Differential Modulation of Mouse Intestinal Organoids with Fecal Luminal Factors from Obese, Allergic, Asthmatic Children
Abstract
1. Introduction
2. Results
2.1. Cohort Description
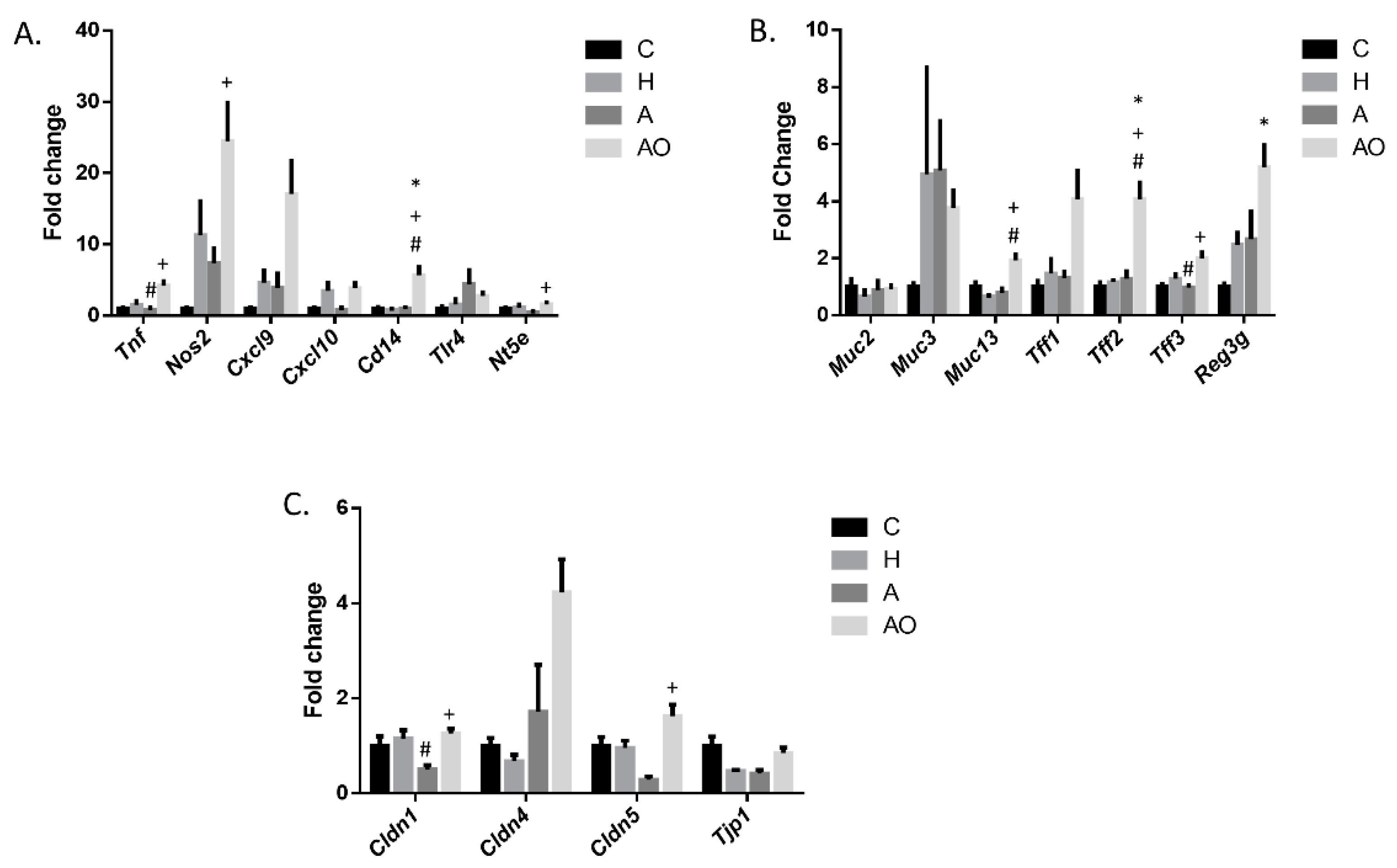
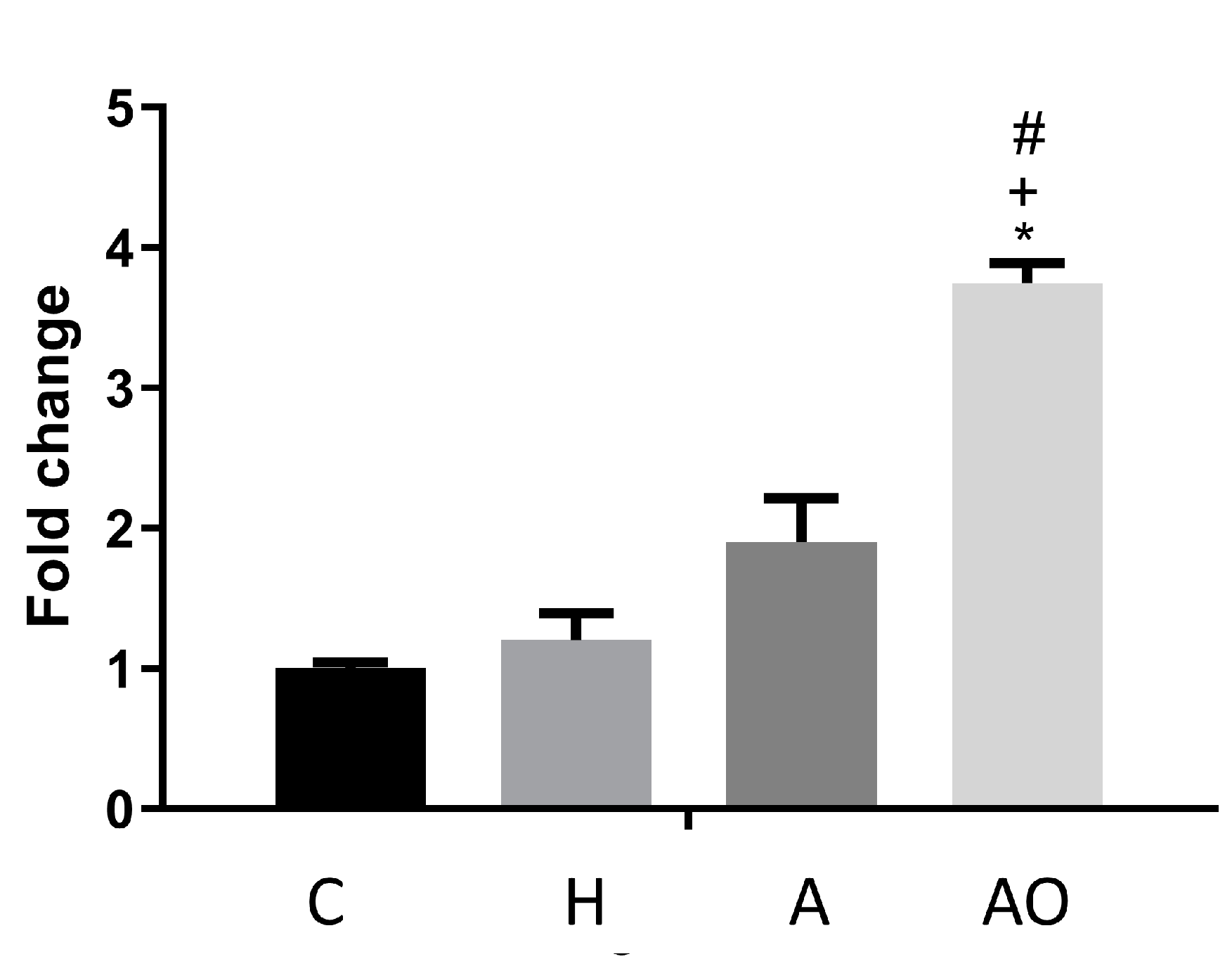
2.2. Fecal Waters from Overweight but Not Normal-Weight Allergic–Asthmatic Children Induce the Expression of Proinflammatory and Intestinal Barrier Function Genes
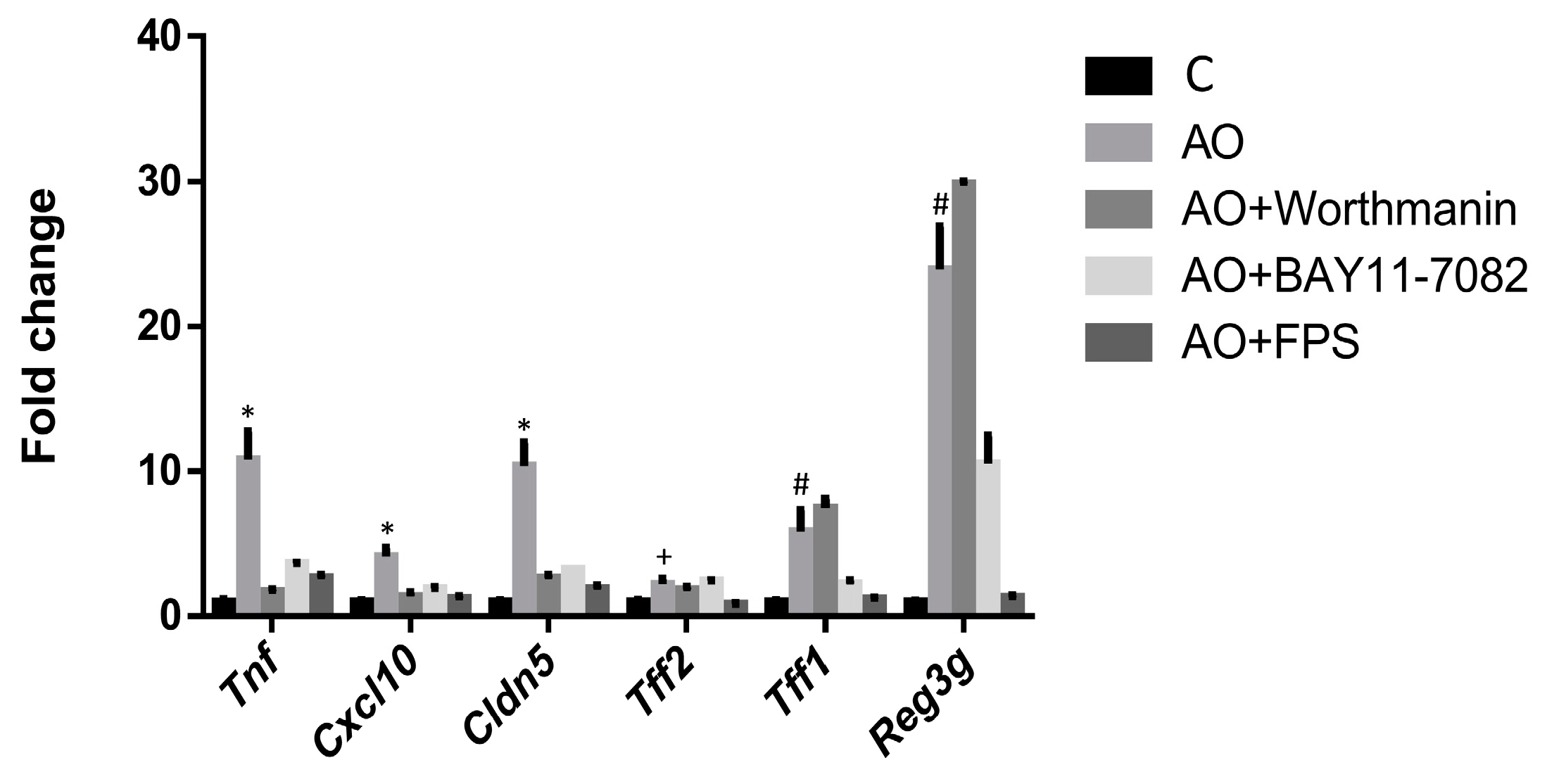
2.3. RAGE, IP3K and NFκB Mediate the Induction of the Expression of Intestinal Barrier Function-Related Genes by Fecal Waters of Obese Allergic Children
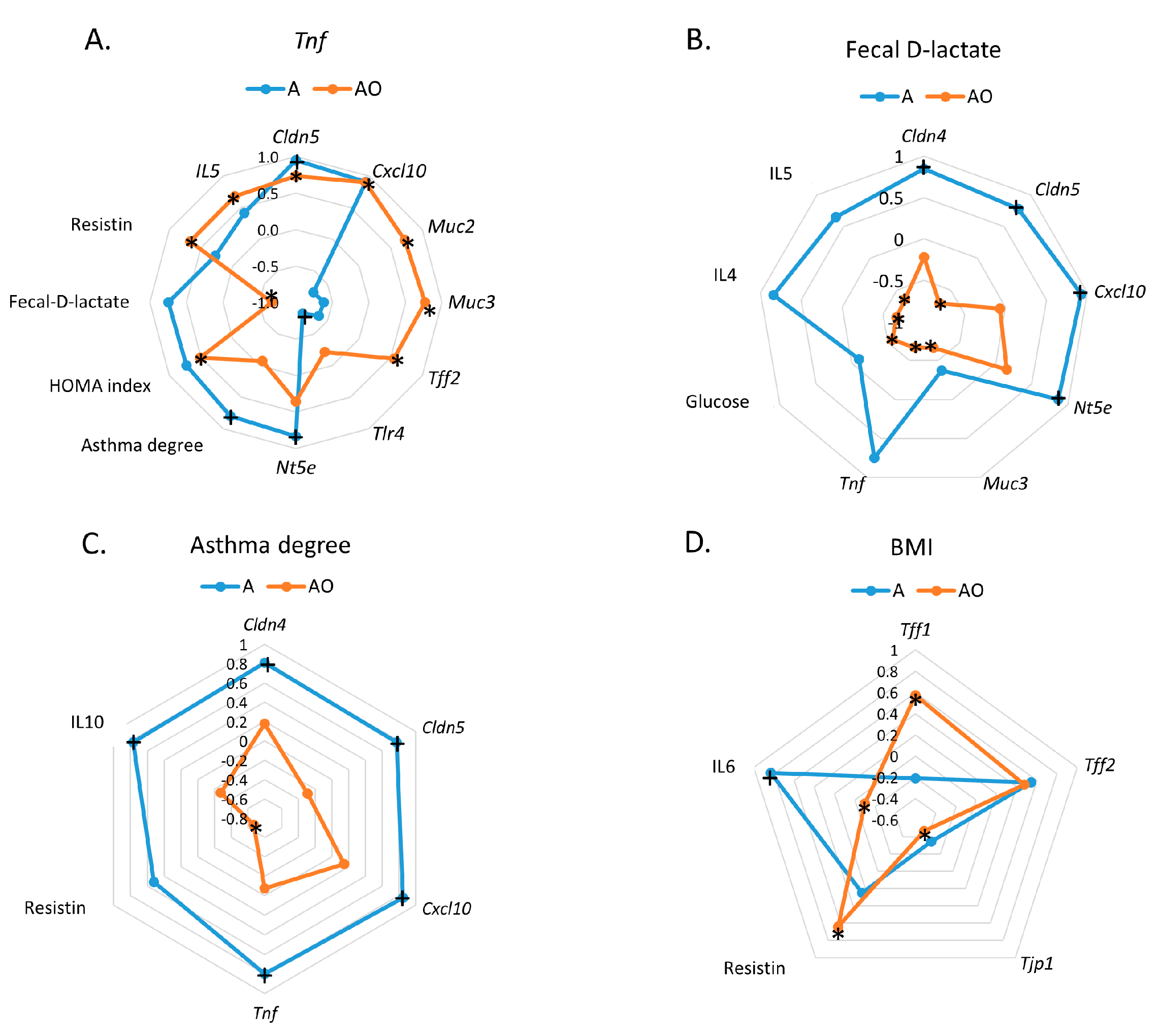
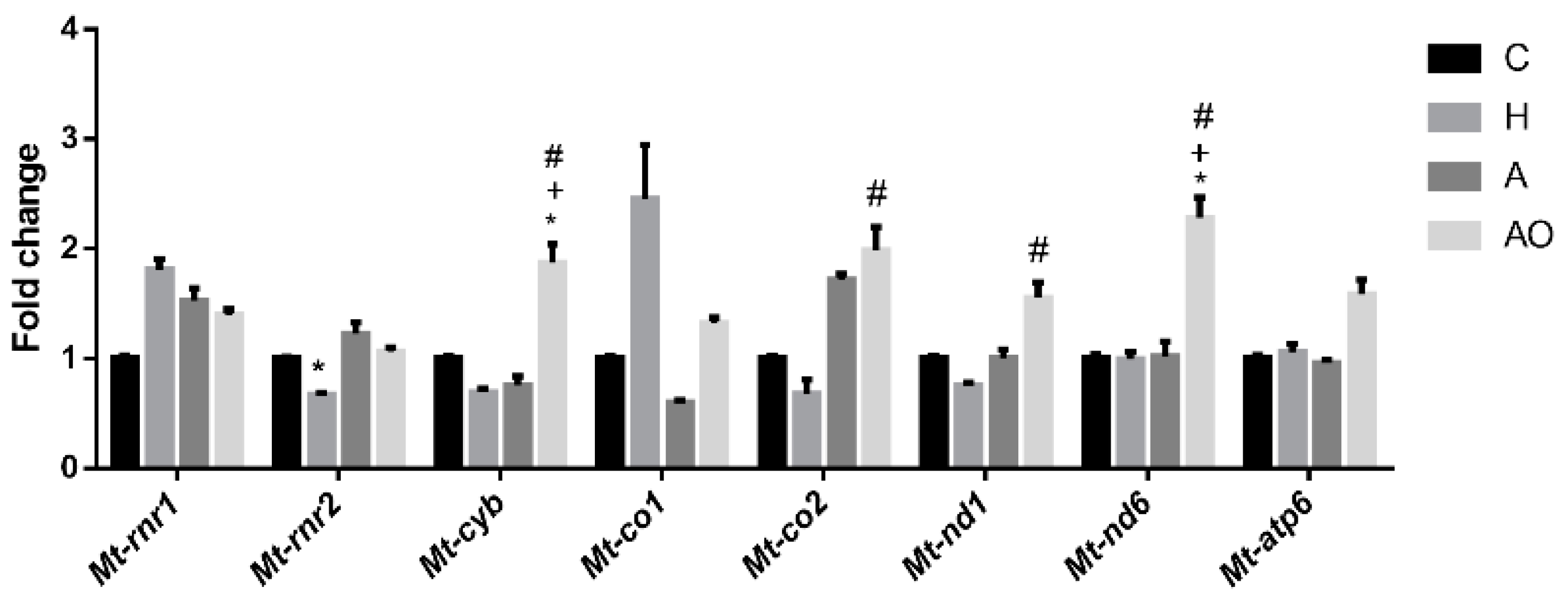
2.4. Fecal Waters from Obese Allergic Children Induce the Expression of Mitochondrial Respiratory Chain Genes
3. Discussion
4. Material and Methods
4.1. Patients
4.2. Organoid Experiments
4.2.1. Fecal Water Preparation
4.2.2. Crypt Isolation and Organoid Culture from Mouse
4.2.3. Analysis of Gene Expression by RT-qPCR
4.3. Data and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lang, J.E. Obesity and childhood asthma. Curr. Opin. Pulm. Med. 2019, 25, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Eder, W.; Ege, M.J.; von Mutius, E. The Asthma Epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Michalovich, D.; Rodriguez-Perez, N.; Smolinska, S.; Pirozynski, M.; Mayhew, D.; Uddin, S.; Van Horn, S.; Sokolowska, M.; Altunbulakli, C.; Eljaszewicz, A.; et al. Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nat. Commun. 2019, 10, 5711. [Google Scholar] [CrossRef]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.Y.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, Z.; Zhang, X.; Huang, P.; Xie, J.; Jiang, Q.; Zhang, Q.; Chung, K.F. Altered gut microbiome compositions are associated with the severity of asthma. J. Thorac. Dis. 2021, 13, 4322–4338. [Google Scholar] [CrossRef]
- Gomez-Llorente, M.A.; Martínez-Cañavate, A.; Chueca, N.; Rico, M.D.; Romero, R.; Anguita-Ruiz, A.; Aguilera, C.M.; Gil-Campos, M.; Mesa, M.D.; Khakimov, B.; et al. A Multi-Omics Approach Reveals New Signatures in Obese Allergic Asthmatic Children. Biomedicines 2020, 8, 359. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, X.; Sun, S.; Che, H. High-Fat Diet-Induced Obesity Aggravates Food Allergy by Intestinal Barrier Destruction and Inflammation. Int. Arch. Allergy Immunol. 2021, 183, 80–92. [Google Scholar] [CrossRef]
- Sanchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martinez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel. Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Dorrestein, P.C.; Mazmanian, S.K.; Knight, R. Finding the missing links among metabolites, microbes, and the host. Immunity 2014, 40, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Stanton, J.E.; Grabrucker, A.M. The use of organoids in food research. Curr. Opin. Food Sci. 2023, 49, 100977. [Google Scholar] [CrossRef]
- Body-Malapel, M.; Djouina, M.; Waxin, C.; Langlois, A.; Gower-Rousseau, C.; Zerbib, P.; Schmidt, A.M.; Desreumaux, P.; Boulanger, E.; Vignal, C. The RAGE signaling pathway is involved in intestinal inflammation and represents a promising therapeutic target for Inflammatory Bowel Diseases. Mucosal Immunol. 2019, 12, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Cuddihey, H.; Cavin, J.B.; Keenan, C.M.; Wallace, L.E.; Vemuri, K.; Makriyannis, A.; MacNaughton, W.K.; Sharkey, K.A. Role of CB(1) receptors in the acute regulation of small intestinal permeability: Effects of high-fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 323, G219–G238. [Google Scholar] [CrossRef] [PubMed]
- Menegati, L.M.; de Oliveira, E.E.; Oliveira, B.d.C.; Macedo, G.C.; de Castro e Silva, F.M. Asthma, obesity, and microbiota: A complex immunological interaction. Immunol. Lett. 2023, 255, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Ulrik, C.S. Obesity and asthma: A coincidence or a causal relationship? A systematic review. Respir. Med. 2013, 107, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.M.; Elbehidy, R.M.; Shokry, D.M.; Elbehidy, E.M. The influence of leptin on Th1/Th2 balance in obese children with asthma. J. Bras. Pneumol. 2013, 39, 562–568. [Google Scholar] [CrossRef]
- Pearce, S.C.; Al-Jawadi, A.; Kishida, K.; Yu, S.; Hu, M.; Fritzky, L.F.; Edelblum, K.L.; Gao, N.; Ferraris, R.P. Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types. BMC Biol. 2018, 16, 19. [Google Scholar] [CrossRef]
- Guerbette, T.; Boudry, G.; Lan, A. Mitochondrial function in intestinal epithelium homeostasis and modulation in diet-induced obesity. Mol. Metab. 2022, 63, 101546. [Google Scholar] [CrossRef]
- Mishra, S.P.; Wang, B.; Jain, S.; Ding, J.; Rejeski, J.; Furdui, C.M.; Kitzman, D.W.; Taraphder, S.; Brechot, C.; Kumar, A.; et al. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut 2023, 72, 1848–1865. [Google Scholar] [CrossRef]
- Aranda, C.J.; Arredondo-Amador, M.; Ocon, B.; Lavin, J.L.; Aransay, A.M.; Martinez-Augustin, O.; Sanchez de Medina, F. Intestinal epithelial deletion of the glucocorticoid receptor NR3C1 alters expression of inflammatory mediators and barrier function. FASEB J. 2019, 33, 14067–14082. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.F.; Wiegerinck, C.L.; de Jonge, H.R.; Bronsveld, I.; Janssens, H.M.; de Winter-de Groot, K.M.; Brandsma, A.M.; de Jong, N.W.; Bijvelds, M.J.; Scholte, B.J.; et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 2013, 19, 939–945. [Google Scholar] [CrossRef] [PubMed]
| A | AO | p Value | |
|---|---|---|---|
| Asthma | 1.889 ± 0.200 | 2.316 ± 0.172 | 0.148 |
| Age | 9.444 ± 0.884 | 8.474 ± 0.486 | 0.306 |
| Weight (Kg) | 31.122 ± 1.980 | 44.887 ± 2.886 | 0.005 |
| Height (m) | 1.383 ± 0.036 | 1.345 ± 0.028 | 0.426 |
| BMI | 16.156 ± 0.452 | 24.363 ± 0.601 * | <0.0001 |
| BMIZscore | −0.489 ± 0.153 | 2.858 ± 0.160 * | <0.0001 |
| Hip perimeter (cm) | 70.714 ± 2.998 | 83.194 ± 2.482 | 0.010 |
| Waist–hip ratio | 0.837 ± 0.019 | 0.862 ± 0.023 | 0.487 |
| Fecal D-Lactate (mM) | 9.100 ± 1.329 | 10.327 ± 1.508 | 0.609 |
| Glucose (mmol/L) | 4.722 ± 0.224 | 4.629 ± 0.087 | 0.4439 |
| Insulin (µUL/mL) | 6.847 ± 1.886 | 8.606 ± 1.137 | 0.1759 |
| HOMA index | 1.144 ± 0.323 | 1.654 ± 0.169 | 0.585 |
| LBP (µg/mL) | 3.171 ± 0.604 | 3.600 ± 0.461 | 0.606 |
| Adiponectin (mg/L) | 7.071 ± 1.718 | 5.631 ± 0.726 | 0.378 |
| Resistin (µg/mL) | 13.671 ± 1.765 | 13.671 ± 1.133 | >0.9999 |
| Adiponectin/resistin ratio | 0.400 ± 0.086 | 0.446 ± 0.071 | 0.706 |
| Leptin (µg/L) | 2.586 ± 0.440 | 8.156 ± 0.555 * | <0.0001 |
| TNF (pg/mL) | 3.800 ± 0.479 | 3.383 ± 0.229 | 0.385 |
| IL-10 (pg/mL) | 3.371 ± 0.341 | 4.114 ± 0.426 | 0.270 |
| IL-13 (pg/mL) | 2.517 ± 0.681 | 1.927 ± 3.373 | 0.419 |
| IL-4 (pg/mL) | 13.917 ± 3.200 | 12.846 ± 2.075 | 0.777 |
| IL-5 (pg/mL) | 1.200 ± 0.145 | 1.421 ± 0.114 | 0.263 |
| IL-6 (pg/mL) | 0.950 ± 0.228 | 1.099 ± 0.220 | 0.683 |
| IL-8 (pg/mL) | 1.957 ± 0.188 | 1.793 ± 0.212 | 0.624 |
| Cd14 | Cldn1 | Cldn4 | Cldn5 | Cxcl10 | Cxcl9 | Muc2 | Muc3 | Muc13 | Nos2 | Nt5e | Tff1 | Tff2 | Tff3 | Tjp1 | Tnf | Tlr4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd14 | 0.573 | 0.758 * | 0.495 | 0.560 | 0.422 | 0.096 | −0.132 | 0.867 * | −0.335 | 0.650 | 0.101 | −0.556 | −0.706 | 0.372 | 0.330 | −0.474 | |
| Cldn1 | 0.573 | 0.922 * | 0.466 | 0.738 | 0.609 | 0.234 | 0.408 | 0.213 | −0.590 | 0.446 | 0.511 | 0.084 | −0.621 | 0.527 | 0.525 | −0.140 | |
| Cldn4 | 0.758 * | 0.922 * | 0.707 | 0.880 * | 0.876 * | −0.139 | 0.004 | 0.511 | −0.634 | 0.854 * | 0.311 | −0.153 | −0.763 * | 0.202 | 0.658 | −0.412 | |
| Cldn5 | 0.495 | 0.466 | 0.707 | 0.942 * | −0.434 | −0.660 | −0.546 | 0.532 | −0.386 | 0.879 * | 0.381 | −0.707 | −0.818 * | −0.395 | 0.949 * | −0.817 * | |
| Cxcl10 | 0.560 | 0.738 | 0.880 * | 0.942 * | −0.464 | −0.542 | −0.416 | 0.511 | −0.509 | 0.943 * | 0.460 | −0.449 | −0.879 * | −0.255 | 0.914 * | −0.690 | |
| Cxcl9 | 0.422 | 0.609 | 0.876 * | −0.434 | −0.464 | 0.894 * | 0.778 * | −0.144 | −0.621 | −0.542 | 0.197 | 0.204 | 0.390 | 0.932 * | −0.468 | 0.050 | |
| Muc2 | 0.096 | 0.234 | −0.139 | −0.660 | −0.542 | 0.894 * | 0.838 * | −0.283 | −0.098 | −0.550 | 0.063 | 0.422 | 0.288 | 0.938 * | −0.722 | 0.288 | |
| Muc3 | −0.132 | 0.408 | 0.004 | −0.546 | −0.416 | 0.778 * | 0.838 * | −0.540 | −0.190 | −0.534 | 0.264 | 0.792 * | 0.545 | 0.802 * | −0.618 | 0.646 | |
| Muc13 | 0.867 * | 0.213 | 0.511 | 0.532 | 0.511 | −0.144 | −0.283 | −0.540 | −0.050 | 0.695 * | −0.127 | −0.711 * | −0.747 * | −0.055 | 0.425 | −0.503 | |
| Nos2 | −0.335 | −0.590 | −0.634 | −0.386 | −0.509 | −0.621 | −0.098 | −0.190 | −0.050 | −0.431 | −0.295 | 0.125 | 0.092 | −0.318 | −0.541 | 0.536 | |
| Nt5e | 0.650 | 0.446 | 0.854 * | 0.879 * | 0.943 * | −0.542 | −0.550 | −0.534 | 0.695 * | −0.431 | 0.023 | −0.510 | −0.808 * | −0.277 | 0.838 * | −0.592 | |
| Tff1 | 0.101 | 0.511 | 0.311 | 0.381 | 0.460 | 0.197 | 0.063 | 0.264 | −0.127 | −0.295 | 0.023 | −0.167 | 0.170 | 0.208 | 0.514 | −0.113 | |
| Tff2 | −0.556 | 0.084 | −0.153 | −0.707 | −0.449 | 0.204 | 0.422 | 0.792 * | −0.711 * | 0.125 | −0.510 | −0.167 | 0.562 | 0.276 | −0.636 | 0.842 * | |
| Tff3 | −0.706 | −0.621 | −0.763 * | −0.818 * | −0.879 * | 0.390 | 0.288 | 0.545 | −0.747 * | 0.092 | −0.808 * | 0.170 | 0.562 | 0.071 | −0.708 | 0.627 | |
| Tjp1 | 0.372 | 0.527 | 0.202 | −0.395 | −0.255 | 0.932 * | 0.938 * | 0.802 * | −0.055 | −0.318 | −0.277 | 0.208 | 0.276 | 0.071 | −0.494 | 0.155 | |
| Tnf | 0.330 | 0.525 | 0.658 | 0.949 * | 0.914 * | −0.468 | −0.722 | −0.618 | 0.425 | −0.541 | 0.838 * | 0.514 | −0.636 | −0.708 | −0.494 | −0.821 * | |
| Tlr4 | −0.474 | −0.140 | −0.412 | −0.817 * | −0.690 | 0.050 | 0.288 | 0.646 | −0.503 | 0.536 | −0.592 | −0.113 | 0.842 * | 0.627 | 0.155 | −0.821 * |
| Cd14 | Cldn1 | Cldn4 | Cldn5 | Cxcl10 | Cxcl9 | Muc2 | Muc3 | Muc13 | Nos2 | Nt5e | Tff1 | Tff2 | Tff3 | Tjp1 | Tnf | Tlr4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd14 | 0.284 | 0.260 | 0.446 | 0.177 | 0.197 | 0.615 * | 0.672 * | 0.509 * | 0.085 | 0.177 | −0.212 | −0.191 | −0.151 | 0.312 | 0.474 | 0.001 | |
| Cldn1 | 0.284 | 0.661 * | 0.658 * | 0.638 * | 0.845 * | 0.007 | 0.452 | 0.243 | 0.248 | 0.810 * | −0.054 | −0.197 | 0.071 | 0.603 * | 0.432 | 0.540 | |
| Cldn4 | 0.260 | 0.661 * | 0.359 | 0.733 * | 0.587 * | 0.120 | 0.539 * | 0.486 * | 0.020 | 0.492 * | 0.443 | 0.374 | 0.498 * | 0.504 * | 0.383 | 0.053 | |
| Cldn5 | 0.446 | 0.658 * | 0.359 | 0.789 * | 0.453 | 0.191 | 0.743 * | 0.156 | 0.576 | 0.370 | 0.108 | −0.219 | 0.045 | 0.028 | 0.737 * | 0.192 | |
| Cxcl10 | 0.177 | 0.638 * | 0.733 * | 0.789 * | 0.549 * | 0.321 | 0.703 * | 0.500 * | 0.330 | 0.436 | 0.475 | 0.475 * | 0.678 * | 0.184 | 0.902 * | −0.075 | |
| Cxcl9 | 0.197 | 0.845 * | 0.587 * | 0.453 | 0.549 * | −0.065 | 0.391 | 0.395 | 0.091 | 0.770 * | −0.113 | 0.220 | 0.306 | 0.570 | 0.351 | 0.357 | |
| Muc2 | 0.615 * | 0.007 | 0.120 | 0.191 | 0.321 | −0.065 | 0.522 * | 0.340 | −0.035 | 0.059 | −0.097 | −0.003 | 0.074 | 0.104 | 0.718 * | −0.186 | |
| Muc3 | 0.672 * | 0.452 | 0.539 * | 0.743 * | 0.703 * | 0.391 | 0.522 * | 0.661 * | 0.432 | 0.398 | 0.395 | 0.365 | 0.401 | 0.097 | 0.770 * | 0.024 | |
| Muc13 | 0.509 * | 0.243 | 0.486 * | 0.156 | 0.500 * | 0.395 | 0.340 | 0.661 * | 0.263 | 0.573 * | 0.222 | 0.093 | 0.616 * | 0.636 * | 0.329 | 0.360 | |
| Nos2 | 0.085 | 0.248 | 0.020 | 0.576 * | 0.330 | 0.091 | −0.035 | 0.432 | 0.263 | 0.022 | 0.206 | −0.030 | 0.370 | −0.005 | 0.149 | 0.576 * | |
| Nt5e | 0.177 | 0.810 * | 0.492 * | 0.370 | 0.436 | 0.770 * | 0.059 | 0.398 | 0.573 * | 0.022 | 0.102 | 0.086 | 0.398 | 0.731 * | 0.357 | 0.413 | |
| Tff1 | −0.212 | −0.054 | 0.443 | 0.108 | 0.475 | −0.113 | −0.097 | 0.395 | 0.222 | 0.206 | 0.102 | 0.675 * | 0.694 * | −0.060 | 0.386 | −0.012 | |
| Tff2 | −0.191 | −0.197 | 0.374 | −0.219 | 0.475 * | 0.220 | −0.003 | 0.365 | 0.093 | −0.030 | 0.086 | 0.675 * | 0.450 | −0.146 | 0.549 * | −0.064 | |
| Tff3 | −0.151 | 0.071 | 0.498 * | 0.045 | 0.678 * | 0.306 | 0.074 | 0.401 | 0.616 * | 0.370 | 0.398 | 0.694 * | 0.450 | 0.242 | 0.357 | 0.277 | |
| Tjp1 | 0.312 | 0.603 * | 0.504 * | 0.028 | 0.184 | 0.570 * | 0.104 | 0.097 | 0.636 * | −0.005 | 0.731 * | −0.060 | −0.146 | 0.242 | −0.043 | 0.578 * | |
| Tnf | 0.474 | 0.432 | 0.383 | 0.737 * | 0.902 * | 0.351 | 0.718 * | 0.770 * | 0.329 | 0.149 | 0.357 | 0.386 | 0.549 * | 0.357 | −0.043 | −0.213 | |
| Tlr4 | 0.001 | 0.540 | 0.053 | 0.192 | −0.075 | 0.357 | −0.186 | 0.024 | 0.360 | 0.576 | 0.413 | −0.012 | −0.064 | 0.277 | 0.578 * | −0.213 |
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| 18S | ACACGGACAGGATTGACAGATTG | GCCAGAGTCTCGTTCGTTATCG |
| Hprt | AGGGATTTGAATCACGTTTG | TTTACTGGCAACATCAACAG |
| Tnf | CGTGGAACTGGCAGAAGAGG | CAGGAATGAGAAGAGGCTGAGAC |
| Nos2 | CATCAACCAGTATTATGGCTC | TTTCCTTTGTTACAGCTTCC |
| Cd14 | GAATCTACCGACCATGGAG | AAGTTGCAGGAACAACTTTC |
| Nt5e | CTATGAGCCTCTTGAAATGG | CTGATATCTTGATCACCAGAG |
| Cxcl9 | GAGGAACCCTAGTGATAAGG | GTTTGATCTCCGTTCTTCAG |
| Cxcl10 | AAAAAGGTCTAAAAGGGCTC | AATTAGGACTAGCCATCCAC |
| Muc13 | CTTTTTGTGTTGCTGTAACG | CAAATGGACACTCTTCACAC |
| Tff2 | CTGTGGAAGATTGTCACTAC | AGATTGATGAAGTCTGGTTG |
| Tff3 | CCTGGTTGCTGGGTCCTCTG | GCCACGGTTGTTACACTGCTC |
| Reg3g | CAGAGGTGGATGGGAGTGGAG | CACAGTGATTGCCTGAGGAAGAG |
| Claudin1 | CAATGCCAGGTATGAATTTG | TCACACATAGTCTTTCCCAC |
| Claudin4 | GACTGTGCAAAGTTACTAGC | ACCAGCAATTTGGATGTAAG |
| Claudin5 | AACAGTTCCTACTGAGATCC | CTTTTTAACACGTCCCTCTG |
| Tjp1 | GGGGCCTACACTGATCAAGA | TGGAGATGAGGCTTCTGCTT |
| Cnr1 | AATGCCATTTAGGTGTTCTG | ATAGGTCTTAGAACCAACCC |
| Tlr4 | GATCAGAAACTCAGCAAAGTC | TGTTTCAATTTCACACCTGG |
| Muc 2 | CCCAGAAGGGACTGTGTATG | TTGTGTTCGCTCTTGGTCAG |
| Muc 3 | AAAGATTACCTCCCATCTCC | TAAAACTAAGCATGCCCTTG |
| Muc 4 | GGATTCCTTCTACGTTACAG | GTAGAGAAATCAGCATCAG |
| mt-Rnr1 | GACACCTTGCCTAGCCACAC | TGGCTGGCACGAAATTTACC |
| mt-Rnr2 | ACTAGCATGAACGGCTAAACG | AAGCTCCATAGGGTCTTCTCG |
| Gene | UniqueAssay ID |
|---|---|
| mt-Atp6 | qMmuCED0051651 |
| mt-Co1 | qMmuCED0061464 |
| mt-Co2 | qMmuCED0027 |
| mt-Cyb | qMmuCED0001514 |
| mtNd6 | qMmuCED0061740 |
| mtNd1 | qMmuCED0003246 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Córdova, S.; Tena-Garitaonaindia, M.; Álvarez-Mercado, A.I.; Gámez-Belmonte, R.; Gómez-Llorente, M.A.; Sánchez de Medina, F.; Martínez-Cañavate, A.; Martínez-Augustin, O.; Gómez-Llorente, C. Differential Modulation of Mouse Intestinal Organoids with Fecal Luminal Factors from Obese, Allergic, Asthmatic Children. Int. J. Mol. Sci. 2024, 25, 866. https://doi.org/10.3390/ijms25020866
Córdova S, Tena-Garitaonaindia M, Álvarez-Mercado AI, Gámez-Belmonte R, Gómez-Llorente MA, Sánchez de Medina F, Martínez-Cañavate A, Martínez-Augustin O, Gómez-Llorente C. Differential Modulation of Mouse Intestinal Organoids with Fecal Luminal Factors from Obese, Allergic, Asthmatic Children. International Journal of Molecular Sciences. 2024; 25(2):866. https://doi.org/10.3390/ijms25020866
Chicago/Turabian StyleCórdova, Samir, Mireia Tena-Garitaonaindia, Ana Isabel Álvarez-Mercado, Reyes Gámez-Belmonte, Mª Amelia Gómez-Llorente, Fermín Sánchez de Medina, Ana Martínez-Cañavate, Olga Martínez-Augustin, and Carolina Gómez-Llorente. 2024. "Differential Modulation of Mouse Intestinal Organoids with Fecal Luminal Factors from Obese, Allergic, Asthmatic Children" International Journal of Molecular Sciences 25, no. 2: 866. https://doi.org/10.3390/ijms25020866
APA StyleCórdova, S., Tena-Garitaonaindia, M., Álvarez-Mercado, A. I., Gámez-Belmonte, R., Gómez-Llorente, M. A., Sánchez de Medina, F., Martínez-Cañavate, A., Martínez-Augustin, O., & Gómez-Llorente, C. (2024). Differential Modulation of Mouse Intestinal Organoids with Fecal Luminal Factors from Obese, Allergic, Asthmatic Children. International Journal of Molecular Sciences, 25(2), 866. https://doi.org/10.3390/ijms25020866







