Induced Pluripotent Stem Cell-Derived Cardiomyocytes Therapy for Ischemic Heart Disease in Animal Model: A Meta-Analysis
Abstract
1. Introduction
2. Results
2.1. Search Results
2.1.1. Characteristics of Included Studies
2.1.2. Intervention Characteristics
2.1.3. Risk of Bias Assessment of Included Studies
2.2. Safety of iPSC-CM Treatment
2.2.1. Mortality
2.2.2. Arrhythmia
2.3. Efficacy of iPSC-CM Treatment
2.3.1. Ejection Fraction (EF)
2.3.2. Fractional Shortening (FS)
2.3.3. Other Cardiac Outcomes
2.3.4. Subgroup Analyses
2.3.5. Meta-Regression
3. Discussion
4. Materials and Methods
4.1. Meta-Analyses
4.1.1. Search Strategy
4.1.2. Inclusion and Exclusion Criteria
4.2. Outcome Definition
4.3. Data Extraction
4.4. Quality Assessment
4.5. Statistical Analyses
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACEi | Angiotensin-converting enzyme inhibitor |
| AE | Adverse event |
| ARB | Angiotensin receptor blockers; |
| CI | Confidence interval |
| EDV | End diastolic volume |
| ESC | Embryonic stem cells |
| ESV | End systolic volume |
| EF | Ejection fraction |
| FAC | Fractional area change |
| FS | Fractional shortening |
| hiPSC | Human induced pluripotent stem cell |
| IHD | Ischemic heart disease |
| iPSC | Induced pluripotent stem cell |
| LVEF | Left ventricular ejection fraction |
| MD | Mean difference |
| MRI | Magnetic resonance imaging |
| MSC | Mesenchymal stem cell |
| OR | Odds ratio |
| PSC | Pluripotent stem cells |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SYRCLE | Systematic Review Centre for Laboratory animal Experimentation |
| SMD | Standardized mean difference |
| RCTs | Randomized controlled trials |
| WMD | Weighted mean difference |
| WT | Wall thickness |
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Bittner, V.; Colantonio, L.D.; Deng, L.; Farkouh, M.E.; Limdi, N.; Monda, K.L.; Rosenson, R.S.; Serban, M.-C.; Somaratne, R.M.; et al. Residual risk for coronary heart disease events and mortality despite intensive medical management after myocardial infarction. J. Clin. Lipidol. 2020, 14, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Omole, A.E.; Fakoya, A.O.J. Ten years of progress and promise of induced pluripotent stem cells: Historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ 2018, 6, e4370. [Google Scholar] [CrossRef]
- Suzuki, H.; Shibata, R.; Kito, T.; Yamamoto, T.; Ishii, M.; Nishio, N.; Ito, S.; Isobe, K.-I.; Murohara, T. Comparative Angiogenic Activities of Induced Pluripotent Stem Cells Derived from Young and Old Mice. PLoS ONE 2012, 7, e39562. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Chen, M.; Yang, B.; Zhang, F.; Cao, K. Intramyocardial Transplantation of Undifferentiated Rat Induced Pluripotent Stem Cells Causes Tumorigenesis in the Heart. PLoS ONE 2011, 6, e19012. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xu, W.; Zhang, H.; Wang, Q.; Yu, J.; Zhang, R.; Chen, Y.; Xia, Y.; Wang, J.; Wang, D. Transplantation of human induced pluripotent stem cell-derived cardiomyocytes improves myocardial function and reverses ventricular remodeling in infarcted rat hearts. Stem Cell Res. Ther. 2020, 11, 73. [Google Scholar] [CrossRef]
- Biagi, D.; Fantozzi, E.T.; Campos-Oliveira, J.C.; Naghetini, M.V.; Ribeiro, A.F.; Rodrigues, S.; Ogusuku, I.; Vanderlinde, R.; Christie, M.L.A.; Mello, D.B.; et al. In Situ Maturated Early-Stage Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Improve Cardiac Function by Enhancing Segmental Contraction in Infarcted Rats. J. Pers. Med. 2021, 11, 374. [Google Scholar] [CrossRef]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, e30–e41. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Stuckey, D.J.; Kidher, E.; Rocco, M.; Jabbour, R.J.; Mansfield, C.A.; Darzi, A.; Harding, S.E.; Stevens, M.M.; Athanasiou, T. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem Cell Rep. 2017, 9, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Minami, I.; Shiozaki, M.; Yu, L.; Yajima, S.; Miyagawa, S.; Shiba, Y.; Morone, N.; Fukushima, S.; Yoshioka, M.; et al. Human Pluripotent Stem Cell-Derived Cardiac Tissue-like Constructs for Repairing the Infarcted Myocardium. Stem Cell Rep. 2017, 9, 1546–1559. [Google Scholar] [CrossRef]
- Miki, K.; Uenaka, H.; Saito, A.; Miyagawa, S.; Sakaguchi, T.; Higuchi, T.; Shimizu, T.; Okano, T.; Yamanaka, S.; Sawa, Y. Bioengineered Myocardium Derived from Induced Pluripotent Stem Cells Improves Cardiac Function and Attenuates Cardiac Remodeling Following Chronic Myocardial Infarction in Rats. Stem Cells Transl. Med. 2012, 1, 430–437. [Google Scholar] [CrossRef]
- Citro, L.; Naidu, S.; Hassan, F.; Kuppusamy, M.L.; Kuppusamy, P.; Angelos, M.G.; Khan, M. Comparison of Human Induced Pluripotent Stem-Cell Derived Cardiomyocytes with Human Mesenchymal Stem Cells following Acute Myocardial Infarction. PLoS ONE 2014, 9, e116281. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Miyagawa, S.; Pearson, J.T.; Fukushima, S.; Saito, A.; Tsuchimochi, H.; Sonobe, T.; Fujii, Y.; Yagi, N.; Astolfo, A.; et al. Functional and Electrical Integration of Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Myocardial Infarction Rat Heart. Cell Transpl. 2015, 24, 2479–2489. [Google Scholar] [CrossRef]
- Ong, S.-G.; Huber, B.C.; Lee, W.H.; Kodo, K.; Ebert, A.D.; Ma, Y.; Nguyen, P.K.; Diecke, S.; Chen, W.-Y.; Wu, J.C.; et al. Microfluidic Single-Cell Analysis of Transplanted Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes After Acute Myocardial Infarction. Circulation 2015, 132, 762–771. [Google Scholar] [CrossRef]
- Winters, A.A.; Bou-Ghannam, S.; Thorp, H.; Hawayek, J.A.; Atkinson, D.L.; Bartlett, C.E.; Silva, F.J.; Hsu, E.W.; Moreno, A.P.; Grainger, D.A.; et al. Evaluation of Multiple Biological Therapies for Ischemic Cardiac Disease. Cell Transpl. 2016, 25, 1591–1607. [Google Scholar] [CrossRef]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K.; et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388–391. [Google Scholar] [CrossRef]
- Lee, W.H.; Chen, W.-Y.; Shao, N.-Y.; Xiao, D.; Qin, X.; Baker, N.; Bae, H.R.; Wei, T.-T.; Wang, Y.; Shukla, P.; et al. Comparison of Non-Coding RNAs in Exosomes and Functional Efficacy of Human Embryonic Stem Cell- versus Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cells 2017, 35, 2138–2149. [Google Scholar] [CrossRef]
- Ishida, M.; Miyagawa, S.; Saito, A.; Fukushima, S.; Harada, A.; Ito, E.; Ohashi, F.; Watabe, T.; Hatazawa, J.; Matsuura, K.; et al. Transplantation of Human-induced Pluripotent Stem Cell-derived Cardiomyocytes Is Superior to Somatic Stem Cell Therapy for Restoring Cardiac Function and Oxygen Consumption in a Porcine Model of Myocardial Infarction. Transplantation 2019, 103, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, Z.; Dong, M. Cardiac repair in a murine model of myocardial infarction with human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2020, 11, 297. [Google Scholar] [CrossRef]
- Samura, T.; Miyagawa, S.; Kawamura, T.; Fukushima, S.; Yokoyama, J.; Takeda, M.; Harada, A.; Ohashi, F.; Sato-Nishiuchi, R.; Toyofuku, T.; et al. Laminin-221 Enhances Therapeutic Effects of Human-Induced Pluripotent Stem Cell–Derived 3-Dimensional Engineered Cardiac Tissue Transplantation in a Rat Ischemic Cardiomyopathy Model. J. Am. Heart Assoc. 2020, 9, e015841. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, J.; Qiang, B.; Romagnuolo, R.; Gagliardi, M.; Keller, G.; Laflamme, M.A.; Li, R.-K.; Nunes, S.S. Transplanted microvessels improve pluripotent stem cell–derived cardiomyocyte engraftment and cardiac function after infarction in rats. Sci. Transl. Med. 2020, 12, eaax2992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Nakada, Y.; Wei, Y.; Bian, W.; Chu, Y.; Borovjagin, A.V.; Xie, M.; Zhu, W.; Nguyen, T.; Zhou, Y.; et al. Cyclin D2 Overexpression Enhances the Efficacy of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes for Myocardial Repair in a Swine Model of Myocardial Infarction. Circulation 2021, 144, 210–228. [Google Scholar] [CrossRef]
- Sun, S.-J.; Lai, W.-H.; Jiang, Y.; Zhen, Z.; Wei, R.; Lian, Q.; Liao, S.-Y.; Tse, H.-F. Immunomodulation by systemic administration of human-induced pluripotent stem cell-derived mesenchymal stromal cells to enhance the therapeutic efficacy of cell-based therapy for treatment of myocardial infarction. Theranostics 2021, 11, 1641–1654. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, H.; Dong, X.; Wang, C.; Na, J.; Zhou, J.; Wang, C. Transferrin improved the generation of cardiomyocyte from human pluripotent stem cells for myocardial infarction repair. Histochem. J. 2021, 52, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, W.; Yu, B.; Yan, X.; Lin, Y.; Zhan, J.; Chen, P.; Song, X.; Yang, P.; Cai, Y. Supramolecular Assemblies of Glycopeptides Enhance Gap Junction Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes via Inducing Spheroids Formation to Optimize Cardiac Repair. Adv. Health Mater. 2023, 12, e2300696. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, S.-J.; Zhen, Z.; Wei, R.; Zhang, N.; Liao, S.-Y.; Tse, H.-F. Myocardial repair of bioengineered cardiac patches with decellularized placental scaffold and human-induced pluripotent stem cells in a rat model of myocardial infarction. Stem Cell Res. Ther. 2021, 12, 13. [Google Scholar] [CrossRef]
- Li, H.; Wang, T.; Feng, Y.; Sun, K.; Huang, G.; Cao, Y.; Xu, A. Optimal transplantation strategy using human induced pluripotent stem cell-derived cardiomyocytes for acute myocardial infarction in nonhuman primates. Medcomm 2023, 4, e289. [Google Scholar] [CrossRef]
- Jabbour, R.J.; Owen, T.J.; Pandey, P.; Reinsch, M.; Wang, B.; King, O.; Couch, L.S.; Pantou, D.; Pitcher, D.S.; Chowdhury, R.A.; et al. In vivo grafting of large engineered heart tissue patches for cardiac repair. J. Clin. Investig. 2021, 6, e144068. [Google Scholar] [CrossRef] [PubMed]
- Mattapally, S.; Zhu, W.; Fast, V.G.; Gao, L.; Worley, C.; Kannappan, R.; Borovjagin, A.V.; Zhang, J. Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice. Am. J. Physiol. Circ. Physiol. 2018, 315, H327–H339. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, S.; Pan, J.; Zhang, K.; Li, X.; Xu, Y.; Jin, C.; He, X.; Shi, J.; Ma, L.; et al. CRISPR/Cas9-edited triple-fusion reporter gene imaging of dynamics and function of transplanted human urinary-induced pluripotent stem cell-derived cardiomyocytes. Eur. J. Nucl. Med. 2021, 48, 708–720. [Google Scholar] [CrossRef]
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012, 126 (Suppl. S1), S29–S37. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, A.; Santoso, M.R.; Mahmoudi, M.; Shukla, P.; Wang, L.; Bennett, M.; Goldstone, A.B.; Wang, M.; Fukushi, M.; Ebert, A.D.; et al. Paracrine Effects of the Pluripotent Stem Cell-Derived Cardiac Myocytes Salvage the Injured Myocardium. Circ. Res. 2017, 121, e22–e36. [Google Scholar] [CrossRef]
- Fan, C.; Fast, V.G.; Tang, Y.; Zhao, M.; Turner, J.F.; Krishnamurthy, P.; Rogers, J.M.; Valarmathi, M.T.; Yang, J.; Zhu, W.; et al. Cardiomyocytes from CCND2-overexpressing human induced-pluripotent stem cells repopulate the myocardial scar in mice: A 6-month study. J. Mol. Cell Cardiol. 2019, 137, 25–33. [Google Scholar] [CrossRef]
- Fan, C.; Tang, Y.; Zhao, M.; Lou, X.; Pretorius, D.; Menasche, P.; Zhu, W.; Zhang, J. CHIR99021 and fibroblast growth factor 1 enhance the regenerative potency of human cardiac muscle patch after myocardial infarction in mice. J. Mol. Cell Cardiol. 2020, 141, 1–10. [Google Scholar] [CrossRef]
- Li, H.; Yu, B.; Yang, P.; Zhan, J.; Fan, X.; Chen, P.; Liao, X.; Ou, C.; Cai, Y.; Chen, M. Injectable AuNP-HA matrix with localized stiffness enhances the formation of gap junction in engrafted human induced pluripotent stem cell-derived cardiomyocytes and promotes cardiac repair. Biomaterials 2021, 279, 121231. [Google Scholar] [CrossRef]
- Stępniewski, J.; Tomczyk, M.; Andrysiak, K.; Kraszewska, I.; Martyniak, A.; Langrzyk, A.; Kulik, K.; Wiśniewska, E.; Jeż, M.; Florczyk-Soluch, U.; et al. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes, in Contrast to Adipose Tissue-Derived Stromal Cells, Efficiently Improve Heart Function in Murine Model of Myocardial Infarction. Biomedicines 2020, 8, 578. [Google Scholar] [CrossRef]
- Yokoyama, J.; Miyagawa, S.; Akagi, T.; Akashi, M.; Sawa, Y. Human induced pluripotent stem cell-derived three-dimensional cardiomyocyte tissues ameliorate the rat ischemic myocardium by remodeling the extracellular matrix and cardiac protein phenotype. PLoS ONE 2021, 16, e0245571. [Google Scholar] [CrossRef]
- Neef, K.; Drey, F.; Lepperhof, V.; Wahlers, T.; Hescheler, J.; Choi, Y.H.; Šarić, T. Co-transplantation of Mesenchymal Stromal Cells and Induced Pluripotent Stem Cell-Derived Cardiomyocytes Improves Cardiac Function After Myocardial Damage. Front. Cardiovasc. Med. 2021, 8, 794690. [Google Scholar] [CrossRef] [PubMed]
- Ja, K.M.M.; Miao, Q.; Tee, N.G.Z.; Lim, S.Y.; Nandihalli, M.; Ramachandra, C.J.; Mehta, A.; Shim, W. iPSC-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium. J. Cell Mol. Med. 2016, 20, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Saludas, L.; Garbayo, E.; Mazo, M.; Pelacho, B.; Abizanda, G.; Iglesias-Garcia, O.; Raya, A.; Prósper, F.; Blanco-Prieto, M.J. Long-Term Engraftment of Human Cardiomyocytes Combined with Biodegradable Microparticles Induces Heart Repair. J. Pharmacol. Exp. Ther. 2019, 370, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, J.; Zhang, P.; Xiong, Q.; Wu, S.C.; Xia, L.; Roy, S.S.; Tolar, J.; O’Connell, T.D.; Kyba, M.; et al. Derivation and high engraftment of patient-specific cardiomyocyte sheet using induced pluripotent stem cells generated from adult cardiac fibroblast. Circ. Heart Fail. 2015, 8, 156–166. [Google Scholar] [CrossRef]
- Iglesias-Garcia, O.; Baumgartner, S.; Macri-Pellizzeri, L.; Rodriguez-Madoz, J.R.; Abizanda, G.; Guruceaga, E.; Albiasu, E.; Corbacho, D.; Benavides-Vallve, C.; Soriano-Navarro, M.; et al. Neuregulin-1β induces mature ventricular cardiac differentiation from induced pluripotent stem cells contributing to cardiac tissue repair. Stem Cells Dev. 2015, 24, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Liang, J.; Huang, W.; Guo, L.; Cai, W.; Wang, L.; Paul, C.; Yang, H.T.; Kim, H.W.; Wang, Y. Electrical Stimulation Enhances Cardiac Differentiation of Human Induced Pluripotent Stem Cells for Myocardial Infarction Therapy. Antioxid. Redox Signal 2018, 28, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.V.; Kensah, G.; Rotaermel, A.; Baraki, H.; Kutschka, I.; Zweigerdt, R.; Martin, U.; Haverich, A.; Gruh, I.; Martens, A. Transplantation of purified iPSC-derived cardiomyocytes in myocardial infarction. PLoS ONE 2017, 12, e0173222. [Google Scholar] [CrossRef]
- Bian, W.; Chen, W.; Nguyen, T.; Zhou, Y.; Zhang, J. miR-199a Overexpression Enhances the Potency of Human Induced-Pluripotent Stem-Cell-Derived Cardiomyocytes for Myocardial Repair. Front. Pharmacol. 2021, 12, 673621. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, M.; Mattapally, S.; Chen, S.; Zhang, J. CCND2 Overexpression Enhances the Regenerative Potency of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Remuscularization of Injured Ventricle. Circ. Res. 2018, 122, 88–96. [Google Scholar] [CrossRef]
- Lou, X.; Zhao, M.; Fan, C.; Fast, V.G.; Valarmathi, M.T.; Zhu, W.; Zhang, J. N-cadherin overexpression enhances the reparative potency of human-induced pluripotent stem cell-derived cardiac myocytes in infarcted mouse hearts. Cardiovasc. Res. 2020, 116, 671–685. [Google Scholar] [CrossRef]
- Qu, X.; Li, J.; Liu, L.; Zhang, J.; Hua, Y.; Suzuki, K.; Harada, A.; Ishida, M.; Yoshida, N.; Okuzaki, D.; et al. ONO-1301 enhances post-transplantation survival of human induced pluripotent stem cell-derived cardiac tissue sheet by promoting angiogenesis. J. Heart Lung Transplant. 2023, 42, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.H.; Wang, S.P.; Gao, Z.H.; Wu, S.N.; Chang, H.Y.; Yang, P.J.; Liu, P.Y.; Liu, Y.W. Efficient Cardiac Differentiation of Human Amniotic Fluid-Derived Stem Cells into Induced Pluripotent Stem Cells and Their Potential Immune Privilege. Int. J. Mol. Sci. 2020, 21, 2359. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, L.L.; Zhang, F.; Bi, W.; Zhang, P.; Yu, X.J.; Rao, S.L.; Wang, S.H.; Li, Q.; Ding, C.; et al. Dual human iPSC-derived cardiac lineage cell-seeding extracellular matrix patches promote regeneration and long-term repair of infarcted hearts. Bioact. Mater. 2023, 28, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Loo, S.; Su, L.; Tan, S.; Tee, G.; Gan, S.U.; Zhang, J.; Chen, X.; Ye, L. Angiopoietin-1 enhanced myocyte mitosis, engraftment, and the reparability of hiPSC-CMs for treatment of myocardial infarction. Cardiovasc. Res. 2021, 117, 1578–1591. [Google Scholar] [CrossRef] [PubMed]
- von Bibra, C.; Shibamiya, A.; Geertz, B.; Querdel, E.; Köhne, M.; Stuedemann, T.; Starbatty, J.; Schmidt, F.N.; Hansen, A.; Hiebl, B.; et al. Human engineered heart tissue transplantation in a guinea pig chronic injury model. J. Mol. Cell Cardiol. 2022, 166, 1–10. [Google Scholar] [CrossRef]
- Munarin, F.; Kant, R.J.; Rupert, C.E.; Khoo, A.; Coulombe, K.L. Engineered human myocardium with local release of angiogenic proteins improves vascularization and cardiac function in injured rat hearts. Biomaterials 2020, 251, 120033. [Google Scholar] [CrossRef]
- Funakoshi, S.; Miki, K.; Takaki, T.; Okubo, C.; Hatani, T.; Chonabayashi, K.; Nishikawa, M.; Takei, I.; Oishi, A.; Narita, M.; et al. Enhanced engraftment, proliferation and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 2016, 6, 19111. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Severino, P.; Mather, P.J.; Pucci, M.; D’amato, A.; Mariani, M.V.; Infusino, F.; Birtolo, L.I.; Maestrini, V.; Mancone, M.; Fedele, F. Advanced Heart Failure and End-Stage Heart Failure: Does a Difference Exist. Diagnostics 2019, 9, 170. [Google Scholar] [CrossRef]
- Hu, S.; Liu, S.; Zheng, Z.; Yuan, X.; Li, L.; Lu, M.; Shen, R.; Duan, F.; Zhang, X.; Li, J.; et al. Isolated Coronary Artery Bypass Graft Combined With Bone Marrow Mononuclear Cells Delivered Through a Graft Vessel for Patients With Previous Myocardial Infarction and Chronic Heart Failure: A Single-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Am. Coll. Cardiol. 2011, 57, 2409–2415. [Google Scholar] [CrossRef]
- Masumoto, H.; Ikuno, T.; Takeda, M.; Fukushima, H.; Marui, A.; Katayama, S.; Shimizu, T.; Ikeda, T.; Okano, T.; Sakata, R.; et al. Human iPS cell-engineered cardiac tissue sheets with cardiomyocytes and vascular cells for cardiac regeneration. Sci. Rep. 2014, 4, 6716. [Google Scholar] [CrossRef] [PubMed]
- La Mantia, D.; Bernardini, C.; Zannoni, A.; Salaroli, R.; Wang, C.; Bencivenni, S.; Forni, M. Efficacy of Stem Cell Therapy in Large Animal Models of Ischemic Cardiomyopathies: A Systematic Review and Meta-Analysis. Animals 2022, 12, 749. [Google Scholar] [CrossRef]
- Zwetsloot, P.P.; Végh, A.M.D.; Jansen of Lorkeers, S.J.; van Hout, G.P.; Currie, G.L.; Sena, E.S.; Gremmels, H.; Buikema, J.W.; Goumans, M.-J.; Macleod, M.R. Cardiac stem cell treatment in myocardial infarction: A systematic review and meta-analysis of preclinical studies. Circ. Res. 2016, 118, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Hau, J.; Schapiro, S.J. (Eds.) Handbook of Laboratory Animal Science: Essential Principles and Practices, 4th ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Manzo, A.; Ootaki, Y.; Ootaki, C.; Kamohara, K.; Fukamachi, K. Comparative study of heart rate variability between healthy human subjects and healthy dogs, rabbits and calves. Lab. Anim. 2009, 43, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Lelovas, P.P.; Kostomitsopoulos, N.G.; Xanthos, T.T. A comparative anatomic and physiologic overview of the porcine heart. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 432–438. [Google Scholar] [PubMed]
- Wollert, K.C.; Meyer, G.P.; Lotz, J.; Ringes-Lichtenberg, S.; Lippolt, P.; Breidenbach, C.; Fichtner, S.; Korte, T.; Hornig, B.; Messinger, D.; et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet 2004, 364, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Meyer, G.P.; Wollert, K.C.; Lotz, J.; Steffens, J.; Lippolt, P.; Fichtner, S.; Hecker, H.; Schaefer, A.; Arseniev, L.; Hertenstein, B.; et al. Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 2006, 113, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, J.; Chen, J.; Luo, R.; He, A.; Xie, X.; Li, J. Optimal temporal delivery of bone marrow mesenchymal stem cells in rats with myocardial infarction. Eur. J. Cardio-Thoracic Surg. 2007, 31, 438–443. [Google Scholar] [CrossRef][Green Version]
- Crisostomo, V.; Baez-Diaz, C.; Maestre, J.; Garcia-Lindo, M.; Sun, F.; Casado, J.G.; Blazquez, R.; Abad, J.L.; Palacios, I.; Rodriguez-Borlado, L.; et al. Delayed administration of allogeneic cardiac stem cell therapy for acute myocardial infarction could ameliorate adverse remodeling: Experimental study in swine. J. Transl. Med. 2015, 13, 156. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Liu, D.; Zhong, Y.; Huang, R.-C. Effects of timing on intracoronary autologous bone marrow-derived cell transplantation in acute myocardial infarction: A meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2017, 8, 231. [Google Scholar] [CrossRef]
- Chen, Y.; Teng, X.; Chen, W.; Yang, J.; Yang, Z.; Yu, Y.; Shen, Z. Timing of transplantation of autologous bone marrow derived mesenchymal stem cells for treating myocardial infarction. Sci. China Life Sci. 2014, 57, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, F.; Ma, J.; Shi, J.; Chen, S.; Liu, Z.; Liu, J. Effect of stem cell transplantation on patients with ischemic heart failure: A systematic review and meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Meng, L.; Xie, J.; Ouyang, J. Direct autologous bone marrow-derived stem cell transplantation for ischemic heart disease: A meta-analysis. Expert Opin. Biol. Ther. 2011, 11, 559–567. [Google Scholar] [CrossRef]
- Tian, T.; Chen, B.; Xiao, Y.; Yang, K.; Zhou, X. Intramyocardial autologous bone marrow cell transplantation for ischemic heart disease: A systematic review and meta-analysis of randomized controlled trials. Atherosclerosis 2014, 233, 485–492. [Google Scholar] [CrossRef]
- Jung, J.W.; Kwon, M.; Choi, J.C.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Familial Occurrence of Pulmonary Embolism after Intravenous, Adipose Tissue-Derived Stem Cell Therapy. Yonsei Med. J. 2013, 54, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Heslop, J.A.; Hammond, T.G.; Santeramo, I.; Piella, A.T.; Hopp, I.; Zhou, J.; Baty, R.; Graziano, E.I.; Marco, B.P.; Caron, A.; et al. Concise Review: Workshop Review: Understanding and Assessing the Risks of Stem Cell-Based Therapies. Stem Cells Transl. Med. 2015, 4, 389–400. [Google Scholar] [CrossRef] [PubMed]
- van der Spoel, T.I.; Jansen of Lorkeers, S.J.; Agostoni, P.; van Belle, E.; Gyöngyösi, M.; Sluijter, J.P.; Cramer, M.J.; Doevendans, P.A.; Chamuleau, S.A. Human relevance of pre-clinical studies in stem cell therapy: Systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc. Res. 2011, 91, 649–658. [Google Scholar] [CrossRef]
- Thompson, M.; Mei, S.H.; Wolfe, D.; Champagne, J.; Fergusson, D.; Stewart, D.J.; Sullivan, K.J.; Doxtator, E.; Lalu, M.; English, S.W.; et al. Cell therapy with intravascular administration of mesenchymal stromal cells continues to appear safe: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 19, 100249. [Google Scholar] [CrossRef]
- Lalu, M.M.; Mazzarello, S.; Zlepnig, J.; Dong, Y.Y.; Montroy, J.; McIntyre, L.; Devereaux, P.; Stewart, D.J.; Mazer, C.D.; Barron, C.C.; et al. Safety and Efficacy of Adult Stem Cell Therapy for Acute Myocardial Infarction and Ischemic Heart Failure (SafeCell Heart): A Systematic Review and Meta-Analysis. Stem Cells Transl. Med. 2018, 7, 857–866. [Google Scholar] [CrossRef]
- Jeong, H.; Yim, H.W.; Park, H.-J.; Cho, Y.; Hong, H.; Kim, N.J.; Oh, I.-H. Mesenchymal Stem Cell Therapy for Ischemic Heart Disease: Systematic Review and Meta-analysis. Int. J. Stem Cells 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated AUGUST 2023); Cochrane: London, UK, 2023. [Google Scholar]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
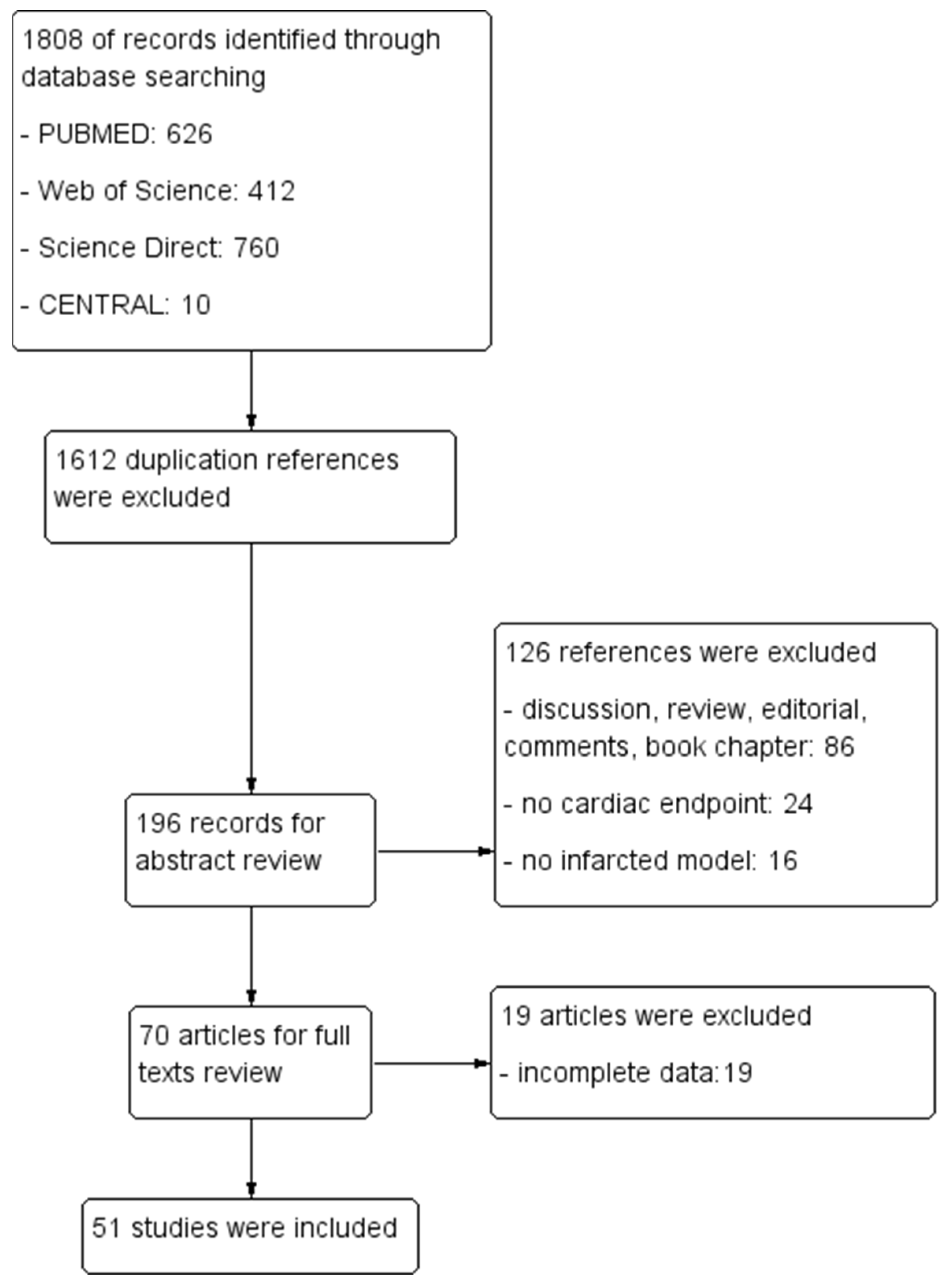

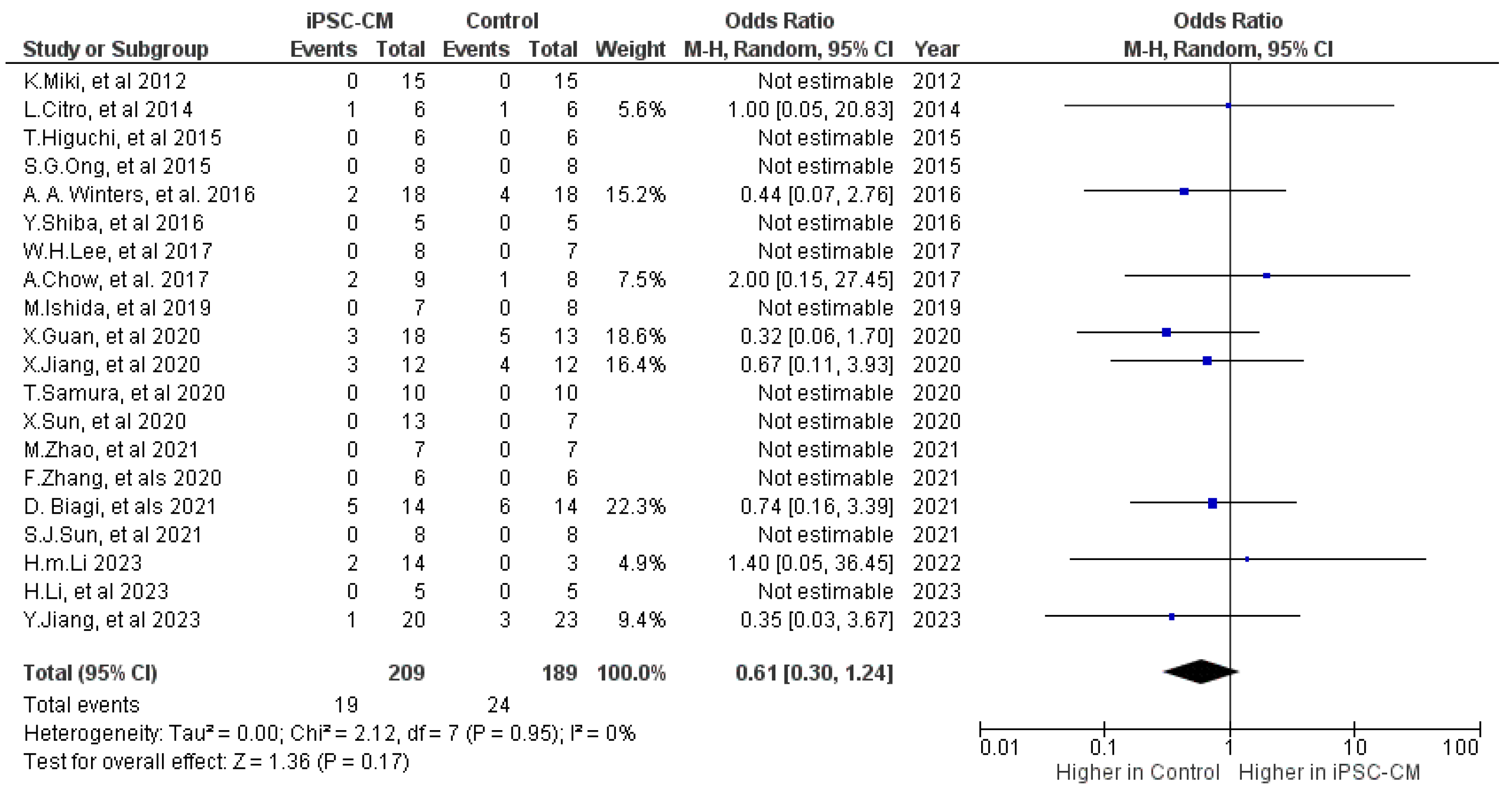
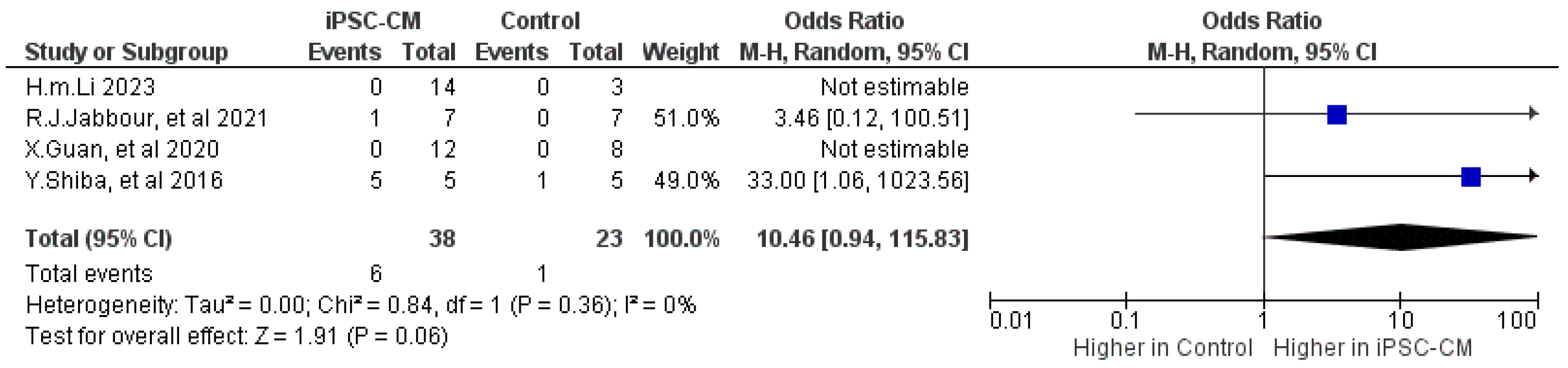

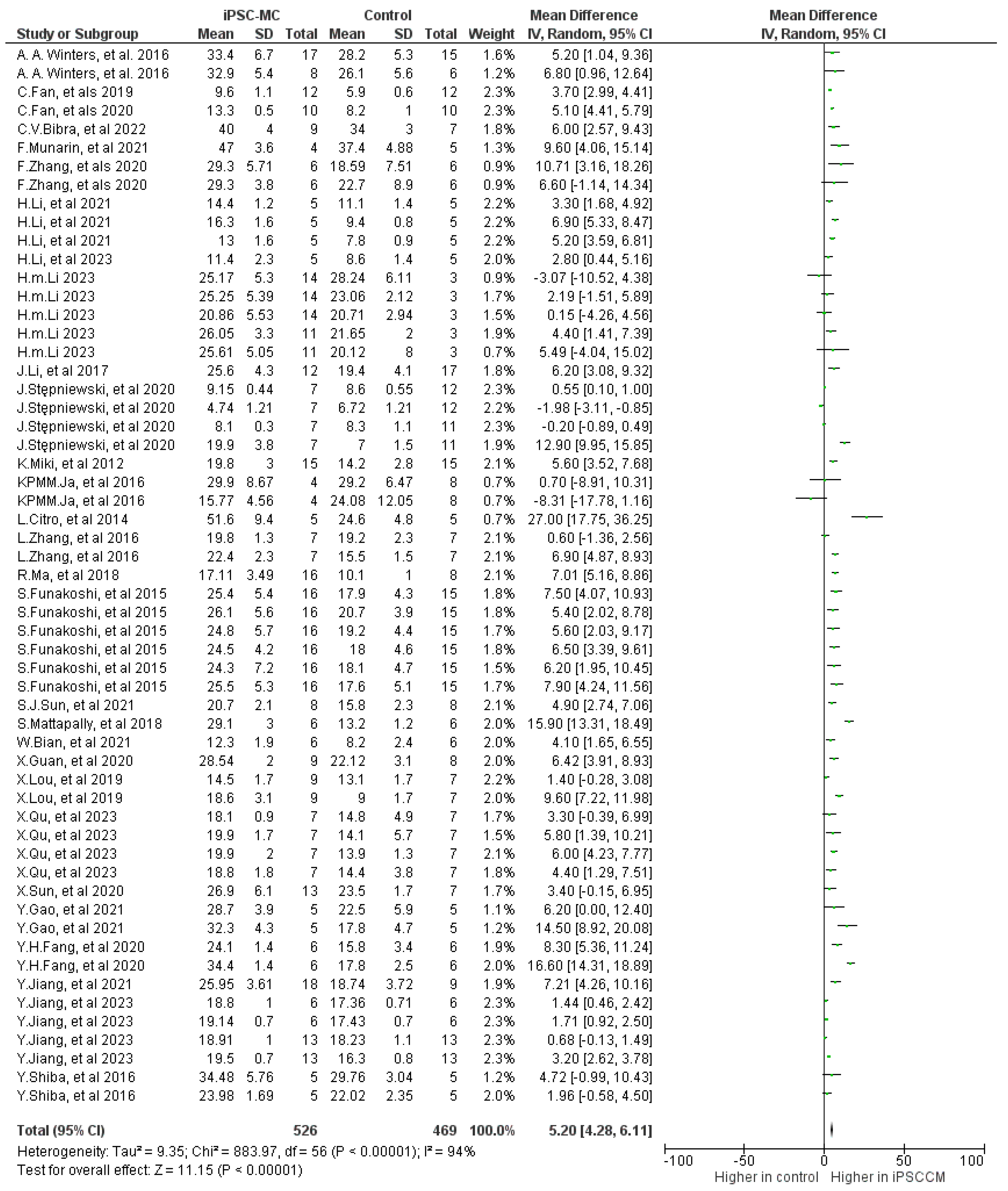

| Outcome | Number of Included Studies | Number of Treatments/Controls | Mean Difference (95% CI) | p | I2 |
|---|---|---|---|---|---|
| LVESV (µL) | 13 | 114/139 | −6.41 (−13.36 to 0.54) | 0.07 | 96% |
| LVEDV (µL) | 16 | 199/191 | −2.66 (−8.6 to 3.27) | 0.3 | 94% |
| LV fibrosis (%) | 30 | 332/276 | −7.62 (−9.72 to −5.52) | <0.001 | 94% |
| Subgroup | Number of Included Studies | Number of Treatments/Controls | Mean Difference (95% CI) | p | Subgroup I2 | Between Group I2 |
|---|---|---|---|---|---|---|
| Animal size | ||||||
| Small | 36 | 676/663 | 8.33 (7.18 to 9.47) | <0.001 | 95% | 0 |
| Big | 5 | 100/52 | 8.38 (4.03 to 12.72) | <0.001 | 0 | |
| Time of follow-up (weeks) | ||||||
| <4 | 24 | 333/309 | 5.38 (4.13 to 6.63) | <0.001 | 91% | 94.7% |
| 4–8 | 36 | 339/318 | 11.23 (9.48 to 12.61) | <0.001 | 91% | |
| >8 | 10 | 104/88 | 7.26 (4.39 to 10.13) | <0.001 | 93% | |
| Delivery method | ||||||
| Intramyocardial | 31 | 549/510 | 8.09 (6.79 to 9.39) | <0.001 | 92% | 90.8% |
| Intravenous | 1 | 25/15 | 2.34 (−0.74 to 5.43) | 0.14 | 1% | |
| Intracoronary | 1 | 9/9 | −5.05 (−10.74 to 0.65 | 0.08 | 0% | |
| Bio-engineered tissue | 14 | 262/265 | 9 (7.2 to 10.80) | <0.001 | 97% | |
| Treatment timing (week) | ||||||
| <1 | 32 | 603/528 | 8.21 (6.93 to 9.49) | <0.001 | 93% | 96.8% |
| 1–4 | 10 | 172/167 | 7.15 (5.66 to 8.63) | <0.001 | 92% | |
| >4 | 2 | 19/20 | 15.28 (13.67 to 16.88) | <0.001 | 0 | |
| Disease model | ||||||
| Permanent injury | 39 | 701/671 | 8.37 (7.24 to 9.50) | <0.001 | 95% | 0 |
| I/R | 4 | 93/44 | 6.78 (2.65 to 10.91) | 0.001 | 93% | |
| Cell origin | ||||||
| Xenogeneic | 37 | 635/575 | 8.75 (7.53 to 9.98) | <0.001 | 94% | 80.2% |
| Allogenic | 6 | 159/140 | 6.23 (4.40 to 8.06) | <0.001 | 93% | |
| Variable | Coefficient | Standard Error | t-Value | p-Value | 95% Confidence Interval |
|---|---|---|---|---|---|
| Animal size: big | −1.649 | 2.342 | −0.704 | 0.483 | −6.304 to 3.007 |
| Animal size: small | 3.513 | 2.916 | 1.205 | 0.232 | −2.283 to 9.308 |
| Treatment timing: acute | −3.108 | 2.266 | −1.372 | 0.174 | −7.611 to 1.396 |
| Treatment timing: chronic | 8.166 | 4.652 | 1.755 | 0.083 | −1.081 to 17.413 |
| Treatment timing: sub-acute | −3.195 | 2.211 | −1.445 | 0.152 | −7.590 to 1.201 |
| Method delivery: Bio-engineered tissue | 3.917 | 2.143 | 1.828 | 0.071 | −0.343 to 8.178 |
| Method delivery: Intracoronary | −8.065 | 3.327 | −2.424 | 0.017 | −14.678 to −1.452 |
| Method delivery: Intramyocardial | 3.824 | 1.641 | 2.331 | 0.022 | 0.563 to 7.085 |
| Method delivery: Intravenous | 2.188 | 2.801 | 0.781 | 0.438 | −3.379 to 7.754 |
| Cell origin: Allogenous | −0.150 | 1.139 | −0.132 | 0.895 | −2.413 to 2.113 |
| Cell origin: Xenogeneic | 2.014 | 0.922 | 2.185 | 0.032 | 0.182 to 3.846 |
| Disease model: IR | 1.962 | 2.619 | 0.749 | 0.456 | −3.244 to 7.168 |
| Disease model: Permanent injury | −0.098 | 2.155 | −0.046 | 0.964 | −4.382 to 4.186 |
| Follow up: <4 weeks | 1.011 | 1.299 | 0.778 | 0.438 | −1.570 to 3.592 |
| Follow up: 4–8 weeks | 1.052 | 1.269 | 0.829 | 0.410 | −1.471 to 3.574 |
| Follow up: >8 weeks | −0.965 | 2.454 | −0.393 | 0.695 | −5.843 to 3.913 |
| No | Registry No | Contact Author | Country | Study Phase | Intervention Model | No Patients | Starting Time | Expected Completion Time |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT03763136 | Jiaxian Wang | China | Phase 1 and Phase 2 | Randomized | 20 | October 2021 | May 2024 |
| 2 | NCT04396899 | Wolfram-Hubertus Zimmermann | Germany | Phase 1 and Phase 2 | Single Group Assignment | 53 | February 2020 | October 2024 |
| 3 | NCT04696328 | Takuji Kawamura | Japan | Phase 1 | Single Group Assignment | 10 | December 2019 | May 2023 |
| 4 | NCT04945018 | Heartseed Inc. | Japan | Phase 1 and Phase 2 | Non-Randomized | 10 | September 2023 | March 2024 |
| 5 | NCT05566600 | Jiaxian Wang | China | Early Phase 1 | Randomized | 32 | October 2022 | July 2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, Q.D.; Saito, Y.; Nakamura, K.; Iida, T.; Yuasa, S. Induced Pluripotent Stem Cell-Derived Cardiomyocytes Therapy for Ischemic Heart Disease in Animal Model: A Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 987. https://doi.org/10.3390/ijms25020987
Vo QD, Saito Y, Nakamura K, Iida T, Yuasa S. Induced Pluripotent Stem Cell-Derived Cardiomyocytes Therapy for Ischemic Heart Disease in Animal Model: A Meta-Analysis. International Journal of Molecular Sciences. 2024; 25(2):987. https://doi.org/10.3390/ijms25020987
Chicago/Turabian StyleVo, Quan Duy, Yukihiro Saito, Kazufumi Nakamura, Toshihiro Iida, and Shinsuke Yuasa. 2024. "Induced Pluripotent Stem Cell-Derived Cardiomyocytes Therapy for Ischemic Heart Disease in Animal Model: A Meta-Analysis" International Journal of Molecular Sciences 25, no. 2: 987. https://doi.org/10.3390/ijms25020987
APA StyleVo, Q. D., Saito, Y., Nakamura, K., Iida, T., & Yuasa, S. (2024). Induced Pluripotent Stem Cell-Derived Cardiomyocytes Therapy for Ischemic Heart Disease in Animal Model: A Meta-Analysis. International Journal of Molecular Sciences, 25(2), 987. https://doi.org/10.3390/ijms25020987







