Theoretical Investigation of Electric Polarizability in Porphyrin–Zinc and Porphyrin–Zinc–Thiazole Complexes Using Small Property-Oriented Basis Sets
Abstract
1. Introduction
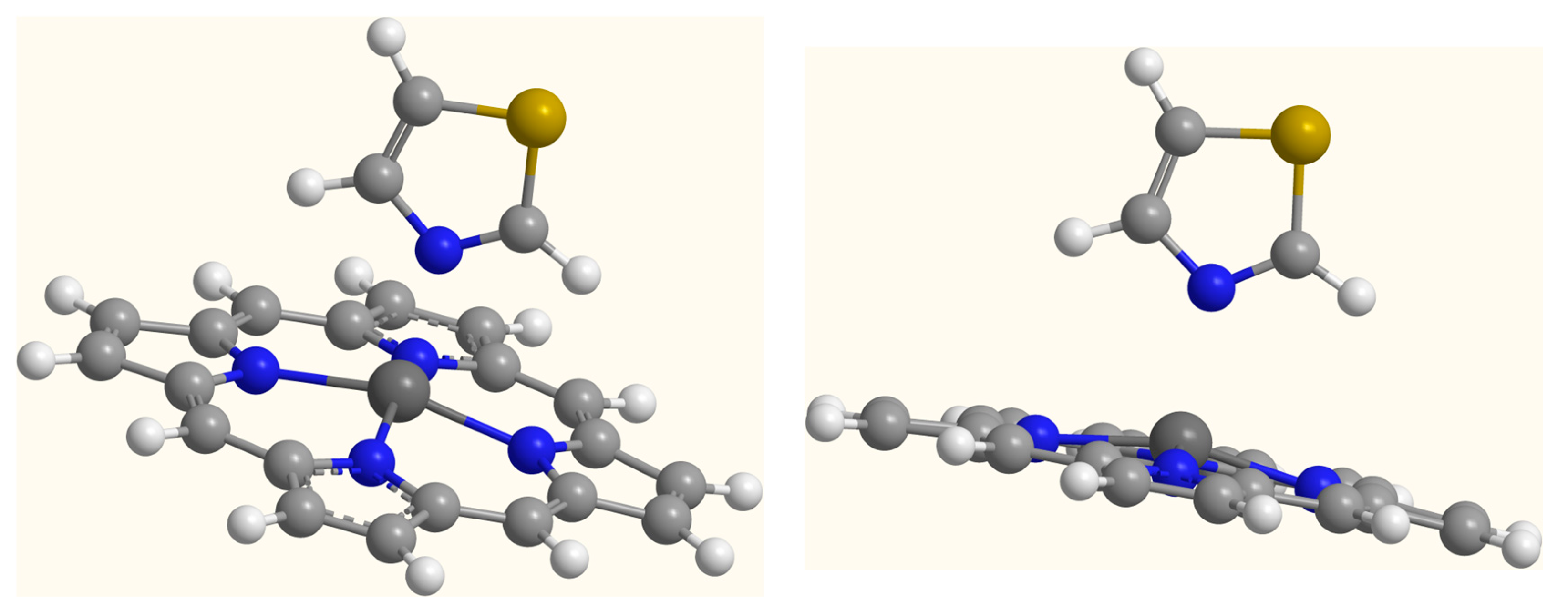
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahoo, S.; Wickramathilaka, K.Y.; Njeri, E.; Silva, D.; Suib, S.L. A review on transition metal oxides in catalysis. Front. Chem. 2024, 12, 1374878. [Google Scholar] [CrossRef] [PubMed]
- Takaya, J. Catalysis using transition metal complexes featuring main group metal and metalloid compounds as supporting ligands. Chem. Sci. 2020, 12, 1964–1981. [Google Scholar] [CrossRef] [PubMed]
- Elattar, R.H.; El-Malla, S.F.; Kamal, A.H.; Mansour, F.R. Applications of metal complexes in analytical chemistry: A review article. Coord. Chem. Rev. 2024, 501, 215568. [Google Scholar] [CrossRef]
- Van Cleave, C.; Crans, D.C. The first-row transition metals in the periodic table of medicine. Inorganics 2019, 7, 111. [Google Scholar] [CrossRef]
- Regueiro Pschepiurca, M.E.; Vadra, N.; Suarez, S.A. Versatile metalloporphyrin-based electrochemical sensing applications: From small gasotransmitters to macromolecules. Eur. J. Inorg. Chem. 2023, 26, e202300005. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, J.; Liu, B.; Yang, J.; Hou, H. Recent advances in metalloporphyrins for environmental and energy applications. Chemosphere 2019, 219, 617–635. [Google Scholar] [CrossRef]
- Nasri, S.; Guergueb, M.; Brahmi, J.; Al-Ghamdi, Y.O.; Loiseau, F.; Nasri, H. Synthesis of a novel zinc(II) porphyrin complex, halide ion reception, catalytic degradation of dyes, and optoelectronic application. Crystals 2023, 13, 238. [Google Scholar] [CrossRef]
- Stracke, J.O.; Hutton, M.; Stewart, M.; Pendás, A.M.; Smith, B.; López-Otin, C.; Murphy, G.; Knäuper, V. Biochemical characterization of the catalytic domain of human matrix metalloproteinase 19. Evidence for a role as a potent basement membrane degrading enzyme. J. Biol. Chem. 2000, 275, 14809–14816. [Google Scholar] [CrossRef]
- Bajju, G.D.; Kundan, S.; Bhagat, M.; Gupta, D.; Kapahi, A.; Devi, G. Synthesis and spectroscopic and biological activities of Zn(II) porphyrin with oxygen donors. Bioinorg. Chem. App. 2014, 2014, 782762. [Google Scholar] [CrossRef]
- Masoudi, M. Synthesis and biological evaluation of 2-(2-hydrazinyl)thiazole derivatives with potential antibacterial and antioxidant activity. J. Sulf. Chem. 2024, 45, 758–770. [Google Scholar] [CrossRef]
- Lemilemu, F.; Bitew, M.; Demissie, T.B.; Eswaramoorthy, R.; Endale, M. Synthesis, antibacterial and antioxidant activities of thiazole-based Schiff base derivatives: A combined experimental and computational study. BMC Chem. 2021, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.T.; Elsharabasy, S.A.; Abdel-Aziem, A. Synthesis and antimicrobial activity of new series of thiazoles, pyridines and pyrazoles based on coumarin moiety. Sci. Rep. 2023, 13, 9912. [Google Scholar] [CrossRef] [PubMed]
- Piechowska, K.; Świtalska, M.; Cytarska, J.; Jaroch, K.; Łuczykowski, K.; Chałupka, J.; Wietrzyk, J.; Misiura, K.; Bojko, B.; Kruszewski, S.; et al. Discovery of tropinone-thiazole derivatives as potent caspase 3/7 activators, and noncompetitive tyrosinase inhibitors with high antiproliferative activity: Rational design, one-pot tricomponent synthesis, and lipophilicity determination. Eur. J. Med. Chem. 2019, 175, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Piechowska, K.; Mizerska-Kowalska, M.; Zdzisińska, B.; Cytarska, J.; Baranowska-Łączkowska, A.; Jaroch, K.; Łuczykowski, K.; Płaziński, W.; Bojko, B.; Kruszewski, S.; et al. Tropinone-derived alkaloids as potent anticancer agents: Synthesis, tyrosinase inhibition, mechanism of action, DFT calculation, and molecular docking studies. Int. J. Mol. Sci. 2020, 21, 9050. [Google Scholar] [CrossRef] [PubMed]
- Donarska, B.; Świtalska, M.; Wietrzyk, J.; Płaziński, W.; Łączkowski, K.Z. Spectrofluorimetric and computational investigation of new phthalimide derivatives towards human neutrophil elastase inhibition and antiproliferative activity. Int. J. Mol. Sci. 2023, 24, 110. [Google Scholar] [CrossRef]
- de Groot, M.J.; Havenith, R.W.; Vinkers, H.M.; Zwaans, R.; Vermeulen, N.P.E.; van Lenthe, J.H. Ab initio calculations on iron-porphyrin model systems for intermediates in the oxidative cycle of cytochrome P450s. J. Comput. Aided Mol. Des. 1998, 12, 183–193. [Google Scholar] [CrossRef]
- Gomila, R.M.; Quiñonero, D.; Frontera, A.; Ballester, P.; Deyà, P.M. Ab initio calculations on zinc porphyrins complexed to amines: Geometrical details and NMR chemical shifts. J. Mol. Struct. THEOCHEM 2000, 531, 381–386. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Pachter, R. Ground state electronic structures and spectra of zinc complexes of porphyrin, tetraazaporphyrin, tetrabenzoporphyrin, and phthalocyanine: A density functional theory study. J. Chem. Phys. 2001, 114, 10757–10767. [Google Scholar] [CrossRef]
- Koseki, J.; Maezono, R.; Tachikawa, M.; Towler, M.D.; Needs, R.J. Quantum Monte Carlo study of porphyrin transition metal complexes. J. Chem. Phys. 2008, 129, 085103. [Google Scholar] [CrossRef]
- Balanaya, M.P.; Kim, D.H. DFT/TD-DFT molecular design of porphyrin analogues for use in dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2008, 10, 5121–5127. [Google Scholar] [CrossRef]
- Durrant, M.C. A computational study of ligand binding affinities in iron(III) porphine and protoporphyrin IX complexes. Dalton Trans. 2014, 43, 9754–9765. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.J.; Song, C.; Ren, X.F. Theoretical study of zinc porphyrin-based dyes for dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2017, 333, 200–207. [Google Scholar] [CrossRef]
- Ishimizu, Y.; Ma, Z.; Hada, M.; Fujii, H. Experimental and theoretical studies of the porphyrin ligand effect on the electronic structure and reactivity of oxoiron(IV) porphyrin π-cation-radical complexes. J. Biol. Inorg. Chem. 2019, 24, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bojorge, N.A.; Zaragoza-Galán, G.; Flores-Holguín, N.R.; Chávez-Rojo, M.A.; Castro-García, C.; Rodríguez-Valdez, L.M. Theoretical analysis of the electronic properties in Zinc-porphyrins derivatives. Theoretical analysis of the electronic properties in Zinc-porphyrins derivatives. J. Mol. Struct. 2019, 1191, 259–270. [Google Scholar] [CrossRef]
- Soury, R.; Chaabene, M.; Haque, A.; Jabli, M.; Alenezi, K.M.; Latif, S.; Abdulaziz, F.; Bchetnia, A.; Philouze, C. Two novel pyrazine Zn(II)-porphyrins complexes: Synthesis, photophysical properties, structure study, DFT-calculation and assessment of an azo dye removal from aqueous solution. J. Solid State Chem. 2022, 310, 123048. [Google Scholar] [CrossRef]
- Sadlej, A.J. Molecular electric polarizabilities. Electronic-field-variant (EFV) gaussian basis set for polarizability calculations. Chem. Phys. Lett. 1977, 47, 50–54. [Google Scholar] [CrossRef]
- Sadlej, A.J. Medium-size polarized basis sets for high-level correlated calculations of molecular electric properties. Collect. Czech. Chem. Commun. 1988, 53, 1995–2016. [Google Scholar] [CrossRef]
- Baranowska, A.; Sadlej, A.J. Explicit time-dependence of basis functions and its consequences. Chem. Phys. Lett. 2004, 398, 270–275. [Google Scholar] [CrossRef]
- Benkova, Z.; Sadlej, A.J.; Oakes, R.E.; Bell, S.E.J. Reduced-size polarized basis sets for calculations of molecular electric properties. I. The basis set generation. J. Comput. Chem. 2005, 26, 145–153. [Google Scholar] [CrossRef]
- Benkova, Z.; Sadlej, A.J.; Oakes, R.E.; Bell, S.E.J. Reduced–size polarized basis sets for calculations of molecular electric properties. III. Second–row atoms. Theor. Chem. Acc. 2005, 113, 238–247. [Google Scholar] [CrossRef]
- Baranowska, A.; Siedlecka, M.; Sadlej, A.J. Reduced-size polarized basis sets for calculations of molecular electric properties. IV. First-row transition metals. Theor. Chem. Acc. 2007, 118, 959–972. [Google Scholar] [CrossRef]
- Pluta, T.; Sadlej, A.J. HyPol basis sets for high-level-correlated calculations of electric dipole hyperpolarizabilities. Chem. Phys. Lett. 1998, 297, 391–401. [Google Scholar] [CrossRef]
- Baranowska, A.; Sadlej, A.J. Polarized basis sets for accurate calculations of static and dynamic electric properties of molecules. J. Comput. Chem. 2010, 31, 552–560. [Google Scholar] [CrossRef]
- Stiehler, J.; Hinze, J. Calculation of static polarizabilities and hyperpolarizabilities for the atoms He through Kr with a numerical RHF method. J. Phys. B At. Mol. Opt. Phys. 1995, 28, 4055–4071. [Google Scholar] [CrossRef]
- Bauschlicher, C.W., Jr.; Maitre, P. Theoretical Study of the First Transition Row Oxides and Sulfides. Theor. Chim. Acta 1995, 90, 189–203. [Google Scholar] [CrossRef]
- Gutsev, G.L.; Andrews, L.; Bauschlicher, C.W., Jr. Similarities and differences in the structure of 3d-metal monocarbides and monoxides. Theor. Chem. Acc. 2003, 109, 298–308. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Rappoport, D.; Furche, F. Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1998, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Aquilante, F.; De Vico, L.; Ferré, N.; Ghigo, G.; Malmqvist, P.-Å.; Neogrády, P.; Pedersen, T.B.; Pitonak, M.; Reiher, M.; Roos, B.O.; et al. MOLCAS 7: The next generation. J. Comput. Chem. 2010, 31, 224–247. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, C.01. 2009. Available online: https://gaussian.com/g09citation/ (accessed on 7 October 2024).

| Atom | Exponent |
|---|---|
| Sc | 0.4120 |
| Ti | 0.5060 |
| V | 0.5740 |
| Cr | 0.0686 |
| Mn | 0.6750 |
| Fe | 0.7440 |
| Co | 0.8490 |
| Ni | 0.9560 |
| Cu | 0.8820 |
| Zn | 1.1000 |
| Atom | ML | ZPol [31] | ZPol-A | Ref. [34] |
|---|---|---|---|---|
| Sc | 0 | 143.46 | 143.84 | 145.03 |
| 1 | 146.68 | 147.00 | 148.13 | |
| 2 | 154.75 | 154.80 | 155.86 | |
| average | 149.26 | 149.49 | 150.68 | |
| Ti | 0 | 125.77 | 126.09 | 127.45 |
| 1 | 126.11 | 126.73 | 128.12 | |
| 2 | 128.36 | 128.67 | 129.48 | |
| 3 | 129.97 | 131.91 | 131.44 | |
| average | 127.81 | 128.67 | 129.36 | |
| V | 0 | 112.74 | 112.96 | 114.95 |
| 1 | 112.36 | 112.79 | 114.30 | |
| 2 | 112.04 | 112.30 | 113.19 | |
| 3 | 110.01 | 111.47 | 111.36 | |
| average | 111.65 | 112.30 | 113.23 | |
| Cr | 0 | 106.26 | 106.78 | 112.88 |
| Mn | 0 | 89.20 | 89.33 | 90.14 |
| Fe | 0 | 76.64 | 76.82 | 77.62 |
| 1 | 77.56 | 77.72 | 78.60 | |
| 2 | 80.48 | 80.60 | 81.62 | |
| average | 78.54 | 78.70 | 79.61 | |
| Co | 0 | 69.52 | 69.72 | 70.59 |
| 1 | 69.75 | 69.96 | 70.96 | |
| 2 | 70.50 | 70.69 | 71.49 | |
| 3 | 71.18 | 71.89 | 72.35 | |
| average | 70.34 | 70.69 | 71.46 | |
| Ni | 0 | 63.99 | 64.13 | 65.39 |
| 1 | 63.87 | 64.05 | 65.16 | |
| 2 | 63.66 | 63.81 | 64.68 | |
| 3 | 62.85 | 63.41 | 63.94 | |
| average | 63.54 | 63.81 | 64.71 | |
| Cu | 0 | 73.46 | 73.68 | 77.19 |
| Zn | 0 | 53.22 | 53.37 | 54.07 |
| Molecule | ZPol 1 | ZPol-A 2 | aVQZ | Ref. 3 |
|---|---|---|---|---|
| ScO | 1.570 | 1.511 | 1.518 | 1.54 |
| TiO | 1.358 | 1.315 | 1.346 | 1.38 |
| VO | 1.317 | 1.295 | 1.345 | 1.42 |
| CrO | 1.537 | 1.562 | 1.579 | 1.53 |
| MnO | 2.042 | 2.062 | 2.004 | 1.96 |
| FeO | 2.019 | 2.055 | 1.954 | – |
| CoO | 1.854 | 1.886 | 1.999 | – |
| NiO | 1.876 | 1.937 | 1.842 | – |
| CuO | 2.065 | 2.109 | 1.949 | 2.01 |
| ZnO | 2.165 | 2.161 | 2.163 | 2.11 |
| RMSE1 | 0.06 | 0.08 | – | – |
| RMSE2 | 0.07 | 0.08 | – | – |
| Basis set on Zn atom | ZPol | ZPol-A | SVPD | TZVPD | aVDZ | aVTZ | aVQZ |
| Porphyrin–zinc complex | 337.85 | 337.67 | 337.02 | 338.33 | 339.15 | 339.24 | 339.96 |
| Porphyrin–zinc–thiazole complex | 382.53 | 382.33 | 382.07 | 383.85 | 385.15 | 385.26 | 386.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuziemski, A.; Łączkowski, K.Z.; Baranowska-Łączkowska, A. Theoretical Investigation of Electric Polarizability in Porphyrin–Zinc and Porphyrin–Zinc–Thiazole Complexes Using Small Property-Oriented Basis Sets. Int. J. Mol. Sci. 2024, 25, 11044. https://doi.org/10.3390/ijms252011044
Kuziemski A, Łączkowski KZ, Baranowska-Łączkowska A. Theoretical Investigation of Electric Polarizability in Porphyrin–Zinc and Porphyrin–Zinc–Thiazole Complexes Using Small Property-Oriented Basis Sets. International Journal of Molecular Sciences. 2024; 25(20):11044. https://doi.org/10.3390/ijms252011044
Chicago/Turabian StyleKuziemski, Arkadiusz, Krzysztof Z. Łączkowski, and Angelika Baranowska-Łączkowska. 2024. "Theoretical Investigation of Electric Polarizability in Porphyrin–Zinc and Porphyrin–Zinc–Thiazole Complexes Using Small Property-Oriented Basis Sets" International Journal of Molecular Sciences 25, no. 20: 11044. https://doi.org/10.3390/ijms252011044
APA StyleKuziemski, A., Łączkowski, K. Z., & Baranowska-Łączkowska, A. (2024). Theoretical Investigation of Electric Polarizability in Porphyrin–Zinc and Porphyrin–Zinc–Thiazole Complexes Using Small Property-Oriented Basis Sets. International Journal of Molecular Sciences, 25(20), 11044. https://doi.org/10.3390/ijms252011044






