Floral Volatile Organic Compounds Change the Composition and Function of the Endophytic Fungal Community in the Flowers of Osmanthus fragrans
Abstract
1. Introduction
2. Results
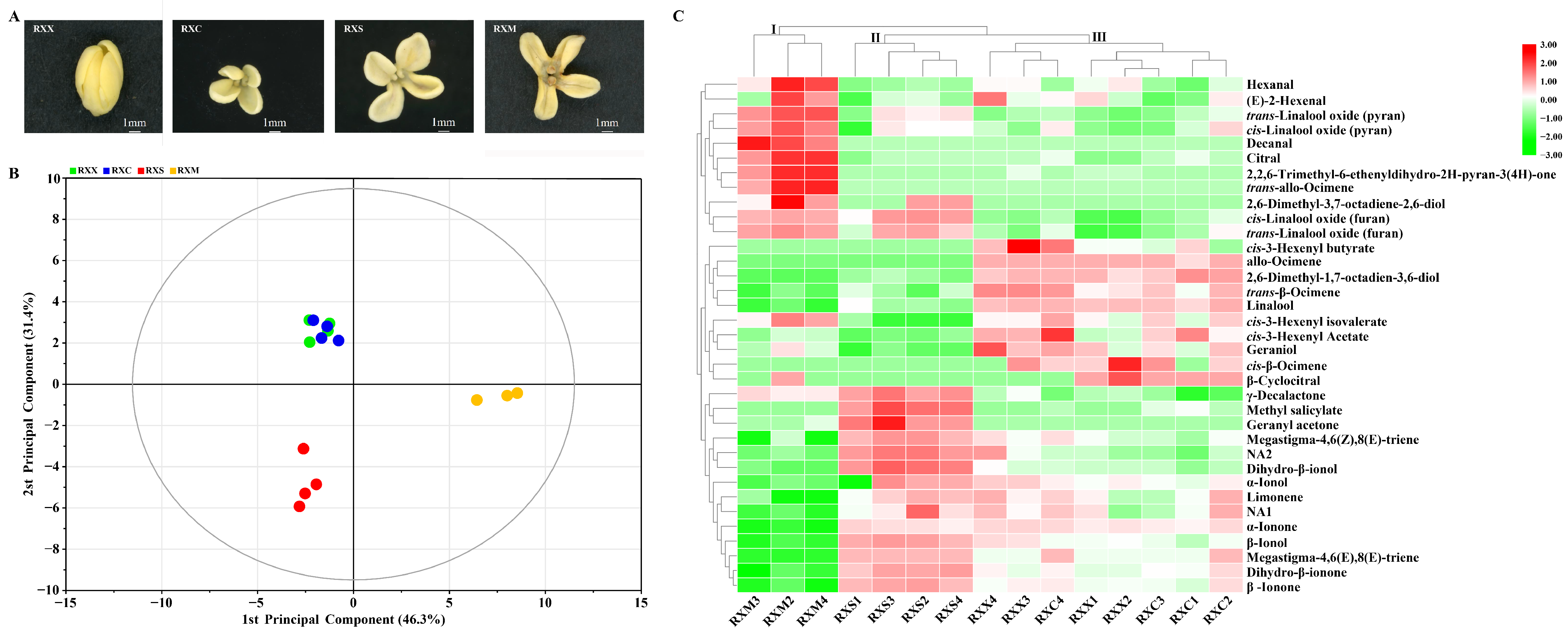
2.1. Identification of O. fragrans ‘Rixiang Gui’ VOCs during Flowering
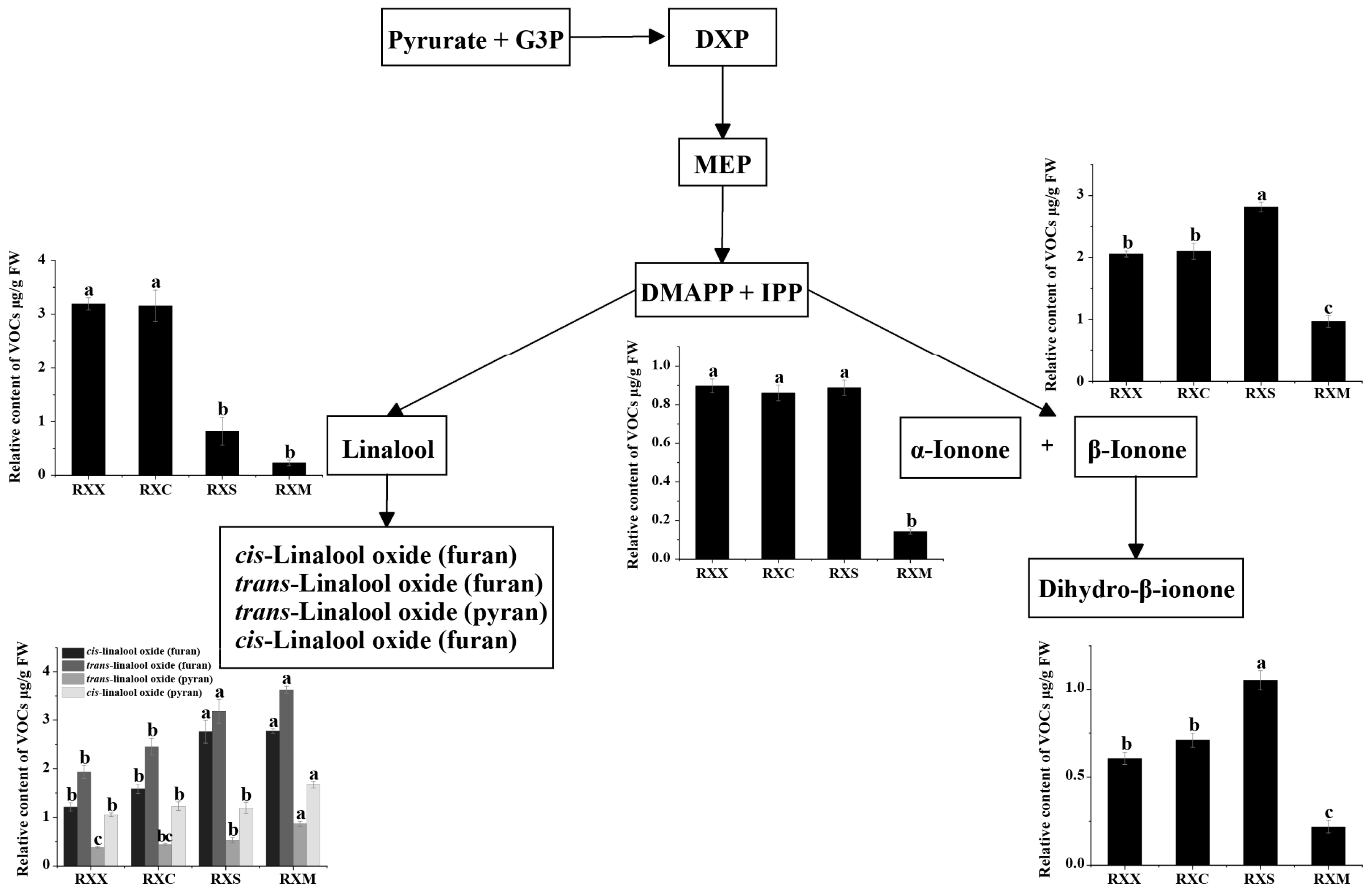
2.2. Differential Release of VOCs at Four Stages of Flowering Development
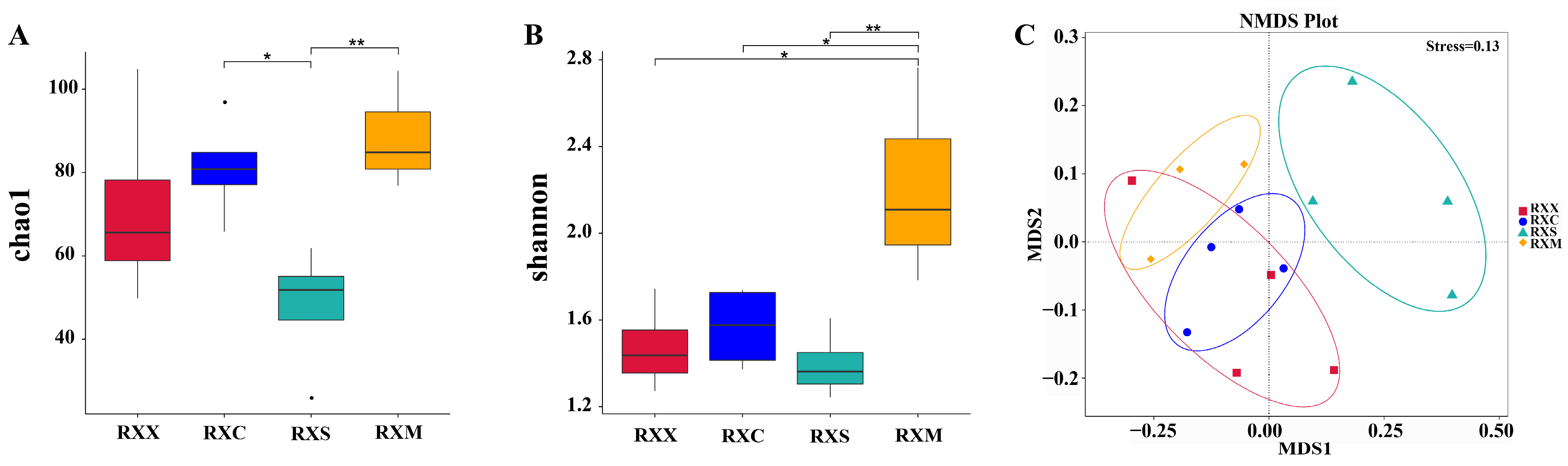
2.3. Alpha Diversity and Community Composition of Endophytic Fungi in O. fragrans ‘Rixiang Gui’ Flowers
2.4. Functional Prediction of the Endophytic Fungi Community at Different Stages of Flowering Development
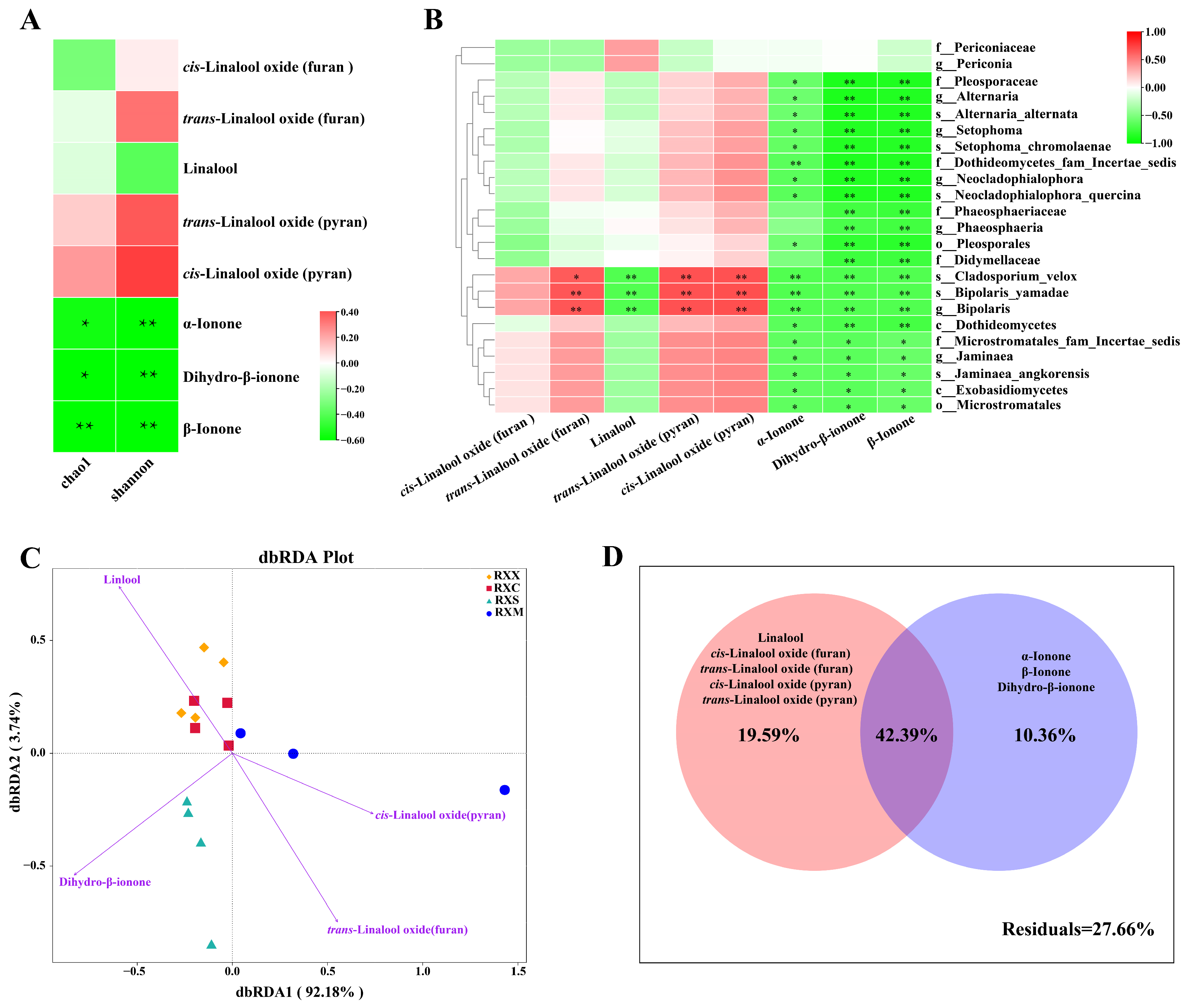
2.5. Linkages between VOCs and Endophytic Fungal Communities in O. fragrans ‘Rixiang Gui’ Flowers
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Detection of VOCs
4.3. DNA Extraction, Sequencing, Library Construction, and Processing
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez, R.J.; White, J.F., Jr.; Arnold, A.E.; Redman, A.R.A. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O. Biotechnological overview of agriculturally important endophytic fungi. Hortic. Environ. Biote. 2021, 62, 507–520. [Google Scholar] [CrossRef]
- Coleman-Derr, D.; Desgarennes, D.; Fonseca-Garcia, C.; Gross, S.; Clingenpeel, S.; Woyke, T.; North, G.; Visel, A.; Partida-Martinez, L.P.; Tringe, S.G. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol. 2016, 209, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gong, X. Niche differentiation rather than biogeography shapes the diversity and composition of microbiome of Cycas panzhihuaensis. Microbiome 2019, 7, 152. [Google Scholar] [CrossRef]
- Huang, A.C.; Jiang, T.; Liu, Y.X.; Bai, Y.C.; Reed, J.; Qu, B.; Goossens, A.; Ntzmann, H.W.; Bai, Y.; Osbourn, A. A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 2019, 364, eaau6389. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; You, C.; Lan, W.Y.; Wang, Y.M.; Yu, B.H.; Peng, Y.I.; Xu, J.R.; Dong, J.Y. Microbial assemblages of Schisandraceae plants and the correlations between endophytic species and the accumulation of secondary metabolites. Plant Soil 2023, 483, 85–107. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]
- Schiestl, F.P. Ecology and evolution of floral volatile-mediated information transfer in plants. New Phytol. 2015, 206, 571–577. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef]
- Rebolleda Gómez, M.; Ashman, T.L. Floral organs act as environmental filters and interact with pollinators to structure the yellow monkeyflower (Mimulus guttatus) floral microbiome. Mol. Ecol. 2019, 28, 5155–5171. [Google Scholar] [CrossRef]
- Keller, A.; McFrederick, Q.S.; Dharampal, P.; Steffan, S.; Danforth, B.N.; Leonhardt, S.D. (More than) Hitchhikers through the network: The shared microbiome of bees and flowers. Curr. Opin. Insect Sci. 2021, 44, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, A.; Bella, P.; Peri, E.; Rostás, M.; Guarino, S.; Lievens, B.; Colazza, S. Nectar-inhabiting bacteria affect olfactory responses of an insect parasitoid by altering nectar odors. Microb. Ecol. 2023, 86, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Bidirectional interaction between phyllospheric microbiotas and plant volatile emissions. Trends Plant Sci. 2016, 21, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, R.; Zhang, J.; Wang, Y.; Kong, P.; Lu, K.; Adnan; Liu, M.; Ao, F.; Zhao, C.; et al. The biochemical and molecular investigation of flower color and scent sheds lights on further genetic modification of ornamental traits in Clivia miniata. Hortic. Res. 2022, 9, uhac114. [Google Scholar] [CrossRef]
- Fu, J.X.; Hou, D.; Wang, Y.G.; Zhang, C.; Bao, Z.Y.; Zhao, H.B.; Hu, S.Q. Identification of floral aromatic volatile compounds in 29 cultivars from four groups of Osmanthus fragrans by gas Chromatography-mass spectrometry. Hortic. Environ. Biote. 2019, 60, 611–623. [Google Scholar] [CrossRef]
- Vannette, R.L. The floral microbiome: Plant, pollinator, and microbial perspectives. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 363–386. [Google Scholar] [CrossRef]
- Wei, N.; Russell, A.L.; Jarrett, A.R.; Ashman, T.L. Pollinators mediate floral microbial diversity and microbial network under agrochemical disturbance. Mol. Ecol. 2021, 30, 2235–2247. [Google Scholar] [CrossRef] [PubMed]
- Boachon, B.; Burdloff, Y.; Ruan, J.X.; Rojo, R.; Junker, R.R.; Vincent, B.; Nicolè, F.; Bringel, F.; Lesot, A.; Henry, L.; et al. A promiscuous CYP706A3 reduces terpene volatile emission from Arabidopsis flowers, affecting florivores and the floral microbiome. Plant Cell 2019, 31, 2947–2972. [Google Scholar] [CrossRef]
- Wei, N.; Whyle, R.L.; Ashman, T.L.; Jamieson, M.A. Genotypic variation in floral volatiles influences floral microbiome more strongly than interactions with herbivores and mycorrhizae in strawberries. Hortic. Res. 2022, 9, uhab005. [Google Scholar] [CrossRef]
- Yao, H.; Sun, X.; He, C.; Maitra, P.; Li, X.C.; Guo, L.D. Phyllosphere epiphytic and endophytic fungal community and network structures differ in a tropical mangrove ecosystem. Microbiome 2019, 7, 57. [Google Scholar] [CrossRef]
- Dong, C.J.; Wang, L.L.; Li, Q.; Shang, Q.M. Epiphytic and endophytic fungal communities of tomato plants. Hortic. Plant J. 2021, 7, 38–48. [Google Scholar] [CrossRef]
- Yue, Y.Z.; Shi, T.T.; Liu, J.W.; Tian, Q.T.; Yang, X.L.; Wang, L.G. Genomic, metabonomic and transcriptomic analyses of sweet osmanthus varieties provide insights into floral aroma formation. Sci. Hortic. 2022, 306, 111442. [Google Scholar] [CrossRef]
- Zou, J.J.; Cai, X.; Zeng, X.L.; Yang, J.; Wang, C.Y. Characterization of aroma-active compounds from Sweet Osmanthus (Osmanthus fragrans) by SDE and SPME coupled with GC-MS and GC-Olfactometry. Int. J. Agric. Biol. 2019, 22, 277–282. [Google Scholar]
- Zhang, Z.Z.; Li, Y.B.; Qi, L.; Wan, X.C. Antifungal activities of major tea leaf volatile constituents toward Colletorichum camelliae Massea. J. Agric. Food Chem. 2006, 54, 3936–3940. [Google Scholar] [CrossRef]
- Nakahara, K.; Alzoreky, N.S.; Yoshihashi, T.; Nguyen, H.T.T.; Trakoontivakorn, G. Chemical composition and antifungal activity of essential oil from Cymbopogon nardus (citronella grass). Jpn. Agric. Res. Q. JARQ 2013, 37, 249–252. [Google Scholar] [CrossRef]
- Ding, W.J.; Ouyang, Q.X.; Li, Y.L.; Shi, T.T.; Li, L.; Yang, X.L.; Ji, K.S.; Wang, L.G.; Yue, Y.Z. Genome-wide investigation of WRKY transcription factors in sweet osmanthus and their potential regulation of aroma synthesis. Tree Physiol. 2020, 40, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.L.; Liu, C.; Zheng, R.R.; Cai, X.; Luo, J.; Zou, J.J.; Wang, C.Y. Emission and accumulation of monoterpene and the key terpene synthase (TPS) associated with monoterpene biosynthesis in Osmanthus fragrans Lour. Front. Plant Sci. 2016, 6, 1232. [Google Scholar] [CrossRef]
- Yun, J.; Cui, C.J.; Zhang, S.H.; Zhu, J.J.; Peng, C.Y.; Cai, H.; Cai, H.M.; Yang, X.G.; Hou, R.Y. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chem. 2021, 360, 130033. [Google Scholar] [CrossRef]
- Liu, D.; Howell, K. Community succession of the grapevine fungal microbiome in the annual growth cycle. Environ. Microbiol. 2021, 23, 1842–1857. [Google Scholar] [CrossRef]
- Wei, R.T.; Ding, Y.T.; Gao, F.F.; Zhang, L.; Wang, L.; Li, H.; Wang, H. Community succession of the grape epidermis microbes of cabernet sauvignon (Vitis vinifera L.) from different regions in China during fruit development. Int. J. Food Microbiol. 2022, 362, 109475. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Burkle, L.A.; Runyon, J.B. Floral volatiles structure plant-pollinator interactions in a diverse community across the growing season. Funct. Ecol. 2019, 33, 2116–2129. [Google Scholar] [CrossRef]
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-beta-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.R.; Loewel, C.; Gross, R.; Dötterl, S.; Keller, A.; Blüthgen, N. Composition of epiphytic bacterial communities differs on petals and leaves. Plant Biol. 2011, 13, 918–924. [Google Scholar] [CrossRef]
- Burdon, R.C.; Junker, R.R.; Scofield, D.G.; Parachnowitsch, A.L. Bacteria colonising Penstemon digitalis show volatile and tissue-specific responses to a natural concentration range of the floral volatile linalool. Chemoecology 2018, 28, 11–19. [Google Scholar] [CrossRef]
- Gundel, P.E.; Rudgers, J.A.; Whitney, K.D. Vertically transmitted symbionts as mechanisms of transgenerational effects. Am. J. Bot. 2017, 104, 787–792. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.J. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, Z.; Friesen, T.L.; Raats, D.; Fahima, T.; Brueggeman, R.S.; Lu, S.; Trick, H.N.; Liu, Z.; Chao, W.; et al. The hijacking of a receptor kinase–driven pathway by a wheat fungal pathogen leads to disease. Sci. Adv. 2016, 2, e1600822. [Google Scholar] [CrossRef]
- Zhu, H.J.; Sun, C.X.; Tong, Y.K.; Wang, D.H.; Chen, S.Y.; Cheng, Z.L.; Li, Q. Insight on the relationship between the compositions and antimicrobial activities of Osmanthus fragrans Lour. (Oleaceae family) essential oils by multivariable analysis. Eur. Food Res. Technol. 2021, 247, 1737–1744. [Google Scholar] [CrossRef]
- Boachon, B.; Junker, R.R.; Miesch, L.; Bassard, J.E.; Höfer, R.; Caillieaudeaux, R.; Seidel, D.E.; Lesot, A.; Heinrich, C.; Ginglinger, J.F.; et al. CYP76C1(Cytochrome P450)-mediated linalool metabolism and the formation of volatile and soluble linalool oxides in Arabidopsis flowers: A strategy for defense against floral antagonists. Plant Cell 2015, 27, 2972–2990. [Google Scholar] [CrossRef]
- Yamagiwa, Y.; Inagaki, Y.; Ichinose, Y.; Toyoda, K.; Hyakumachi, M.; Shiraishi, T. Talaromyces wortmannii FS2 emits beta-caryphyllene, which promotes plant growth and induces resistance. J. Gen. Plant Pathol. 2011, 77, 336–341. [Google Scholar] [CrossRef]
- Amirzakariya, B.Z.; Shakeri, A. Bioactive terpenoids derived from plant endophytic fungi: An updated review (2011–2020). Phytochemistry 2022, 197, 113130. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, C.L.; Teixeira Duarte, M.C.; Baesa Ribeiro, B.; de Magalhães, P.M. Linalool production from the leaves of Bursera aloexylon and its antimicrobial activity. Fitoterapia 2007, 78, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Li, C.; Zhang, L.Y.; Zhang, L.; Lin, J.; Jiang, N.; Wang, Q.; Xu, Q.; Zheng, H.R.; Gu, L.W.; et al. Mechanism of antifungal activity and therapeutic action of β-ionone on Aspergillus fumigatus keratitis via suppressing LOX1 and JNK/p38 MAPK activation. Int. Immunopharmacol. 2022, 110, 108992. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Wang, Q.F.; Li, H.S.; Wang, X.Y.; Zhang, R.M.; Yang, X.Y.; Jiang, Q.W.; Shi, Q.H. Revealing the mechanisms for linalool antifungal activity against Fusarium oxysporum and its efficient control of fusarium wilt in tomato plants. Int. J. Mol. Sci. 2023, 24, 458. [Google Scholar] [CrossRef]
- Fu, J.; Hou, D.; Zhang, C.; Bao, Z.; Zhao, H.; Hu, S. The emission of the floral scent of four Osmanthus fragrans cultivars in response to different temperatures. Molecules 2017, 22, 430. [Google Scholar] [CrossRef]
- Cai, X.; Yang, J.; Zeng, X.; Zou, J.J. Light and Temperature Effects on the Color and Scent of Osmanthus fragrans ‘Houban Yingui’. Hortic. Sci. Technol. 2022, 40, 595–604. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Shi, M.; Ye, Y.; Yue, Y.; Wang, L.; Yang, X. Floral Volatile Organic Compounds Change the Composition and Function of the Endophytic Fungal Community in the Flowers of Osmanthus fragrans. Int. J. Mol. Sci. 2024, 25, 857. https://doi.org/10.3390/ijms25020857
Shi T, Shi M, Ye Y, Yue Y, Wang L, Yang X. Floral Volatile Organic Compounds Change the Composition and Function of the Endophytic Fungal Community in the Flowers of Osmanthus fragrans. International Journal of Molecular Sciences. 2024; 25(2):857. https://doi.org/10.3390/ijms25020857
Chicago/Turabian StyleShi, Tingting, Man Shi, Yunfang Ye, Yuanzheng Yue, Lianggui Wang, and Xiulian Yang. 2024. "Floral Volatile Organic Compounds Change the Composition and Function of the Endophytic Fungal Community in the Flowers of Osmanthus fragrans" International Journal of Molecular Sciences 25, no. 2: 857. https://doi.org/10.3390/ijms25020857
APA StyleShi, T., Shi, M., Ye, Y., Yue, Y., Wang, L., & Yang, X. (2024). Floral Volatile Organic Compounds Change the Composition and Function of the Endophytic Fungal Community in the Flowers of Osmanthus fragrans. International Journal of Molecular Sciences, 25(2), 857. https://doi.org/10.3390/ijms25020857






