High Ki-67 Expression Predicting a Risk Factor for the Progression of Disease within 24 Months and Microenvironment in Follicular Lymphoma
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Comparison of Characteristics between Patients with FL with and without POD24
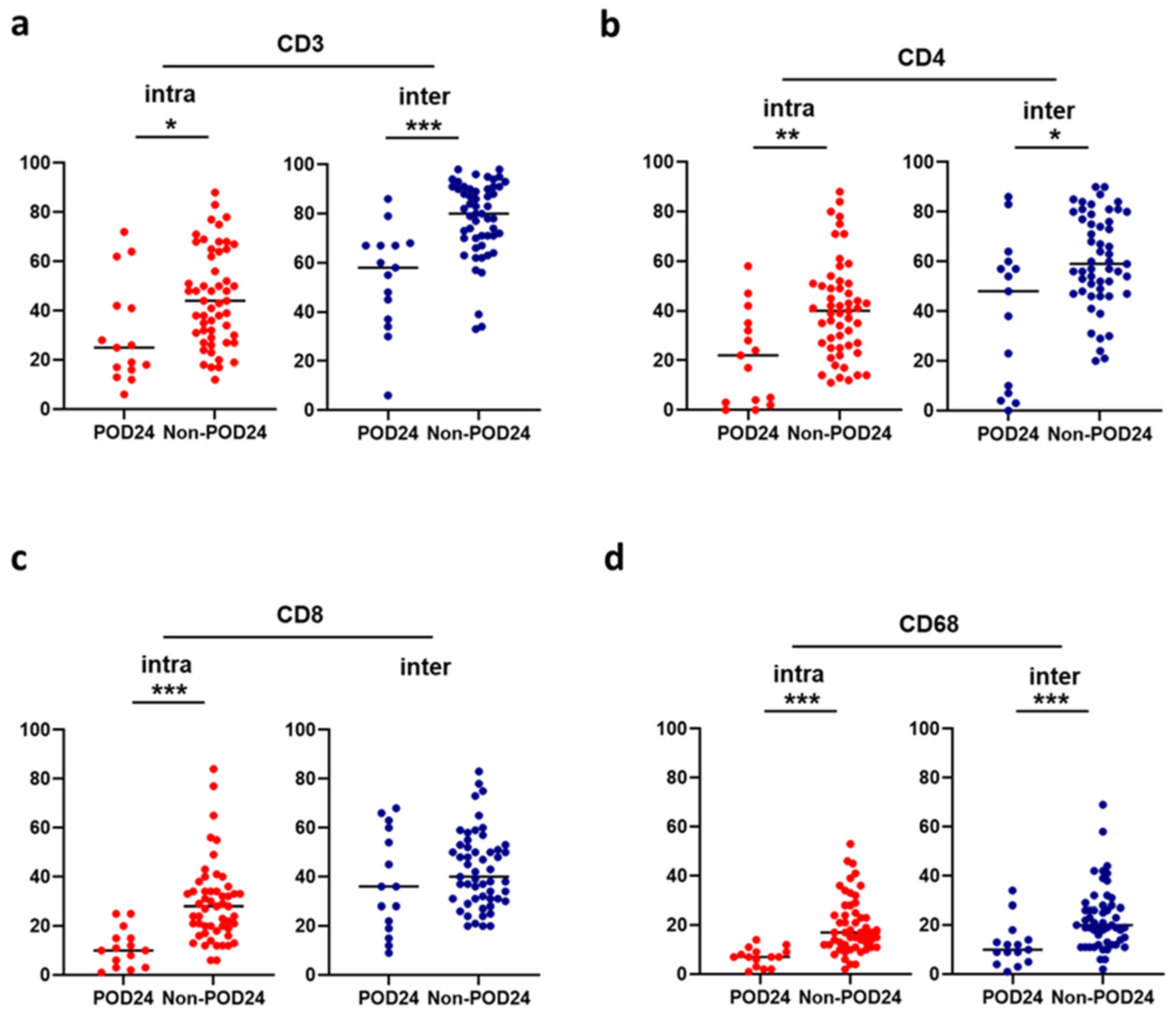
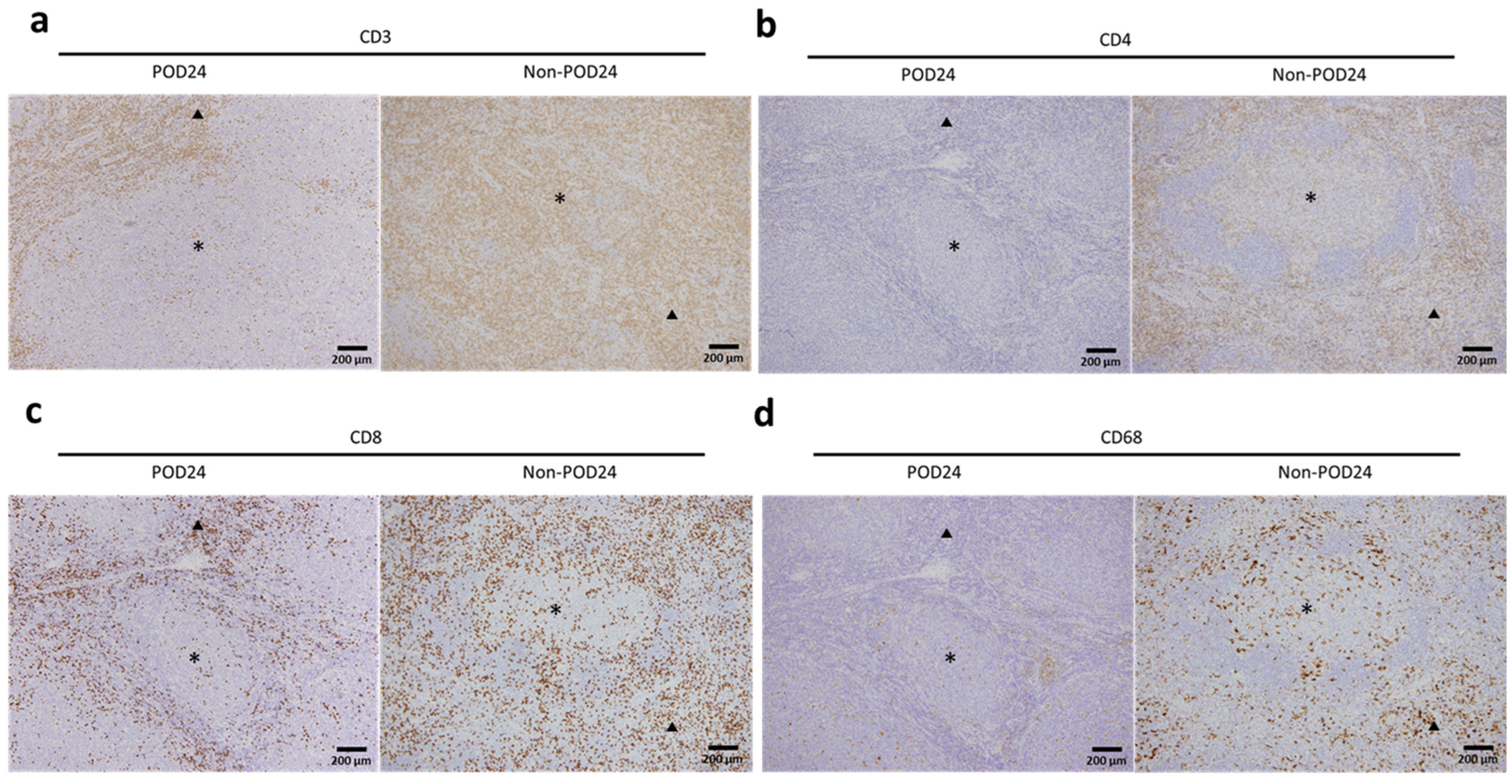
2.3. Association between the Microenvironment and POD24
2.4. Relationship between Microenvironment Components and Clinical Outcomes
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Histology and Immunohistochemistrical Analysis
4.3. Definitions and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casulo, C.; Byrtek, M.; Dawson, K.L.; Zhou, X.; Farber, C.M.; Flowers, C.R.; Hainsworth, J.D.; Maurer, M.J.; Cerhan, J.R.; Link, B.K.; et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: An analysis from the national LymphoCare study. J. Clin. Oncol. 2015, 33, 2516–2522. [Google Scholar] [CrossRef]
- Casulo, C.; Dixon, J.G.; Le-Rademacher, J.; Hoster, E.; Hochster, H.S.; Hiddemann, W.; Marcus, R.; Kimby, E.; Herold, M.; Sebban, C.; et al. Validation of POD24 as a robust early clinical end point of poor survival in FL from 5225 patients on 13 clinical trials. Blood 2022, 139, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.L.; Kridel, R.; Moccia, A.A.; Savage, K.J.; Villa, D.R.; Scott, D.W.; Gerrie, A.S.; Ferguson, D.; Cafferty, F.; Slack, G.W.; et al. Early progression after Bendamustine-rituximab is associated with high risk of transformation in advanced stage follicular lymphoma. Blood 2019, 134, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Solal-Céligny, P.; Roy, P.; Colombat, P.; White, J.; Armitage, J.O.; Arranz-Saez, R.; Au, W.Y.; Bellei, M.; Brice, P.; Caballero, D.; et al. Follicular lymphoma international prognostic index. Blood 2004, 104, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Federico, M.; Bellei, M.; Marcheselli, L.; Luminari, S.; Lopez-Guillermo, A.; Vitolo, U.; Pro, B.; Pileri, S.; Pulsoni, A.; Soubeyran, P.; et al. Follicular lymphoma international prognostic index 2: A new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J. Clin. Oncol. 2009, 27, 4555–4562. [Google Scholar] [CrossRef] [PubMed]
- Bachy, E.; Maurer, M.J.; Habermann, T.M.; Gelas-Dore, B.; Maucort-Boulch, D.; Estell, J.A.; Van den Neste, E.; Bouabdallah, R.; Gyan, E.; Feldman, A.L.; et al. A simplified scoring system in de novo follicular lymphoma treated initially with immunochemotherapy. Blood. 2018, 132, 49–58. [Google Scholar] [CrossRef]
- Mir, F.; Mattiello, F.; Grigg, A.; Herold, M.; Hiddemann, W.; Marcus, R.; Seymour, J.F.; Bolen, C.R.; Knapp, A.; Nielsen, T.; et al. Follicular Lymphoma Evaluation Index (FLEX): A new clinical prognostic model that is superior to existing risk scores for predicting progression-free survival and early treatment failure after frontline immunochemotherapy. Am. J. Hematol. 2020, 95, 1503–1510. [Google Scholar] [CrossRef]
- Casulo, C. Follicular lymphoma: Is there an optimal way to define risk? Hematol. Am. Soc. Hematol. Educ. Program. 2021, 2021, 313–319. [Google Scholar] [CrossRef]
- Pastore, A.; Jurinovic, V.; Kridel, R.; Hoster, E.; Staiger, A.M.; Szczepanowski, M.; Pott, C.; Kopp, N.; Murakami, M.; Horn, H.; et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: A retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015, 16, 1111–1122. [Google Scholar] [CrossRef]
- Jurinovic, V.; Kridel, R.; Staiger, A.M.; Szczepanowski, M.; Horn, H.; Dreyling, M.H.; Rosenwald, A.; Ott, G.; Klapper, W.; Zelenetz, A.D.; et al. Clinicogenetic risk models predict early progression of follicular lymphoma after first-line immunochemotherapy. Blood 2016, 128, 1112–1120. [Google Scholar] [CrossRef]
- Huet, S.; Tesson, B.; Jais, J.P.; Feldman, A.L.; Magnano, L.; Thomas, E.; Traverse-Glehen, A.; Albaud, B.; Carrère, M.; Xerri, L.; et al. A gene-expression profiling score for prediction of outcome in patients with follicular lymphoma: A retrospective training and validation analysis in three international cohorts. Lancet Oncol. 2018, 19, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Mondello, P.; Fama, A.; Larson, M.C.; Feldman, A.L.; Villasboas, J.C.; Yang, Z.Z.; Galkin, I.; Svelolkin, V.; Postovalova, E.; Bagaev, A.; et al. Lack of intrafollicular memory CD4 + T cells is predictive of early clinical failure in newly diagnosed follicular lymphoma. Blood Cancer J. 2021, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sevilla, J.J.; Fernández-Rodríguez, C.; Bento, L.; Diez-Feijóo, R.; Pinzón, S.; Gibert, J.; Fernández-Ibarrondo, L.; Lafuente, M.; Ferrer, A.; Sánchez-González, B.; et al. Evaluation of 4 prognostic indices in follicular lymphoma treated in first line with immunochemotherapy. Blood Adv. 2023, 7, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, T.; Lejeune, M.; Salvadó, M.T.; Lopez, C.; Jaén, J.; Bosch, R.; Pons, L.E. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J. Clin. Oncol. 2006, 24, 5350–5357. [Google Scholar] [CrossRef]
- Canioni, D.; Salles, G.; Mounier, N.; Brousse, N.; Keuppens, M.; Morchhauser, F.; Lamy, T.; Sonet, A.; Rousselet, M.C.; Foussard, C.; et al. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J. Clin. Oncol. 2008, 26, 440–446. [Google Scholar] [CrossRef]
- Tobin, J.W.D.; Keane, C.; Gunawardana, J.; Mollee, P.; Birch, S.; Hoang, T.; Lee, J.; Li, L.; Huang, L.; Murigneux, V.; et al. Progression of disease within 24 months in follicular lymphoma is associated with reduced intratumoral immune infiltration. J. Clin. Oncol. 2019, 37, 3300–3309. [Google Scholar] [CrossRef]
- Han, G.; Deng, Q.; Marques-Piubelli, M.L.; Dai, E.; Dang, M.; Ma, M.C.J.; Li, X.; Yang, H.; Henderson, J.; Kudryashova, O.; et al. Follicular lymphoma microenvironment characteristics associated with tumor cell mutations and MHC Class II expression. Blood Cancer Discov. 2022, 3, 428–443. [Google Scholar] [CrossRef]
- Sander, B.; de Jong, D.; Rosenwald, A.; Xie, W.; Balagué, O.; Calaminici, M.; Carreras, J.; Gaulard, P.; Gribben, J.; Hagenbeek, A.; et al. The reliability of immunohistochemical analysis of the tumor microenvironment in follicular lymphoma: A validation study from the Lunenburg Lymphoma Biomarker Consortium. Haematologica 2014, 99, 715–725. [Google Scholar] [CrossRef][Green Version]
- Wang, S.A.; Wang, L.; Hochberg, E.P.; Muzikansky, A.; Harris, N.L.; Hasserjian, R.P. Low histologic grade follicular lymphoma with high proliferation index: Morphologic and clinical features. Am. J. Surg. Pathol. 2005, 29, 1490–1496. [Google Scholar] [CrossRef] [PubMed]
- Sohani, A.R.; Maurer, M.J.; Giri, S.; Pitcher, B.; Chadburn, A.; Said, J.W.; Bartlett, N.L.; Czuczman, M.S.; Martin, P.; Rosenbaum, C.A.; et al. Biomarkers for risk stratification in patients with previously untreated follicular lymphoma receiving anti-CD20-based biological therapy. Am. J. Surg. Pathol. 2021, 45, 384–393. [Google Scholar] [CrossRef]
- Hu, J.; Gao, F.; Zhao, J.; Song, W.; Wang, Y.; Zheng, Y.; Wang, L.; Han, W.; Ma, L.; Wang, J.; et al. The prognostic index PRIMA-PI combined with Ki67 as a better predictor of progression of disease within 24 months in follicular lymphoma. Front. Oncol. 2023, 13, 1090610. [Google Scholar]
- Nasir, A.; Hegerova, L.; Yousaf, H.; Forster, C.L.; Shanley, R.; Linden, M.A.; Bachanova, V.; Yohe, S. Digital and manual interfollicular Ki-67 are associated with a progression-free survival in patients with low-grade follicular lymphoma. Am. J. Clin. Pathol. 2024, 161, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Klapper, W.; Hoster, E.; Rölver, L.; Schrader, C.; Janssen, D.; Tiemann, M.; Bernd, H.W.; Determann, O.; Hansmann, M.L.; Möller, P.; et al. Tumor sclerosis but not cell proliferation or malignancy grade is a prognostic marker in advanced-stage follicular lymphoma: The German Low Grade Lymphoma Study Group. J. Clin. Oncol. 2007, 25, 3330–3336. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.E.; Cho, J.; Kim, W.S.; Kim, S.J. Impact of transformation on the survival of patients diagnosed with follicular lymphoma that progressed within 24 months. J. Cancer 2021, 12, 2488–2497. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Z.; Kim, H.J.; Villasboas, J.C.; Price-Troska, T.; Jalali, S.; Wu, H.; Luchtel, R.A.; Polley, M.C.; Novak, A.J.; Ansell, S.M. Mass cytometry analysis reveals that specific intratumoral CD4+ T cell subsets correlate with patient survival in follicular lymphoma. Cell Rep. 2019, 26, 2178–2193.e3. [Google Scholar] [CrossRef]
- Milcent, B.; Josseaume, N.; Petitprez, F.; Riller, Q.; Amorim, S.; Loiseau, P.; Toubert, A.; Brice, P.; Thieblemont, C.; Teillaud, J.L.; et al. Recovery of central memory and naive peripheral T cells in Follicular Lymphoma patients receiving rituximab-chemotherapy based regimen. Sci. Rep. 2019, 9, 13471. [Google Scholar] [CrossRef]
- Nath, K.; Law, S.C.; Sabdia, M.B.; Gunawardana, J.; de Long, L.M.; Sester, D.; Shanavas, M.; Tsang, H.; Tobin, J.W.D.; Halliday, S.J.; et al. Intratumoral T cells have a differential impact on FDG-PET parameters in follicular lymphoma. Blood Adv. 2021, 5, 2644–2649. [Google Scholar] [CrossRef]
- Roider, T.; Baertsch, M.A.; Fitzgerald, D.; Vöhringer, H.; Brinkmann, B.J.; Czernilofsky, F.; Knoll, M.; Llaó-Cid, L.; Mathioudaki, A.; Faßbender, B.; et al. Multimodal and spatially resolved profiling identifies distinct patterns of T cell infiltration in nodal B cell lymphoma entities. Nat. Cell Biol. 2024, 26, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Kastenschmidt, J.M.; Schroers-Martin, J.G.; Sworder, B.J.; Sureshchandra, S.; Khodadoust, M.S.; Liu, C.L.; Olsen, M.; Kurtz, D.M.; Diehn, M.; Wagar, L.E.; et al. A human lymphoma organoid model for evaluating and targeting the follicular lymphoma tumor immune microenvironment. Cell Stem Cell 2024, 31, 410–420.e4. [Google Scholar] [CrossRef]
- Rai, S.; Inoue, H.; Sakai, K.; Hanamoto, H.; Matsuda, M.; Maeda, Y.; Haeno, T.; Watatani, Y.; Kumode, T.; Serizawa, K.; et al. Decreased expression of T-cell-associated immune markers predicts poor prognosis in patients with follicular lymphoma. Cancer Sci. 2022, 113, 660–673. [Google Scholar] [CrossRef]
- Liu, L.; Yu, X.; Li, Z.; He, X.; Zha, J.; Lin, Z.; Hong, Y.; Zheng, H.; Lai, Q.; Ding, K.; et al. Revealing the evolution of the tumor immune microenvironment in follicular lymphoma patients progressing within 24 months using single-cell imaging mass cytometry. J. Hematol. Oncol. 2022, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Nastoupil, L.; Feugier, P.; Schiano de Colella, J.M.; Tilly, H.; Palomba, M.L.; Bachy, E.; Fruchart, C.; Libby, E.N.; Casasnovas, R.O.; et al. Six-year results from RELEVANCE: Lenalidomide plus rituximab (R2) versus rituximab-chemotherapy Followed by rituximab maintenance in untreated advanced follicular lymphoma. J. Clin. Oncol. 2022, 40, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Bachy, E.; Houot, R.; Feugier, P.; Bouabdallah, K.; Bouabdallah, R.; Virelizier, E.N.; Maerevoet, M.; Fruchart, C.; Snauwaert, S.; Le Gouill, S.; et al. Obinutuzumab plus lenalidomide in advanced, previously untreated follicular lymphoma in need of systemic therapy: A LYSA study. Blood 2022, 139, 2338–2346. [Google Scholar] [CrossRef]
- Gribben, J.G.; Fowler, N.; Morschhauser, F. Mechanisms of action of lenalidomide in B-cell non-Hodgkin lymphoma. J. Clin. Oncol. 2015, 33, 2803–2811. [Google Scholar] [CrossRef]
- Xerri, L.; Huet, S.; Venstrom, J.M.; Szafer-Glusman, E.; Fabiani, B.; Canioni, D.; Chassagne-Clément, C.; Dartigues-Cuilléres, P.; Charlotte, F.; Laurent, C.; et al. Rituximab treatment circumvents the prognostic impact of tumor-infiltrating T-cells in follicular lymphoma patients. Hum. Pathol. 2017, 64, 128–136. [Google Scholar] [CrossRef]
- Gouni, S.; Marques-Piubelli, M.L.; Strati, P. Follicular lymphoma and macrophages: Impact of approved and novel therapies. Blood Adv. 2021, 5, 4303–4312. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.J.; Postovalova, E.; Varlamova, A.; Bagaev, A.; Sorokina, M.; Kudryashova, O.; Meerson, M.; Polyakova, M.; Galkin, I.; Svekolkin, V.; et al. Multi-omic profiling of follicular lymphoma reveals changes in tissue architecture and enhanced stromal remodeling in high-risk patients. Cancer Cell 2024, 42, 444–463.e10. [Google Scholar] [CrossRef]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 blockade by Hu5F9-G4 and rituximab in non-Hodgkin’s lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Brice, P.; Bastion, Y.; Lepage, E.; Brousse, N.; Haïoun, C.; Moreau, P.; Straetmans, N.; Tilly, H.; Tabah, I.; Solal-Céligny, P. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: A randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 1997, 15, 1110–1117. [Google Scholar] [CrossRef]
- Cheson, B.D.; Horning, S.J.; Coiffier, B.; Shipp, M.A.; Fisher, R.I.; Connors, J.M.; Lister, T.A.; Vose, J.; Grillo-López, A.; Hagenbeek, A.; et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI sponsored international working group. J. Clin. Oncol. 1999, 17, 1244. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Shiozawa, E.; Shimada, S.; Sasaki, Y.; Abe, M.; Murai, S.; Baba, Y.; Arai, N.; Okamoto, N.; Kabasawa, N.; et al. Ki-67 expression of immunohistochemistry using computerized image analysis is a useful prognostic marker in follicular lymphomas. Int. J. Clin. Exp. Pathol. 2018, 11, 3366–3374. [Google Scholar] [PubMed]
- Wahlin, B.E.; Aggarwal, M.; Montes-Moreno, S.; Gonzalez, L.F.; Roncador, G.; Sanchez-Verde, L.; Christensson, B.; Sander, B.; Kimby, E. A unifying microenvironment model in follicular lymphoma: Outcome is predicted by programmed death-1–positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin. Cancer Res. 2010, 16, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Sweetenham, J.W.; Goldman, B.; LeBlanc, M.L.; Cook, J.R.; Tubbs, R.R.; Press, O.W.; Maloney, D.G.; Fisher, R.I.; Rimsza, L.M.; Braziel, R.M.; et al. Prognostic value of regulatory T cells, lymphoma-associated macrophages, and MUM-1 expression in follicular lymphoma treated before and after the introduction of monoclonal antibody therapy: A Southwest Oncology Group Study. Ann. Oncol. 2010, 21, 1196–1202. [Google Scholar] [CrossRef]
- Blaker, Y.N.; Spetalen, S.; Brodtkorb, M.; Lingjaerde, O.C.; Beiske, K.; Østenstad, B.; Sander, B.; Wahlin, B.E.; Melen, C.M.; Myklebust, J.H.; et al. The tumour microenvironment influences survival and time to transformation in follicular lymphoma in the rituximab era. Br. J. Haematol. 2016, 175, 102–114. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]

| Parameter | n (%) | |
|---|---|---|
| Number of patients | 101 (100) | |
| Age | ||
| <60 years | 28 (28) | |
| ≥60 years | 73 (72) | |
| Sex | ||
| Female | 50 (50) | |
| Male | 51 (50) | |
| ECOG PS | ||
| 0–1 | 94 (93) | |
| 2–4 | 7 (7) | |
| Ann Arbor | ||
| Stage I/II | 22 (22) | |
| Stage III/IV | 79 (78) | |
| Histological findings | ||
| Grade 1–2 | 80 (79) | |
| Grade 3a | 21 (21) | |
| FLIPI | ||
| Low risk | 26 (26) | |
| Intermediate risk | 34 (34) | |
| High risk | 41 (41) | |
| Hb | ||
| <LLN (g/dL) | 21 (21) | |
| ≥LLN (g/dL) | 80 (79) | |
| LDH | ||
| <ULN (U/L) | 69 (68) | |
| ≥ULN (U/L) | 32 (32) | |
| sIL-2R | ||
| <ULN (U/mL) | 28 (28) | |
| ≥ULN (U/mL) | 72 (71) | |
| Data missing | 1 (1) | |
| β2-MG | ||
| <ULN (mg/L) | 37 (37) | |
| ≥ULN (mg/L) | 25 (25) | |
| Data missing | 39 (39) | |
| Ki-67 | ||
| Low (<30%) | 70 (69) | |
| High (≥30%) | 30 (30) | |
| Data missing | 1 (1) | |
| High tumor burden by GELF criteria | 39 (39) | |
| POD24 | ||
| POD24 | 15 (15) | |
| Non-POD24 | 86 (85) | |
| First treatment | ||
| Rituximab + chemotherapy | 80 (79) | |
| Rituximab monotherapy | 9 (9) | |
| Other | 12 (12) | |
| Factor | POD24 | Non-POD24 | p-Value | ||
|---|---|---|---|---|---|
| (n = 15), n (%) | (n = 86), n (%) | ||||
| Age | |||||
| <60 years | 3 (20) | 25 (29) | 0.55 | ||
| ≥60 years | 12 (80) | 61 (71) | |||
| Median (range), years | 65 (48–83) | 65 (30–86) | 0.53 | ||
| Sex | |||||
| Female | 7 (47) | 43 (50) | 1.00 | ||
| Male | 8 (53) | 43 (50) | |||
| Performance status (ECOG) | |||||
| 0–1 | 13 (87) | 81 (94) | 0.28 | ||
| 2–4 | 2 (13) | 5 (6) | |||
| Ann Arbor | |||||
| Stage I/II | 3 (20) | 19 (22) | 1.00 | ||
| Stage III/IV | 12 (80) | 67 (78) | |||
| Histological findings | |||||
| Grade 1–2 | 11 (73) | 69 (80) | 0.51 | ||
| Grade 3a | 4 (27) | 17 (20) | |||
| FLIPI | |||||
| Low risk | 2 (13) | 24 (28) | 0.36 | ||
| Intermediate risk | 7 (47) | 27 (31) | |||
| High risk | 6 (40) | 35 (41) | |||
| Hemoglobin | |||||
| <LLN (g/dL) | 4 (27) | 17 (20) | 0.37 | ||
| ≥LLN (g/dL) | 11 (73) | 69 (80) | |||
| LDH | |||||
| <ULN (U/L) | 7 (47) | 62 (72) | 0.071 | ||
| ≥ULN (U/L) | 8 (53) | 24 (28) | |||
| sIL-2R | |||||
| <ULN (U/mL) | 2 (13) | 26 (30) |  | 0.22 | |
| ≥ULN (U/mL) | 13 (87) | 59 (69) | |||
| data missing | 0 (0) | 1 (1) | |||
| β2-MG | |||||
| <ULN (mg/L) | 2 (13) | 35 (41) |  | 0.052 | |
| ≥ULN (mg/L) | 6 (40) | 19 (22) | |||
| Data missing | 7 (47) | 32 (37) | |||
| Ki-67 | |||||
| Low (<30%) | 4 (27) | 66 (77) |  | 2.1 × 10−4 | |
| High (≥30%) | 11 (73) | 19 (22) | |||
| Data missing | 0 (0) | 1 (1) | |||
| High tumor burden by GELF criteria | 8 (53) | 31 (36) | 0.25 | ||
| First treatment group | |||||
| Rituximab + chemotherapy | 11 (73) | 69 (80) | 0.68 | ||
| Rituximab monotherapy | 2 (13) | 7 (8) | |||
| Other | 2 (13) | 10 (12) | |||
| n | POD24 Univariate | POD24 Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| Age | ||||||||
| <60 years | 28 | 1 | ||||||
| ≥60 years | 73 | 1.67 | 0.47–5.93 | 0.43 | ||||
| Sex | ||||||||
| Female | 50 | 1 | ||||||
| Male | 51 | 1.06 | 0.38–2.91 | 0.92 | ||||
| ECOG PS | ||||||||
| 0–1 | 94 | 1 | ||||||
| 2–4 | 7 | 2.31 | 0.52–10.24 | 0.27 | ||||
| Ann Arbor | ||||||||
| Stage I/II | 22 | 1 | ||||||
| Stage III/IV | 79 | 1.26 | 0.36–4.47 | 0.72 | ||||
| Histological findings | ||||||||
| Grade 1–2 | 80 | 1 | ||||||
| Grade 3a | 21 | 1.42 | 0.45–4.47 | 0.55 | ||||
| FLIPI | ||||||||
| Low/Int | 60 | 1 | ||||||
| High | 41 | 1.14 | 0.41–3.21 | 0.80 | ||||
| Hb | ||||||||
| <LLN (g/dL) | 21 | 1 | ||||||
| ≥LLN (g/dL) | 80 | 0.62 | 0.20–1.96 | 0.42 | ||||
| LDH | ||||||||
| <ULN (U/L) | 69 | 1 | 1 | |||||
| ≥ULN (U/L) | 32 | 2.78 | 1.01–7.67 | 0.049 | 1.87 | 0.66–5.27 | 0.24 | |
| sIL-2R | ||||||||
| <ULN (U/mL) | 28 | 1 | ||||||
| ≥ULN (U/mL) | 72 | 2.84 | 0.64–12.57 | 0.17 | ||||
| Ki-67 | ||||||||
| Low (<30%) | 70 | 1 | 1 | |||||
| High (≥30%) | 30 | 7.17 | 2.28–22.55 | 7.4 × 10−4 | 6.29 | 1.96–20.22 | 0.0020 | |
| High tumor burden | ||||||||
| No | 62 | 1 | ||||||
| Yes | 39 | 2.00 | 0.72–5.51 | 0.18 | ||||
| POD24 | PFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |||
| CD3+ cells in intrafollicular | 1.01 | 0.97–1.05 | 0.59 | 1.01 | 0.99–1.04 | 0.41 | 1.01 | 0.96–1.06 | 0.80 | ||
| in interfollicular | 0.95 | 0.92–0.99 | 0.022 | 0.95 | 0.93–0.98 | 0.0012 | 0.96 | 0.92–1.01 | 0.14 | ||
| CD4+ cells in intrafollicular | 1.02 | 0.96–1.09 | 0.50 | 1.01 | 0.98–1.05 | 0.54 | 0.98 | 0.91–1.06 | 0.66 | ||
| in interfollicular | 0.98 | 0.93–1.03 | 0.39 | 0.98 | 0.95–1.01 | 0.25 | 1.01 | 0.96–1.07 | 0.68 | ||
| CD8+ cells in intrafollicular | 0.91 | 0.83–1.00 | 0.053 | 0.92 | 0.86–0.97 | 0.0038 | 0.95 | 0.86–1.05 | 0.30 | ||
| in interfollicular | 1.03 | 0.97–1.09 | 0.28 | 1.03 | 0.99–1.07 | 0.093 | 1.06 | 0.99–1.14 | 0.064 | ||
| CD68+ cells in intrafollicular | 0.85 | 0.72–0.99 | 0.045 | 0.95 | 0.90–1.02 | 0.14 | 0.91 | 0.80–1.03 | 0.13 | ||
| in interfollicular | 0.99 | 0.92–1.07 | 0.87 | 1.01 | 0.97–1.06 | 0.61 | 1.05 | 0.97–1.14 | 0.22 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narita, H.; Kuroiwa, K.; Kawaguchi, Y.; Murai, S.; Sasaki, Y.; Homma, M.; Kawamata, N.; Hayashi, H.; Nagao, K.; Okamura, R.; et al. High Ki-67 Expression Predicting a Risk Factor for the Progression of Disease within 24 Months and Microenvironment in Follicular Lymphoma. Int. J. Mol. Sci. 2024, 25, 11057. https://doi.org/10.3390/ijms252011057
Narita H, Kuroiwa K, Kawaguchi Y, Murai S, Sasaki Y, Homma M, Kawamata N, Hayashi H, Nagao K, Okamura R, et al. High Ki-67 Expression Predicting a Risk Factor for the Progression of Disease within 24 Months and Microenvironment in Follicular Lymphoma. International Journal of Molecular Sciences. 2024; 25(20):11057. https://doi.org/10.3390/ijms252011057
Chicago/Turabian StyleNarita, Hinako, Kai Kuroiwa, Yukiko Kawaguchi, So Murai, Yosuke Sasaki, Mayumi Homma, Natsuki Kawamata, Hidenori Hayashi, Kazuki Nagao, Reiko Okamura, and et al. 2024. "High Ki-67 Expression Predicting a Risk Factor for the Progression of Disease within 24 Months and Microenvironment in Follicular Lymphoma" International Journal of Molecular Sciences 25, no. 20: 11057. https://doi.org/10.3390/ijms252011057
APA StyleNarita, H., Kuroiwa, K., Kawaguchi, Y., Murai, S., Sasaki, Y., Homma, M., Kawamata, N., Hayashi, H., Nagao, K., Okamura, R., Uesugi, Y., Sasaki, Y., Shimada, S., Watanuki, M., Arai, N., Yanagisawa, K., Shiozawa, E., Yamochi, T., & Hattori, N. (2024). High Ki-67 Expression Predicting a Risk Factor for the Progression of Disease within 24 Months and Microenvironment in Follicular Lymphoma. International Journal of Molecular Sciences, 25(20), 11057. https://doi.org/10.3390/ijms252011057





