Embers of the Past: Early Childhood Traumas Interact with Variation in P2RX7 Gene Implicated in Neuroinflammation on Markers of Current Suicide Risk
Abstract
1. Introduction
2. Results
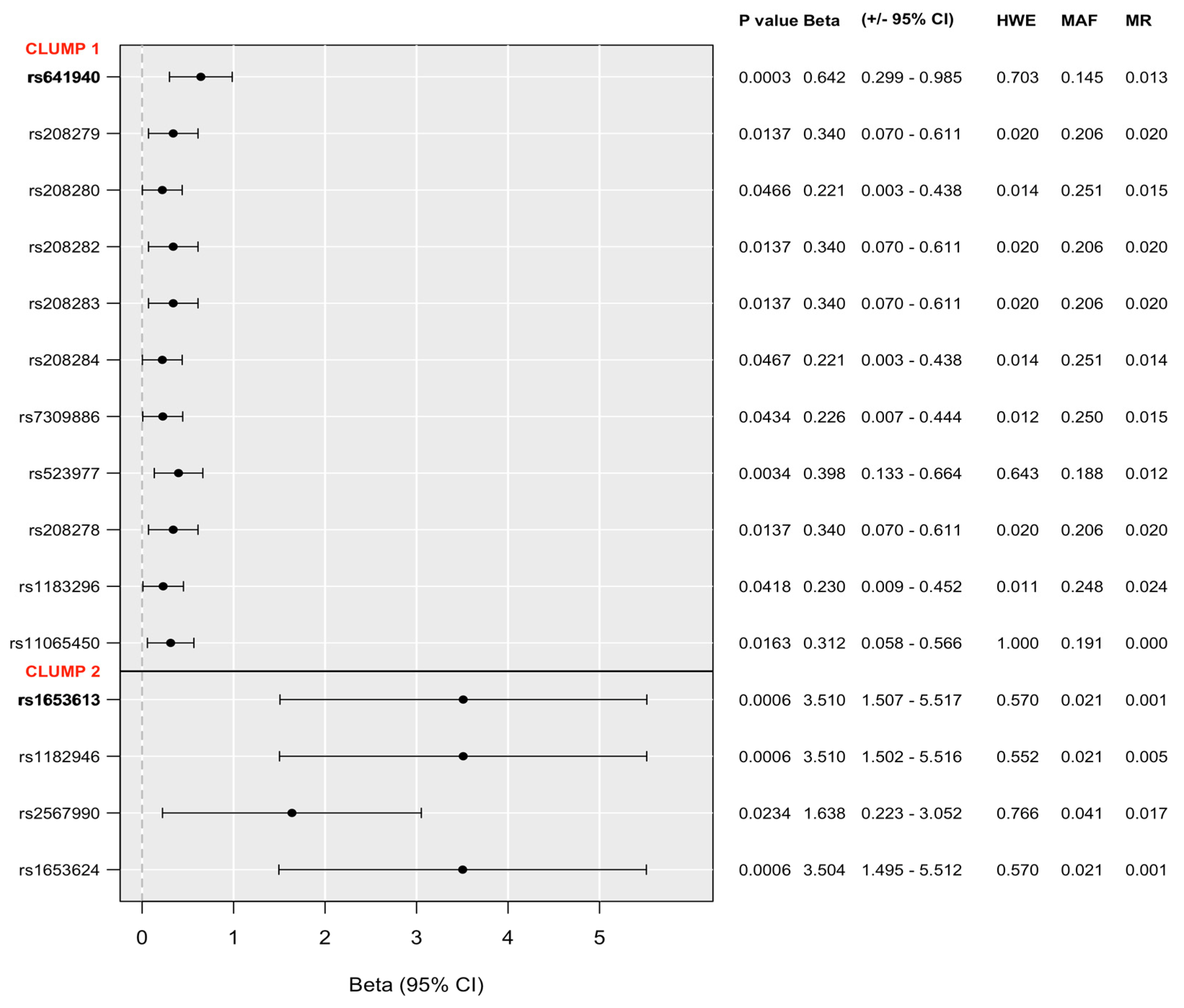
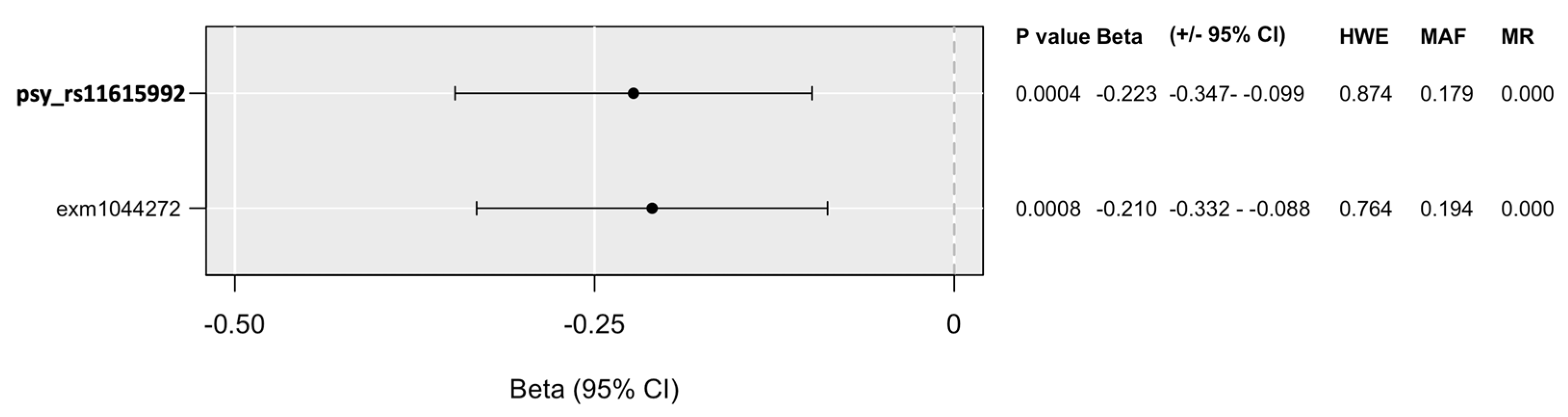
2.1. Main Effects of Variation in P2RX7 on Lifetime Suicide Attempts, Current Suicidal Ideation, Current Hopelessness, and Current Thoughts of Death
2.2. Gene x Environment Effects of Variation in P2RX7 on Lifetime Suicide Attempts, Current Suicidal Ideation, Current Hopelessness, and Current Thoughts of Death: Interaction with Childhood Adversities (CHA)
2.3. Gene x Environment Effects of Variation in P2RX7 on Lifetime Suicide Attempts, Current Suicidal Ideation, Current Hopelessness, and Current Thoughts of Death: Interaction with Recent Life Events (RLE)
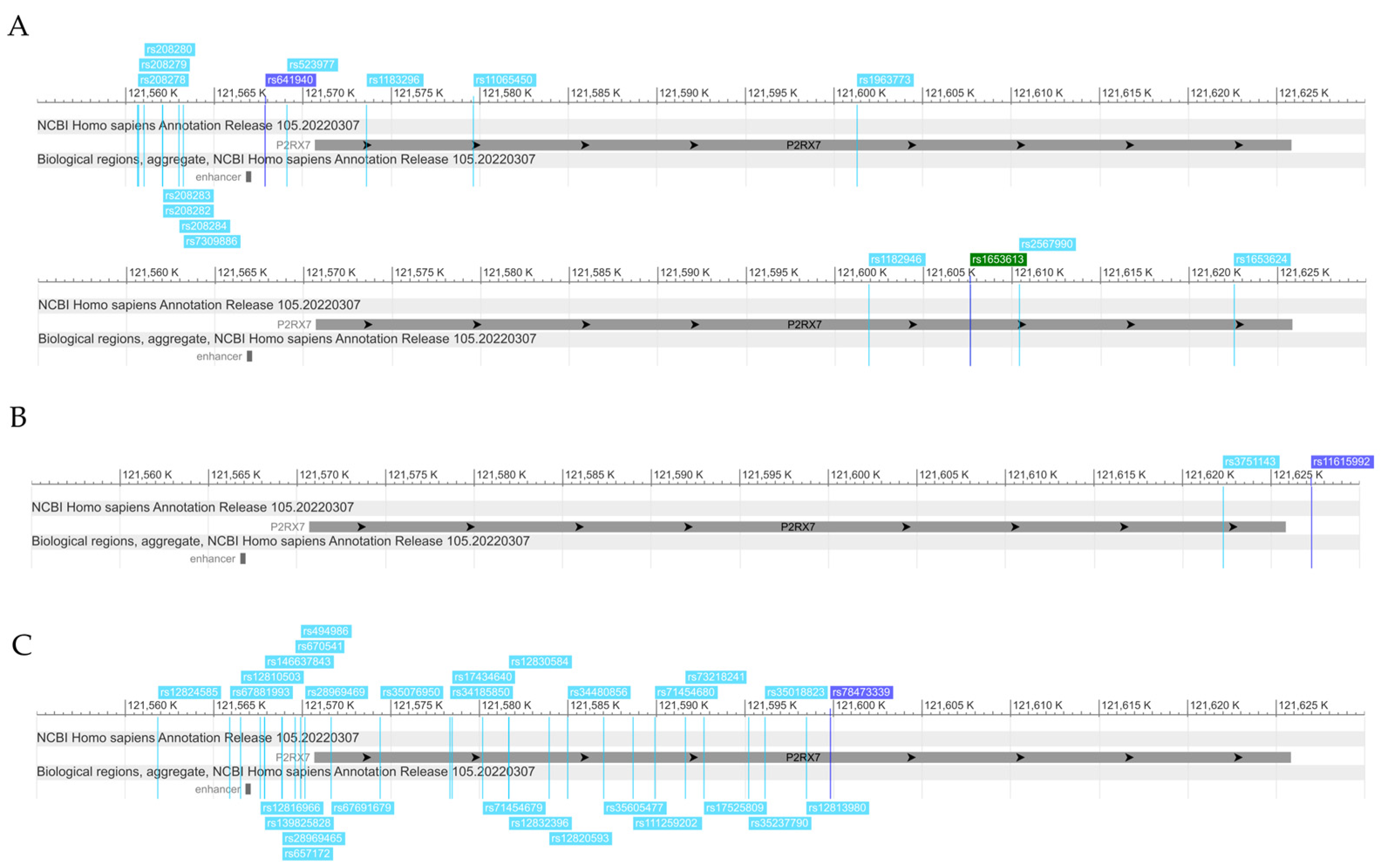
2.4. In Silico Characterization and Functional Prediction of Identified Top SNPs

3. Discussion
3.1. The Potential Role of Neuroinflammation, P2X7 Receptors and the P2RX7 Gene on Suicidal Behaviour
3.2. P2X7 Receptors and the P2RX7 Gene as a Potential Focus of Attention in Understanding and Treatment of Neuropsychiatric Disorders and Suicide
3.3. Effects of P2RX7 Variation on Suicidal Behaviour in Interaction with Childhood Adversity
3.4. Limitations
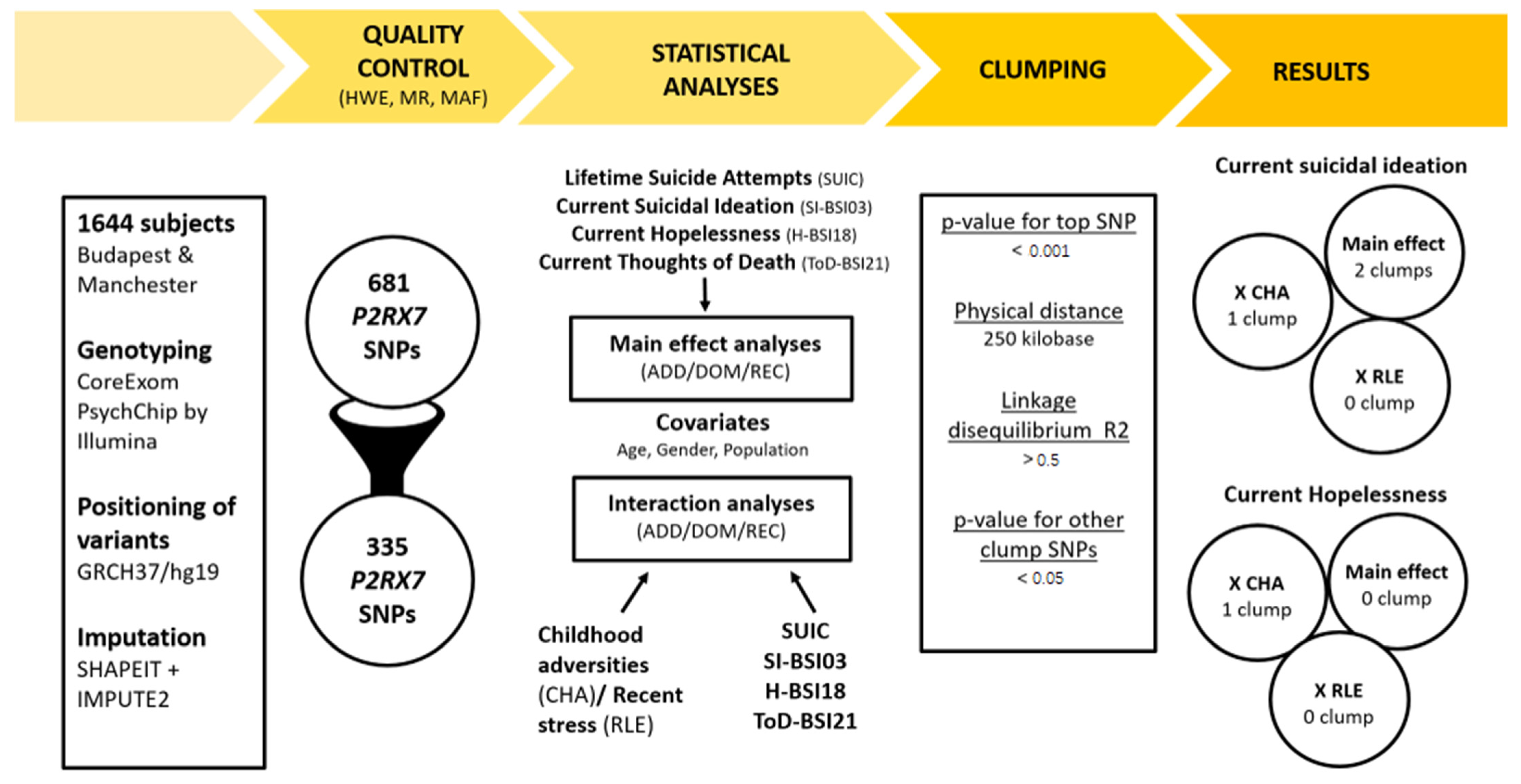
4. Methods
4.1. Study Population
4.2. Phenotypes
4.3. Genotyping
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Suicide. Available online: https://www.who.int/news-room/fact-sheets/detail/suicide (accessed on 14 December 2023).
- Gonda, X.; Dome, P.; Serafini, G.; Pompili, M. How to save a life: From neurobiological underpinnings to psychopharmacotherapies in the prevention of suicide. Pharmacol. Ther. 2023, 244, 108390. [Google Scholar] [CrossRef]
- Bould, H.; Mars, B.; Moran, P.; Biddle, L.; Gunnell, D. Rising suicide rates among adolescents in England and Wales. Lancet 2019, 394, 116–117. [Google Scholar] [CrossRef] [PubMed]
- Wisłowska-Stanek, A.; Kołosowska, K.; Maciejak, P. Neurobiological Basis of Increased Risk for Suicidal Behaviour. Cells 2021, 10, 2519. [Google Scholar] [CrossRef] [PubMed]
- Klonsky, E.D.; May, A.M.; Saffer, B.Y. Suicide, Suicide Attempts, and Suicidal Ideation. Annu. Rev. Clin. Psychol. 2016, 12, 307–330. [Google Scholar] [CrossRef] [PubMed]
- Rihmer, Z.; Gonda, X. The effect of pharmacotherapy on suicide rates in bipolar patients. CNS Neurosci. Ther. 2012, 18, 238–242. [Google Scholar] [CrossRef]
- Benard, V.; Vaiva, G.; Masson, M.; Geoffroy, P.A. Lithium and suicide prevention in bipolar disorder. Encephale 2016, 42, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Michel, K.; Gysin-Maillart, A.; Breit, S.; Walther, S.; Pavlidou, A. Psychopharmacological treatment is not associated with reduced suicide ideation and reattempts in an observational follow-up study of suicide attempters. J. Psychiatr. Res. 2021, 140, 180–186. [Google Scholar] [CrossRef]
- Gonda, X.; Serafini, G.; Dome, P. Fight the fire: Association of cytokine genomic markers and suicidal behavior may pave the way for future therapies. J. Pers. Med. 2023, 13, 1078. [Google Scholar] [CrossRef]
- Levey, D.F.; Polimanti, R.; Cheng, Z.; Zhou, H.; Nuñez, Y.Z.; Jain, S.; He, F.; Sun, X.; Ursano, R.J.; Kessler, R.C.; et al. Genetic associations with suicide attempt severity and genetic overlap with major depression. Transl. Psychiatry 2019, 9, 22. [Google Scholar] [CrossRef]
- Mullins, N.; Bigdeli, T.B.; Børglum, A.D.; Coleman, J.R.I.; Demontis, D.; Mehta, D.; Power, R.A.; Ripke, S.; Stahl, E.A.; Starnawska, A.; et al. GWAS of Suicide Attempt in Psychiatric Disorders and Association with Major Depression Polygenic Risk Scores. Am. J. Psychiatry 2019, 176, 651–660. [Google Scholar] [CrossRef]
- Liu, R.T.; Miller, I. Life events and suicidal ideation and behavior: A systematic review. Clin. Psychol. Rev. 2014, 34, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; Ma, Z.; Wang, G.; Jia, C.; Niu, L.; Zhou, L. The pattern of stressful life events prior to suicide among the older adults in rural China: A national case-control psychological autopsy study. BMC Geriatr. 2020, 20, 473. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Bellis, M.A.; Hardcastle, K.A.; Sethi, D.; Butchart, A.; Mikton, C.; Jones, L.; Dunne, M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e356–e366. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, I.; Austin, J.L.; Gooding, P. Association of Childhood Maltreatment With Suicide Behaviors Among Young People: A Systematic Review and Meta-analysis. JAMA Netw Open 2020, 3, e2012563. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.; Woo, J.; Maes, M.S.; Zai, C.C. Suicide epigenetics, a review of recent progress. J. Affect. Disord. 2020, 265, 423–438. [Google Scholar] [CrossRef]
- Rohleder, N. Stress and inflammation–The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 2019, 105, 164–171. [Google Scholar] [CrossRef]
- Hanisch, U.K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Brisch, R.; Wojtylak, S.; Saniotis, A.; Steiner, J.; Gos, T.; Kumaratilake, J.; Henneberg, M.; Wolf, R. The role of microglia in neuropsychiatric disorders and suicide. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 929–945. [Google Scholar] [CrossRef]
- Andrejew, R.; Oliveira-Giacomelli, Á.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 Receptor: Central Hub of Brain Diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef]
- Bryleva, E.Y.; Brundin, L. Kynurenine pathway metabolites and suicidality. Neuropharmacology 2017, 112 Pt B, 324–330. [Google Scholar] [CrossRef]
- Amitai, M.; Taler, M.; Ben-Baruch, R.; Lebow, M.; Rotkopf, R.; Apter, A.; Fennig, S.; Weizman, A.; Chen, A. Increased circulatory IL-6 during 8-week fluoxetine treatment is a risk factor for suicidal behaviors in youth. Brain Behav. Immun. 2020, 87, 301–308. [Google Scholar] [CrossRef]
- Brundin, L.; Bryleva, E.Y.; Thirtamara Rajamani, K. Role of Inflammation in Suicide: From Mechanisms to Treatment. Neuropsychopharmacology 2017, 42, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Serafini, G.; Costanza, A.; Aguglia, A.; Amerio, A.; Trabucco, A.; Escelsior, A.; Sher, L.; Amore, M. The role of inflammation in the pathophysiology of depression and suicidal behavior: Implications for treatment. Med. Clin. 2023, 107, 1–29. [Google Scholar]
- Bokor, J.; Sutori, S.; Torok, D.; Gal, Z.; Eszlari, N.; Gyorik, D.; Baksa, D.; Petschner, P.; Serafini, G.; Pompili, M.; et al. Inflamed Mind: Multiple Genetic Variants of. Front. Psychiatry 2021, 12, 746206. [Google Scholar] [CrossRef]
- Serafini, G.; Parisi, V.M.; Aguglia, A.; Amerio, A.; Sampogna, G.; Fiorillo, A.; Pompili, M.; Amore, M. A specific inflammatory profile underlying suicide risk? Systematic review of the main literature findings. Int. J. Environ. Res. Public Health 2020, 17, 2393. [Google Scholar] [CrossRef]
- Cha, C.B.; Franz, P.J.; Guzmán, E.M.; Glenn, C.R.; Kleiman, E.M.; Nock, M.K. Annual Research Review: Suicide among youth-epidemiology, (potential) etiology, and treatment. J. Child. Psychol. Psychiatry 2018, 59, 460–482. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ohgidani, M.; Kuwano, N.; Chrétien, F.; Lorin de la Grandmaison, G.; Onaya, M.; Tominaga, I.; Setoyama, D.; Kang, D.; Mimura, M.; et al. Suicide and Microglia: Recent Findings and Future Perspectives Based on Human Studies. Front. Cell Neurosci. 2019, 13, 31. [Google Scholar] [CrossRef]
- Steiner, J.; Bielau, H.; Brisch, R.; Danos, P.; Ullrich, O.; Mawrin, C.; Bernstein, H.G.; Bogerts, B. Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 2008, 42, 151–157. [Google Scholar] [CrossRef]
- Torres-Platas, S.G.; Cruceanu, C.; Chen, G.G.; Turecki, G.; Mechawar, N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014, 42, 50–59. [Google Scholar] [CrossRef]
- Meyer, J.H.; Cervenka, S.; Kim, M.J.; Kreisl, W.C.; Henter, I.D.; Innis, R.B. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 2020, 7, 1064–1074. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Biber, K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 2016, 64, 1772–1787. [Google Scholar] [CrossRef]
- Sperlágh, B.; Illes, P. P2X7 receptor: An emerging target in central nervous system diseases. Trends Pharmacol. Sci. 2014, 35, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.S.; Casarotto, P.C.; Hiroaki-Sato, V.A.; Sartim, A.G.; Guimarães, F.S.; Joca, S.R. Antidepressant- and anticompulsive-like effects of purinergic receptor blockade: Involvement of nitric oxide. Eur. Neuropsychopharmacol. 2013, 23, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Xin, Y.; JiaWen, W.; ShuQin, Z.; GuiLian, Z.; HaiQin, W.; Zhen, G.; HongWei, R.; YongNan, L. The P2X7 receptor in activated microglia promotes depression- and anxiety-like behaviors in lithium -pilocarpine induced epileptic rats. Neurochem. Int. 2020, 138, 104773. [Google Scholar] [CrossRef] [PubMed]
- Kristof, Z.; Eszlari, N.; Sutori, S.; Gal, Z.; Torok, D.; Baksa, D.; Petschner, P.; Sperlagh, B.; Anderson, I.M.; Deakin, J.F.W.; et al. P2RX7 gene variation mediates the effect of childhood adversity and recent stress on the severity of depressive symptoms. PLoS ONE 2021, 16, e0252766. [Google Scholar] [CrossRef]
- Kristof, Z.; Gal, Z.; Torok, D.; Eszlari, N.; Sutori, S.; Erdelyi-Hamza, B.; Petschner, P.; Sperlagh, B.; Anderson, I.M.; Deakin, J.F.W. Variation along P2RX7 interacts with early traumas on severity of anxiety suggesting a role for neuroinflammation. Sci. Rep. 2023, 13, 7757. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Clarke, T.-K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef]
- McGuffin, P.; Knight, J.; Breen, G.; Brewster, S.; Boyd, P.R.; Craddock, N.; Gill, M.; Korszun, A.; Maier, W.; Middleton, L. Whole genome linkage scan of recurrent depressive disorder from the depression network study. Hum. Mol. Genet. 2005, 14, 3337–3345. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, Y.; Li, M.; Zhang, Z.; Li, T.; Luo, X.-J. Genome-wide Mendelian randomization identifies actionable novel drug targets for psychiatric disorders. Neuropsychopharmacology 2023, 48, 270–280. [Google Scholar] [CrossRef]
- Shi, S.; White, M.; Borsetti, H.; Pendergast, J.S.; Hida, A.; Ciarleglio, C.; De Verteuil, P.; Cadar, A.; Cala, C.; McMahon, D. Molecular analyses of circadian gene variants reveal sex-dependent links between depression and clocks. Transl. Psychiatry 2016, 6, e748. [Google Scholar] [CrossRef]
- The International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009, 460, 748–752. [Google Scholar] [CrossRef]
- Lam, M.; Chen, C.-Y.; Li, Z.; Martin, A.R.; Bryois, J.; Ma, X.; Gaspar, H.; Ikeda, M.; Benyamin, B.; Brown, B.C. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat. Genet. 2019, 51, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.D.; Huang, X.; Fox, K.R.; Franklin, J.C. Depression and hopelessness as risk factors for suicide ideation, attempts and death: Meta-analysis of longitudinal studies. Br. J. Psychiatry 2018, 212, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.E.; Johnson, E.; Lawrence, J.M.; Rossom, R.C.; Ahmedani, B.; Lynch, F.L.; Beck, A.; Waitzfelder, B.; Ziebell, R.; Penfold, R.B. Predicting suicide attempts and suicide deaths following outpatient visits using electronic health records. Am. J. Psychiatry 2018, 175, 951–960. [Google Scholar] [CrossRef] [PubMed]
- McHugh, C.M.; Corderoy, A.; Ryan, C.J.; Hickie, I.B.; Large, M.M. Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open 2019, 5, e18. [Google Scholar] [CrossRef] [PubMed]
- von Mücke-Heim, I.A.; Deussing, J.M. The P2X7 receptor in mood disorders: Emerging target in immunopsychiatry, from bench to bedside. Neuropharmacology 2022, 224, 109366. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.A.; de Melo Reis, R.A.; de Souza, C.A.; de Freitas, M.S.; Teixeira, P.C.; Neto Moreira Ferreira, D.; Xavier, R.F. The P2X7 receptor: Shifting from a low- to a high-conductance channel—An enigmatic phenomenon? Biochim. Biophys. Acta 2014, 1838, 2578–2587. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Schmalzing, G.; Markwardt, F. The Elusive P2X7 Macropore. Trends Cell Biol. 2018, 28, 392–404. [Google Scholar] [CrossRef]
- Kaczmarek-Hajek, K.; Zhang, J.; Kopp, R.; Grosche, A.; Rissiek, B.; Saul, A.; Bruzzone, S.; Engel, T.; Jooss, T.; Krautloher, A.; et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. eLife 2018, 7, e36217. [Google Scholar] [CrossRef]
- Illes, P.; Khan, T.M.; Rubini, P. Neuronal P2X7 Receptors Revisited: Do They Really Exist? J. Neurosci. 2017, 37, 7049–7062. [Google Scholar] [CrossRef]
- Csölle, C.; Baranyi, M.; Zsilla, G.; Kittel, A.; Gölöncsér, F.; Illes, P.; Papp, E.; Vizi, E.S.; Sperlágh, B. Neurochemical Changes in the Mouse Hippocampus Underlying the Antidepressant Effect of Genetic Deletion of P2X7 Receptors. PLoS ONE 2013, 8, e66547. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 1051, 17–53. [Google Scholar] [PubMed]
- Black, C.; Miller, B.J. Meta-Analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal Versus Nonsuicidal Patients. Biol. Psychiatry 2015, 78, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Ganança, L.; Oquendo, M.A.; Tyrka, A.R.; Cisneros-Trujillo, S.; Mann, J.J.; Sublette, M.E. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology 2016, 63, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.P.; Franklin, J.C.; Ribeiro, J.D.; Fox, K.R.; Bentley, K.H.; Kleiman, E.M.; Nock, M.K. Biological risk factors for suicidal behaviors: A meta-analysis. Transl. Psychiatry 2016, 6, e887. [Google Scholar] [CrossRef] [PubMed]
- Schiweck, C.; Claes, S.; Van Oudenhove, L.; Lafit, G.; Vaessen, T.; de Beeck, G.O.; Berghmans, R.; Wijkhuijs, A.; Müller, N.; Arolt, V.; et al. Childhood trauma, suicide risk and inflammatory phenotypes of depression: Insights from monocyte gene expression. Transl. Psychiatry 2020, 10, 296. [Google Scholar] [CrossRef]
- Lund-Sørensen, H.; Benros, M.E.; Madsen, T.; Sørensen, H.J.; Eaton, W.W.; Postolache, T.T.; Nordentoft, M.; Erlangsen, A. A Nationwide Cohort Study of the Association Between Hospitalization with Infection and Risk of Death by Suicide. JAMA Psychiatry 2016, 73, 912–919. [Google Scholar] [CrossRef]
- Lengvenyte, A.; Conejero, I.; Courtet, P.; Olié, E. Biological bases of suicidal behaviours: A narrative review. Eur. J. Neurosci. 2021, 53, 330–351. [Google Scholar] [CrossRef]
- Keaton, S.A.; Madaj, Z.B.; Heilman, P.; Smart, L.; Grit, J.; Gibbons, R.; Postolache, T.T.; Roaten, K.; Achtyes, E.D.; Brundin, L. An inflammatory profile linked to increased suicide risk. J. Affect. Disord. 2019, 247, 57–65. [Google Scholar] [CrossRef]
- Batty, G.D.; Bell, S.; Stamatakis, E.; Kivimäki, M. Association of Systemic Inflammation with Risk of Completed Suicide in the General Population. JAMA Psychiatry 2016, 73, 993–995. [Google Scholar] [CrossRef]
- Dwivedi, Y. MicroRNAs in depression and suicide: Recent insights and future perspectives. J. Affect. Disord. 2018, 240, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Shinko, Y.; Otsuka, I.; Okazaki, S.; Horai, T.; Boku, S.; Takahashi, M.; Ueno, Y.; Sora, I.; Hishimoto, A. Chemokine alterations in the postmortem brains of suicide completers. J. Psychiatr. Res. 2020, 120, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Borisov, A.S.; Broadwell, S.D.; Capuron, L.; Woolwine, B.J.; Jacobson, I.M.; Nemeroff, C.B.; Miller, A.H. Depression during pegylated interferon-alpha plus ribavirin therapy: Prevalence and prediction. J. Clin. Psychiatry 2005, 66, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Constant, A.; Castera, L.; Dantzer, R.; Couzigou, P.; de Ledinghen, V.; Demotes-Mainard, J.; Henry, C. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: Evidence for an overlap between manic/hypomanic and depressive symptoms. J. Clin. Psychiatry 2005, 66, 1050–1057. [Google Scholar] [CrossRef]
- Dieperink, E.; Ho, S.B.; Tetrick, L.; Thuras, P.; Dua, K.; Willenbring, M.L. Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. Gen. Hosp. Psychiatry 2004, 26, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.; Brouwer, J.T.; van der Mast, R.C.; Schalm, S.W. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J. Hepatol. 1994, 21, 241–243. [Google Scholar] [CrossRef]
- Lehrer, S.; Rheinstein, P.H. Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce suicidal ideation and depression. Discov. Med. 2019, 28, 205–212. [Google Scholar] [PubMed]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Erhardt, A.; Lucae, S.; Unschuld, P.G.; Ising, M.; Kern, N.; Salyakina, D.; Lieb, R.; Uhr, M.; Binder, E.B.; Keck, M.E.; et al. Association of polymorphisms in P2RX7 and CaMKKb with anxiety disorders. J. Affect. Disord. 2007, 101, 159–168. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zeng, R.; Gorodeski, G. Endogenously expressed truncated P2X7 receptor lacking the C-terminus is preferentially upregulated in epithelial cancer cells and fails to mediate ligand-induced pore formation and apoptosis. Nucleosides Nucleotides Nucleic Acids 2006, 25, 1271–1276. [Google Scholar] [CrossRef]
- Adinolfi, E.; Cirillo, M.; Woltersdorf, R.; Falzoni, S.; Chiozzi, P.; Pellegatti, P.; Callegari, M.G.; Sandona, D.; Markwardt, F.; Schmalzing, G. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010, 24, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-H.; Roger, S.; Baldwin, S. Insights into the molecular mechanisms underlying mammalian P2X7 receptor functions and contributions in diseases, revealed by structural modeling and single nucleotide polymorphisms. Front. Pharmacol. 2013, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Syed Mortadza, S.A.; Yan, J.; Zhang, L.; Wang, L.; Yin, Y.; Li, C.; Chalon, S.; Emond, P.; Belzung, C.; et al. ATP-activated P2X7 receptor in the pathophysiology of mood disorders and as an emerging target for the development of novel antidepressant therapeutics. Neurosci. Biobehav. Rev. 2018, 87, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Galfalvy, H.; Haghighi, F.; Hodgkinson, C.; Goldman, D.; Oquendo, M.A.; Burke, A.; Huang, Y.Y.; Giegling, I.; Rujescu, D.; Bureau, A.; et al. A genome-wide association study of suicidal behavior. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 168, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Afifi, T.O.; Henriksen, C.A.; Asmundson, G.J.; Sareen, J. Childhood maltreatment and substance use disorders among men and women in a nationally representative sample. Can. J. Psychiatry 2012, 57, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Chuang, D.M.; Lee, Y. Adverse childhood experiences, gender, and HIV risk behaviors: Results from a population-based sample. Prev. Med. Rep. 2016, 4, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Lindert, J.; von Ehrenstein, O.S.; Grashow, R.; Gal, G.; Braehler, E.; Weisskopf, M.G. Sexual and physical abuse in childhood is associated with depression and anxiety over the life course: Systematic review and meta-analysis. Int. J. Public Health 2014, 59, 359–372. [Google Scholar] [CrossRef]
- Cloitre, M.; Stovall-McClough, K.C.; Nooner, K.; Zorbas, P.; Cherry, S.; Jackson, C.L.; Gan, W.; Petkova, E. Treatment for PTSD related to childhood abuse: A randomized controlled trial. Am. J. Psychiatry 2010, 167, 915–924. [Google Scholar] [CrossRef]
- Brodsky, B.S.; Stanley, B. Adverse childhood experiences and suicidal behavior. Psychiatr. Clin. N. Am. 2008, 31, 223–235. [Google Scholar] [CrossRef]
- Marshall, B.D.; Galea, S.; Wood, E.; Kerr, T. Longitudinal associations between types of childhood trauma and suicidal behavior among substance users: A cohort study. Am. J. Public Health 2013, 103, e69–e75. [Google Scholar] [CrossRef]
- Danese, A.; Lewis, S.L. Psychoneuroimmunology of Early-Life Stress: The Hidden Wounds of Childhood Trauma? Neuropsychopharmacology 2017, 42, 99–114. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.B.; Green, J.A.; Ferguson, E.; O’Carroll, R.E.; O’Connor, R.C. Effects of childhood trauma on cortisol levels in suicide attempters and ideators. Psychoneuroendocrinology 2018, 88, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, C.P.; Glaser, R.; Kiecolt-Glaser, J.K. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun. 2013, 27, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.S.; Sluyter, R.; Gu, B.J.; Stokes, L.; Fuller, S.J. The human P2X7 receptor and its role in innate immunity. Tissue Antigens 2011, 78, 321–332. [Google Scholar] [CrossRef]
- Lord, B.; Ameriks, M.K.; Wang, Q.; Fourgeaud, L.; Vliegen, M.; Verluyten, W.; Haspeslagh, P.; Carruthers, N.I.; Lovenberg, T.W.; Bonaventure, P.; et al. A novel radioligand for the ATP-gated ion channel P2X7: [3H] JNJ-54232334. Eur. J. Pharmacol. 2015, 765, 551–559. [Google Scholar] [CrossRef]
- He, Y.; Taylor, N.; Fourgeaud, L.; Bhattacharya, A. The role of microglial P2X7: Modulation of cell death and cytokine release. J. Neuroinflamm. 2017, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Falzoni, S.; Donvito, G.; Di Virgilio, F. Detecting adenosine triphosphate in the pericellular space. Interface Focus 2013, 3, 20120101. [Google Scholar] [CrossRef]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef]
- von Muecke-Heim, I.A.; Ries, C.; Urbina, L.; Deussing, J.M. P2X7R antagonists in chronic stress-based depression models: A review. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 1343–1358. [Google Scholar] [CrossRef]
- Urbina-Treviño, L.; von Mücke-Heim, I.A.; Deussing, J.M. P2X7 Receptor-Related Genetic Mouse Models—Tools for Translational Research in Psychiatry. Front. Neural Circuits 2022, 16, 876304. [Google Scholar] [CrossRef] [PubMed]
- Turecki, G. The molecular bases of the suicidal brain. Nat. Rev. Neurosci. 2014, 15, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Labonté, B.; Suderman, M.; Maussion, G.; Navaro, L.; Yerko, V.; Mahar, I.; Bureau, A.; Mechawar, N.; Szyf, M.; Meaney, M.J.; et al. Genome-wide epigenetic regulation by early-life trauma. Arch. Gen. Psychiatry 2012, 69, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Labonté, B.; Suderman, M.; Maussion, G.; Lopez, J.P.; Navarro-Sánchez, L.; Yerko, V.; Mechawar, N.; Szyf, M.; Meaney, M.J.; Turecki, G. Genome-wide methylation changes in the brains of suicide completers. Am. J. Psychiatry 2013, 170, 511–520. [Google Scholar] [CrossRef]
- Weder, N.; Zhang, H.; Jensen, K.; Yang, B.Z.; Simen, A.; Jackowski, A.; Lipschitz, D.; Douglas-Palumberi, H.; Ge, M.; Perepletchikova, F.; et al. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 417–424.e5. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.; Deleva, V.; Deng, X.; Sequeira, A.; Pomarenski, A.; Klempan, T.; Ernst, N.; Quirion, R.; Gratton, A.; Szyf, M.; et al. Alternative splicing, methylation state, and expression profile of tropomyosin-related kinase B in the frontal cortex of suicide completers. Arch. Gen. Psychiatry 2009, 66, 22–32. [Google Scholar] [CrossRef]
- Roth, T.L.; Lubin, F.D.; Funk, A.J.; Sweatt, J.D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry 2009, 65, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Ghosh, A.K.; Ghosh, B.; Bhattacharyya, S.; Mondal, A.C. Decreased mRNA and Protein Expression of BDNF, NGF, and their Receptors in the Hippocampus from Suicide: An Analysis in Human Postmortem Brain. Clin. Med. Insights Pathol. 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Stein, D.J.; Vasconcelos, M.F.; Albrechet-Souza, L.; Ceresér, K.M.M.; de Almeida, R.M.M. Microglial Over-Activation by Social Defeat Stress Contributes to Anxiety- and Depressive-Like Behaviors. Front. Behav. Neurosci. 2017, 11, 207. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Trojan, E.; Głombik, K.; Budziszewska, B.; Kubera, M.; Lasoń, W.; Popiołek-Barczyk, K.; Mika, J.; Wędzony, K.; Basta-Kaim, A. Prenatal stress is a vulnerability factor for altered morphology and biological activity of microglia cells. Front. Cell. Neurosci. 2015, 9, 82. [Google Scholar] [CrossRef]
- Delpech, J.C.; Wei, L.; Hao, J.; Yu, X.; Madore, C.; Butovsky, O.; Kaffman, A. Early life stress perturbs the maturation of microglia in the developing hippocampus. Brain Behav. Immun. 2016, 57, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.; Ochoa-Zarzosa, A.; Torner, L. Maternal separation activates microglial cells and induces an inflammatory response in the hippocampus of male rat pups, independently of hypothalamic and peripheral cytokine levels. Brain Behav. Immun. 2016, 55, 39–48. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.; Pakan, J.M.P.; Yilmazer-Hanke, D.; McDermott, K.W. Acute in utero exposure to lipopolysaccharide induces inflammation in the pre- and postnatal brain and alters the glial cytoarchitecture in the developing amygdala. J Neuroinflammation 2017, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Delpech, J.C. Dynamic cross-talk between microglia and peripheral monocytes underlies stress-induced neuroinflammation and behavioral consequences. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79 Pt A, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, G.; Chase, D.; Pegg, E.; Downey, D.; Toth, Z.G.; Stones, K.; Platt, H.; Mekli, K.; Payton, A.; Elliott, R.; et al. CNR1 gene is associated with high neuroticism and low agreeableness and interacts with recent negative life events to predict current depressive symptoms. Neuropsychopharmacology 2009, 34, 2019–2027. [Google Scholar] [CrossRef]
- Lazary, J.; Lazary, A.; Gonda, X.; Benko, A.; Molnar, E.; Juhasz, G.; Bagdy, G. New evidence for the association of the serotonin transporter gene (SLC6A4) haplotypes, threatening life events, and depressive phenotype. Biol. Psychiatry 2008, 64, 498–504. [Google Scholar] [CrossRef]
- Juhasz, G.; Dunham, J.S.; McKie, S.; Thomas, E.; Downey, D.; Chase, D.; Lloyd-Williams, K.; Toth, Z.G.; Platt, H.; Mekli, K.; et al. The CREB1-BDNF-NTRK2 Pathway in Depression: Multiple Gene-Cognition-Environment Interactions. Biol. Psychiatry 2011, 69, 762–771. [Google Scholar] [CrossRef]
- Derogatis, L.R.; Melisaratos, N. The brief symptom inventory: An introductory report. Psychol. Med. 1983, 13, 595–605. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Fink, L.; Handelsman, L.; Foote, J.; Lovejoy, M.; Wenzel, K.; Sapareto, E.; Ruggiero, J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 1994, 151, 1132–1136. [Google Scholar]
- Brugha, T.; Bebbington, P.; Tennant, C.; Hurry, J. The List of Threatening Experiences: A subset of 12 life event categories with considerable long-term contextual threat. Psychol. Med. 1985, 15, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Brugha, T.S.; Cragg, D. The List of Threatening Experiences: The reliability and validity of a brief life events questionnaire. Acta Psychiatr. Scand. 1990, 82, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.; Smith, N.; Curtis, C.; Huckett, L.; Mill, J.; Craig, I.W. DNA from buccal swabs recruited by mail: Evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav. Genet. 2003, 33, 67–72. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristof, Z.; Gal, Z.; Torok, D.; Eszlari, N.; Sutori, S.; Sperlagh, B.; Anderson, I.M.; Deakin, B.; Bagdy, G.; Juhasz, G.; et al. Embers of the Past: Early Childhood Traumas Interact with Variation in P2RX7 Gene Implicated in Neuroinflammation on Markers of Current Suicide Risk. Int. J. Mol. Sci. 2024, 25, 865. https://doi.org/10.3390/ijms25020865
Kristof Z, Gal Z, Torok D, Eszlari N, Sutori S, Sperlagh B, Anderson IM, Deakin B, Bagdy G, Juhasz G, et al. Embers of the Past: Early Childhood Traumas Interact with Variation in P2RX7 Gene Implicated in Neuroinflammation on Markers of Current Suicide Risk. International Journal of Molecular Sciences. 2024; 25(2):865. https://doi.org/10.3390/ijms25020865
Chicago/Turabian StyleKristof, Zsuliet, Zsofia Gal, Dora Torok, Nora Eszlari, Sara Sutori, Beata Sperlagh, Ian M. Anderson, Bill Deakin, Gyorgy Bagdy, Gabriella Juhasz, and et al. 2024. "Embers of the Past: Early Childhood Traumas Interact with Variation in P2RX7 Gene Implicated in Neuroinflammation on Markers of Current Suicide Risk" International Journal of Molecular Sciences 25, no. 2: 865. https://doi.org/10.3390/ijms25020865
APA StyleKristof, Z., Gal, Z., Torok, D., Eszlari, N., Sutori, S., Sperlagh, B., Anderson, I. M., Deakin, B., Bagdy, G., Juhasz, G., & Gonda, X. (2024). Embers of the Past: Early Childhood Traumas Interact with Variation in P2RX7 Gene Implicated in Neuroinflammation on Markers of Current Suicide Risk. International Journal of Molecular Sciences, 25(2), 865. https://doi.org/10.3390/ijms25020865






