Abstract
Several Ranunculaceae species are used in folk medicine to eliminate pathologies associated with oxidative stress as well as parasitic infections; however, a number of studies confirming their pharmacological properties is limited. In this study, 19 ethanolic extracts obtained from 16 Ranunculaceae species were assayed for in vitro antioxidant, antiproliferative, and antiparasitic potential. The maximum antioxidant potential in both oxygen radical absorbance capacity (ORAC) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays was observed for Aconitum toxicum extract [half-maximal inhibitory concentration (IC50) 18.7 and 92.6 μg/mL]. Likewise, Anemone transsilvanica extract exerted the most promising antiproliferative activity against Caco-2 (IC50 46.9 μg/mL) and HT29 (IC50 70.2 μg/mL) cell lines in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Additionally, a dual antioxidant and cytotoxicity effect was demonstrated for Aconitum moldavicum and Caltha palustris extracts. Whilst the efficacy of extracts was modest against Trypanosoma brucei (IC50 ranging from 88.8 to 269.3 µg/mL), several extracts exhibited high potency against Leishmania infantum promastigotes (Aconitum vulparia IC50 18.8 µg/mL). We also tested them against the clinically relevant intracellular stage and found extract of A. vulparia to be the most effective (IC50 29.0 ± 1.1 µg/mL). All tested extracts showed no or low toxicity against FHs 74Int normal cell line (IC50 ranging from 152.9 to >512 µg/mL). In conclusion, we suggest the above-mentioned plant extracts as potential candidates for development of novel plant-based antioxidant and/or antiproliferative and/or antileishmanial compounds.
1. Introduction
An interest in plants with medical potential and pharmaceutical usage has risen dramatically in recent years due to their diverse bioactive properties, which include anticancer, antioxidant, and antiparasitic effects. About 1300 medicinal plants are used in Europe, of which 90% are harvested from wild resources [1]. Ranunculaceae (buttercup family) is one of the largest flowering plant families comprising about 60 genera and 2500 species distributed worldwide, with most found in the temperate and cold areas of the northern hemisphere [2]. In Romania, the family is represented by 23 genera and approximately 110 species including rare and endemic plants, distributed in all regions of the country [3]. Although many species belonging to the family are well-known for being highly poisonous, a number of them have been used for centuries as medicines, spices, and vegetables after cautious processing to reduce their toxicity [4]. In folk medicine, Ranunculaceae species have been used in heat-clearing, detoxification, and as a natural remedy for malaria and various ailments related to oxidative stress such as asthma, arthritis, bronchitis, cancer, gout, and rheumatism [5,6,7]. At present, a broad spectrum of pharmacological activities, including anti-inflammatory, analgesic, antimicrobial, antiparasitic, and antitumor properties have been reported for various Ranunculaceae species [4]. Currently, a number of derivatives of compounds isolated from species belonging to the family (e.g., cimifugaside, hellebrigenin, hydrastine, and thymoquinone) are used as treatment for several conditions, such as cancer, cardiac dysfunctions, and various types of inflammation [8,9,10].
It has been observed that many members of the family exhibit a strong free radical scavenging activity on top of the various secondary metabolites (e.g., diterpenoid alkaloids, triterpenoid saponins, thymoquinone) isolated from Aconitum, Anemone, Consolida, Delphinium, and Nigella species. Moreover, they are also described as one of the most promising natural compounds for treating multiple types of cancer (e.g., breast cancer, colon cancer, epidermoid carcinoma, gastric carcinoma, and prostate cancer among others) via modulating multiple signal pathways involved in cancer initiation and progression [4,11,12,13,14,15,16,17]. Several studies of crude extracts and isolated compounds of Consolida species support its utilization as anthelmintic herbals and reveal their high potential as treatment options for protozoal infections, in particular against Leishmania sp., Plasmodium sp., and Trypanosoma spp. [18,19]. A set of flavonol glycosides, bisbenzyl isoquinoline alkaloids, and diterpenoid alkaloids such as 15,22-O-diacetyl-19-oxo-dihydroatisine, azitine, and isoazitine obtained from Aconitum, Delphinium, and Consolida species displayed promising antileishmanial and antitrypanocidal properties [18,20,21]. Although the high potential of Ranunculaceae species as medicinal plants has been recognized, many of the family members, including endemic plants used in folk medicine, remain uncharacterized regarding their biological activities.
Thus, the main aim of this study was to investigate 19 ethanolic extracts obtained from 16 species belonging to the Ranunculaceae family, namely, Aconitum moldavicum Hacq., A. toxicum Rchb, A. variegatum L., A. vulparia Rchb., Anemone transsilvanica (Fuss) Heuff., Caltha palustris L., Hepatica nobilis Mill., Ranunculus acris L., R. bulbosus L., R. carpaticus Herbich, R. platanifolius L., R. polyanthemos L., R. repens L., R. sardous Crantz, R. serpens subsp. nemorosus (DC.) G. López, and Trollius europaeus L. for their antioxidant, cytotoxicity, and antiprotozoal effects in vitro.
2. Results and Discussion
In this study, 19 crude extracts of 16 Ranunculaceae species used in folk medicine (Table 1) were assayed for their in vitro antioxidant, antiproliferative and antiprotozoal activities. The complete results of 2,2-diphenyl-1-picrylhydrazyl (DPPH), oxygen radical absorbance capacity (ORAC), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and resazurin assays of tested plant extracts are summarized in Table 2.

Table 1.
Ethnobotanical data on tested Ranunculaceae medicinal plants.

Table 2.
In vitro antioxidant, antiproliferative, and antiparasitic activity of Ranunculaceae crude extracts.
2.1. Antioxidant Effect
Oxidative stress is caused by an imbalance between production and accumulation of reactive oxygen species (ROS) that participate in cell-signaling pathways controlling programmed cell death, gene expression, and mechanisms of immune defense and play a critical role in maintaining homeostasis in living organisms [22,23]. Excessive ROS accumulation in the cells/tissues leads to their damage (oxidative stress), and can be responsible, to varying degrees, for the development of various human non-communicable diseases (NCDs) such as cancer, diabetes, metabolic disorders, atherosclerosis, and cardiovascular diseases [24,25]. Considerable evidence shows that numerous medicinal and edible plant species and various compounds they produce, mainly phenolics, are potent scavengers of ROS [26,27,28]. Furthermore, many species in the Ranunculaceae family are rich in bioactive compounds which help scavenge free radicals and reduce oxidative stress in cells. The most notable of these include flavonoids, phenolic acids, alkaloids, saponins, and tannins [29,30,31,32,33].
In the present study, the most promising free radical scavenging potential has been observed for A. toxicum (herb) extract in both assays, the ORAC with half-maximal inhibitory concentration (IC50) = 18.7 ± 6.6 μg/mL, and the DPPH IC50 = 92.6 ± 16.6 μg/mL. Furthermore, based on the results of the ORAC test, A. toxicum extract showed higher antioxidant potential than positive control Trolox (IC50 = 22.4 ± 7.3 μg/mL). Additionally, A. toxicum extract exhibited relatively low toxicity toward the FHs 74Int human epithelial cell line with an IC50 of 274.9 μg/mL. Likewise, significant antioxidant activity was detected for A. moldavicum (IC50 = 23.1 ± 4.6 μg/mL) and A. variegatum (IC50 = 26.4 ± 7.7 μg/mL) extracts in the ORAC assay. The promising antioxidant potential of several Aconitum species (including A. toxicum, A. variegatum, and A. vulparia) has been already described [26,31,32,33,34,35]; however, to the best of our knowledge, this is the first report concerning the antioxidant activity of A. moldavicum. According to the previous reports, the flavonol glycosides are described as the major groups of natural compounds responsible for the antioxidant ability of Aconitum species [32,33]. Previously, two flavonol glycosides isolated from methanolic extracts of Aconitum burnatii demonstrated scavenging abilities comparable to those of vitamin C and Trolox [36]. Additionally, the same study characterized three new flavonol glycosides from the methanolic extracts of Aconitum variegatum (herb) [35]. In a similar study using bioassay-guided fractionation on several methanolic extracts of Italian Aconitum species (A. napellus subsp. tauricum, A. napellus subsp. neomontanum, A. paniculatum, A. vulparia), 13 flavonol glycosides were isolated, with two fractions revealing the highest activity in the DPPH assay, showing IC50 values of 2.0 μg/mL and 2.6 μg/mL, respectively [33]. Furthermore, the phytochemical study of Aconitum anthora identified four flavonol glycosides, two of which were isolated for the first time [31]. Similar 3,7-O-glycosides were identified in Aconitum chiisanense Nakai [37], A. napellus ssp. tauricum (Wulfer) Gayer [32], and A. napellus ssp. neomontanum (Wulfer) Gayer [38]. The comparison of data from previous work on the chemical profile of Aconitum species has shown that the highest antioxidant potential could be attributed to several flavonols isolated from A. napellus subspecies. Furthermore, comparing our data with the aforementioned studies suggests that flavonol glycosides contribute to the antioxidant potential of A. toxicum, A. moldavicum, and A. variegatum extracts, although this hypothesis should be verified through further chemical analysis of the extracts examined in this investigation.
In addition, based on the results of the ORAC assay in the present study, the extracts of Anemone transsilvanica (leaves), C. palustris, H. nobilis(herb), R. acris, R. platanifolis, R. repens, R. serpens subsp. nemorosus, and T. europaeus exhibited interesting free radical scavenging ability with IC50 values in the range from 35.7 to 47.7 μg/mL. The rest of the tested plant extracts exerted medium to weak inhibition of AAPH in the range from 56.4 to 238.6 μg/mL. In the DPPH assay, all extracts (except A. toxicum) showed only weak (IC50 in the range from 104.0 to 240.5 μg/mL) or no antioxidant potential (IC50 > 256 μg/mL). These results are in accordance with the findings of other authors, who previously reported the antioxidant activity of C. palustris, R. acris, R. bulbosus, R. sardous, and T. europaeus [34,35,39]. To the best of our knowledge, the antioxidant potential of A. transsilvanica, H. nobilis, R. carpaticus, R. platanifolius, R. polyanthemos, R. repens, and R. serpens was evaluated for the first time in present study.
Previously, various triterpenoids isolated from Anemone cathayensis demonstrated radical scavenging activity in vitro comparable to that of natural antioxidants (65.9–78.3% at concentrations of 0.05–0.1 mg/mL) [17]. Furthermore, over the last few decades, various studies have investigated the chemical components and pharmacological activities of the Ranunculus species [29]. Consequently, the phytochemical analysis of several related Ranunculus species has already been described. The phytochemical screening of R. arvensis [40], R. japonicus [41], and R. ternatus [42,43] revealed that these species are rich in glycoside ranunculin, protoanemonin, flavonoids, and saponins.
Finally, various studies suggest that species belonging to the Ranunculus genus (e.g., R. arvensis, R. marginatus var. trachycarpus, R. sprunerianus Boiss) exhibit potent antioxidant activity, largely due to the presence of bioactive compounds such as flavonoids and polyphenols [13,44]. However, in a previous report by Neag et al. [39] on extracts of R. ficaria, R. bulbosus, R. sardous, and R. sceleratus, no direct or linear correlation was found between the content of polyphenols (flavonoids and phenolic acids) and the obtained antioxidant capacity, suggesting that other classes of bioactive compounds may also contribute to the antioxidant effect of the Ranunculus species.
Moreover, we should keep in mind that the choice of solvent used for extraction [45] and seasonal variations in climate directly affect the growth cycle of plants and the concentration of bioactive compounds [46]. In fact, the timing of collection (winter and spring) has been shown to play a major role in the variability of fractions/extracts and consequently is likely to influence their therapeutic efficacy and bioactive potential. For example, Hrichi et al. [47] demonstrated that winter leaves of Convolvulus althaeoides L. contained the highest amounts of polyphenolic compounds, while spring-collected leaves harbored the highest pigment content. Therefore, many factors must be considered when comparing the antioxidant activity and chemical composition of various plant extracts.
2.2. Anticancer Activity
The anticancer activity of plants has been a major focus of research, as many medicinal plants contain bioactive compounds that can inhibit the growth of cancer cells, induce apoptosis, or affect cancer pathways. The anticancer activity of plant extracts typically refers to the ability of the extract or an isolated compound to inhibit tumor cell proliferation or induce cell death [11]. Previous studies, as reviewed by Hao et al. [11], have revealed that at least 17 genera of the Ranunculaceae (buttercup family) are enriched with anticancer phytometabolites, including alkaloids, terpenoids, saponins, and polysaccharides [4,11].
Based on the MTT assay performed in this study, the extracts obtained from leaves and roots of A. transsilvanica demonstrated the most promising antiproliferative effect to both Caco-2 (IC50 = 46.9 ± 5.9 and 65.8 ± 2.6 μg/mL) and HT29 (IC50 = 86.5 ± 4.5 and 70.2 ± 9.4 μg/mL) human cancer cell lines. The antiproliferative potential of the above-mentioned extracts was also confirmed by automatic time-lapse fluorescent image capture (Figure 1). Although these extracts do not induce significant cell death at a tested concentration of 100 μg/mL (Figure 2a,b), A. transsilvanica extracts (leaves and roots) significantly inhibit the proliferation of Caco-2 and HT29 cell lines treated for 72 h as compared to non-treated cells (Figure 2c,d). A. transsilvanica is an endemic species from Romania, and regrettably, its chemical composition has not been previously characterized. However, other authors have described that saponins are the most abundant compounds found in the Anemone species, along with significant amounts of ranunculin, anemonin, and protoanemonin [48,49,50]. Han et al. [51] suggested that triterpenoid saponins isolated from Anemone flaccida are involved in the suppression of hepatocellular carcinoma through multiple signaling cascades related to tumorigenesis and tumor metabolism, demonstrating high potential applications in cancer therapy. Furthermore, there is evidence that Raddeanin A, an oleanane-type triterpenoid saponin described as the main bioactive constituent extracted from the root of Anemone raddeana [52], inhibits proliferation and induces apoptosis in various human tumor cells, including gastric cancer cells, hepatocellular carcinoma cells, and non-small-cell lung carcinoma cells [53,54,55,56]. Further research indicates that Raddeanin A is an effective inhibitor of breast cancer-induced osteolysis [57]. Accordingly, it is tempting to assume that triterpene saponins are involved in A. transsilvanica bioactivity.
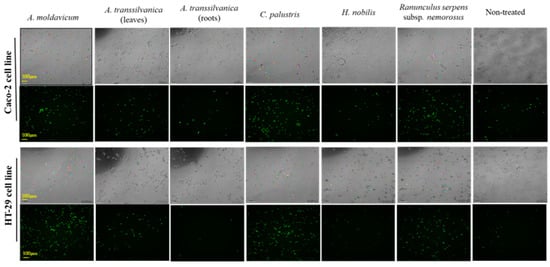
Figure 1.
Brightfield and fluorescent image capture (100×) of Caco-2 and HT29 cell lines exposed for 72 h at A. moldavicum, A. transsilvanica (leaves and roots extracts), C. palustris, H. nobilis, and R. serpens subsp. nemorosus extracts (100 μg/mL) using Lumascope 720 (Etaluma, Carlsbad, CA, USA). The Green dead cell stain SYTOX (15 nM/well) was added together with the extracts to each well and automated brightfield and fluorescent image capture was performed every 2 h.
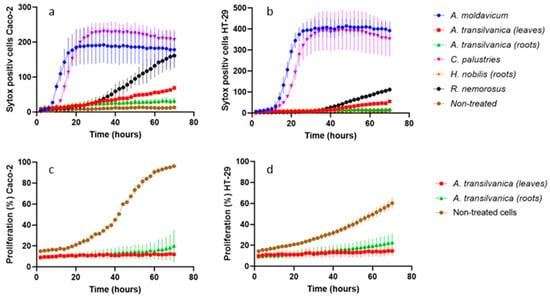
Figure 2.
Numbers of SYTOX Green positive Caco-2 (a) and HT29 (b) cells were quantified based on fluorescent image captures using Lumaquant 8.8 software. The percent of proliferation (%) of the Caco-2 (c) and HT29 (d) cell lines was calculated based on percentage area of cells over time from brightfield image captures using Lumaquant 8.8 software. The adenocarcinoma cell lines were treated with A. moldavicum, A. transsilvanica (leaves and roots extracts), C. palustris, H. nobilis, and R. serpens subsp. nemorosus ethanolic extracts in a concentration of 100 μg/mL for 72 h. The results are expressed as mean (n = 3) +/− SD.
On the other hand, A. moldavicum and C. palustris extracts (100 μg/mL) were demonstrated to be the most effective in inducing cell death in both adenocarcinoma cell lines tested within the first 5 to 10 h of extract exposure (Figure 2a,b). As far as the results of the MTT assay, the IC50 values calculated for extracts obtained from A. moldavicum and C. palustris were 160.7 ± 7.5 and 85.8 ± 7.3 μg/mL for Caco-2, and 77.5 ± 10.4 and 148.8 ± 0.5 μg/mL for HT29 cells, respectively. Furthermore, A. moldavicum and C. palustris extracts demonstrated relatively low toxicity against a normal epithelial cell line with an IC50 of 291.2 ± 7.2 and 277.4 ± 16.9 μg/mL, respectively.
Antiproliferative activity was also detected for R. serpens subsp. nemorosus extract against tested Caco-2 (IC50 = 83.3 ± 4.6 μg/mL) and HT29 (IC50 = 67.4 ± 1.8 μg/mL) cells. Moreover, based on the results of cell death assay, R. serpens subsp. nemorosus extract exposure significantly induced cell death in Caco-2 cells (Figure 2a). The root extracts of H. nobilis exhibited the maximum selective cytotoxicity toward Caco-2 adenocarcinoma cells, with an IC50 of 46.9 ± 5.9 μg/mL and a selectivity index at 7.1 in the MTT assay. The remaining extracts exhibited weak antiproliferative activities (IC50 ranging from 94.1 to 387.7 μg/mL) or were ineffective (IC50 > 512 μg/mL). Toxicity assessment in normal cells revealed that extracts of A. toxicum (roots), A. variegatum, A. vulparia, R. platanifolius, R. polyanthemos, R. repens, and T. europaeus did not affect the growth of the FHs 74Int cell line at a concentration of 512 μg/mL. The remaining tested extracts demonstrated low toxicity with IC50 ranging from 152.9 to 463.6 μg/mL. Although promising anti-cancer activities have been already reported for several genera of the family including Aconitum, Anemone, and Ranunculus [11]; to the best of our knowledge, this is the first report on the antiproliferative activity of A. moldavicum, A. transsilvanica, and R. serpens subsp. nemorosus.
The data obtained in previous studies indicate that several Aconitum alkaloids (e.g., aconitine, lappaconitine, and taipeinine) exhibit anti-cancer activities [58,59]. Furthermore, diterpenoid alkaloids (e.g., C18-, C19-, C20-, and bis-diterpenoid alkaloid) isolated from Aconitum species and Delphinium plants are described as the most promising natural compounds for cancer treatment [12]. However, we should be aware that these compounds serve as a double-edged sword because they could be potentially toxic while having beneficial medicinal effects. Although there is increasing evidence that has shown the benefits of using Aconitum and Delphinium herbs in the treatment of various illnesses, it is of high importance to point out that these plants have a high toxicity level in the raw form and are marked as “very poisonous that must be used with extreme care” [60]. Nevertheless, further analysis should be carried out to determine the chemical composition of extracts and to ultimately assign the active compound from the extract mixture.
2.3. Antileishmanial Effect
The Ranunculaceae crude extracts were tested for their in vitro effects on extracellular and intracellular forms of Leishmania infantum. The parasitic disease leishmaniasis is a major health problem worldwide affecting millions of people, especially in developing nations [61]. L. infantum causes visceral leishmaniasis, the most severe form, involving infections of the liver, spleen, and bone marrow and which is associated with high fatality. Current medications are having considerable side effects and are expensive to access. Furthermore, emerging resistant strains limit the application of current treatments in endemic areas. Therefore, the development of effective and affordable chemotherapeutic agents offering novel cures for leishmaniasis is an urgent research priority [62].
Among all tested extracts, A. transsilvanica (leaves) and H. nobilis (herb and roots) extracts exhibit the highest potency against L. infantum promastigotes with IC50 = 18.6 ± 6.5, 19.5 ± 6.4 and 22.1 ± 5.8 μg/mL, respectively (Figure 3). Additionally, C. palustris (IC50 = 30.7 ± 1.8 μg/mL), A. toxicum (herb extract IC50 = 34.7 ± 20.7 μg/mL), A. transsilvanica (roots IC50 = 41.0 ± 24.0 μg/mL), R. platanifolius (IC50 = 41 ± 11.1 μg/mL), and R. repens (IC50 = 47 ± 11.1 μg/mL) extracts demonstrated significant antileishmanial activity against the promastigote form of L. infantum (Figure 3). Furthermore, the selectivity index of the above-mentioned extracts ranged from 7.9 to 23.8, indicating a relatively large window between cytotoxicity and antiparasitic activity. The remaining extracts exhibit an IC50 value ranging from 55 to 310 μg/mL. IC50 values obtained after 72 h of extract exposure for L. infantum promastigote form are summarized in Table 2. Due to the intracellular nature of Leishmania infection, effective chemical compounds must be able to eliminate the parasite inside the host cell. Therefore, other than sufficient selectivity, which is an essential feature of drug candidates, anti-leishmanial compounds should possess the ability to penetrate the mammalian host cell to clear the intracellular form of the parasite. Evaluating all the extracts at a concentration of 76.8 µg/mL, we identified two Aconitum species (A. toxicum, and A. vulparia) extracts successfully limiting intracellular parasite growth without having a significant toxic effect on the murine macrophage host cell J774 (Figure 4).
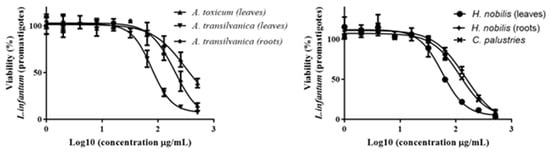
Figure 3.
Dose-response (IC50) of A. toxicum, A. transilvanica (leaves and roots), H. nobilis (herb and roots), and C. palustries extracts against L. infantum promastigotes.
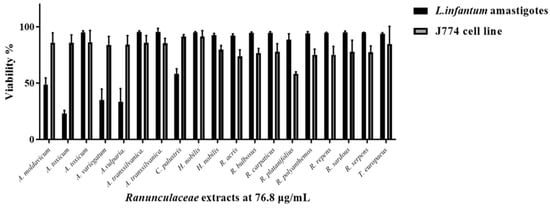
Figure 4.
Single-point parasite rescue assay for L. infantum intracellular amastigotes. A bar graph shows antiparasitic activity of 19 Ranunculaceae ethanolic extracts at a concentration of 76.8 μg/mL against L. infantum amastigotes and cytotoxicity against J774 macrophage host cells, expressed as % cell viability +/− standard deviation.
Detailed dose-response analysis (Figure 5a) showed that the A. vulparia extract was the most effective against the intracellular amastigote form of L. infantum with an IC50 of 29 ± 1 µg/mL in the rescue assay and the A. toxicum extract resulted in an IC50 equal to 40 ± 1 µg/mL (Figure 5b). Cytotoxicity for J774 host macrophages, monitored in parallel, revealed selectivity indexes of 4.7 and 4.2, respectively, with IC50 of 124 ± 1 µg/mL (A. vulparia) and IC50 of 190 ± 1.1 µg/mL (A. toxicum) extracts.
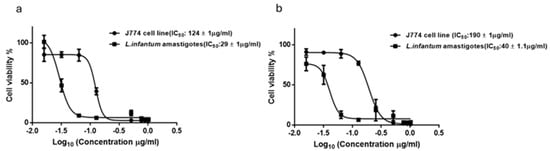
Figure 5.
Dose-response analysis (IC50) of A. vulparia (a) and A. toxicum herb (b) extracts for L. infantum intracellular amastigotes. Derived IC50 values against amastigotes and J774 host macrophages for each extract are indicated in the legend (n = 3).
These results indicate that the active leishmanicidal compound(s) of these extracts are efficiently entering the host cell. The modest therapeutic window may be optimized through further studies aimed at identifying the exact effective fraction of these extracts. Recent studies have shown that alkaloids and berberine compounds isolated from various plants are important sources of drug candidates against leishmaniasis. Regarding the Ranunculaceae species, there are shreds of evidence that extracts and essential oils isolated from Nigella sativa seeds possess in vitro and in vivo antileishmanial activity [63,64,65]. Additionally, diterpenpene alkaloids such as 15,22-O-diacetyl-19-oxo-dihydroatisine, azitine, and isoazitine obtained from Aconitum, Delphinium, and Consolida species, exhibit leishmanicidal activities against the promastigotes form of L. infantum [20]. Furthermore, several flavonoid derivatives from Aconitum napellus subsp. lusitanicum, Consolida oliveriana, Delphinium gracile, and D. staphisagria exhibit a leishmanicidal effect against promastigote as well as amastigote forms of L. donovani, L. braziliensis, and L. infantum at similar concentrations as the reference drug (Glucantime) [66,67,68]. However, to the best of our knowledge, this is the first report demonstrating the antileishmanial activity of extracts of A. transsilvanica, A. toxicum, A. vulparia, and H. nobilis against the promastigote form of L. infantum. Significantly, we demonstrate that A. toxicum and A. vulparia extracts have the ability to eliminate the clinically relevant, intracellular form from host macrophages.
Among the extracts studied in the current study, A. toxicum and A.vulparia extracts are promising candidates for further investigations in animal models of Leishmania infection. Furthermore, combinational therapy of these candidates alongside considerably toxic generic drugs like Amphotericine B or Miltefosine could be considered in animal model studies to reduce the required dosage to less toxic levels [69] as well as lowering the risk of developing resistance [70]. Moreover, the possibility of an immunomodulatory effect of these herbal extracts or their derivatives [71], warranting further investigation, could potentially improve drug response by providing activation and dominance of beneficial TH1 (T helper 1) immune responses and activation of M1 type macrophages which support an ideal healing profile for Leishmania infection [29,72]. Also notably, the origin of these candidate extracts from the genus Ranunculus, widely accepted as traditional wound healers [73], may potentiate the curative effect when applied as topical medications either in the form of cream or as a bioactive compound in wound dressing bandages for cutaneous leishmaniasis [74].
2.4. Antitrypanosomal Effect
Extracts were also tested for in vitro potency against Trypanosoma brucei, the causative agent of human and animal African trypanosomiasis, also known as sleeping sickness in humans and nagana in animals, exerting a considerable impact on sub-Saharan African economics and public health. The parasite is transmitted by the blood-feeding tsetse fly vector (Glossina sp.), and the resulting infection is usually fatal unless treated. As far as the antitrypanosomal activity of the Ranunculaceae species is considered, there are just a few reports in this direction. Herrmann et al. [75] reported the strong activity of Coptis chinensis methanolic extract against T. brucei with an IC50 of 0.4 µg/mL. Another study, by Marin et al. [21], shows that several flavonoid glycosides isolated from Aconitum napellus subsp. lusitanicum, Consolida oliveriana, and Delphinium gracile exhibit higher trypanocidal activities than the reference drug benznidazole against the intracellular forms of Trypanosoma cruzi.
In the current study, the ethanolic extracts showed only moderate or low activity against T. brucei. Out of all the tested extracts, A. toxicum and C. palustris exhibited the lowest IC50 values of 88.8 ± 25 and 83 µg/mL. Additionally, A. moldavicum (IC50 = 89.9 ± 1.6 μg/mL), R. acris (IC50 = 91.4 ± 3.7 μg/mL), and R. platanifolius (IC50 = 98.3 ± 0.4 μg/mL) extracts revealed similar antitrypanosomal potential (Figure 6). The remaining extracts displayed an IC50 ranging from 102.6 to 269.3 against T. brucei. To the best of our knowledge, this is the first report concerning the antitrypanosomal effect of all plants tested in the present study. However, further analyses should be carried out to identify the active compound(s) from the extract mixture.
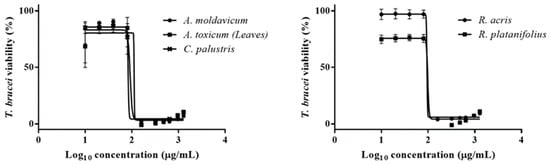
Figure 6.
Dose-response analyses (IC50) of A. moldavicum, A. toxicum (herb), C. palustries, R. acris, and R. platanifolius extracts against bloodstream form of T. brucei. Corresponding IC50s are listed in Table 2.
2.5. Combined Antioxidant and Antiproliferative Activity
Previously it was reported that the progression of cancer is strongly related to oxidative stress [76]. It is considered that reactive oxygen species (ROS) act as secondary messengers and in overdose can initiate DNA damage (e.g., oxidized DNA bases), which might ultimately lead to carcinogenesis and tumor promotion [77,78]. Currently, many studies combine chemotherapy with certain types of antioxidants (e.g., curcuminoid, β-carotene, glutathione, glutamine, resveratrol, selenium, vitamins C and E) due to their potential to decrease the toxic effect of medication [79,80]. In fact, much attention has been focused on the antioxidative and antiproliferative compounds isolated from various plant species, to provide clues for modern drug discovery [81,82,83]. Taking into account all of the aforementioned, we also assessed extracts for exerting dual antioxidant and anticancer activity. Among all tested plant extracts, combined antioxidant and antiproliferative effects were observed for A. moldavicum and C. palustris together with their relatively low toxicity toward FHs 74Int cell lines. Although extracts of A. moldavicum are locally used to treat cough, neuralgia, and rheumatism, their efficacies have not been scientifically validated. According to Borcsa et al. [84], the chemical composition of A. moldavicum is mainly characterized by high amounts of diterpenoid and norditerpene alkaloids. Additionally, the presence of certain flavonoids was also reported in various Aconitum species [31,36], but studies on the flavonoids content of A. moldavicum are lacking. Therefore, it remains unclear if alkaloids, flavonoids, or phenolic compounds play a role in the observed combinatory activities of A. moldavicum. Notably, the results obtained in our research for C. palustris are in agreement with the findings of Mubashir et al. [85], who reported a broad spectrum of bioactivities for the respective root methanolic extract, including antioxidant and antiproliferative effects.
A favorable role of antioxidant activity has also been recognized for the treatment of parasitic infections, by means of selectively reducing oxidative damage in the host caused by reactive oxygen or nitrogen species [86]. While the generation of the latter radicals is an integral part of the host immune response, several antiprotozoal frontline treatment options rely on nitrodrugs, which function as prodrugs that are selectively activated by radical formation inside the parasite cell. This mode of action is relevant for benznidazole, a frontline treatment for chagas disease [87], nifurtimox, a potent trypanocidal [88,89], and also for nitroimidazoles, which are currently the only treatment option for trichomonaiasis [90]. In this context, future studies could address the suitability of our plant extracts with antioxidant properties for combination therapy with nitrodrugs.
3. Materials and Methods
3.1. Plant Material
The selection of plant species was based on previously reported data on traditional use for the treatment of diseases likely to be associated with oxidative stress (e.g., chronic inflammation, asthma, arthritis, gout, and rheumatism), cancer, and parasitic diseases (e.g., malaria) [5,6,7]. Plants were collected in Romania from their natural habitats during their main flowering seasons in June–July 2016 and May–August 2017. Except A. transsilvanica, which is flowering in early spring, all collected herbs were in the flowering stage during collection. Plants were collected in five mountain areas, namely, Mt. Almajului, Mt. Intorsurii, Mt. Piatra Craiului, Mt. Postavaru, Mt. Stamba, and one city area in Cluj-Napoca. Identification of specimens was performed in the field and the voucher specimens have been deposited in the Herbarium collection (CLA) at the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania. The botanical names, voucher specimen numbers, location and date of plant collection, and traditional uses of plant species are given in Table 1.
3.2. Sample Preparation
Plant materials were air-dried at room temperature and finely ground into powder using an electric mill GM100 (Retsch, Haan, Germany) and 5 g of each powdered sample was macerated in 150 mL of 80% ethanol (EtOH) for 24 h at room temperature using a laboratory shaker GFL3005 (GFL, Burgwedel, Germany). Extracts were subsequently filtered and evaporated to dryness using a rotary evaporator R-200 (Buchi, Flawil, Switzerland) in vacuo at 40 °C. Dried residues were dissolved in 100% dimethylsulphoxide (DMSO) to obtain a concentration of 51.2 mg/mL stock solution of each extract, which was stored at –80 °C until tested. Dry residue extraction yield was calculated as follows: extraction yield % = weight of the dry extract after evaporation/weight of the dry plant material used for extraction × 100%. Dried residue extraction yields (%) are shown in Table 2.
3.3. Chemicals and Reagents
The following chemicals and reagents, purchased from Sigma–Aldrich (Prague, Czech Republic), were used in this study: 2,2-azobis(2-methylpropionamidine) dihydrochloride (AAPH), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), Dulbecco’s modified Eagle’s medium (DMEM), human epidermal growth factor, fetal calf serum (FCS), fluorescein (FL), non-essential amino acids, penicillin-streptomycin solutions, RPMI medium, thiazolyl blue tetrazolium bromide (MTT), and vinorelbine. DMSO, EtOH, and methanol were purchased from Penta (Prague, Czech Republic). Hybri-Care Medium 46-X was purchased from American Type Culture Collection (ATCC; Rockville, MD, USA). Medium 199 (M199), Minimum Essential Media (MEM), fetal bovine serum (FCS), BME vitamins, Phorbol 12-myristate 13-acetate (PMA), and SYTOX Green death cell stain (S34860) were purchased from Thermo Fisher Scientific (Prague, Czech Republic). Amikin was prepared from Medopharm (Kodambakkam Chennai, Tamilnadu, India) and resazurin was obtained from Chem Cruz (Dallas, TX, USA).
3.4. Cell Cultures
Human colon adenocarcinoma cell lines Caco-2 and HT29 (ATCC, Rockville, MD, USA) were maintained in DMEM supplemented with FCS (10%), 1 mM sodium bicarbonate, 1 mM sodium pyruvate, non-essential amino acids (1%), and penicillin-streptomycin solution (100 U/mL). Normal small intestinal cell line FHs 74Int (ATCC, Rockville, MD, USA) was maintained in Hybri-Care medium 46-X supplemented with 10% FCS, penicillin-streptomycin solution (100 U/mL) and 30 ng/mL human epidermal growth factor. Murine macrophage cell line (J774) was cultivated in vitro in RPMI supplemented with 10% FCS, 1% MEM and penicillin-streptomycin solution (100 U/mL), and PMA (50 µg/mL). Cell cultures were incubated in a 5% CO2 atmosphere at 37 °C using an MCO 170AIC-PE CO2 incubator (Panasonic Corporation, Osaka, Japan).
3.5. Parasite Strains and Culture
Leishmania infantum (MHOM/BR/76/M4192) promastigotes were cultivated in vitro in M199 media supplemented with NaHCO3 (1 mM), BME Vitamins (1%), human urine (0.1%; collected from a healthy person), Amikin (0.1%), and 10% FCS. Addition of human urine to leishmania culture is popular for improving cell growth and has become a widely used media component [91,92]. The culture was kept in Roux flasks with a surface area of 25 cm2 at 27 °C in an air atmosphere using an MCO 170AIC-PE CO2 incubator (PHCbi, Tokyo, Japan). The bloodstream form of Trypanosoma brucei brucei (T. brucei), derived from Lister strain 427 (Molteno Institute Trypanosomal antigen type (MITat) 1.2) were cultured in HMI-9 complete medium (HMI-9 supplemented with 10% FCS, 100 U/mL penicillin, and 100 U/mL streptomycin at 37 °C with 5% CO2 in a humid atmosphere) [93].
3.6. Antioxidant Activity
3.6.1. Oxygen Radical Absorbance Capacity (ORAC) Assay
The ORAC method previously described by Ou et al. in 2001 [94] and slightly modified by Tauchen et al. [95] and Rondevaldova et al. [96] was used for the evaluation of the ability of the extracts to protect FL from AAPH-induced damage. Firstly, the serial dilutions (final concentration range: 256 to 1 μg/mL) of each extract were prepared in phosphate buffer in black absorbance 96-well microtiter plates using the automated pipetting platform Freedom EVO 100 (Tecan, Mannedorf, Switzerland). Blank and positive control wells received phosphate buffer and synthetic antioxidant (Trolox) dilution, respectively. After, the addition of 150 μL FL (480 μM) prepared in 75 mM phosphate buffer (pH 7.0) to each of the well plates was incubated at 37 °C for 10 min. Subsequently, the reaction was started by applying 25 μL AAPH (153 mM). The plates were incubated for another 90 min at 37 °C. Immediately, the fluorescence changes were measured using a Cytation 3 microplate reader, using Gen5 software, version 3.11 (BioTek, Winooski, VT, USA) with λexc: 485 nm and λem: 528 nm. The fluorescence signals were used to monitor the rate of FL quenching.
3.6.2. DPPH Radical Scavenging Assay
The ability of extracts to neutralize DPPH was determined using a slightly modified method previously described by Sharma and Bhat [97]. Serial dilutions of each sample were prepared in absolute methanol (100 μL) in 96-well microtiter plates using the automated pipetting platform Freedom EVO 100 (Tecan, Mannedorf, Switzerland). Blank and positive control wells received methanol and Trolox dilution, respectively. The reaction was started by adding 75 μL of absolute methanol and 25 μL of freshly prepared 1 mM DPPH to each well, creating a range of concentrations from 0.125 to 256 μg/mL. The mixture was kept in the dark at room temperature for 30 min. Absorbance was measured at 517 nm using a Cytation 3 microplate reader, using Gen5 software, version 3.11 (BioTek, Winooski, VT, USA).
3.7. Cytotoxicity Activity
3.7.1. Cell Viability Assay
A modified method based on the metabolization of yellow MTT to blue formazan by mitochondrial dehydrogenases in living cells previously described by Mosmann in 1983 [98] was used to test cell viability. Caco-2, HT29, and FHs 74Int cell lines were preincubated in a 96-well plate at a density of 2.5 × 103 (Caco-2 and HT29) and 2.5 × 105 (FHs 74Int) cells per well for 24 h in a humidified atmosphere of 5% CO2 in air at 37 °C. After 24 h, the cells were treated with two-fold serial diluted samples (16 to 512 μg/mL) for 72 h. Subsequently, fresh DMEM or Hybri-Care medium containing MTT reagent (1 mg/mL) was added to each well, and plates were incubated for an additional 2 h at 37 °C. The media were then removed, and the intracellular formazan product was dissolved in 100 μL of DMSO. The absorbance was measured at 555 nm using a Tecan Infinite M200 spectrometer (Tecan Group, Mannedorf, Switzerland). Vinorelbine was used as a positive control (at a concentration range of 0.001 to 16 μg/mL).
3.7.2. Cell Death Assay
Cell death and the percent of the proliferation of Caco-2 and HT29 cell lines treated with the most promising extracts were evaluated using the automated brightfield and fluorescent image capture with a Lumascope 720 microscope (Etaluma, Carlsbad, CA, USA). Firstly, human colon adenocarcinoma cell lines were preincubated at 37 °C in 96-well plates at a density of 5 × 103 cells per well for 24 h with 5% CO2. Subsequently, cells were treated with the six extracts, namely, A. moldavicum, A. transsilvanica (leaves and root extracts), C. palustris, H. nobilis, and R. serpens subsp. nemorosus at concentration of 100 μg/mL. The Green dead cell stain SYTOX (15 nM/well) was added together with the extracts to each well and automated brightfield and fluorescent image capture was performed every 2 h at 100× on a LumascopeTM 720. The number of SYTOX positive cells, as well as the percent of proliferation, were quantified using Lumaquant 8.8 software (Etaluma, Carlsbad, CA, USA).
3.8. Antiparasitic Activity
3.8.1. Resazurin Assay
The growth inhibitory effects of the extracts against L. infantum (promastigote form) and T. brucei (blood stream form) were evaluated using a resazurin-based viability assay. Firstly, each extract was dispensed into a white, tissue culture-treated, 384 microtiter plates (BD Falcon, Franklin Lakes, NJ, USA) using an ECHO 550 acoustic dispenser (Labcyte, San Jose, CA, USA) to prepare for a final concentration range of 1 to 1024 μg/mL. Subsequently, L. infantum and T. brucei cells in medium were added at a density of 5 × 105 and 103 cells/well, respectively, to a volume of 100 μL/well. The plates were incubated at 27 °C (L. infantum) or 37 °C in a humid, 5% CO2 atmosphere (T. brucei) for 72 h. Then, resazurin solution (500 µM in PBS) was added in the ratio of 1:10 (vol/vol) to each well and incubated overnight in order to assess the viability of L. infantum and T. brucei. The fluorescence intensity of the samples was measured with a plate reader (Biotek, Winooski, VT, USA) at λexc: 550 nm and λem: 590 nm. IC50 values were calculated from dose-response curves based on non-linear regression in GrapPad Prism (San Diego, CA, USA). Amphotericin (L. infantum) and the phenoxybenzoxaborole [99] AN3057 (T. brucei) were used as a positive control.
3.8.2. Parasite Rescue Assay
Extracts were evaluated against intracellular amastigote stage of L. infantum using a parasite rescue assay described previously by Zahedifard et al. [100]. First, murine macrophage cells (J774) were seeded (5 × 104 cell/well) for 24 h to differentiate. Afterward, the cells were washed with warm (37 °C) serum-free RPMI. Stationary phase promastigotes of L. infantum were diluted in RPMI 2% FCS and added in the ratio of 10:1 (parasite: cell) (5 × 105 cell/well). After 24 h incubation, cells were washed five times with serum-free RPMI to remove extracellular parasites and exposed to extracts at concentrations (ranging from 16 to 1024 μg/mL). The extract dilutions were prepared in RPMI (2% FCS) using an ECHO 550 acoustic dispenser (Labcyte, San Jose, CA, USA). After 48 h, three washing steps were performed, and afterward, cells were lysed with 20 µL of 0.05% SDS in RPMI for 30 s and liberated parasites incubated at 26 °C with M199 for another 72 h. Lastly, the viability of these cells was measured based on resazurin assay as described above. Percent of viability was normalized using non-treated and no cell controls [101].
3.9. Statistical Analysis
All tests were carried out in triplicate. ORAC, DPPH, and MTT assays were performed in three independent experiments, while resazurin and parasite rescue assays were repeated twice (in biological duplicate, each in technical triplicate). Results were expressed as an IC50 in μg/mL for the above-mentioned assays. In ORAC and DPPH, the concentration of the extract ((IC50 (μg/mL))required to inhibit 50% of the radical was calculated using linear regression analysis. For MTT, resazurin, and parasite rescue assays, the IC50 (μg/mL) was determined as the concentration of the respective extract required to reduce 50% of cell viability when compared to the non-treated control. The selectivity of anticancer and antiparasitic extracts was determined in order to evaluate their safety. Thus, the selectivity index (SI) was calculated as the ratio of the geometric mean of IC50 values of normal small intestinal cell line FHs 74Int to IC50 values calculated for colon adenocarcinoma cell lines Caco-2 and HT29 cells or the IC50 values calculated for L. infantum and T. brucei, respectively. Higher SI is thus indicative of a safer extract. Statistical analysis was performed in Magellan software, version 7.2 (Tecan Group, Mannedorf, Switzerland) GraphPad Prism 6.0 software (San Diego, CA, USA) and Microsoft Office Excel 2016 (Microsoft, Redmond, WA, USA).
4. Conclusions
In summary, the main objective of this study was to determine the in vitro antioxidant, anticancer, and antiparasitic potential of ethanolic extracts obtained from 16 Ranunculaceae species. Our studies revealed a significant antioxidant potential of extracts of A. toxicum, comparable to the prototypic antioxidant Trolox. Furthermore, A. transsilvanica and R. serpens subsp. nemorosus extracts produced a selective antiproliferative effect against carcinogenic and non-carcinogenic cell lines. The results also showed that A. moldavicum and C. palustris extracts exhibit strong cytotoxicity potential against adenocarcinoma cell lines combined with free radical scavenging ability. A further interesting finding of the study is the selective cytotoxic effect of A. vulparia and A. toxicum extracts against the intracellular form of L. infantum. It remains a major challenge to target intracellular pathogens, and our results indicate that the active leishmanicidal compounds of these extracts are efficiently entering the host cell. Ultimately, the above-mentioned plant extracts can be considered as prospective materials for the further development of novel plant-based anti-leishmanicidal, antioxidant, and/or antiproliferative compounds. However, further research should be carried out with the aim of isolating the bioactive compounds responsible for their biological effects.
Author Contributions
Conceptualization, L.K., M.Z., D.P., J.R. and C.D.H.; methodology, all authors; software, all authors; validation, all authors; formal analysis, all authors; investigation, C.D.H., F.Z. and I.D.; writing—original draft preparation, C.D.H.; writing—review and editing, all authors; visualization, all authors; supervision, L.K., J.R. and M.Z.; project administration, L.K. and M.Z.; funding acquisition, L.K. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Internal Grant Agency of the Faculty of Tropical AgriSciences of the Czech University of Life Sciences Prague, grant number IGA 20243109, Ministry of Education, Youth and Sport of the Czech Republic, project OPVVV 16_019/0000759, UEFISCDI Romania, grant number PN-III-565, and METROFOOD-CZ research infrastructure project, MEYS grant number LM2023064.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data underlying this article are available in the article.
Acknowledgments
The authors are grateful to Dan Gurean (Faculty of Silviculture and Forest Engineering, Transylvania University, Brasov), and Sabin Bădărău (Faculty of Environmental Science and Engineering, Babeş-Bolyai University, Cluj-Napoca) for assistance in plant collection and preparation of plant material in the field. We are also grateful to Ladislav Andera for his help regarding the cell death assay using the Lumascope 720 microscope (Etaluma, Carlsbad, CA, USA).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, S.-L.; Yu, H.; Luo, H.-M.; Wu, Q.; Li, C.-F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Heywood, V.H.; Brummitt, R.K.; Culham, A.; Seberg, O. Flowering Plant Families of the World, 1st ed.; Firefly Books: Richmond Hill, ON, Canada, 2007; pp. 273–276. [Google Scholar]
- Cristea, V. Plante Vasculare: Diversitate, Sistematica, Ecologie Si Importanta; Presa Universitara Clujeana: Cluj Napoca, Romania, 2014; pp. 32–38. [Google Scholar]
- Hao, D.-C.; Xiao, P.-G.; Ma, H.-Y.; Peng, Y.; He, C.-N. Mining chemodiversity from biodiversity: Pharmacophylogeny of medicinal plants of Ranunculaceae. Chin. J. Nat. Med. 2015, 13, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Alexan, M.; Bojor, O.; Craciun, F. Flora Medicinala a Romaniei, 2nd ed.; Ceres: Bucuresti, Romania, 1991; pp. 23–41. [Google Scholar]
- Neblea, M.; Marian, M.; Duţa, M. Medicinal plant diversity in the Flora of the west part of Bucegi mountains (Romania). Acta Hortic. 2012, 955, 41–49. [Google Scholar] [CrossRef]
- Tamas, M. Botanica Farmaceutica: Sistematica-Cormobionta, 3rd ed.; Medicala Universitara: Cluj Napoca, Romania, 2005; pp. 40–44. [Google Scholar]
- Darshan, S.; Doreswamy, R. Patented antiinflammatory plant drug development from traditional medicine. Phytother. Res. 2004, 18, 343–357. [Google Scholar] [CrossRef]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef]
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531. [Google Scholar] [CrossRef]
- Hao, D.C.; He, C.N.; Shen, J.; Xiao, P.G. Anticancer chemodiversity of Ranunculaceae medicinal plants: Molecular mechanisms and functions. Curr. Genom. 2017, 18, 39–59. [Google Scholar] [CrossRef]
- Ren, M.Y.; Yu, Q.T.; Shi, C.Y.; Luo, J.B. Anticancer activities of C18-, C19-, C20-, and bis-diterpenoid alkaloids derived from genus Aconitum. Molecules 2017, 22, 267. [Google Scholar] [CrossRef]
- Bhatti, M.Z.; Ali, A.; Ahmad, A.; Saeed, A.; Malik, S.A. Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Res. Notes 2015, 8, 279. [Google Scholar] [CrossRef]
- Munir, N.; Ijaz, W.; Altaf, I.; Naz, S. Evaluation of antifungal and antioxidant potential of two medicinal plants: Aconitum heterophyllum and Polygonum bistorta. Asian Pac. J. Trop. Biomed. 2014, 4, S639–S643. [Google Scholar] [CrossRef]
- Shoieb, A.M.; Elgayyar, M.; Dudrick, P.S.; Bell, J.L.; Tithof, P.K. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int. J. Oncol. 2003, 22, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Zhao, X.; Qian, Y.; Wang, Q. Antioxidant and anticancer activities of methanolic extract of Trollius chinensis Bunge. Afr. J. Pharm. Pharmacol. 2013, 7, 1015–1019. [Google Scholar] [CrossRef][Green Version]
- Wang, J.L.; Liu, K.; Gong, W.Z.; Wang, Q.; Xu, D.T.; Liu, M.F.; Bi, K.L.; Song, Y.F. Anticancer, antioxidant, and antimicrobial activities of anemone (Anemone cathayensis). Food Sci. Biotechnol. 2012, 21, 551–557. [Google Scholar] [CrossRef]
- Yin, T.; Cai, L.; Ding, Z. A systematic review on the chemical constituents of the genus Consolida (Ranunculaceae) and their biological activities. RSC Adv. 2020, 10, 35072–35089. [Google Scholar] [CrossRef] [PubMed]
- Castano Osorio, J.C.; Giraldo Garcia, A.M. Antiparasitic phytotherapy perspectives, scope and current development. Infectio 2019, 23, 189–204. [Google Scholar] [CrossRef]
- Mishra, B.B.; Singh, R.K.; Srivastava, A.; Tripathi, V.J.; Tiwari, V.K. Fighting against Leishmaniasis: Search of alkaloids as future true potential anti-Leishmanial agents. Mini Rev. Med. Chem. 2009, 9, 107–123. [Google Scholar] [CrossRef]
- Marin, C.; Diaz, J.G.; Maiques, D.I.; Ramirez-Macias, I.; Rosales, M.J.; Guitierrez-Sanchez, R.; Canas, R.; Sanchez-Moreno, M. Antitrypanosomatid activity of flavonoid glycosides isolated from Delphinium gracile, D. staphisagria, Consolida oliveriana and from Aconitum napellus subsp. lusitanicum. Phytochem. Lett. 2017, 19, 196–209. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Swiętek, M.; Lu, Y.C.; Konefal, R.; Ferreira, L.P.; Cruz, M.M.; Ma, Y.H.; Horak, D. Scavenging of reactive oxygen species by phenolic compound-modified maghemite nanoparticles. Beilstein J. Nanotechnol. 2019, 20, 1073–1088. [Google Scholar] [CrossRef]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U. Antioxidants and human diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef]
- Taniyama, Y.; Griendling, K.K. Reactive oxygen species in the vasculature. Hypertension 2003, 42, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 20, e00370. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Rondevaldova, J.; Tauchen, J.; Mascellani, A.; Tulkova, J.; Magdalita, P.M.; Tulin, E.E.; Kokoska, L. Antioxidant activity and total phenolic content of underutilized edible tree species of the Philippines. Horticulturae 2024, 10, 1051. [Google Scholar] [CrossRef]
- Goo, Y.-K. Therapeutic potential of Ranunculus species (Ranunculaceae): A literature review on traditional medicinal herbs. Plants 2022, 11, 1599. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Mariani, C.; Braca, A.; Vitalini, S.; De Tommasi, N.; Visioli, F.; Fico, G. Flavonoid characterization and in vitro antioxidant activity of Aconitum anthora L. (Ranunculaceae). Phytochemistry 2008, 69, 1220–1226. [Google Scholar] [CrossRef]
- Fico, G.; Braca, A.; Bilia, A.R.; Tome, F.; Morelli, I. New flavonol glycosides from the flowers of Aconitum napellus ssp. tauricum. Planta Medica 2001, 67, 287–290. [Google Scholar] [CrossRef]
- Braca, A.; Fico, G.; Morelli, I.; De Simone, F.; Tome, F.; De Tommasi, N. Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species. J. Ethnopharmacol. 2003, 86, 63–67. [Google Scholar] [CrossRef]
- Malik, J.; Tauchen, J.; Landa, P.; Kutil, Z.; Marsik, P.; Kloucek, P.; Havlik, J.; Kokoska, L. In vitro antiinflammatory and antioxidant potential of root extracts from Ranunculaceae species. S. Afr. J. Bot. 2017, 109, 128–137. [Google Scholar] [CrossRef]
- Sutan, N.A.; Manolescu, D.S.; Fiarescu, I.; Neblea, A.M.; Sutan, C.; Ducu, C.; Soare, L.C.; Negrea, D.; Avramescu, S.M.; Fiarescu, R.C. Phytosynthesis of gold and silver nanoparticles enhance in vitro antioxidant and mitostimulatory activity of Aconitum toxicum Reichenb. rhizomes alcoholic extracts. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 93, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Braca, A.; Passarella, D.; Fico, G. New flavonol glycosides from Aconitum burnatii Gayer and Aconitum variegatum L. Fitoterapia 2010, 81, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Whang, W.K.; Kim, I.H. New flavonoids from the aerial parts of Aconitum chiisanense. Planta Med. 1997, 63, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Fico, G.; Braca, A.; De Tommasi, N.; Tome, F.; Morelli, I. Flavonoids from Aconitum napellus subsp. neomontanum. Phytochemistry 2001, 57, 543–546. [Google Scholar] [CrossRef]
- Neag, T.; Toma, C.C.; Olah, N.; Ardelean, A. Polyphenols profile and antioxidant activity of some Romanian Ranunculus species. Stud. Univ. Babes-Bolyai Chem. 2017, 62, 75–88. [Google Scholar] [CrossRef]
- Khan, M.Z.; Jan, S.; Khan, F.U.; Noor, W.; Khan, Y.M.; Shah, A.; Chaudhary, M.I.; Ali, F.; Khan, K.; Ullah, W.; et al. Phytochemical screening and biological activities of Ranunculus arvensis. Int. J. Biosci. 2017, 11, 15–21. [Google Scholar]
- Rui, W.; Chen, H.; Tan, Y.; Zhong, Y.; Feng, Y. Rapid analysis of the main components of the total glycosides of Ranunculus japonicus by UPLC/Q-TOF-MS. Nat. Prod. Commun. 2010, 5, 783–788. [Google Scholar] [CrossRef]
- Deng, K.Z.; Xiong, Y.; Zhou, B.; Guan, Y.M.; Luo, Y.M. Chemical constituents from the roots of Ranunculus ternatus and their inhibitory effects on Mycobacterium tuberculosis. Molecules 2013, 18, 11859–11865. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Tian, J.K. Two new indolopyridoquinazoline alkaloidal glycosides from Ranunculus ternatus. Chem. Pharm. Bull. 2007, 55, 1267–1269. [Google Scholar] [CrossRef]
- Kaya, G.I.; Somer, N.U.; Konyalioglu, S.; Yalcin, H.T.; Yavaşoglu, N.U.K.; Sarikaya, B.; Onur, M.A. Antioxidant and antibacterial activities of Ranunculus marginatus var. trachycarpus and R. sprunerianus. Turk. J. Biol. 2010, 34, 139–146. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Medicinal plants: Factors of influence on the content of secondary metabolites. Química Nova 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Hrichi, S.; Chaabane-Banaoues, R.; Giuffrida, D.; Mangraviti, D.; Oulad El Majdoub, Y.; Rigano, F.; Mondello, L.; Babba, H.; Mighri, Z.; Cacciola, F. Effect of seasonal variation on the chemical composition and antioxidant and antifungal activities of Convolvulus althaeoides L. leaf extracts. Arab. J. Chem. 2020, 13, 5651–5668. [Google Scholar] [CrossRef]
- Sun, Y.X.; Liu, J.C.; Liu, D.Y. Phytochemicals and bioactivities of Anemone raddeana Regel: A review. Pharmazie 2011, 66, 813–821. [Google Scholar] [PubMed]
- Pei, C.; Fenge, W.; Lisheng, D. Advances in the studies on the chemical constituents and biologic activities for Anemone species. Nat. Prod. Res. Dev. 2004, 16, 581–584. [Google Scholar]
- Han, L.-T.; Li, J.; Huang, F.; Yu, S.-G.; Fang, N.-B. Triterpenoid saponins from Anemone flaccida induce apoptosis activity in HeLa cells. J. Asian Natl. Prod. Res. 2009, 11, 122–127. [Google Scholar] [CrossRef]
- Han, L.-T.; Fang, Y.; Li, M.M.; Yang, H.B.; Huang, F. The antitumor effects of triterpenoid saponins from the Anemone flaccida and the underlying mechanism. Evid. Based Complement. Altern. Med. 2013, 2013, 517931. [Google Scholar] [CrossRef]
- Luan, X.; Guan, Y.; Wang, C.; Zhao, M.; Lu, Q.; Tang, Y.; Liu, Y.; Yu, D.; Wang, X.; Qi, H.; et al. Determination of Raddeanin A in rat plasma by liquid chromatography–tandem mass spectrometry: Application to a pharmacokinetic study. J. Chromatogr. B 2013, 923–924, 43–47. [Google Scholar] [CrossRef]
- Naz, I.; Ramchandani, S.; Khan, M.R.; Yang, M.H.; Ahn, K.S. Anticancer potential of Raddeanin A, a natural triterpenoid isolated from Anemone raddeana Regel. Molecules 2020, 25, 1035. [Google Scholar] [CrossRef]
- Xue, G.; Zou, X.; Zhou, J.-Y.; Sun, W.; Wu, J.; Xu, J.; Wang, R.-P. Raddeanin A induces human gastric cancer cells apoptosis and inhibits their invasion in vitro. Biochem. Biophys. Res. Comm. 2013, 439, 196–202. [Google Scholar] [CrossRef][Green Version]
- Guan, Y.Y.; Liu, H.-J.; Luan, X.; Xu, J.-R.; Lu, Q.; Liu, Y.-R.; Gao, Y.-G.; Zhao, M.; Chen, H.-Z.; Fang, C. Raddeanin A, a triterpenoid saponin isolated from Anemone raddeana, suppresses the angiogenesis and growth of human colorectal tumor by inhibiting VEGFR2 signaling. Phytomedicine 2015, 22, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Yu, Y.; Zhang, Y.-F.; Li, Z.-M.; Cai, G.-Z.; Gong, J.-Y. Synergy of Raddeanin A and cisplatin induced therapeutic effect enhancement in human hepatocellular carcinoma. Biochem. Biophys. Res. Comm. 2017, 485, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mo, J.; Zhao, C.; Huang, K.; Feng, M.; He, W.; Wang, J.; Chen, S.; Xie, Z.; Ma, J.; et al. Raddeanin A suppresses breast cancer-associated osteolysis through inhibiting osteoclasts and breast cancer cells. Cell Death Dis. 2018, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Chouhan, R.; Sultan, P.; Hassan, Q.P.; Gandhi, S.G. A comprehensive review of phytochemistry, pharmacology and toxicology of the genus Aconitum L. Adv. Trad. Med. 2023, 23, 299–320. [Google Scholar] [CrossRef]
- Mi, L.; Li, Y.; Sun, M.; Zhang, P.; Li, Y.; Yang, H. A systematic review of pharmacological activities, toxicological mechanisms and pharmacokinetic studies on Aconitum alkaloids. Chin. J. Nat. Med. 2021, 19, 505–520. [Google Scholar] [CrossRef]
- Chan, Y.-T.; Wang, N.; Feng, Y. The toxicology and detoxification of Aconitum: Traditional and modern views. Chin. Med. 2021, 16, 61. [Google Scholar] [CrossRef]
- WHO. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 16 September 2024).
- No, J.H. Visceral leishmaniasis: Revisiting current treatments and approaches for future discoveries. Acta Trop. 2016, 155, 113–123. [Google Scholar] [CrossRef]
- Jabbar, E.A.K.; AL-Aboody, B.A.; Jarullah, B.A.; Noori, N. Isolation and molecular diagnosis of Leishmania major and study activity of aqueous extract of plant Nigella sativa against the parasite in vitro. Int. J. Pharm. Qual. Assur. 2019, 10, 47–50. [Google Scholar]
- Al-Turkmani, M.O.; Mokrani, L.; Soukkarieh, C. Antileishmanial apoptotic activity of Nigella sativa L. essential oil and thymoquinone triggers on Leishmania tropica. Indian J. Exp. Biol. 2020, 58, 699–705. [Google Scholar]
- Bafghi, A.F.; Vahidi, A.R.; Anvari, M.H.; Barzegar, K.; Ghafourzadeh, M. The in vivo antileishmanial activity of alcoholic extract from Nigella sativa seeds. Afr. J. Microbiol. Res. 2011, 5, 1504–1510. [Google Scholar]
- Bapela, M.J.; Kaiser, M.; Meyer, J.J.M. Antileishmanial activity of selected South African plant species. S. Afr. J. Bot. 2017, 108, 342–345. [Google Scholar] [CrossRef]
- Ramírez-Macias, I.; Marin, C.; Diaz, J.G.; Rosales, M.J.; Gutierrez-Sanchez, R.; Sanchez-Moreno, M. Leishmanicidal activity of nine novel flavonoids from Delphinium staphisagria. Sci. World J. 2012, 2012, 203646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shyaula, S.L.; Tamang, T.; Ghouri, N.; Adhikari, A.; Marasini, S.; Bajracharya, G.B.; Manandhar, M.D.; Choudhary, M.I. Antileishmanial diterpenoid alkaloids from Aconitum spicatum (Bruhl) Stapf. Nat. Prod. Res. 2016, 30, 2590–2593. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Singh, J.; Singh, V.K.; Agrawal, N.; Kumar, R. Current and emerging therapies for the treatment of leishmaniasis. Expert Opin. Orphan Drugs 2024, 12, 19–32. [Google Scholar] [CrossRef]
- Wijnant, G.; Dumetz, F.; Dirkx, L.; Bulte, D.; Cuypers, B.; Van Bocxlaer, K.; Hendrickx, S. Tackling drug resistance and other causes of treatment failure in Leishmaniasis. Front. Trop. Dis. 2022, 3, 837460. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B.; Chen, Y.; Wang, Q.; Ahmed, A.F.; Zhang, Y.; Kang, W. The immunomodulatory effects of active ingredients from Nigella sativa in RAW264.7 cells through NF-κB/MAPK signaling pathways. Front. Nutr. 2022, 9, 899797. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-derived nutraceuticals and immune system modulation: An evidence-based overview. Vaccines 2020, 8, 468. [Google Scholar] [CrossRef]
- Costa-da-Silva, A.C.; Nascimento, D.d.O.; Ferreira, J.R.M.; Guimaraes-Pinto, K.; Freire-de-Lima, L.; Morrot, A.; Decote-Ricardo, D.; Filardy, A.A.; Freire-de-Lima, C.G. Immune responses in Leishmaniasis: An overview. Trop. Med. Infect. Dis. 2022, 7, 54. [Google Scholar] [CrossRef]
- Cedillo-Cortezano, M.; Martinez-Cuevas, L.R.; López, J.A.M.; Barrera López, I.L.; Escutia-Perez, S.; Petricevich, V.L. Use of medicinal plants in the process of wound healing: A literature review. Pharmaceuticals 2024, 17, 303. [Google Scholar] [CrossRef]
- Herrmann, F.; Romero, M.R.; Blazquez, A.G.; Kaufmann, D.; Ashour, M.L.; Kahl, S.; Marin, J.J.; Efferth, T.; Wink, M. Diversity of pharmacological properties in Chinese and European medicinal plants: Cytotoxicity, antiviral and antitrypanosomal screening of 82 herbal drugs. Diversity 2011, 3, 547–580. [Google Scholar] [CrossRef]
- Kou, X.; Kirberger, M.; Yang, Y.; Chen, N. Natural products for cancer prevention associated with Nrf2–ARE pathway. Food Sci. Hum. Wellness 2013, 2, 22–28. [Google Scholar] [CrossRef]
- Gerhauser, C.; Klimo, K.; Heiss, E.; Neumann, I.; Gamal-Eldeen, A.; Knauft, J.; Liu, G.Y.; Sitthimonchai, S.; Frank, N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat. Res. 2003, 523, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Hensley, K.; Robinson, K.A.; Gabbita, S.P.; Salsman, S.; Floyd, R.A. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Tarlovsky, V. Role of antioxidants in cancer therapy. Nutrition 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Das, L.; Vinayak, M. Long term effect of curcumin in restoration of tumour suppressor p53 and phase-II antioxidant enzymes via activation of Nrf2 signaling and modulation of inflammation in prevention of cancer. PLoS ONE 2015, 10, e0124000. [Google Scholar] [CrossRef]
- Ji, C.C.; Tang, H.F.; Hu, Y.Y.; Zhang, Y.; Zheng, M.H.; Qin, H.Y.; Li, S.Z.; Wang, X.Y.; Fei, Z.; Cheng, G. Saponin 6 derived from Anemone taipaiensis induces U87 human malignant glioblastoma cell apoptosis via regulation of Fas and Bcl 2 family proteins. Mol. Med. Rep. 2016, 14, 380–386. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Chen, W.; Jiao, Y.; Hou, J.; Wu, Q.; Lu, Y.; Qi, X. Pulsatilla saponin A, an active molecule from Pulsatilla chinensis, induces cancer cell death and inhibits tumor growth in mouse xenograft models. J. Surg. Res. 2014, 188, 387–395. [Google Scholar] [CrossRef]
- Borcsa, B.; Fodor, L.; Csupor, D.; Forgo, P.; Molnar, A.; Hohmann, J. Diterpene alkaloids from the roots of Aconitum moldavicum and assessment of Nav 1.2 sodium channel activity of aconitum alkaloids. Planta Medica 2014, 80, 231–236. [Google Scholar] [CrossRef]
- Mubashir, S.; Dar, M.Y.; Lone, B.A.; Zargar, M.I.; Shah, W.A. Anthelmintic, antimicrobial, antioxidant and cytotoxic activity of Caltha palustris var. alba Kashmir, India. Chin. J. Nat. Med. 2014, 12, 567–572. [Google Scholar] [CrossRef]
- Sanchez-Villamil, J.P.; Bautista-Nino, P.K.; Serrano, N.C.; Rincon, M.Y.; Garg, N.J. Potential role of antioxidants as adjunctive therapy in Chagas disease. Oxid. Med. Cell. Longev. 2020, 2020, 9081813. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.S.; Wilkinson, S.R. Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob. Agents Chemother. 2012, 56, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, S.; Foth, B.J.; Kelner, A.; Sokolova, A.Y.; Berriman, M.; Fairlamb, A.H. Nitroheterocyclic drug resistance mechanisms in Trypanosoma brucei. J. Antimicrob. Chemother. 2016, 71, 625–634. [Google Scholar] [CrossRef]
- Wyllie, S.; Roberts, A.J.; Norval, S.; Patterson, S.; Foth, B.J.; Berriman, M.; Read, K.D.; Fairlamb, A.H. Activation of bicyclic nitro-drugs by a novel nitroreductase (NTR2) in Leishmania. PLoS Pathog. 2016, 12, e1005971. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D.; Kolarich, D.; Binder, M.; Stadlmann, J.; Altmann, F.; Duchene, M. Trichomonas vaginalis: Metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system: Implications for nitroimidazole toxicity and resistance. Mol. Microbiol. 2009, 72, 518–536. [Google Scholar] [CrossRef]
- Howard, H.K.; Pharoah, M.M.; Ashall, F.; Miles, M.A. Human urine stimulates growth of Leishmania in vitro. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 477–479. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Bagirova, M.; Elcicek, S.; Koc, R.C.; Oztel, O.N. Effect of human urine on cell cycle and infectivity of Leismania species promastigotes in vitro. Am. J. Trop. Med. Hyg. 2011, 85, 639–643. [Google Scholar] [CrossRef]
- Hirumi, H.; Hirumi, K. Axenic culture of African trypanosome bloodstream forms. Parasitol. Today 1994, 10, 80–84. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agr. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Tauchen, J.; Huml, L.; Bortl, L.; Doskocil, I.; Jarosova, V.; Marsik, P.; Frankova, A.; Clavo Peralta, Z.M.; Chuspe Zans, M.E.; Havlik, J.; et al. Screening of medicinal plants traditionally used in Peruvian Amazon for in vitro antioxidant and anticancer potential. Nat. Prod. Res. 2019, 33, 2718–2721. [Google Scholar] [CrossRef]
- Rondevaldova, J.; Novy, P.; Tauchen, J.; Drabek, O.; Kotikova, Z.; Dajcl, J.; Mascellani, A.; Chrun, R.; Nguon, S.; Kokoska, L. Determination of antioxidants, minerals and vitamins in Cambodian underutilized fruits and vegetables. J. Food Meas. Charact. 2023, 17, 716–731. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zoltner, M.; Leung, K.F.; Scullion, P.; Hutchinson, S.; Del Pino, R.C.; Vincent, I.M.; Zhang, Y.K.; Freund, Y.R.; Alley, M.R.; et al. Host-parasite co-metabolic activation of antitrypanosomal aminomethyl-benzoxaboroles. PLoS Pathog. 2018, 14, e1006850. [Google Scholar] [CrossRef] [PubMed]
- Zahedifard, F.; Bansal, M.; Sharma, N.; Kumar, S.; Shen, S.; Singh, P.; Rathi, B.; Zoltner, M. Phenotypic screening reveals a highly selective phthalimide-based compound with antileishmanial activity. PLoS Negl. Trop. Dis. 2024, 18, e0012050. [Google Scholar] [CrossRef]
- Jain, S.K.; Sahu, R.; Walker, L.A.; Tekwani, B.L. A parasite rescue and transformation assay for antileishmanial screening against intracellular Leishmania donovani amastigotes in THP1 human acute monocytic leukemia cell line. J. Vis. Exp. 2012, 70, e4054. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).