The Role of Mast Cells in the Remodeling Effects of Molecular Hydrogen on the Lung Local Tissue Microenvironment under Simulated Pulmonary Hypertension
Abstract
:1. Introduction
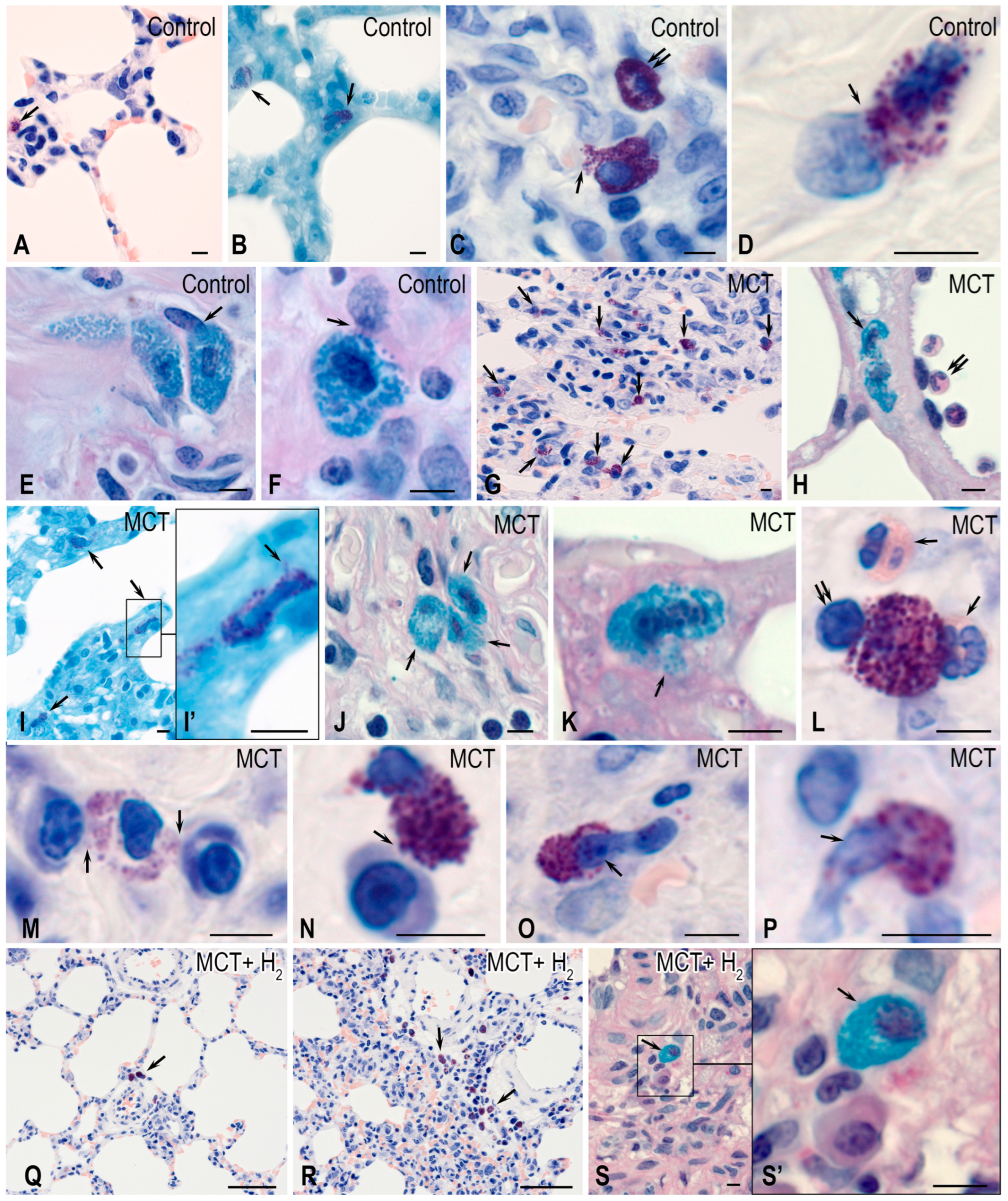
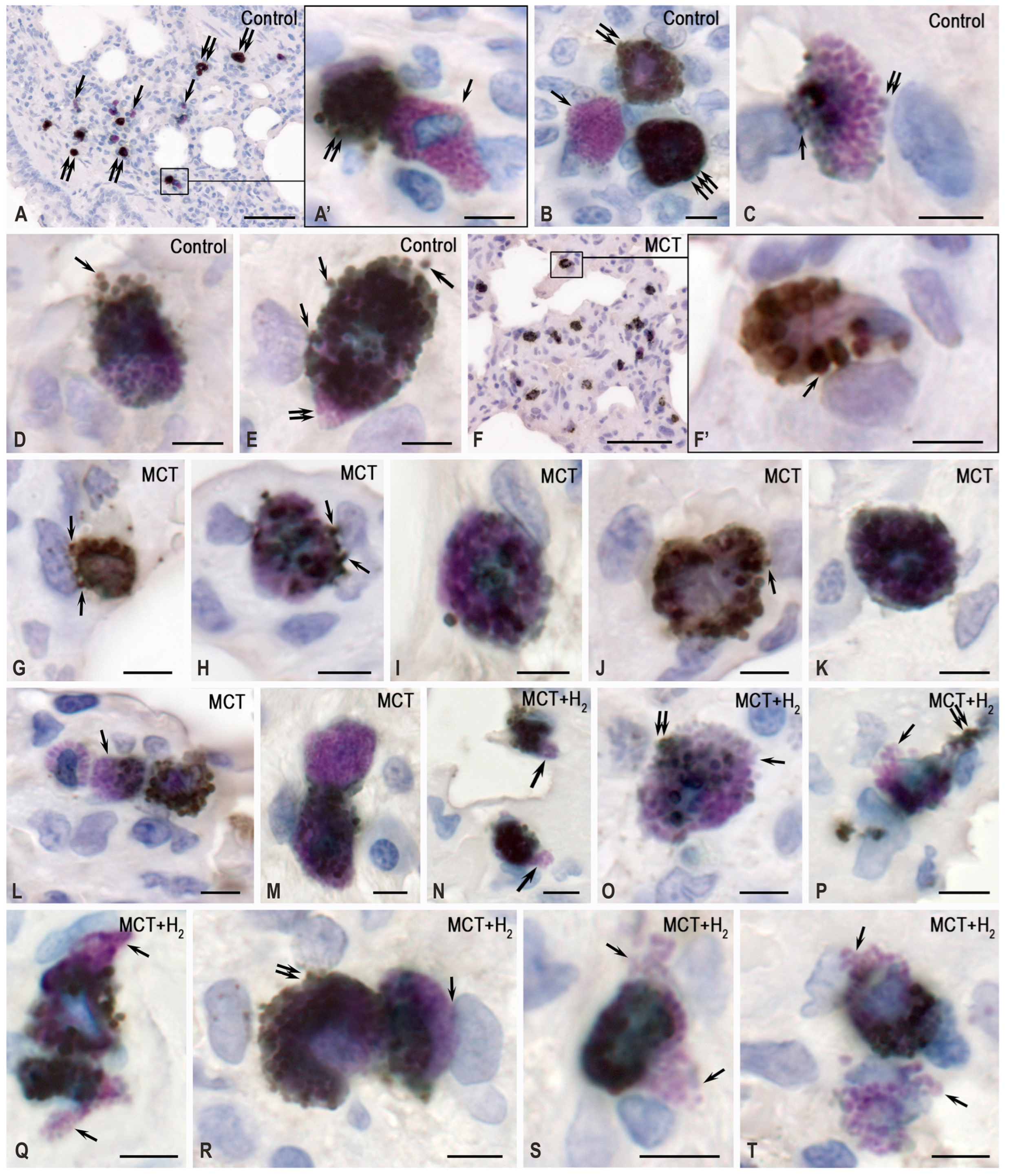
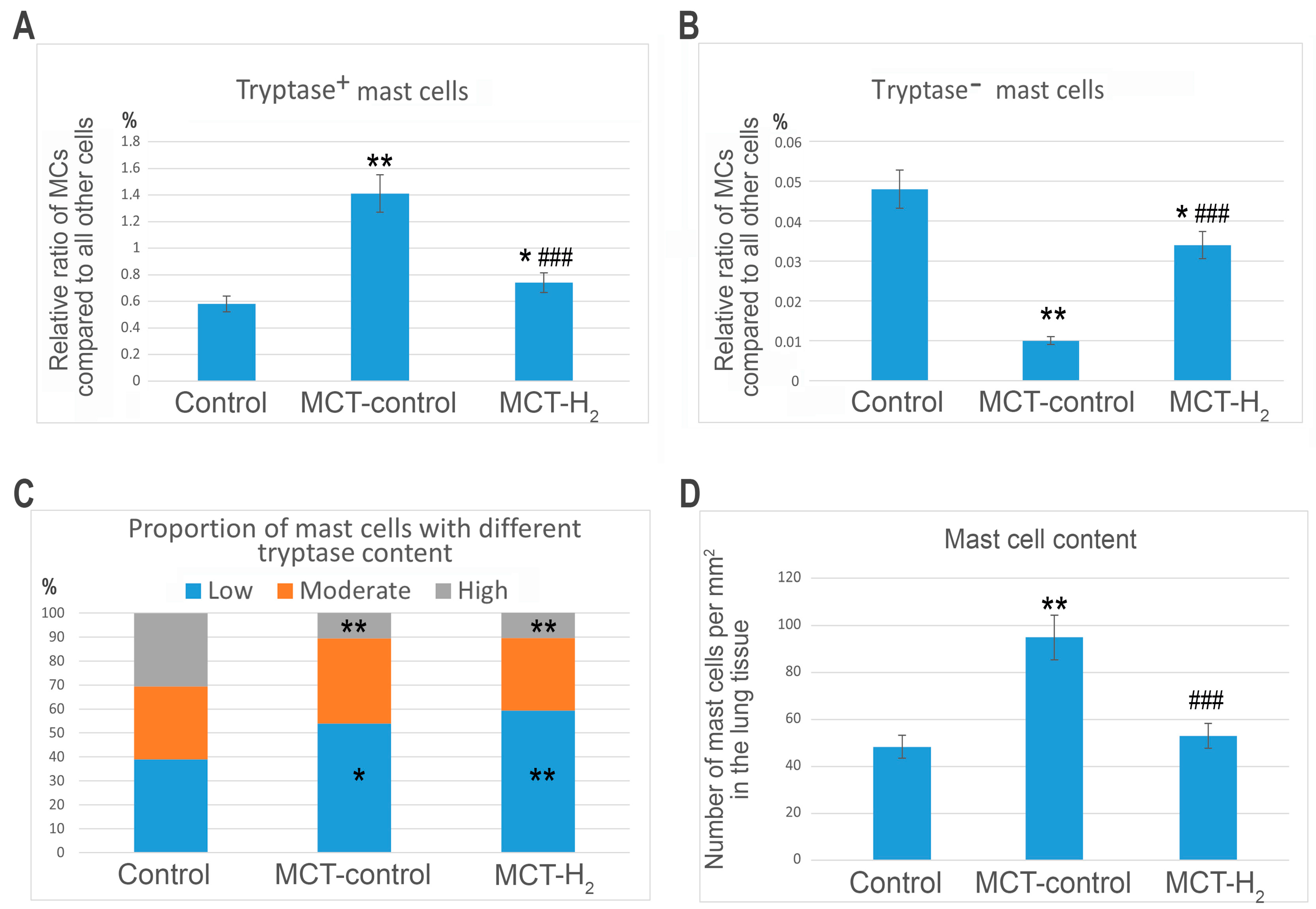
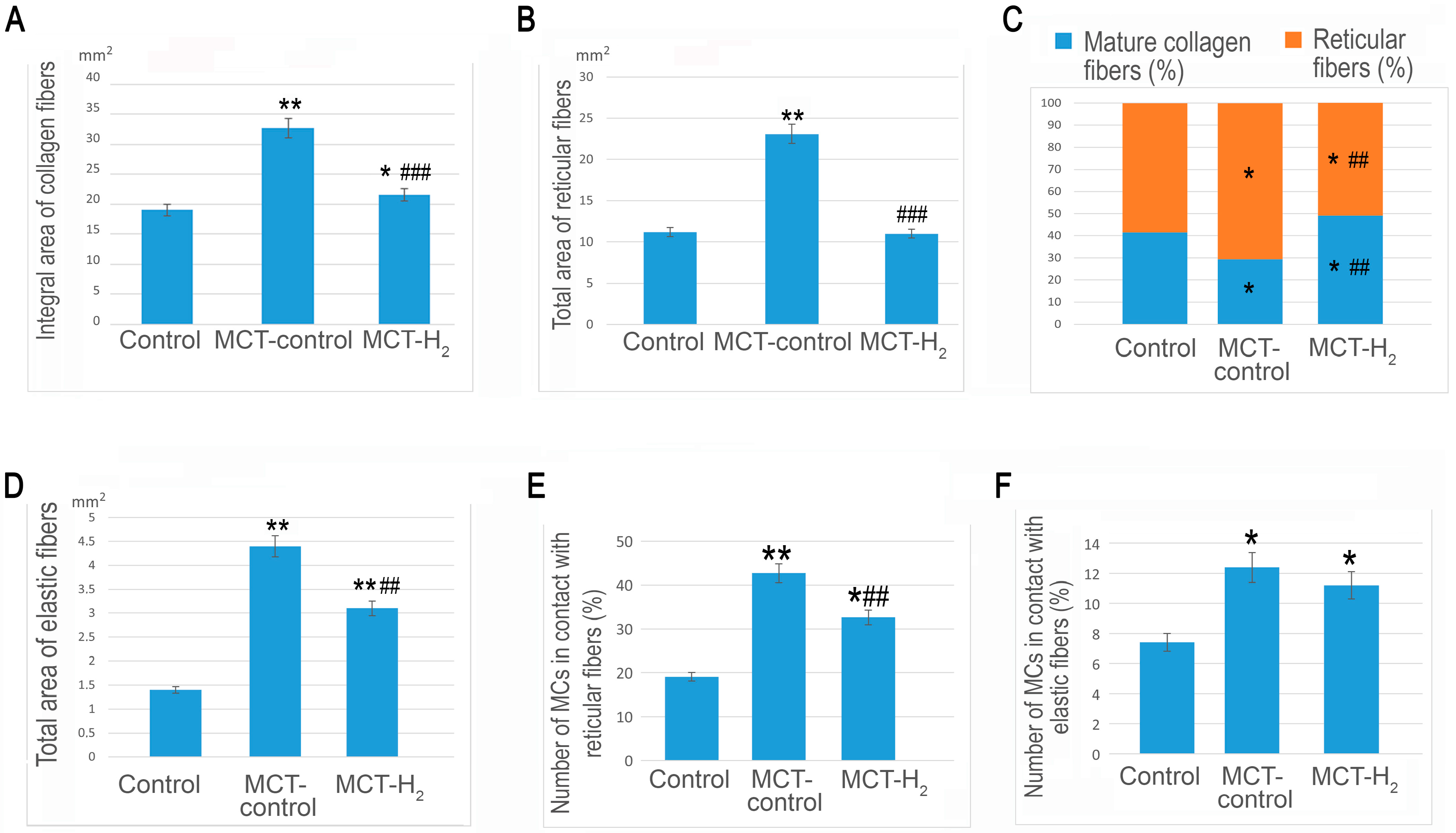
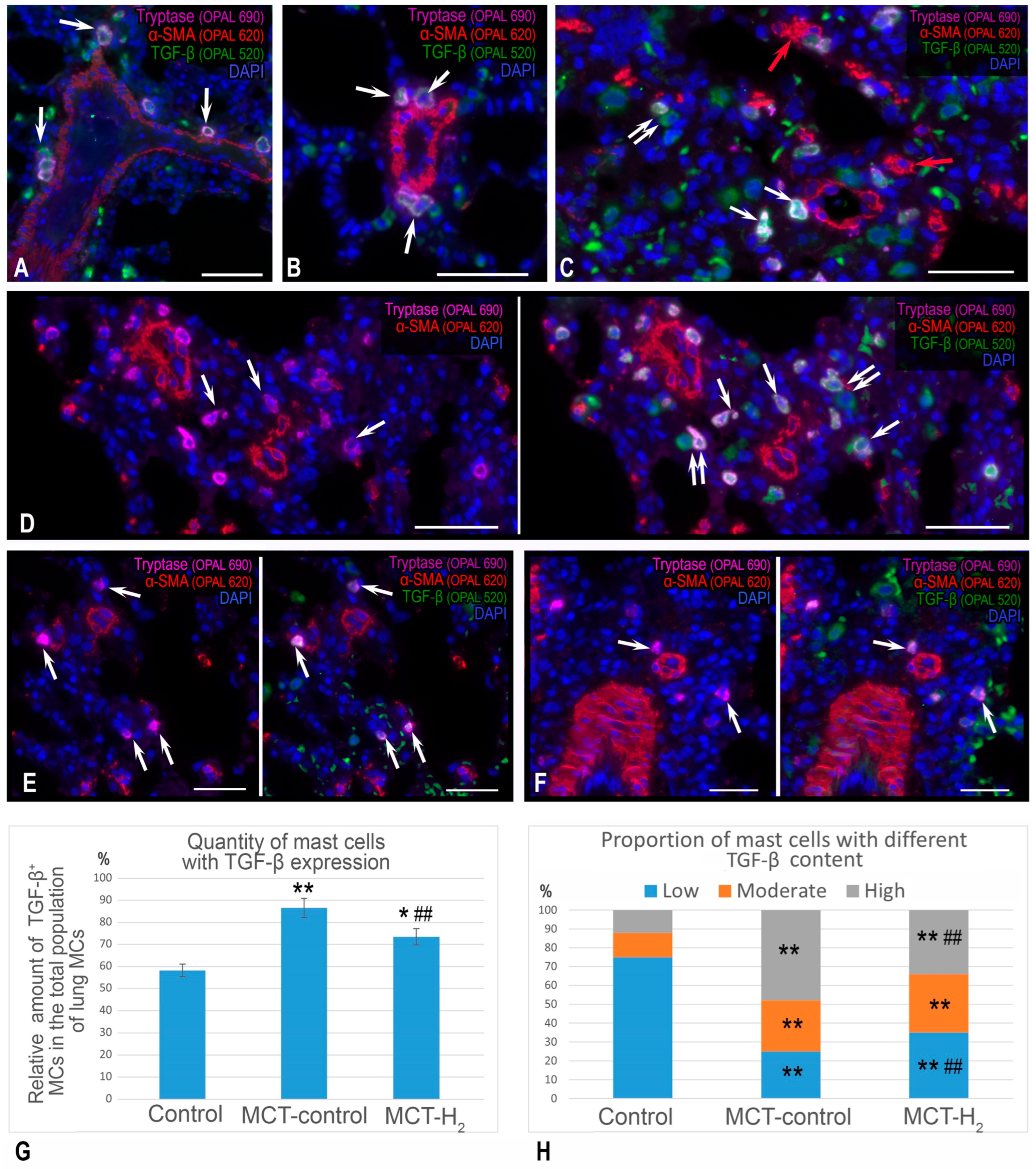
2. Results
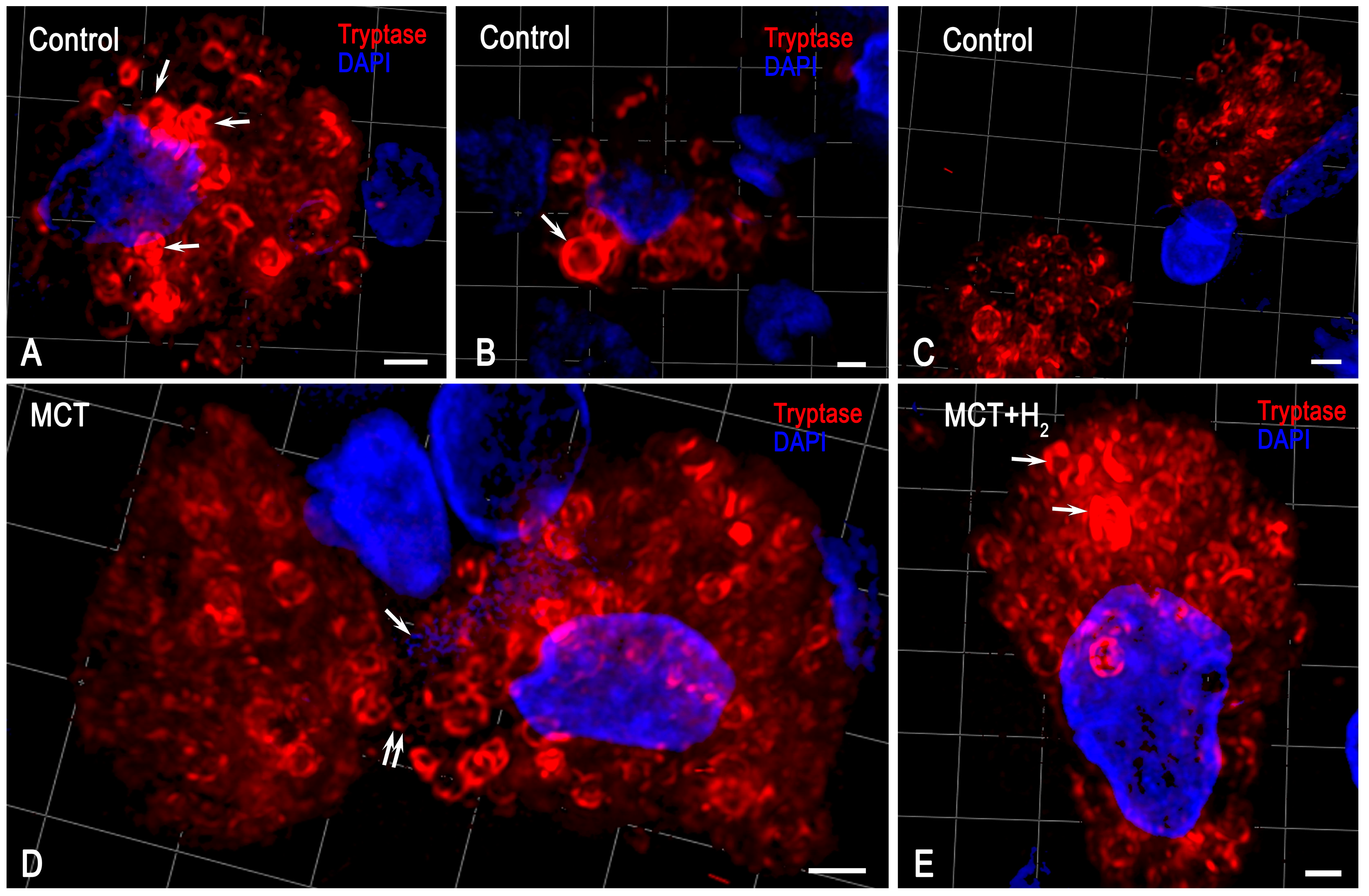
3. Discussion
4. Materials and Methods
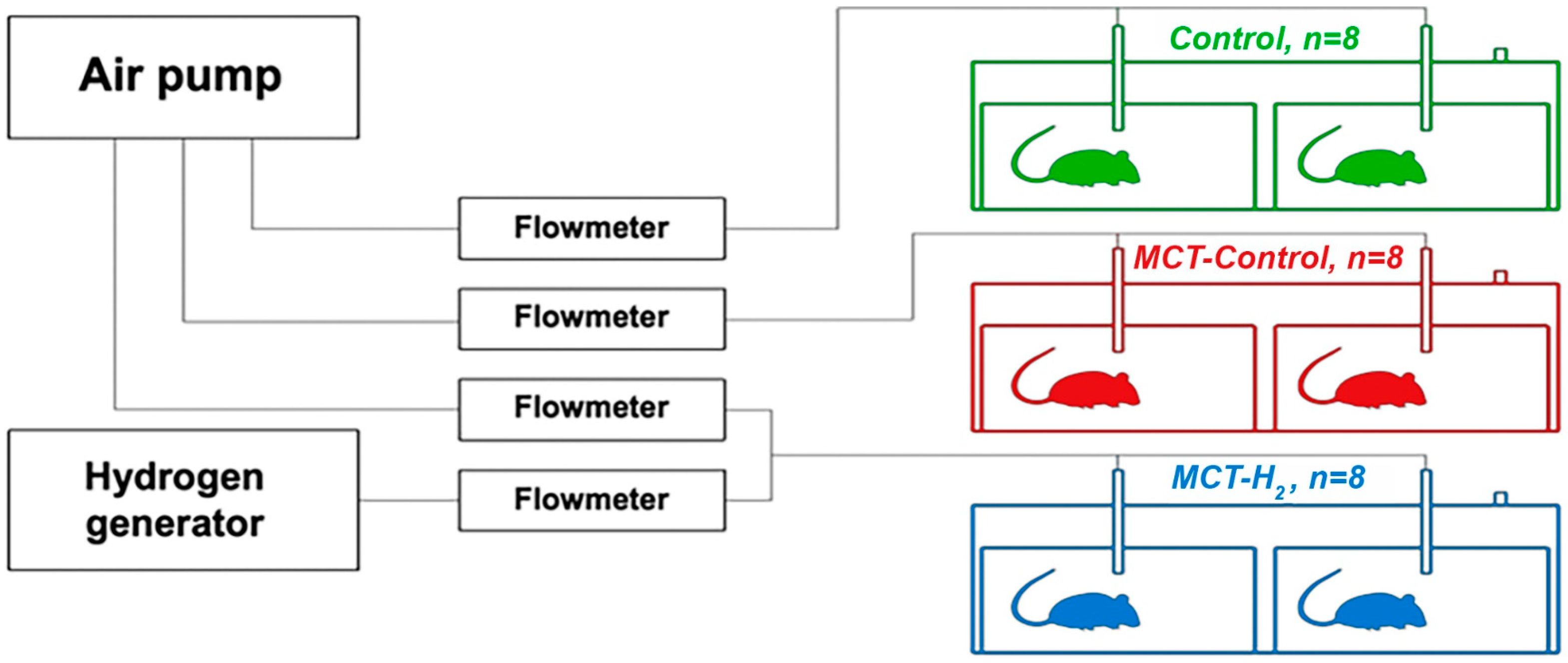
4.1. Experimental Design
4.2. Histoprocessing
4.3. Tissue Probe Staining
4.4. Quantitative Analysis
4.5. Image Acquisition
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albano, G.D.; Gagliardo, R.P.; Montalbano, A.M.; Profita, M. Overview of the Mechanisms of Oxidative Stress: Impact in Inflammation of the Airway Diseases. Antioxidants 2022, 11, 2237. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Garcia, V.; Estupinan-Jimenez, J.R.; Vivas-Mejia, P.E.; Gonzalez-Villasana, V.; Vazquez-Guillen, J.M.; Resendez-Perez, D. A vicious circle in breast cancer: The interplay between inflammation, reactive oxygen species, and microRNAs. Front. Oncol. 2022, 12, 980694. [Google Scholar] [CrossRef] [PubMed]
- Otoupalova, E.; Smith, S.; Cheng, G.; Thannickal, V.J. Oxidative Stress in Pulmonary Fibrosis. Compr. Physiol. 2020, 10, 509–547. [Google Scholar] [CrossRef]
- Lesmana, R.; Parameswari, C.; Mandagi, G.F.; Wahyudi, J.F.; Permana, N.J.; Radhiyanti, P.T.; Gunadi, J.W. The Role of Exercise-Induced Reactive Oxygen Species (ROS) Hormesis in Aging: Friend or Foe. Cell. Physiol. Biochem. 2022, 56, 692–706. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Kloska, D.; Grochot-Przeczek, A.; Feelisch, M.; Cuadrado, A.; van Goor, H. Personalized redox medicine in inflammatory bowel diseases: An emerging role for HIF-1alpha and NRF2 as therapeutic targets. Redox Biol. 2023, 60, 102603. [Google Scholar] [CrossRef]
- Kuropatkina, T.; Atiakshin, D.; Sychev, F.; Artemieva, M.; Samoilenko, T.; Gerasimova, O.; Shishkina, V.; Gufranov, K.; Medvedeva, N.; LeBaron, T.W.; et al. Hydrogen Inhalation Reduces Lung Inflammation and Blood Pressure in the Experimental Model of Pulmonary Hypertension in Rats. Biomedicines 2023, 11, 3141. [Google Scholar] [CrossRef]
- Yu, Y.; Blokhuis, B.R.; Garssen, J.; Redegeld, F.A. Non-IgE mediated mast cell activation. Eur. J. Pharmacol. 2016, 778, 33–43. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Ye, F.; Du, Y.; Tang, Z.X. Research progress of mast cell activation-related receptors and their functions. Sheng Li Xue Bao 2019, 71, 645–656. [Google Scholar]
- Elieh Ali Komi, D.; Wohrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Dileepan, K.N.; Raveendran, V.V.; Sharma, R.; Abraham, H.; Barua, R.; Singh, V.; Sharma, R.; Sharma, M. Mast cell-mediated immune regulation in health and disease. Front. Med. 2023, 10, 1213320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lin, Z.; Wang, M.; Wang, L.; Ji, Y.; Yang, J.; Yang, Y.; Zhu, G.; Liu, T. Identification and verification of PTPN3 as a novel biomarker in predicting cancer prognosis, immunity, and immunotherapeutic efficacy. Eur. J. Med. Res. 2024, 29, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.G.; Kim, A.R.; Lee, D.; An, S.B.; Shim, Y.A.; Jang, M.H. Degranulation of Mast Cells as a Target for Drug Development. Cells 2023, 12, 1506. [Google Scholar] [CrossRef]
- Xu, H.; Bin, N.R.; Sugita, S. Diverse exocytic pathways for mast cell mediators. Biochem. Soc. Trans. 2018, 46, 235–247. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Metz, M.; Kolkhir, P.; Kocaturk, E.; Scheffel, J.; Frischbutter, S.; Terhorst-Molawi, D.; Fox, L.; Maurer, M. Chronic urticaria and the pathogenic role of mast cells. Allergol. Int. 2023, 72, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Daubeuf, F.; Pejler, G.; Abrink, M.; Frossard, N. Mast cells contribute to bleomycin-induced lung inflammation and injury in mice through a chymase/mast cell protease 4-dependent mechanism. J. Immunol. 2014, 192, 1847–1854. [Google Scholar] [CrossRef]
- Bonnekoh, H.; Scheffel, J.; Kambe, N.; Krause, K. The role of mast cells in autoinflammation. Immunol. Rev. 2018, 282, 265–275. [Google Scholar] [CrossRef]
- Varricchi, G.; Marone, G. Mast Cells: Fascinating but Still Elusive after 140 Years from Their Discovery. Int. J. Mol. Sci. 2020, 21, 464. [Google Scholar] [CrossRef]
- Galli, S.J.; Gaudenzio, N.; Tsai, M. Mast Cells in Inflammation and Disease: Recent Progress and Ongoing Concerns. Annu. Rev. Immunol. 2020, 38, 49–77. [Google Scholar] [CrossRef]
- Raj, S.; Unsworth, L.D. Targeting active sites of inflammation using inherent properties of tissue-resident mast cells. Acta Biomater. 2023, 159, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Update on Mast Cell Proteases as Drug Targets. Immunol. Allergy Clin. N. Am. 2023, 43, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Salton, F.; Braga, L.; Wade, B.; Confalonieri, P.; Volpe, M.C.; Baratella, E.; Maiocchi, S.; Confalonieri, M. The History and Mystery of Alveolar Epithelial Type II Cells: Focus on Their Physiologic and Pathologic Role in Lung. Int. J. Mol. Sci. 2021, 22, 2566. [Google Scholar] [CrossRef]
- Trombetta, A.C.; Soldano, S.; Contini, P.; Tomatis, V.; Ruaro, B.; Paolino, S.; Brizzolara, R.; Montagna, P.; Sulli, A.; Pizzorni, C.; et al. A circulating cell population showing both M1 and M2 monocyte/macrophage surface markers characterizes systemic sclerosis patients with lung involvement. Respir. Res. 2018, 19, 186. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015, 555, 289–317. [Google Scholar] [CrossRef]
- LeBaron, T.W.; Sharpe, R.; Ohno, K. Electrolyzed-Reduced Water: Review I. Molecular Hydrogen Is the Exclusive Agent Responsible for the Therapeutic Effects. Int. J. Mol. Sci. 2022, 23, 14750. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Sharpe, R.; Ohno, K. Electrolyzed-Reduced Water: Review II: Safety Concerns and Effectiveness as a Source of Hydrogen Water. Int. J. Mol. Sci. 2022, 23, 14508. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, J.; Zhang, Y. Role of Molecular Hydrogen in Ageing and Ageing-Related Diseases. Oxid. Med. Cell Longev. 2022, 2022, 2249749. [Google Scholar] [CrossRef]
- Huang, L. Molecular hydrogen: A therapeutic antioxidant and beyond. Med. Gas Res. 2016, 6, 219–222. [Google Scholar] [CrossRef]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef]
- Noda, M.; Uemura, Y.; Yoshii, Y.; Horita, T.; Takemi, S.; Sakata, I.; Sakai, T. Circulating messenger for neuroprotection induced by molecular hydrogen. Can. J. Physiol. Pharmacol. 2019, 97, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Higashida, K.; Muraoka, I. Application of Molecular Hydrogen as a Novel Antioxidant in Sports Science. Oxid. Med. Cell Longev. 2020, 2020, 2328768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Fu, Z. Molecular hydrogen is a potential protective agent in the management of acute lung injury. Mol. Med. 2022, 28, 27. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Fujita, Y.; Ito, M.; Masuda, A.; Ohno, K.; Ichihara, M.; Kojima, T.; Nozawa, Y.; Ito, M. Molecular hydrogen suppresses FcepsilonRI-mediated signal transduction and prevents degranulation of mast cells. Biochem. Biophys. Res. Commun. 2009, 389, 651–656. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef]
- Atiakshin, D.; Kostin, A.; Volodkin, A.; Nazarova, A.; Shishkina, V.; Esaulenko, D.; Buchwalow, I.; Tiemann, M.; Noda, M. Mast Cells as a Potential Target of Molecular Hydrogen in Regulating the Local Tissue Microenvironment. Pharmaceuticals 2023, 16, 817. [Google Scholar] [CrossRef]
- Manaenko, A.; Lekic, T.; Ma, Q.; Zhang, J.H.; Tang, J. Hydrogen inhalation ameliorated mast cell-mediated brain injury after intracerebral hemorrhage in mice. Crit. Care Med. 2013, 41, 1266–1275. [Google Scholar] [CrossRef]
- Kajisa, T.; Yamaguchi, T.; Hu, A.; Suetake, N.; Kobayashi, H. Hydrogen water ameliorates the severity of atopic dermatitis-like lesions and decreases interleukin-1beta, interleukin-33, and mast cell infiltration in NC/Nga mice. Saudi Med. J. 2017, 38, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Soboleva, M.; Nikityuk, D.; Alexeeva, N.; Klochkova, S.; Kostin, A.; Shishkina, V.; Buchwalow, I.; Tiemann, M. Mast Cells in Regeneration of the Skin in Burn Wound with Special Emphasis on Molecular Hydrogen Effect. Pharmaceuticals 2023, 16, 348. [Google Scholar] [CrossRef]
- Hugle, T. Beyond allergy: The role of mast cells in fibrosis. Swiss Med. Wkly. 2014, 144, w13999. [Google Scholar] [CrossRef]
- Bradding, P.; Pejler, G. The controversial role of mast cells in fibrosis. Immunol. Rev. 2018, 282, 198–231. [Google Scholar] [CrossRef] [PubMed]
- Legere, S.A.; Haidl, I.D.; Legare, J.F.; Marshall, J.S. Mast Cells in Cardiac Fibrosis: New Insights Suggest Opportunities for Intervention. Front. Immunol. 2019, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Morozov, S.; Dlin, V.; Kostin, A.; Volodkin, A.; Ignatyuk, M.; Kuzovleva, G.; Baiko, S.; Chekmareva, I.; Chesnokova, S.; et al. Renal Mast Cell-Specific Proteases in the Pathogenesis of Tubulointerstitial Fibrosis. J. Histochem. Cytochem. 2024, 72, 495–515. [Google Scholar] [CrossRef] [PubMed]
- Rabelo Melo, F.; Santosh Martin, S.; Sommerhoff, C.P.; Pejler, G. Exosome-mediated uptake of mast cell tryptase into the nucleus of melanoma cells: A novel axis for regulating tumor cell proliferation and gene expression. Cell Death Dis. 2019, 10, 659. [Google Scholar] [CrossRef]
- Alanazi, S.; Rabelo Melo, F.; Pejler, G. Tryptase Regulates the Epigenetic Modification of Core Histones in Mast Cell Leukemia Cells. Front. Immunol. 2021, 12, 804408. [Google Scholar] [CrossRef]
- von Kockritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.I.; Takefuji, Y. Molecular Hydrogen Protects against Various Tissue Injuries from Side Effects of Anticancer Drugs by Reducing Oxidative Stress and Inflammation. Biomedicines 2024, 12, 1591. [Google Scholar] [CrossRef]
- Kura, B.; Bagchi, A.K.; Singal, P.K.; Barancik, M.; LeBaron, T.W.; Valachova, K.; Soltes, L.; Slezak, J. Molecular hydrogen: Potential in mitigating oxidative-stress-induced radiation injury (1). Can. J. Physiol. Pharmacol. 2019, 97, 287–292. [Google Scholar] [CrossRef]
- Alwazeer, D.; Liu, F.F.; Wu, X.Y.; LeBaron, T.W. Combating Oxidative Stress and Inflammation in COVID-19 by Molecular Hydrogen Therapy: Mechanisms and Perspectives. Oxid. Med. Cell Longev. 2021, 2021, 5513868. [Google Scholar] [CrossRef]
- Zheng, C.M.; Hou, Y.C.; Liao, M.T.; Tsai, K.W.; Hu, W.C.; Yeh, C.C.; Lu, K.C. Potential role of molecular hydrogen therapy on oxidative stress and redox signaling in chronic kidney disease. Biomed. Pharmacother. 2024, 176, 116802. [Google Scholar] [CrossRef]
- Slezak, J.; Kura, B.; LeBaron, T.W.; Singal, P.K.; Buday, J.; Barancik, M. Oxidative Stress and Pathways of Molecular Hydrogen Effects in Medicine. Curr. Pharm. Des. 2021, 27, 610–625. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Tang, L.; Wang, G.; Lin, J.; Liao, W.; Pan, W.; Xu, J. Molecular Hydrogen Protects Human Melanocytes from Oxidative Stress by Activating Nrf2 Signaling. J. Investig. Dermatol. 2020, 140, 2230–2241.e2239. [Google Scholar] [CrossRef]
- Kura, B.; Slezak, J. The Protective Role of Molecular Hydrogen in Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2024, 25, 7884. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Varricchi, G.; de Paulis, A.; Marone, G.; Galli, S.J. Future Needs in Mast Cell Biology. Int. J. Mol. Sci. 2019, 20, 4397. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Mortaz, E.; Amani, S.; Tiotiu, A.; Folkerts, G.; Adcock, I.M. The Role of Mast Cells in IgE-Independent Lung Diseases. Clin. Rev. Allergy Immunol. 2020, 58, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Caraffa, A.; Mastrangelo, F.; Tettamanti, L.; Ronconi, G.; Frydas, I.; Kritas, S.K.; Theoharides, T.C. Critical role of inflammatory mast cell in fibrosis: Potential therapeutic effect of IL-37. Cell Prolif. 2018, 51, e12475. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Meurer, S.K.; Liedtke, C.; Huber, M. Mast Cells in Liver Fibrogenesis. Cells 2019, 8, 1429. [Google Scholar] [CrossRef]
- Omelyanenko, N.P.; Slutsky, L.I. Connective Tissue (Histophysiology and Biochemistry), RAS and RAMS ed.; Mironov, S.P., Ed.; Izvestia Publishing House: Moscow, Russia, 2009; Volume 1. [Google Scholar]
- Mienaltowski, M.J.; Birk, D.E. Structure, physiology, and biochemistry of collagens. Adv. Exp. Med. Biol. 2014, 802, 5–29. [Google Scholar] [CrossRef]
- Wu, K.; Li, G. Investigation of the Lag Phase of Collagen Fibrillogenesis Using Fluorescence Anisotropy. Appl. Spectrosc. 2015, 69, 1121–1128. [Google Scholar] [CrossRef]
- Colige, A.; Vandenberghe, I.; Thiry, M.; Lambert, C.A.; Van Beeumen, J.; Li, S.W.; Prockop, D.J.; Lapiere, C.M.; Nusgens, B.V. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J. Biol. Chem. 2002, 277, 5756–5766. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, S.; Khademhosseini, A.; Smit, T.H. Mechanisms of lamellar collagen formation in connective tissues. Biomaterials 2016, 97, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Birk, D.E.; Zycband, E.I.; Winkelmann, D.A.; Trelstad, R.L. Collagen fibrillogenesis in situ. Discontinuous segmental assembly in extracellular compartments. Ann. N. Y. Acad. Sci. 1990, 580, 176–194. [Google Scholar] [CrossRef]
- Birk, D.E.; Fitch, J.M.; Babiarz, J.P.; Doane, K.J.; Linsenmayer, T.F. Collagen fibrillogenesis in vitro: Interaction of types I and V collagen regulates fibril diameter. J. Cell Sci. 1990, 95(Pt. 4), 649–657. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Biol. 2018, 149, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Yi, T. Mast cell tryptases in allergic inflammation and immediate hypersensitivity. Curr. Opin. Immunol. 2021, 72, 94–106. [Google Scholar] [CrossRef]
- Cairns, J.A.; Walls, A.F. Mast cell tryptase stimulates the synthesis of type I collagen in human lung fibroblasts. J. Clin. Investig. 1997, 99, 1313–1321. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cells and collagen fibrillogenesis. Histochem. Cell Biol. 2020, 154, 21–40. [Google Scholar] [CrossRef]
- Hellman, L.; Akula, S.; Fu, Z.; Wernersson, S. Mast Cell and Basophil Granule Proteases—In Vivo Targets and Function. Front. Immunol. 2022, 13, 918305. [Google Scholar] [CrossRef]
- Buchwalow, I.; Samoilova, V.; Boecker, W.; Tiemann, M. Non-specific binding of antibodies in immunohistochemistry: Fallacies and facts. Sci. Rep. 2011, 1, 28. [Google Scholar] [CrossRef]
- Buchwalow, I.B.; Böcker, W. Immunohistochemistry: Basics and Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Buchwalow, I.; Samoilova, V.; Boecker, W.; Tiemann, M. Multiple immunolabeling with antibodies from the same host species in combination with tyramide signal amplification. Acta Histochem. 2018, 120, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Nikolaeva, E.; Gritsevskaya, D.; Semyachkina, A.; Kostin, A.; Volodkin, A.; Morozov, S.; Dlin, V.; Ignatyuk, M.; Mikhaleva, L.; et al. Skin mast cells in Marfan syndrome: Specific emphasis on connective tissue remodeling. Arch. Dermatol. Res. 2024, 316, 271. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.A.; Shishkina, V.V.; Gerasimova, O.A.; Meshkova, V.Y.; Samodurova, N.Y.; Samoilenko, T.V.; Buchwalow, I.B.; Samoilova, V.E.; Tiemann, M. Combined histochemical approach in assessing tryptase expression in the mast cell population. Acta Histochem. 2021, 123, 151711. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]


| Antibodies | Host | Catalogue Nr. | Dilution | Source |
|---|---|---|---|---|
| Tryptase | Mouse monoclonal | #ab2378 | 1:2500 | AbCam, Cambridge, UK |
| TGF-β | Rabbit monoclonal | #ab215715 | 1:200 | AbCam, Cambridge, UK |
| Alpha SMA | Mouse monoclonal | #ab124964 | 1:2500 | AbCam, Cambridge, UK |
| Antibodies and Other Reagents | Source | Dilution | Label |
|---|---|---|---|
| Goat anti-mouse IgG Ab (#ab97035) | AbCam, Cambridge, UK | 1/300 | Cy3 |
| Goat anti-rabbit IgG Ab (#ab150077): | AbCam, Cambridge, UK | 1/300 | Alexa Fluor 488 |
| Secondary antibodies conjugated with horseradish peroxidase (Opal Polymer HRP Ms + Rb (#ARH1001EA)) | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 480 Reagent Pack (#FP1500001KT) |
| Secondary antibodies conjugated with horseradish peroxidase (Opal Polymer HRP Ms + Rb (#ARH1001EA)) | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 570 Reagent Pack (#FP1488001KT) |
| Secondary antibodies conjugated with horseradish peroxidase (Opal Polymer HRP Ms + Rb (#ARH1001EA)) | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 690 Reagent Pack (#FP1497001KT) |
| R-Universal buffer for antigen unmasking/epitope recovery on formalin-fixed, paraffin-embedded sections (#AP0530-500) | Aptum Biologics Ltd., Southampton, SO16 8AD, UK | 1:10 | w/o |
| AmpliStain™ anti-Mouse 1-Step HRP (#AS-M1-HRP) | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| AmpliStain™ anti-Rabbit 1-Step HRP (#AS-R1-HRP) | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| 4′,6-diamidino-2-phenylindole (DAPI, #D9542-5MG) | Sigma, Hamburg, Germany | 5 µg/mL | w/o |
| VECTASHIELD®® Mounting Medium (#H-1000) | Vector Laboratories, Burlingame, CA, USA | ready-to-use | w/o |
| DAB Peroxidase Substrat Kit (#SK-4100) | Vector Laboratories, Burlingame, CA, USA | ready-to-use | DAB |
| Toluidine blue (Biovitrum, #07-002) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Giemsa solution (Biovitrum, #21-023) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Silver impregnation (Biovitrum, #21-026) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Weigert for elastic fibers (Biovitrum, #21-030) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Weiger—Van Gieson (Biovitrum, #21-020) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Brilliant cresyl blue (Biovitrum, #20-041) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Mayer’s hematoxylin (Biovitrum, #05-002) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atiakshin, D.; Kostin, A.; Alekhnovich, A.; Volodkin, A.; Ignatyuk, M.; Klabukov, I.; Baranovskii, D.; Buchwalow, I.; Tiemann, M.; Artemieva, M.; et al. The Role of Mast Cells in the Remodeling Effects of Molecular Hydrogen on the Lung Local Tissue Microenvironment under Simulated Pulmonary Hypertension. Int. J. Mol. Sci. 2024, 25, 11010. https://doi.org/10.3390/ijms252011010
Atiakshin D, Kostin A, Alekhnovich A, Volodkin A, Ignatyuk M, Klabukov I, Baranovskii D, Buchwalow I, Tiemann M, Artemieva M, et al. The Role of Mast Cells in the Remodeling Effects of Molecular Hydrogen on the Lung Local Tissue Microenvironment under Simulated Pulmonary Hypertension. International Journal of Molecular Sciences. 2024; 25(20):11010. https://doi.org/10.3390/ijms252011010
Chicago/Turabian StyleAtiakshin, Dmitrii, Andrey Kostin, Alexander Alekhnovich, Artem Volodkin, Michael Ignatyuk, Ilya Klabukov, Denis Baranovskii, Igor Buchwalow, Markus Tiemann, Marina Artemieva, and et al. 2024. "The Role of Mast Cells in the Remodeling Effects of Molecular Hydrogen on the Lung Local Tissue Microenvironment under Simulated Pulmonary Hypertension" International Journal of Molecular Sciences 25, no. 20: 11010. https://doi.org/10.3390/ijms252011010
APA StyleAtiakshin, D., Kostin, A., Alekhnovich, A., Volodkin, A., Ignatyuk, M., Klabukov, I., Baranovskii, D., Buchwalow, I., Tiemann, M., Artemieva, M., Medvedeva, N., LeBaron, T. W., Noda, M., & Medvedev, O. (2024). The Role of Mast Cells in the Remodeling Effects of Molecular Hydrogen on the Lung Local Tissue Microenvironment under Simulated Pulmonary Hypertension. International Journal of Molecular Sciences, 25(20), 11010. https://doi.org/10.3390/ijms252011010








