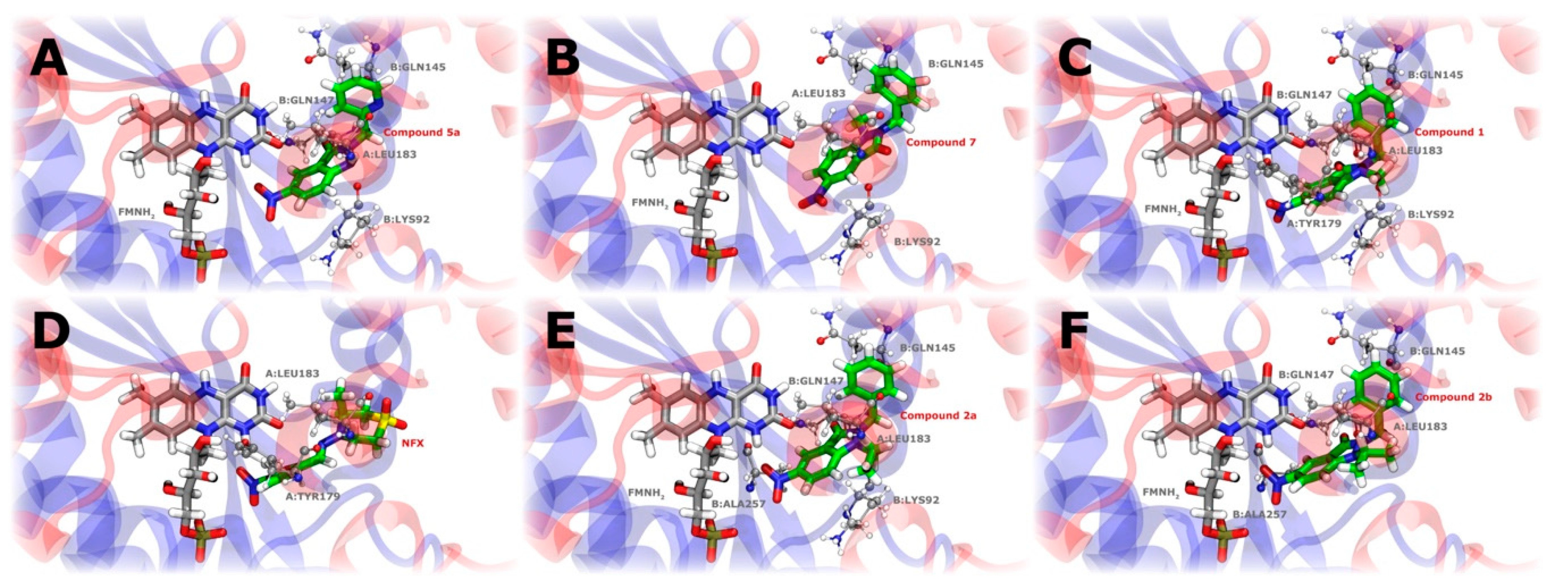
2.4.6. Interaction of Nitroreductase Receptor on T. cruzi and Compounds
2.4.7. General Discussion
3. Materials and Methods
3.1. Chemistry
3.1.1. General Methods
3.1.2. Preparation of 1,2-Disubstituted Indazolinones
3.1.3. Preparation of 5-Nitroindazole
3.2. Cyclic Voltammetry (CV)
3.3. ESR Spectroscopy
3.4. Biologic Assays
3.4.1. Cytotoxicity Assay
3.4.2. Epimastigotes Viability Study
3.4.3. Trypomastigote Viability Study
3.4.4. Plasmatic Membrane Permeability Assay
3.4.5. Analysis of ATP Levels
3.4.6. Intracellular Generation of Reactive Oxygen Species (ROS)
3.4.7. Determination of the Effect on Mitochondrial Membrane Potential (Δψm) in Dm28c Trypomastigote of T. cruzi
3.4.8. ESR Studies in Parasite Media
3.4.9. Interaction of Nitro Compounds with TcNTR through Docking Calculations
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Molina, J.A.; Molina, I. Chagas Disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Ferreira, L.L.G.; de Moraes, J.; Andricopulo, A.D. Approaches to Advance Drug Discovery for Neglected Tropical Diseases. Drug Discov. Today 2022, 27, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Bhardwaj, M.; Agrawal, N.; Bhardwaj, A. A Comprehensive Review on Potential Candidates for the Treatment of Chagas Disease. Chem. Biol. Drug Des. 2023, 102, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Scarim, C.B.; Jornada, D.H.; Chelucci, R.C.; de Almeida, L.; dos Santos, J.L.; Chung, M.C. Current Advances in Drug Discovery for Chagas Disease. Eur. J. Med. Chem. 2018, 155, 824–838. [Google Scholar] [CrossRef]
- Kawaguchi, W.H.; Cerqueira, L.B.; Fachi, M.M.; Campos, M.L.; Reason, I.J.M.; Pontarolo, R. Efficacy and Safety of Chagas Disease Drug Therapy and Treatment Perspectives. In Chagas Disease–Basic Investigations and Challenges; InTech: London, UK, 2018. [Google Scholar]
- Pereira, P.C.M.; Navarro, E.C. Challenges and Perspectives of Chagas Disease: A Review. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 34. [Google Scholar] [CrossRef]
- Tarleton, R.L. Effective Drug Discovery in Chagas Disease. Trends Parasitol. 2023, 39, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.S.; Bot, C.; Wilkinson, S.R. Nifurtimox Activation by Trypanosomal Type I Nitroreductases Generates Cytotoxic Nitrile Metabolites. J. Biol. Chem. 2011, 286, 13088–13095. [Google Scholar] [CrossRef]
- Wilkinson, S.R.; Taylor, M.C.; Horn, D.; Kelly, J.M.; Cheeseman, I. A Mechanism for Cross-Resistance to Nifurtimox and Benznidazole in Trypanosomes. Proc. Natl. Acad. Sci. USA 2008, 105, 5022–5027. [Google Scholar] [CrossRef]
- Cirqueira, M.L.; Bortot, L.O.; Bolean, M.; Aleixo, M.A.A.; Luccas, P.H.; Costa-Filho, A.J.; Ramos, A.P.; Ciancaglini, P.; Nonato, M.C. Trypanosoma Cruzi Nitroreductase: Structural Features and Interaction with Biological Membranes. Int. J. Biol. Macromol. 2022, 221, 891–899. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Olea-Azar, C.; Arán, V.J.; Diaz-Urrutia, C. ESR and Electrochemical Study of 1,2-Disubstituted 5-Nitroindazolin-3-Ones and 2-Substituted 3-Alkoxy-5-Nitro-2H-Indazoles: Reactivity and Free Radical Production Capacity in the Presence of Biological Systems. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 75, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Venegas, B.; Olea-Azar, C.; Arán, V.J.; Speisky, H. Indazoles: A New Top Seed Structure in the Search of Efficient Drugs against Trypanosoma Cruzi. Future Med. Chem. 2013, 5, 1843–1859. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Urrutia, C.A.; Olea-Azar, C.A.; Zapata, G.A.; Lapier, M.; Mura, F.; Aguilera-Venegas, B.; Arán, V.J.; López-Múñoz, R.A.; Maya, J.D. Biological and Chemical Study of Fused Tri- and Tetracyclic Indazoles and Analogues with Important Antiparasitic Activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 95, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.C.; Rolón, M.; Montero-Torres, A.; Fonseca-Berzal, C.; Escario, J.A.; Gómez-Barrio, A.; Gálvez, J.; Marrero-Ponce, Y.; Arán, V.J. Synthesis, Biological Evaluation and Chemometric Analysis of Indazole Derivatives. 1,2-Disubstituted 5-Nitroindazolinones, New Prototypes of Antichagasic Drug. Eur. J. Med. Chem. 2012, 58, 214–227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fonseca-Berzal, C.; Da Silva, P.B.; Da Silva, C.F.; Vasconcelos, M.; Batista, M.M.; Escario, J.A.; Arán, V.J.; Gómez-Barrio, A.; Soeiro, M.N.C. Exploring the Potential Activity Spectrum of Two 5-Nitroindazolinone Prototypes on Different Trypanosoma Cruzi Strains. Parasitol. Open 2015, 1, e1. [Google Scholar] [CrossRef][Green Version]
- Fonseca-Berzal, C.; Escario, J.A.; Arán, V.J.; Gómez-Barrio, A. Further Insights into Biological Evaluation of New Anti-Trypanosoma Cruzi 5-Nitroindazoles. Parasitol. Res. 2014, 113, 1049–1056. [Google Scholar] [CrossRef]
- Fonseca-Berzal, C.; Ibáñez-Escribano, A.; de Castro, S.; Escario, J.A.; Gómez-Barrio, A.; Arán, V.J. 5-Nitroindazole-Based Compounds: Further Studies for Activity Optimization as Anti-Trypanosoma Cruzi Agents. Acta Trop. 2022, 234, 106607. [Google Scholar] [CrossRef]
- Arán, V.J.; Flores, M.; Muñoz, P.; Páez, J.A.; Sánchez-Verdú, P.; Stud, M. Analogues of Cytostatic, Fused Indazolinones: Synthesis, Conformational Analysis and Cytostatic Activity Against HeLa Cells of Some 1-Substituted Indazolols, 2-Substituted Indazolinones, and Related Compounds. Liebigs Ann. 1996, 1996, 683–691. [Google Scholar] [CrossRef]
- Fonseca-Berzal, C.; Ibáñez-Escribano, A.; Reviriego, F.; Cumella, J.; Morales, P.; Jagerovic, N.; Nogal-Ruiz, J.J.; Escario, J.A.; da Silva, P.B.; Soeiro, M. de N.C.; et al. Antichagasic and Trichomonacidal Activity of 1-Substituted 2-Benzyl-5-Nitroindazolin-3-Ones and 3-Alkoxy-2-Benzyl-5-Nitro-2H-Indazoles. Eur. J. Med. Chem. 2016, 115, 295–310. [Google Scholar] [CrossRef]
- Fonseca-Berzal, C.; Ibáñez-Escribano, A.; Vela, N.; Cumella, J.; Nogal-Ruiz, J.J.; Escario, J.A.; da Silva, P.B.; Batista, M.M.; Soeiro, M.D.N.C.; Sifontes-Rodríguez, S.; et al. Antichagasic, Leishmanicidal, and Trichomonacidal Activity of 2-Benzyl-5-Nitroindazole-Derived Amines. ChemMedChem 2018, 13, 1246–1259. [Google Scholar] [CrossRef]
- Muzart, J. N,N-Dimethylformamide: Much More than a Solvent. Tetrahedron 2009, 65, 8313–8323. [Google Scholar] [CrossRef]
- Olea-Azar, C.; Cerecetto, H.; Gerpe, A.; González, M.; Arán, V.J.; Rigol, C.; Opazo, L. ESR and Electrochemical Study of 5-Nitroindazole Derivatives with Antiprotozoal Activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Olea-Azar, C.; Barriga, G.; Folch, C.; Gerpe, A.; Cerecetto, H.; González, M. Comparative Spectroscopic and Electrochemical Study of Nitroindazoles: 3-Alcoxy, 3-Hydroxy and 3-Oxo Derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 70, 557–563. [Google Scholar] [CrossRef]
- Maria Aravena, C.; Claudio Olea, A.; Cerecetto, H.; González, M.; Maya, J.D.; Rodríguez-Becerra, J. Potent 5-Nitrofuran Derivatives Inhibitors of Trypanosoma Cruzi Growth: Electrochemical, Spectroscopic and Biological Studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Venegas, B.; Olea-Azar, C.; Norambuena, E.; Arán, V.J.; Mendizábal, F.; Lapier, M.; Maya, J.D.; Kemmerling, U.; López-Muñoz, R. ESR, Electrochemical, Molecular Modeling and Biological Evaluation of 4-Substituted and 1,4-Disubstituted 7-Nitroquinoxalin-2-Ones as Potential Anti-Trypanosoma Cruzi Agents. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, M.L.; Hamilton, R.G.; Nicholson, R.S. Theory of Cyclic Voltammetry for a Dimerization Reaction Initiated Electrochemically. Anal. Chem. 1969, 41, 260–267. [Google Scholar] [CrossRef]
- Nicholson, R.S.; Shain, Irving. Theory of Stationary Electrode Polarography. Single Scan and Cyclic Methods Applied to Reversible, Irreversible, and Kinetic Systems. Anal. Chem. 1964, 36, 706–723. [Google Scholar] [CrossRef]
- Robledo-O’Ryan, N.; Matos, M.J.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Moncada-Basualto, M.; Mura, F.; Lapier, M.; Maya, J.D.; Olea-Azar, C. Synthesis, Antioxidant and Antichagasic Properties of a Selected Series of Hydroxy-3-Arylcoumarins. Bioorg Med. Chem. 2017, 25, 621–632. [Google Scholar] [CrossRef]
- Livertoux, M.-H.; Lagrange, P.; Minn, A. The Superoxide Production Mediated by the Redox Cycling of Xenobiotics in Rat Brain Microsomes Is Dependent on Their Reduction Potential. Brain Res. 1996, 725, 207–216. [Google Scholar] [CrossRef]
- Duling, D.R. Simulation of Multiple Isotropic Spin-Trap EPR Spectra. J. Magn. Reson. B 1994, 104, 105–110. [Google Scholar] [CrossRef]
- Gerpe, A.; Aguirre, G.; Boiani, L.; Cerecetto, H.; González, M.; Olea-Azar, C.; Rigol, C.; Maya, J.D.; Morello, A.; Piro, O.E.; et al. Indazole N-Oxide Derivatives as Antiprotozoal Agents: Synthesis, Biological Evaluation and Mechanism of Action Studies. Bioorg. Med. Chem. 2006, 14, 3467–3480. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.; Arán, V.J.; Boiani, L.; Olea-Azar, C.; Lavaggi, M.L.; González, M.; Cerecetto, H.; Maya, J.D.; Carrasco-Pozo, C.; Cosoy, H.S. New Potent 5-Nitroindazole Derivatives as Inhibitors of Trypanosoma Cruzi Growth: Synthesis, Biological Evaluation, and Mechanism of Action Studies. Bioorg. Med. Chem. 2009, 17, 8186–8196. [Google Scholar] [CrossRef] [PubMed]
- Mollineda-Diogo, N.; Chaviano-Montes de Oca, C.S.; Sifontes-Rodríguez, S.; Espinosa-Buitrago, T.; Monzote-Fidalgo, L.; Meneses-Marcel, A.; Morales-Helguera, A.; Perez-Castillo, Y.; Arán-Redó, V. Antileishmanial Activity of 5-Nitroindazole Derivatives. Ther. Adv. Infect. Dis. 2023, 10, 20499361231208296. [Google Scholar] [CrossRef]
- Menna-Barreto, R.F.S. Cell Death Pathways in Pathogenic Trypanosomatids: Lessons of (over)Kill. Cell Death Dis. 2019, 10, 93. [Google Scholar] [CrossRef]
- Bethencourt-Estrella, C.J.; Delgado-Hernández, S.; López-Arencibia, A.; San Nicolás-Hernández, D.; Tejedor, D.; García-Tellado, F.; Lorenzo-Morales, J.; Piñero, J.E. In Vitro Activity and Mechanism of Cell Death Induction of Cyanomethyl Vinyl Ethers Derivatives against Trypanosoma Cruzi. Int. J. Parasitol. Drugs Drug Resist. 2023, 22, 72–80. [Google Scholar] [CrossRef]
- Bethencourt-Estrella, C.J.; Delgado-Hernández, S.; López-Arencibia, A.; San Nicolás-Hernández, D.; Tejedor, D.; García-Tellado, F.; Lorenzo-Morales, J.; Piñero, J.E. In Vitro Activity and Cell Death Mechanism Induced by Acrylonitrile Derivatives against Leishmania Amazonensis. Bioorg. Chem. 2022, 124, 105872. [Google Scholar] [CrossRef]
- Correa, I.T.S.; da Costa-Silva, T.A.; Tempone, A.G. Bioenergetics Impairment of Trypanosoma Cruzi by the Antihypertensive Manidipine: A Drug Repurposing Strategy. Acta Trop. 2021, 214, 105768. [Google Scholar] [CrossRef]
- Docampo, R. The Origin and Evolution of the Acidocalcisome and Its Interactions with Other Organelles. Mol. Biochem. Parasitol. 2016, 209, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Martínez, J.; Salgado, F.; Liempi, A.; Kemmerling, U.; Mera-Adasme, R.; Olea-Azar, C.; Moncada-Basualto, M.; Borges, F.; Uriarte, E.; Matos, M.J. Synthesis and Study of the Trypanocidal Activity of Catechol-Containing 3-Arylcoumarins, Inclusion in β-Cyclodextrin Complexes and Combination with Benznidazole. Arab. J. Chem. 2022, 15, 103641. [Google Scholar] [CrossRef]
- Marco Antônio Salgado Martins, T.; de Figueiredo Peloso, E.; Costa-Silva, H.M.; Rajão, M.A.; Van Houten, B.; Machado, C.R.; Ramos Gadelha, F. Mitochondrial Behavior during Nuclear and Mitochondrial DNA Repair in Trypanosoma Cruzi Epimastigotes. Exp. Parasitol. 2020, 219, 108016. [Google Scholar] [CrossRef]
- Barriga-Gonzalez, G.; Olea-Azar, C.; Zuniga-Lopez, C.M.; Folch-Cano, C.; Aguilera-Venegas, B.; Porcal, W.; Gonzalez, M.; Cerecetto, H. Spin Trapping: An Essential Tool for the Study of Diseases Caused by Oxidative Stress. Curr. Top. Med. Chem. 2015, 15, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, G.N.; Skelly, J.V.; Neidle, S. Crystal Structure of FMN-Dependent Nitroreductase from Escherichia Coli B: A Prodrug-Activating Enzyme. J. Med. Chem. 2000, 43, 3624–3631. [Google Scholar] [CrossRef]
- García-Estrada, C.; Pérez-Pertejo, Y.; Domínguez-Asenjo, B.; Holanda, V.N.; Murugesan, S.; Martínez-Valladares, M.; Balaña-Fouce, R.; Reguera, R.M. Further Investigations of Nitroheterocyclic Compounds as Potential Antikinetoplastid Drug Candidates. Biomolecules 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Martins Alho, M.; García-Sánchez, R.N.; Nogal-Ruiz, J.J.; Escario, J.A.; Gómez-Barrio, A.; Martínez-Fernández, A.R.; Arán, V.J. Synthesis and Evaluation of 1,1′-Hydrocarbylenebis(Indazol-3-Ols) as Potential Antimalarial Drugs. ChemMedChem 2009, 4, 78–87. [Google Scholar] [CrossRef]
- Arán, V.J.; Asensio, J.L.; Ruiz, J.R.; Stud, M. Reactivity of 1,1-Disubstituted Indazol-3-Ylio Oxides: Synthesis of Some Substituted Indazolols and Indazolinones. J. Chem. Soc. Perkin 1 1993, 1119–1127. [Google Scholar] [CrossRef]
- Muro, B.; Reviriego, F.; Navarro, P.; Marín, C.; Ramírez-Macías, I.; Rosales, M.J.; Sánchez-Moreno, M.; Arán, V.J. New Perspectives on the Synthesis and Antichagasic Activity of 3-Alkoxy-1-Alkyl-5-Nitroindazoles. Eur. J. Med. Chem. 2014, 74, 124–134. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Vieites, M.; Otero, L.; Santos, D.; Toloza, J.; Figueroa, R.; Norambuena, E.; Olea-Azar, C.; Aguirre, G.; Cerecetto, H.; González, M.; et al. Platinum(II) Metal Complexes as Potential Anti-Trypanosoma Cruzi Agents. J. Inorg. Biochem. 2008, 102, 1033–1043. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Saugar, J.M.; Dellisanti, M.; Barra, D.; Simmaco, M.; Rivas, L. Temporins, Small Antimicrobial Peptides with Leishmanicidal Activity. J. Biol. Chem. 2005, 280, 984–990. [Google Scholar] [CrossRef]
- Grecco, S.S.; Costa-Silva, T.A.; Jerz, G.; de Sousa, F.S.; Alves Conserva, G.A.; Mesquita, J.T.; Galuppo, M.K.; Tempone, A.G.; Neves, B.J.; Andrade, C.H.; et al. Antitrypanosomal Activity and Evaluation of the Mechanism of Action of Dehydrodieugenol Isolated from Nectandra Leucantha (Lauraceae) and Its Methylated Derivative against Trypanosoma Cruzi. Phytomedicine 2017, 24, 62–67. [Google Scholar] [CrossRef]
- Moncada-Basualto, M.; Matsuhiro, B.; Mansilla, A.; Lapier, M.; Maya, J.D.; Olea-Azar, C. Supramolecular Hydrogels of β-Cyclodextrin Linked to Calcium Homopoly-l-Guluronate for Release of Coumarins with Trypanocidal Activity. Carbohydr. Polym. 2019, 204, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Rostán, S.; Pozo-Martínez, J.; Arcos, M.A.; Moncada-Basualto, M.; Aguilera, E.; Alvarez, N.; Olea-Azar, C.; Mahler, G.; Otero, L. Pt(II) and Pd(II) Complexes with Coumarin-Thiosemicarbazone Hybrid Ligands and Triphenylphosphine Coligand as Potential Anti T. Cruzi Agents. J. Mol. Struct. 2024, 1313, 138711. [Google Scholar] [CrossRef]
- Lin, Z.; Akin, H.; Rao, R.; Hie, B.; Zhu, Z.; Lu, W.; Smetanin, N.; Verkuil, R.; Kabeli, O.; Shmueli, Y.; et al. Evolutionary-Scale Prediction of Atomic-Level Protein Structure with a Language Model. Science (1979) 2023, 379, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res 2023, 51, D523–D531. [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform 2011, 3, 33. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully Automated Protein–Ligand Interaction Profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef]
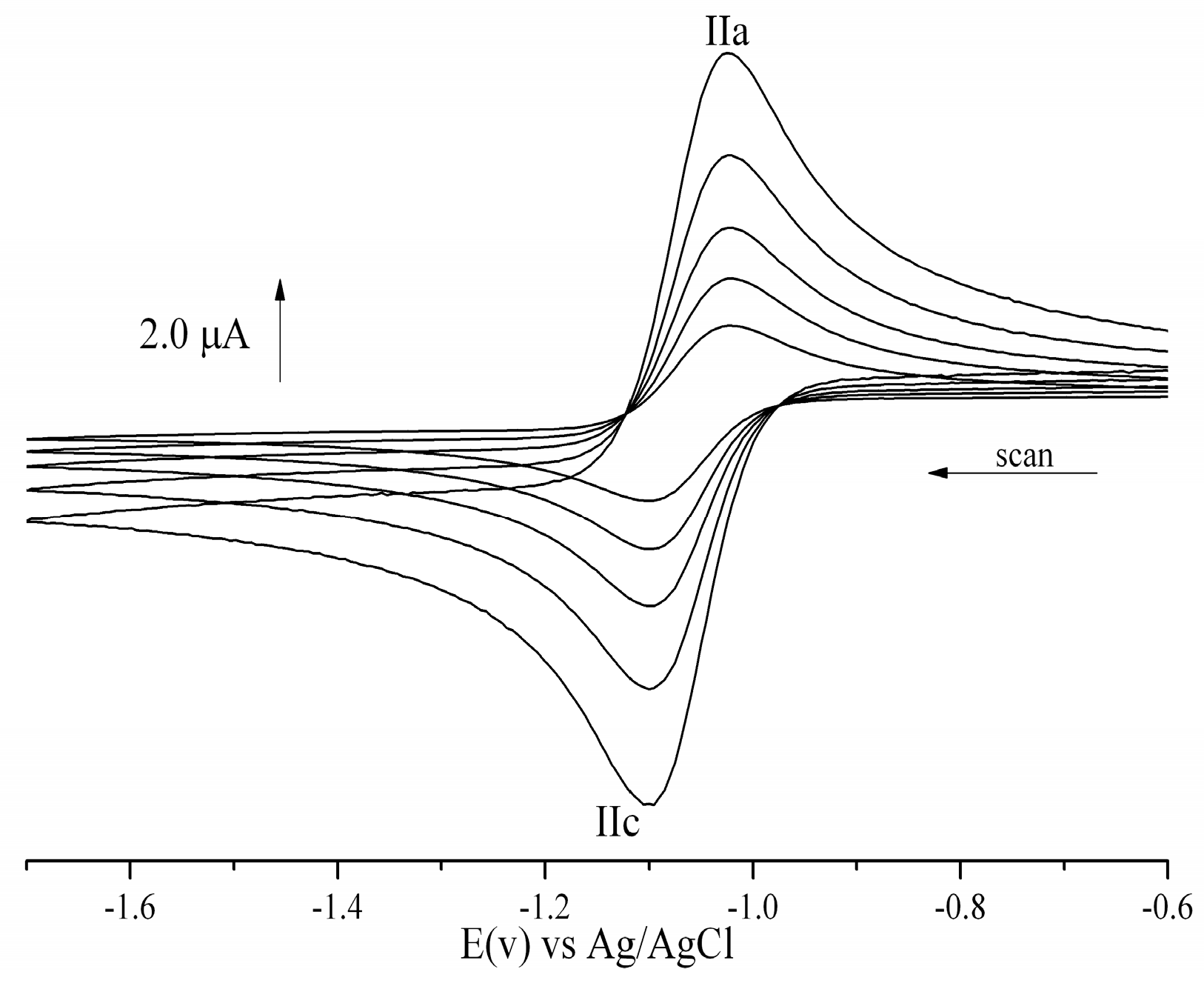
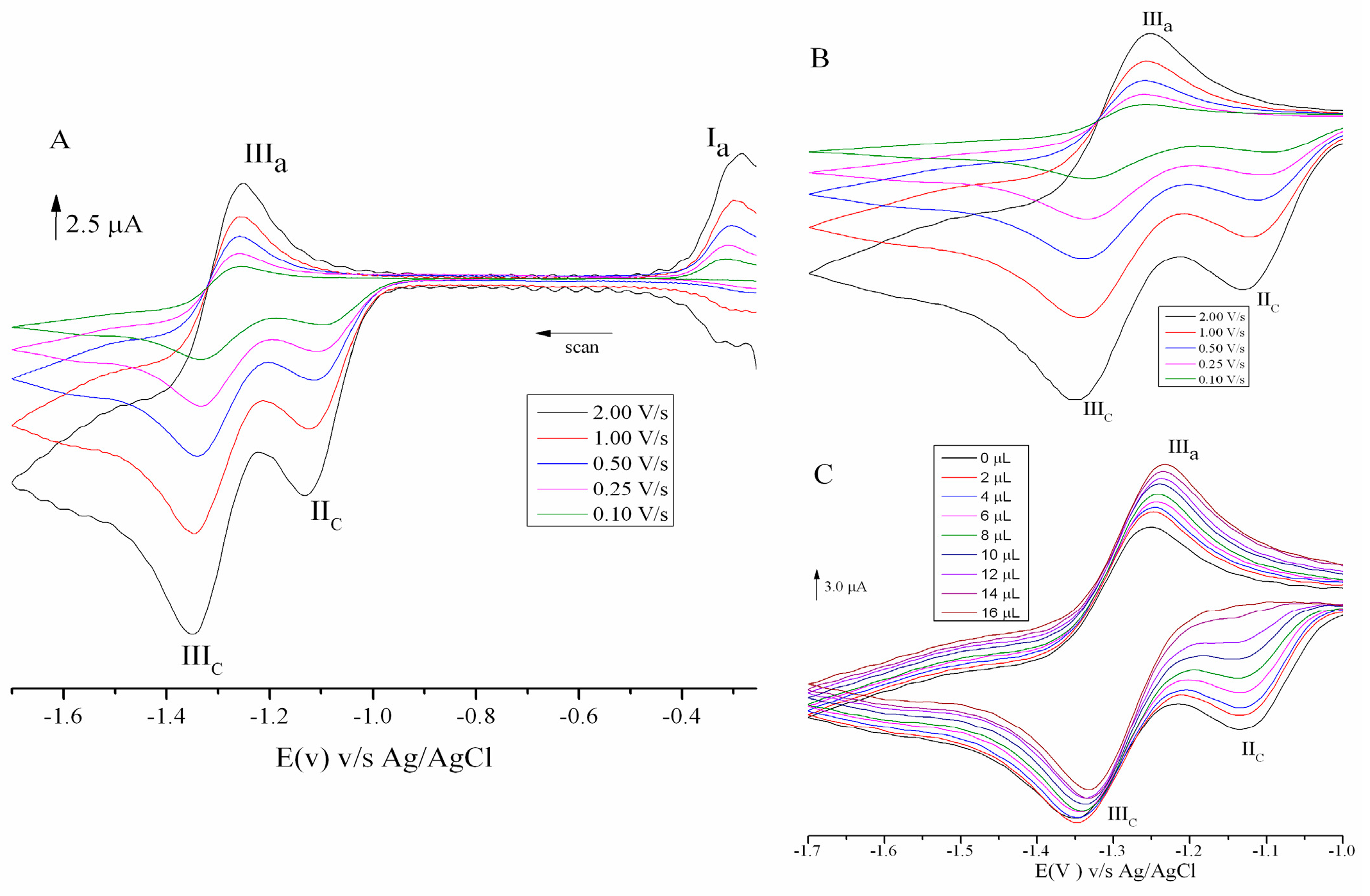
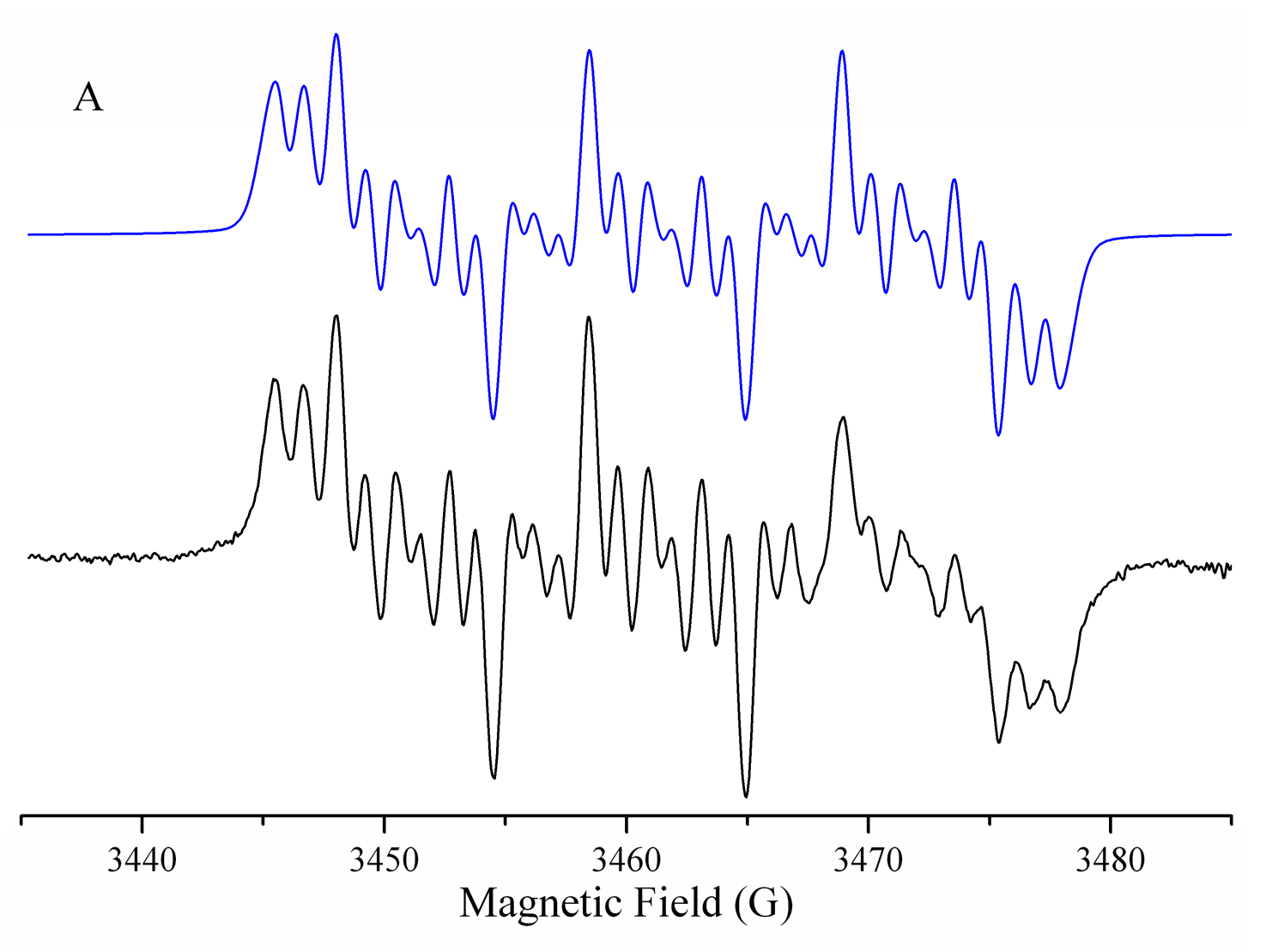
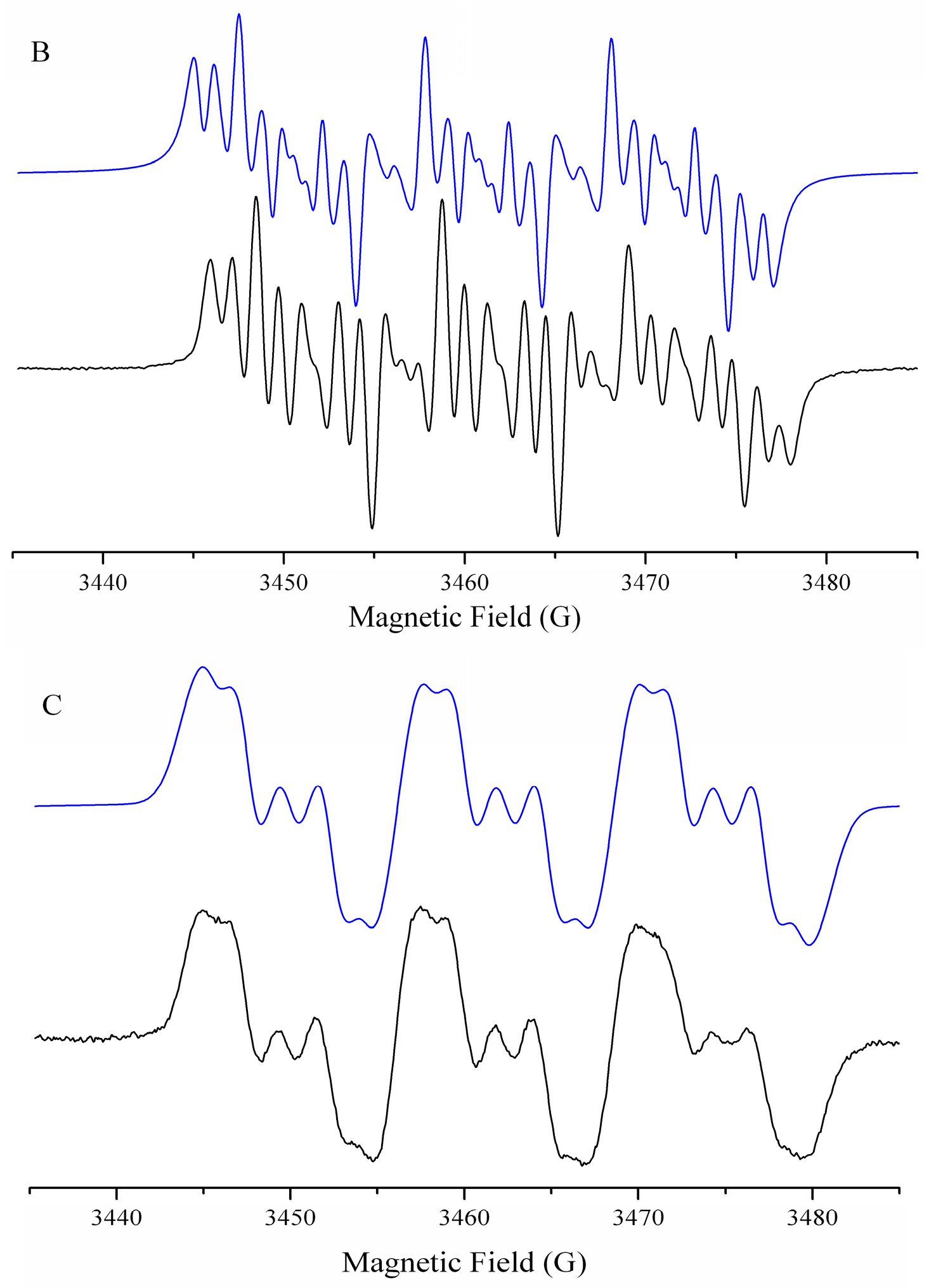

| Compound | −EpcIII (V) | −EpaIII (V) | −E1/2 (V) | Ipa/Ipc | Compound | −EpcIII (V) | −EpaIII (V) | −E1/2 (V) | Ipa/Ipc |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.12 | 1.02 | 1.07 | 0.94 | 13 | 1.13 | 0.99 | 1.06 | 0.97 |
| 3a | 1.13 | 0.95 | 1.04 | 0.99 | 14 | 1.20 | 0.99 | 1.10 | 0.94 |
| 3b | 1.13 | 1.03 | 1.08 | 1.00 | 15 | 1.16 | 1.04 | 1.10 | 0.96 |
| 4 | 1.37 | 1.27 | 1.32 | 0.63 | 16 | 1.15 | 1.03 | 1.09 | 0.91 |
| 5a | 1.36 | 1.32 | 1.34 | 0.62 | 17 | 1.37 | 0.82 | 1.10 | 0.96 |
| 5b | 1.12 | 1.04 | 1.08 | 0.97 | 18 | 1.12 | 1.02 | 1.07 | 0.91 |
| 6 | 1.22 | 0.94 | 1.08 | 0.99 | 19 | 1.35 | 1.28 | 1.32 | 0.65 |
| 7 | 1.20 | 0.98 | 1.09 | 1.00 | 20 | 1.38 | 1.28 | 1.33 | 0.67 |
| 8 | 1.14 | 0.99 | 1.07 | 1.00 | 21 | 1.37 | 1.27 | 1.32 | 0.56 |
| 9 | 1.10 | 1.02 | 1.06 | 0.96 | 22 | 1.38 | 1.26 | 1.33 | 0.59 |
| 10 | 1.17 | 0.96 | 1.07 | 0.93 | 23 | 1.37 | 1.25 | 1.31 | 0.78 |
| 11 | 1.21 | 0.99 | 1.10 | 0.89 | NFX | 0.91 | 0.85 | 0.88 | 1.01 |
| 12 | 1.17 | 0.96 | 1.07 | 1.00 | BNZ | 1.08 | 0.93 | 1.00 | 0.98 |
| Compounds | aN1 | aN2 | aH1 | aH2 | aH3 |
|---|---|---|---|---|---|
| 1 | 10.28 | 1.42 | 4.57 | 2.42 | 0.97 |
| 3a | 10.42 | 1.39 | 4.60 | 2.39 | 1.05 |
| 3b | 11.01 | 1.26 | 5.30 | 2.31 | 1.01 |
| 4 | 11.04 | 1.28 | 5.30 | 2.29 | 0.95 |
| 5a | 11.67 | 1.20 | 5.25 | 2.37 | 1.01 |
| 5b | 11.51 | 1.21 | 5.01 | 2.31 | 1.06 |
| 6 | 10.95 | 1.23 | 4.97 | 2.28 | 1.05 |
| 7 | 11.09 | 1.20 | 5.31 | 2.27 | 0.98 |
| 8 | 11.15 | 1.17 | 5.19 | 2.41 | 0.95 |
| 9 | 11.27 | 1.28 | 5.21 | 2.38 | 0.86 |
| 10 | 10.11 | 1.25 | 5.18 | 2.43 | 1.96 |
| 11 | 11.04 | 1.27 | 5.24 | 2.28 | 1.05 |
| 12 | 11.17 | 1.21 | 5.19 | 2.40 | 1.11 |
| 13 | 11.32 | 1.24 | 5.20 | 2.37 | 1.04 |
| 14 | 11.41 | 1.18 | 5.17 | 2.29 | 0.94 |
| 15 | 11.36 | 1.21 | 5.15 | 2.37 | 1.01 |
| 16 | 11.06 | 1.23 | 5.20 | 2.41 | 1.04 |
| 17 | 11.12 | 1.18 | 5.26 | 2.39 | 1.01 |
| 18 | 11.09 | 1.21 | 5.21 | 2.41 | 1.10 |
| 19 | 12.29 | 4.55 | 2.03 | 2.03 | |
| 20 | 12.43 | 4.69 | 2.08 | 2.08 | |
| 21 | 12.36 | 4.77 | 1.77 | 1.77 | |
| 22 | 12.31 | 4.62 | 2.09 | 2.09 | |
| 23 | 12.33 | 4.70 | 2.05 | 2.05 |
| IC50 (µM) | ||||||
|---|---|---|---|---|---|---|
| RAW 264.7 | Epimastigote | Trypomastigote | SI * Epimastigote | SI * Trypomastigote | iLogP ** | |
| 1 | 37.8 ± 1.2 | 10.1 ± 0.6 | >100 | 3.7 | - | 2.14 |
| 3a | 26.1 ± 0.8 | 8.9 ± 1.3 | 18.5 ± 0.9 | 2.9 | 1.4 | 1.05 |
| 3b | >200 | 28.6 ± 2.1 | 41.8 ± 1.1 | >22.5 | >4.8 | 1.49 |
| 4 | 97.5 ± 1.2 | 46.9 ± 3.4 | 63.4 ± 1.4 | 2.1 | 1.5 | 1.73 |
| 5a | 68.9 ± 0.7 | 1.1 ± 0.3 | 5.4 ± 0.7 | 62.6 | 12.8 | 1.78 |
| 5b | 76.5 ± 1.2 | 52.2 ± 1.3 | 86.5 ± 1.2 | 1.5 | 0.9 | 2.03 |
| 6 | 83.9 ± 0.9 | 32.9 ± 0.7 | 56.4 ± 1.5 | 2.6 | 1.5 | 2.09 |
| 7 | 95.1 ± 1.1 | 29.5 ± 1.5 | 41.2 ± 0.8 | 3.2 | 2.3 | 2.30 |
| 8 | 116.4 ± 1.7 | 36.4 ± 0.8 | 57.8 ± 1.3 | 3.2 | 2.0 | 2.21 |
| 9 | 175.5 ± 2.3 | 43.7 ± 2.0 | 61.6 ± 1.4 | 4.0 | 2.8 | 2.10 |
| 10 | >200 | 40.3 ± 2.0 | 54.2 ± 1.1 | >5.0 | >3.7 | 2.41 |
| 11 | 68.8 ± 1.2 | 70.1 ± 2.1 | 67.8 ± 1.7 | 1.0 | 1.0 | 2.39 |
| 12 | 72.4 ± 1.6 | 30.3 ± 1.1 | 52.5 ± 1.3 | 2.4 | 1.4 | 2.23 |
| 13 | >200 | 79.8 ± 2.3 | 43.6 ± 1.1 | >2.5 | >4.6 | 2.47 |
| 14 | 172.2 ± 2.3 | 83.9 ± 1.9 | 48.6 ± 1.2 | 2.1 | 3.5 | 2.44 |
| 15 | 36.3 ± 1.1 | >100 | >100 | - | - | 2.23 |
| 16 | >200 | >100 | >100 | - | - | 2.35 |
| 17 | 165.7 ± 2.3 | 35.4 ± 1.1 | 89.3 ± 1.0 | 4.7 | 1.9 | 2.19 |
| 18 | >200 | 68.7 ± 1.7 | >100 | >2.9 | - | 2.39 |
| 19 | >200 | >100 | >100 | - | - | 1.29 |
| 20 | >200 | >100 | >100 | - | - | 1.72 |
| 21 | >200 | 84.3 ± 2.1 | 91.2 ± 2.2 | >2.4 | >2.1 | 2.33 |
| 22 | >200 | 6.2 ± 0.9 | 15.3 ± 1.2 | >32.3 | >13.1 | 1.93 |
| 23 | >200 | >100 | >100 | - | - | 1.38 |
| NFX | 263.4 ± 2.2 | 17.4 ± 1.3 | 21.5 ± 1.4 | 15.1 | 12.3 | 0.71 |
| Compounds | Docking Score (kcal/mol) | Compounds | Docking Score (kcal/mol) |
|---|---|---|---|
| 1 | −6.6 | 12 | −5.9 |
| 2a | −7.4 | 13 | −6.0 |
| 2b | −6.9 | 14 | −6.5 |
| 3a | −5.3 | 15 | −5.2 |
| 3b | −5.2 | 16 | −5.5 |
| 4 | −6.4 | 17 | −6.1 |
| 5a | −7.3 | 18 | −4.6 |
| 5b | −6.6 | 19 | −5.8 |
| 6 | −6.3 | 20 | −5.6 |
| 7 | −6.8 | 21 | −6.0 |
| 8 | −4.5 | 22 | −6.4 |
| 9 | −5.9 | 23 | −5.0 |
| 10 | −5.9 | NFX | −6.5 |
| 11 | −6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozo-Martínez, J.; Arán, V.J.; Zúñiga-Bustos, M.; Parra-Magna, S.; Rocha-Valderrama, E.; Liempi, A.; Castillo, C.; Olea-Azar, C.; Moncada-Basualto, M. In Vitro Evaluation of New 5-Nitroindazolin-3-one Derivatives as Promising Agents against Trypanosoma cruzi. Int. J. Mol. Sci. 2024, 25, 11107. https://doi.org/10.3390/ijms252011107
Pozo-Martínez J, Arán VJ, Zúñiga-Bustos M, Parra-Magna S, Rocha-Valderrama E, Liempi A, Castillo C, Olea-Azar C, Moncada-Basualto M. In Vitro Evaluation of New 5-Nitroindazolin-3-one Derivatives as Promising Agents against Trypanosoma cruzi. International Journal of Molecular Sciences. 2024; 25(20):11107. https://doi.org/10.3390/ijms252011107
Chicago/Turabian StylePozo-Martínez, Josué, Vicente J. Arán, Matías Zúñiga-Bustos, Sebastián Parra-Magna, Esteban Rocha-Valderrama, Ana Liempi, Christian Castillo, Claudio Olea-Azar, and Mauricio Moncada-Basualto. 2024. "In Vitro Evaluation of New 5-Nitroindazolin-3-one Derivatives as Promising Agents against Trypanosoma cruzi" International Journal of Molecular Sciences 25, no. 20: 11107. https://doi.org/10.3390/ijms252011107
APA StylePozo-Martínez, J., Arán, V. J., Zúñiga-Bustos, M., Parra-Magna, S., Rocha-Valderrama, E., Liempi, A., Castillo, C., Olea-Azar, C., & Moncada-Basualto, M. (2024). In Vitro Evaluation of New 5-Nitroindazolin-3-one Derivatives as Promising Agents against Trypanosoma cruzi. International Journal of Molecular Sciences, 25(20), 11107. https://doi.org/10.3390/ijms252011107






