Preoperative Factors for Lymphovascular Invasion in Prostate Cancer: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
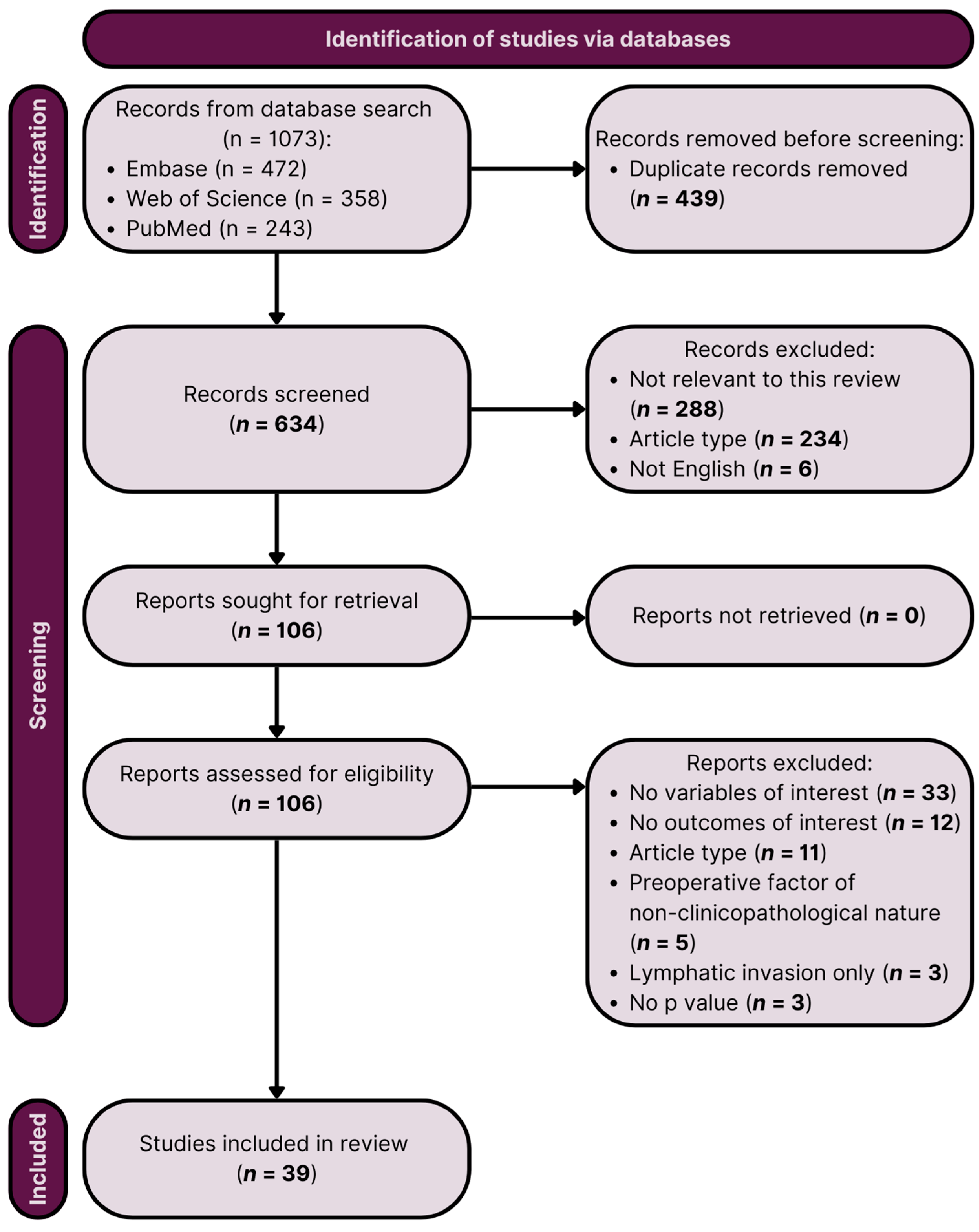
2.1. Study Characteristics
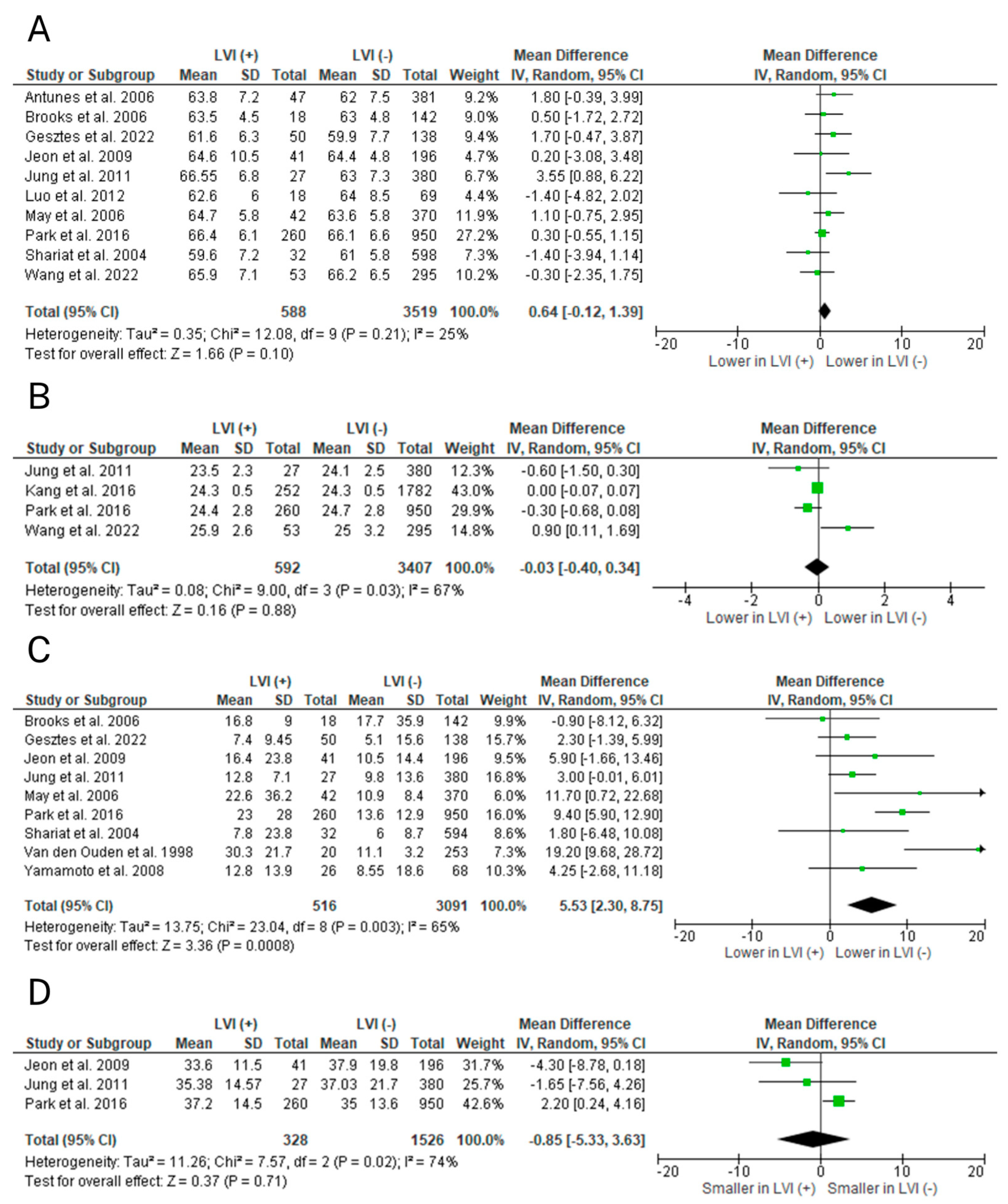
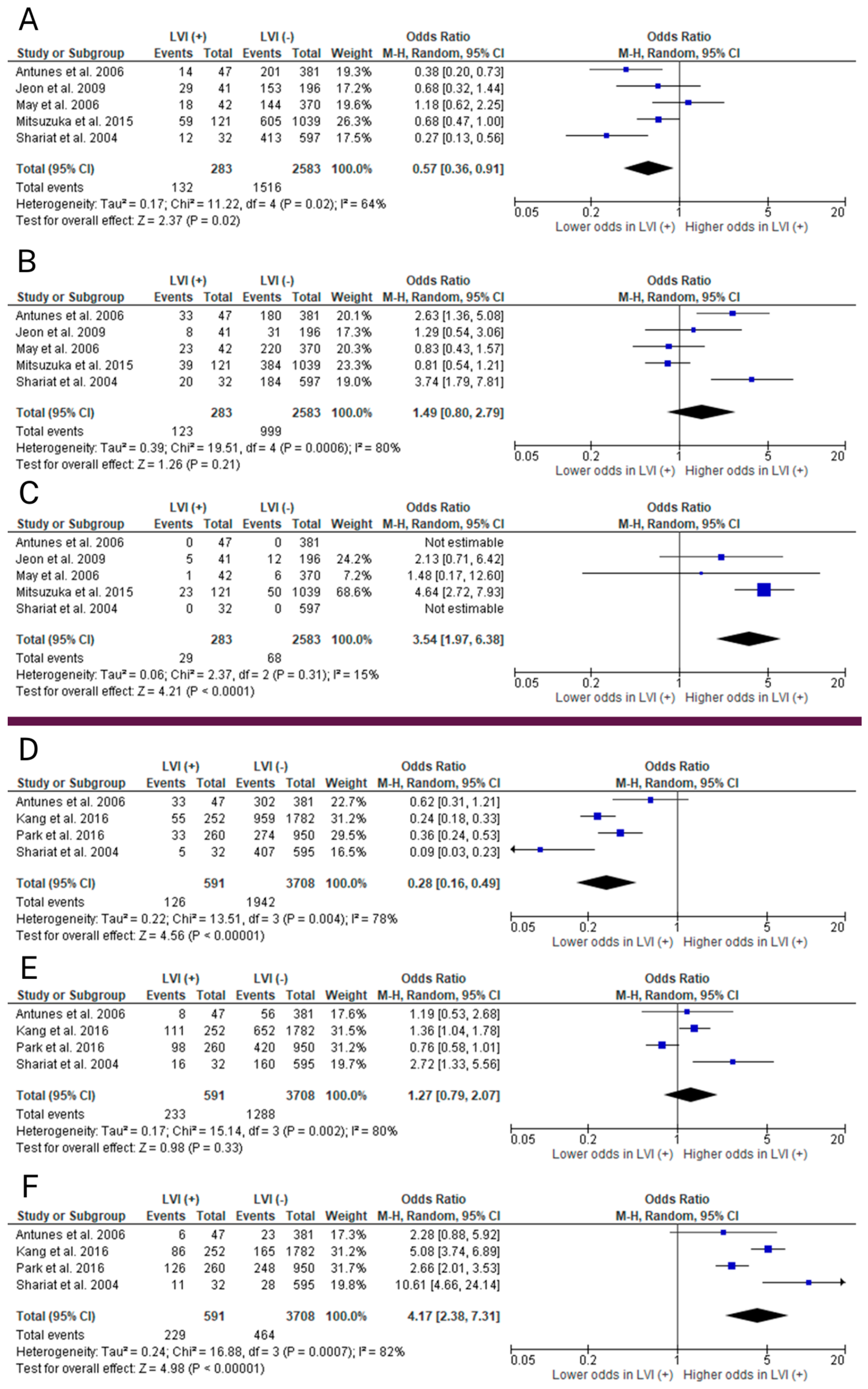
2.2. Meta-Analyses
2.2.1. Age
2.2.2. BMI
2.2.3. Clinical T Stage
2.2.4. Gleason Score
2.2.5. Preoperative PSA
2.2.6. Prostate Volume
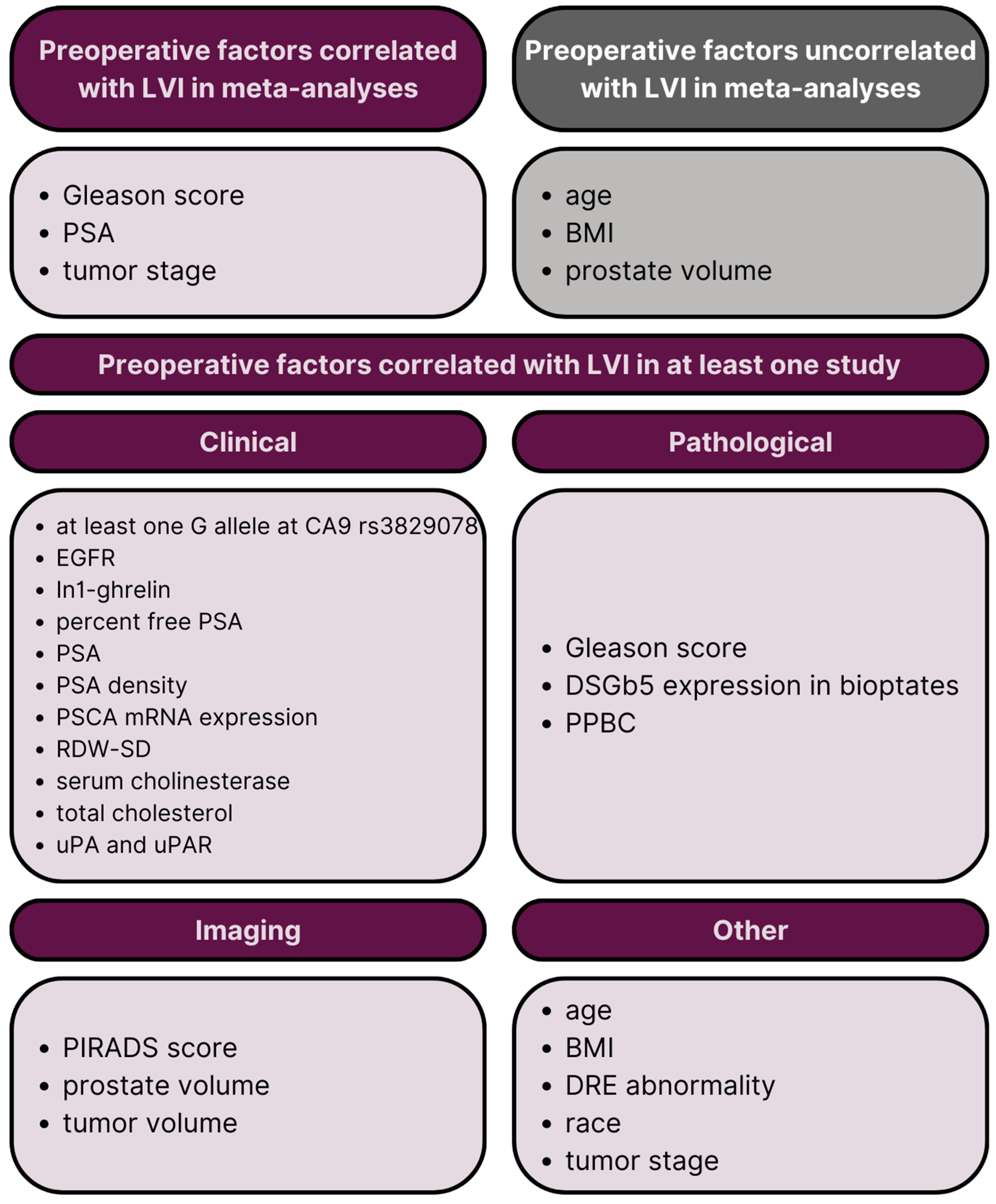
2.3. Results of the Systematic Review
2.3.1. Preoperative Clinical Factors
2.3.2. Preoperative Pathological Factors
2.3.3. Preoperative Imaging-Related Factors
2.3.4. Other Preoperative Factors
3. Discussion
4. Materials and Methods
4.1. Search Strategy
- PubMed: ‘prostate cancer’ AND (‘microvascular invasion’ or ‘lymphovascular invasion’) using Medical Subject Headings (MeSH) terms;
- Embase: (‘prostate cancer’/exp OR ‘prostate cancer’ OR ((‘prostate’/exp OR prostate) AND (‘cancer’/exp OR cancer))) AND (lymphovascular OR microvascular) AND invasion;
- Web of Science: (ALL = (prostate cancer)) AND ALL = (lymphovascular invasion OR microvascular invasion).
4.2. Inclusion Criteria
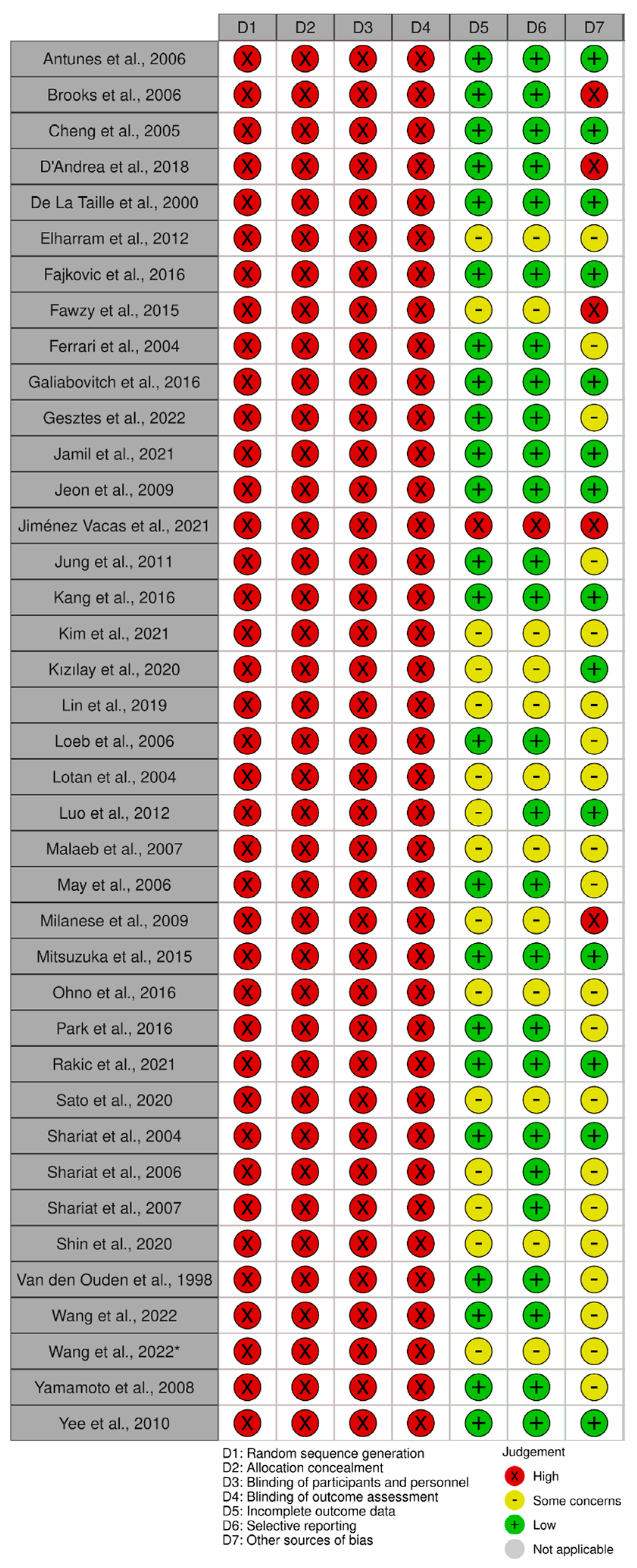
4.3. Study Eligibility and Quality/Risk of Bias Assessment
- Population: patients with PCa who underwent RP.
- Intervention: RP and final histopathological examination; patients with LVI in final specimens.
- Comparison: patients without LVI in final histopathology.
- Outcome: preoperative clinicopathological factors and their correlation with LVI in RP specimens.
- (1)
- Selection of the study population;
- (2)
- Comparability of the groups;
- (3)
- Ascertainment of the outcome.
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Mahmud, A.; Bass, B.; Brundage, M. Prognostic Significance of Lymphovascular Invasion in Radical Prostatectomy Specimens. BJU Int. 2012, 110, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, H.; Pan, X.W.; Xu, D.F.; Cui, X.G.; Chen, J.; Hong, Y.; Gao, Y.; Yin, L.; Ye, J.Q.; et al. The Prognostic Value of Lymphovascular Invasion in Radical Prostatectomy: A Systematic Review and Meta-Analysis. Asian J. Androl. 2016, 18, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, L.; Wu, B.; Zha, Z.; Zhao, H.; Jun, Y.; Jiang, Y. The Impact of Lymphovascular Invasion in Patients with Prostate Cancer following Radical Prostatectomy and Its Association with Their Clinicopathological Features: An Updated PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 2018, 97, e13537. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Larcher, A.; Abdollah, F.; Capitanio, U.; Gallina, A.; Suardi, N.; Bianchi, M.; Sun, M.; Freschi, M.; Salonia, A.; et al. Updated Nomogram Predicting Lymph Node Invasion in Patients with Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection: The Essential Importance of Percentage of Positive Cores. Eur. Urol. 2012, 61, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Ploussard, G.; Valerio, M.; Mattei, A.; Fiori, C.; Fossati, N.; Stabile, A.; Beauval, J.B.; Malavaud, B.; Roumiguié, M.; et al. A Novel Nomogram to Identify Candidates for Extended Pelvic Lymph Node Dissection Among Patients with Clinically Localized Prostate Cancer Diagnosed with Magnetic Resonance Imaging-Targeted and Systematic Biopsies. Eur. Urol. 2019, 75, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Cagiannos, I.; Karakiewicz, P.; Eastham, J.A.; Ohori, M.; Rabbani, F.; Gerigk, C.; Reuter, V.; Graefen, M.; Hammerer, P.G.; Erbersdobler, A.; et al. A Preoperative Nomogram Identifying Decreased Risk of Positive Pelvic Lymph Nodes in Patients with Prostate Cancer. J. Urol. 2003, 170, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Cimino, S.; Reale, G.; Castelli, T.; Favilla, V.; Giardina, R.; Russo, G.I.; Privitera, S.; Morgia, G. Comparison between Briganti, Partin and MSKCC Tools in Predicting Positive Lymph Nodes in Prostate Cancer: A Systematic Review and Meta-Analysis. Scand. J. Urol. 2017, 51, 345–350. [Google Scholar] [CrossRef]
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Milan; EAU Guidelines Office: Arnhem, The Netherlands, 2023; ISBN 978-94-92671-19-6. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 1 June 2023).
- Jiménez-Vacas, J.M.; Montero-Hidalgo, A.J.; Gómez-Gómez, E.; Fuentes-Fayos, A.C.; Ruiz-Pino, F.; Guler, I.; Camargo, A.; Anglada, F.J.; Carrasco-Valiente, J.; Tena-Sempere, M.; et al. In1-Ghrelin Splicing Variant as a Key Element in the Pathophysiological Association between Obesity and Prostate Cancer. J. Clin. Endocrinol. Metab. 2021, 106, E4956–E4968. [Google Scholar] [CrossRef]
- Antunes, A.A.; Srougi, M.; Dall’Oglio, M.F.; Crippa, A.; Paranhos, M.; Cury, J.; Nesrallah, L.J.; Leite, K.R. Microvascular Invasion Is an Independent Prognostic Factor in Patients with Prostate Cancer Treated with Radical Prostatectomy. Int. Braz. J. Urol. 2006, 32, 668–675. [Google Scholar] [CrossRef]
- Cheng, L.; Jones, T.D.; Lin, H.; Eble, J.N.; Zeng, G.; Carr, M.D.; Koch, M.O. Lymphovascular Invasion Is an Independent Prognostic Factor in Prostatic Adenocarcinoma. J. Urol. 2005, 174, 2181–2185. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.L.; Chiang, P.H.; Chen, Y.T.; Cheng, Y.T. Lymphovascular Invasion Is a Pathological Feature Related to Aggressive Cancer Behavior and Predicts Early Recurrence in Prostate Cancer. Kaohsiung J. Med. Sci. 2012, 28, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Ohori, M.; Nakashima, J.; Okubo, H.; Satake, N.; Hashimoto, T.; Tachibana, M. Association between Preoperative Serum Total Cholesterol Level and Biochemical Recurrence in Prostate Cancer Patients Who Underwent Radical Prostatectomy. Mol. Clin. Oncol. 2016, 4, 1073–1077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, M.; Shimada, S.; Watanabe, M.; Kawasaki, Y.; Sato, T.; Morozumi, K.; Mitsuzuka, K.; Ito, A. Expression of Ganglioside Disialosyl Globopentaosyl Ceramide in Prostate Biopsy Specimens as a Predictive Marker for Recurrence after Radical Prostatectomy. Tohoku J. Exp. Med. 2020, 252, 1–8. [Google Scholar] [CrossRef]
- Shin, T.J.; Jung, W.; Ha, J.Y.; Kim, B.H.; Kim, Y.H. The Significance of the Visible Tumor on Preoperative Magnetic Resonance Imaging in Localized Prostate Cancer. Prostate Int. 2021, 9, 6–11. [Google Scholar] [CrossRef]
- Wang, F.; Liang, J.; Yang, F.; Liu, F.; Han, S.; Xing, N. Preoperative Red Cell Distribution Width Is Associated with Postoperative Lymphovascular Invasion in Prostate Cancer Patients Treated with Radical Prostatectomy: A Retrospective Study. Front. Endocrinol. 2022, 13, 1020655. [Google Scholar] [CrossRef]
- Wang, F.; Liu, F.; Liang, J.; Yang, F.; Xing, N. Preoperative Platelet Count Correlates With Postoperative Perineural Invasion on Specimen in Patients Treated With Radical Prostatectomy. Front. Oncol. 2022, 12, 906936. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kawakami, S.; Yonese, J.; Fujii, Y.; Ohkubo, Y.; Suyama, T.; Komai, Y.; Kijima, T.; Ishikawa, Y.; Fukui, I. Lymphovascular Invasion Is an Independent Predictor of Prostate-Specific Antigen Failure after Radical Prostatectomy in Patients with PT3aN0 Prostate Cancer. Int. J. Urol. 2008, 15, 895–899. [Google Scholar] [CrossRef]
- De La Taille, A.; Rubin, M.A.; Buttyan, R.; Olsson, C.A.; Bagiella, E.; Burchardt, M.; Wellisch, O.M.; Katz, A.E. Is Microvascular Invasion on Radical Prostatectomy Specimens a Useful Predictor of PSA Recurrence for Prostate Cancer Patients? Eur. Urol. 2000, 38, 79–84. [Google Scholar] [CrossRef]
- Ferrari, M.K.; McNeal, J.E.; Malhotra, S.M.; Brooks, J.D. Vascular Invasion Predicts Recurrence after Radical Prostatectomy: Stratification of Risk Based on Pathologic Variables. Urology 2004, 64, 749–753. [Google Scholar] [CrossRef]
- Galiabovitch, E.; Hovens, C.M.; Peters, J.S.; Costello, A.J.; Battye, S.; Norden, S.; Ryan, A.; Corcoran, N.M. Routinely Reported ‘Equivocal’ Lymphovascular Invasion in Prostatectomy Specimens Is Associated with Adverse Outcomes. BJU Int. 2017, 119, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Gesztes, W.; Schafer, C.; Young, D.; Fox, J.; Jiang, J.; Chen, Y.; Kuo, H.C.; Mwamukonda, K.B.; Dobi, A.; Burke, A.P.; et al. Focal P53 Protein Expression and Lymphovascular Invasion in Primary Prostate Tumors Predict Metastatic Progression. Sci. Rep. 2022, 12, 5404. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.G.; Bae, J.; Yi, J.S.; Hwang, I.S.; Lee, S.E.; Lee, E. Perineural Invasion Is a Prognostic Factor for Biochemical Failure after Radical Prostatectomy. Int. J. Urol. 2009, 16, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Lee, J.W.; Arkoncel, F.R.P.; Cho, N.H.; Yusoff, N.A.M.; Kim, K.J.; Song, J.M.; Kim, S.J.; Rha, K.H. Significance of Perineural Invasion, Lymphovascular Invasion, and High-Grade Prostatic Intraepithelial Neoplasia in Robot-Assisted Laparoscopic Radical Prostatectomy. Ann. Surg. Oncol. 2011, 18, 3828–3832. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Oh, J.J.; Lee, S.; Hong, S.K.; Lee, S.E.; Byun, S.S. Perineural Invasion and Lymphovascular Invasion Are Associated with Increased Risk of Biochemical Recurrence in Patients Undergoing Radical Prostatectomy. Ann. Surg. Oncol. 2016, 23, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, M.U.; Chae, H.K.; Nam, W.; Kim, S.W.; Yu, H.; Kim, H.G.; Kang, G.H.; Park, J.Y. Overweight and Obesity as Risk Factors for Biochemical Recurrence of Prostate Cancer after Radical Prostatectomy. Int. J. Clin. Oncol. 2022, 27, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.P.; Albert, P.S.; O’Connell, J.; McLeod, D.G.; Poggi, M.M. Lymphovascular Invasion in Prostate Cancer: Prognostic Significance in Patients Treated with Radiotherapy after Radical Prostatectomy. Cancer 2006, 106, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, D.; Soria, F.; Abufaraj, M.; Gust, K.; Foerster, B.; Vartolomei, M.D.; Kimura, S.; Mari, A.; Briganti, A.; Remzi, M.; et al. Clinical Value of Cholinesterase in the Prediction of Biochemical Recurrence after Radical Prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 528.e7–528.e13. [Google Scholar] [CrossRef]
- Shariat, S.F.; Khoddami, S.M.; Saboorian, H.; Koeneman, K.S.; Sagalowsky, A.I.; Cadeddu, J.A.; McConnell, J.D.; Holmes, M.N.; Roehrborn, C.G. Lymphovascular Invasion Is a Pathological Feature of Biologically Aggressive Disease in Patients Treated with Radical Prostatectomy. J. Urol. 2004, 171, 1122–1127. [Google Scholar] [CrossRef]
- Shariat, S.F.; Roehrborn, C.G.; McConnell, J.D.; Park, S.; Alam, N.; Wheeler, T.M.; Slawin, K.M. Association of the Circulating Levels of the Urokinase System of Plasminogen Activation with the Presence of Prostate Cancer and Invasion, Progression, and Metastasis. J. Clin. Oncol. 2007, 25, 349–355. [Google Scholar] [CrossRef]
- Fajkovic, H.; Mathieu, R.; Lucca, I.; Hiess, M.; Hübner, N.; Al Awamlh, B.A.H.; Lee, R.; Briganti, A.; Karakiewicz, P.; Lotan, Y.; et al. Validation of Lymphovascular Invasion Is an Independent Prognostic Factor for Biochemical Recurrence after Radical Prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 233.e1–233.e6. [Google Scholar] [CrossRef]
- Kızılay, F.; Çelik, S.; Sözen, S.; Özveren, B.; Eskiçorapçı, S.; Özgen, M.; Özen, H.; Akdoğan, B.; Aslan, G.; Narter, F.; et al. Correlation of Prostate-Imaging Reporting and Data Scoring System Scoring on Multiparametric Prostate Magnetic Resonance Imaging with Histopathological Factors in Radical Prostatectomy Material in Turkish Prostate Cancer Patients: A Multicenter Study of T. Prostate Int. 2020, 8, 10–15. [Google Scholar] [CrossRef]
- Loeb, S.; Roehl, K.A.; Yu, X.; Antenor, J.A.V.; Han, M.; Gashti, S.N.; Yang, X.J.; Catalona, W.J. Lymphovascular Invasion in Radical Prostatectomy Specimens: Prediction of Adverse Pathologic Features and Biochemical Progression. Urology 2006, 68, 99–103. [Google Scholar] [CrossRef]
- Lotan, Y.; Shariat, S.F.; Khoddami, S.M.; Saboorian, H.; Koeneman, K.S.; Cadeddu, J.A.; Sagalowsky, A.I.; McConnell, J.D.; Roehrborn, C.G. The Percent of Biopsy Cores Positive for Cancer Is a Predictor of Advanced Pathological Stage and Poor Clinical Outcomes in Patients Treated with Radical Prostatectomy. J. Urol. 2004, 171, 2209–2214. [Google Scholar] [CrossRef]
- Malaeb, B.S.; Rashid, H.H.; Lotan, Y.; Khoddami, S.M.; Shariat, S.F.; Sagalowsky, A.I.; McConnell, J.D.; Roehrborn, C.G.; Koeneman, K.S. Prostate Cancer Disease-Free Survival after Radical Retropubic Prostatectomy in Patients Older than 70 Years Compared to Younger Cohorts. Urol. Oncol. Semin. Orig. Investig. 2007, 25, 291–297. [Google Scholar] [CrossRef]
- May, M.; Kaufmann, O.; Hammermann, F.; Loy, V.; Siegsmund, M. Prognostic Impact of Lymphovascular Invasion in Radical Prostatectomy Specimens. BJU Int. 2007, 99, 539–544. [Google Scholar] [CrossRef]
- Mitsuzuka, K.; Narita, S.; Koie, T.; Kaiho, Y.; Tsuchiya, N.; Yoneyama, T.; Kakoi, N.; Kawamura, S.; Tochigi, T.; Ohyama, C.; et al. Lymphovascular Invasion Is Significantly Associated with Biochemical Relapse after Radical Prostatectomy Even in Patients with PT2N0 Negative Resection Margin. Prostate Cancer Prostatic Dis. 2015, 18, 25–30. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, Y.; Yu, H.; Choi, I.Y.; Byun, S.S.; Kwak, C.; Chung, B.H.; Lee, H.M.; Kim, C.S.; Lee, J.Y. Is Lymphovascular Invasion a Powerful Predictor for Biochemical Recurrence in PT3 N0 Prostate Cancer? Results from the K-CaP Database. Sci. Rep. 2016, 6, 25419. [Google Scholar] [CrossRef][Green Version]
- Elharram, M.; Margel, D.; Finelli, A.; Trachtenberg, J.; Evans, A.; van der Kwast, T.H.; Sweet, J.M.; Fleshner, N. Perineural Invasion on Prostate Biopsy Does Not Predict Adverse Pathological Outcome. Can. J. Urol. 2012, 19, 6567–6572. [Google Scholar]
- Fawzy, M.S.; Mohamed, R.H.; Elfayoumi, A.R.R. Prostate Stem Cell Antigen (PSCA) MRNA Expression in Peripheral Blood in Patients with Benign Prostatic Hyperplasia and/or Prostate Cancer. Med. Oncol. 2015, 32, 74. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, S.S.; Yang, C.K.; Li, J.R.; Chen, C.S.; Hung, S.C.; Chiu, K.Y.; Cheng, C.L.; Ou, Y.C.; Yang, S.F. Genetic Polymorphism and Carbonic Anhydrase 9 Expression Can Predict Nodal Metastatic Prostate Cancer Risk in Patients with Prostate-Specific Antigen Levels ≤10 Ng/Ml at Initial Biopsy. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 814.e9–814.e16. [Google Scholar] [CrossRef]
- Milanese, G.; Dellabella, M.; Fazioli, F.; Pierpaoli, E.; Polito, M.; Siednius, N.; Montironi, R.; Blasi, F.; Muzzonigro, G. Increased Urokinase-Type Plasminogen Activator Receptor and Epidermal Growth Factor Receptor in Serum of Patients With Prostate Cancer. J. Urol. 2009, 181, 1393–1400. [Google Scholar] [CrossRef]
- Shariat, S.F.; Abdel-Aziz, K.F.; Roehrborn, C.G.; Lotan, Y. Pre-Operative Percent Free Psa Predicts Clinical Outcomes in Patients Treated with Radical Prostatectomy with Total PSA Levels below 10 Ng/Ml. Eur. Urol. 2006, 49, 293–302. [Google Scholar] [CrossRef]
- Van den Ouden, D.; Kranse, R.; Hop, W.C.J.; Van der Kwast, T.H.; Schröder, F.H. Microvascular Invasion in Prostate Cancer: Prognostic Significance in Patients Treated by Radical Prostatectomy for Clinically Localized Carcinoma. Urol. Int. 1998, 60, 17–24. [Google Scholar] [CrossRef]
- Yee, D.S.; Shariat, S.F.; Lowrance, W.T.; Maschino, A.C.; Savage, C.J.; Cronin, A.M.; Scardino, P.T.; Eastham, J.A. Prognostic Significance of Lymphovascular Invasion in Radical Prostatectomy Specimens. BJU Int. 2011, 108, 502–507. [Google Scholar] [CrossRef]
- Rakic, N.; Jamil, M.; Keeley, J.; Sood, A.; Vetterlein, M.; Dalela, D.; Arora, S.; Modonutti, D.; Bronkema, C.; Novara, G.; et al. Evaluation of Lymphovascular Invasion as a Prognostic Predictor of Overall Survival after Radical Prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 495.e1–495.e6. [Google Scholar] [CrossRef]
- Jamil, M.; Rakic, N.; Sood, A.; Keeley, J.; Modonutti, D.; Novara, G.; Jeong, W.; Menon, M.; Rogers, C.G.; Abdollah, F. Impact of Lymphovascular Invasion on Overall Survival in Patients With Prostate Cancer following Radical Prostatectomy: Stage-per-Stage Analysis. Clin. Genitourin. Cancer 2021, 19, e319–e325. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Al Qa’qa’, S.; Downes, M.R.; Jain, R.; van der Kwast, T. Morphologic Pattern, Frequency, and Spatial Distribution of Lymphovascular Invasion Foci in Radical Prostatectomy Specimens. Int. J. Surg. Pathol. 2022, 31, 939–948. [Google Scholar] [CrossRef]
- Małkiewicz, B.; Kiełb, P.; Karwacki, J.; Czerwińska, R.; Długosz, P.; Lemiński, A.; Nowak, Ł.; Krajewski, W.; Szydełko, T. Utility of Lymphadenectomy in Prostate Cancer: Where Do We Stand? J. Clin. Med. 2022, 11, 2343. [Google Scholar] [CrossRef]
- Bernard, B.; Gershman, B.; Karnes, R.J.; Sweeney, C.J.; Vapiwala, N. Approach to Oligometastatic Prostate Cancer. Am. Soc. Clin. Oncol. Educ. B. 2016, 35, 119–129. [Google Scholar] [CrossRef]
- Freeman, A. Perineural and Lymphovascular Invasion on Prostatic Biopsy: Pathological Assessment and Significance. Surg. Oncol. 2009, 18, 200–202. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, S.X.; Wu, S.; Dahl, D.M.; Blute, M.L.; De Zhong, W.; Zhou, X.; Wu, C.L. Clinicopathological Characteristics of Localized Prostate Cancer in Younger Men Aged ≤ 50 Years Treated with Radical Prostatectomy in the PSA Era: A Systematic Review and Meta-Analysis. Cancer Med. 2020, 9, 6473–6484. [Google Scholar] [CrossRef]
- Orzechowska, M.J.; Anusewicz, D.; Bednarek, A.K. Age- and Stage-Dependent Prostate Cancer Aggressiveness Associated with Differential Notch Signaling. Int. J. Mol. Sci. 2023, 24, 164. [Google Scholar] [CrossRef]
- Milonas, D.; Venclovas, Z.; Jievaltas, M. Age and Aggressiveness of Prostate Cancer: Analysis of Clinical and Pathological Characteristics after Radical Prostatectomy for Men with Localized Prostate Cancer. Cent. Eur. J. Urol. 2019, 72, 240–246. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Rev. Española Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. The Ottawa Hospital Research Institute Clinical Epidemiology Program. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 December 2023).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
| Study | Country | Journal | Study Design | Study Period | LVI+ and LVI− [n] | LVI+ [n] | LVI+ [%] | LVI− [n] | Definition of LVI | Preoperative Predictors of LVI (p-Value) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antunes et al., 2006 [11] | Brazil | International Brazilian Journal of Urology | retrospective, single-center | 1993–2000 | 428 | 47 | 11.0 | 381 | presence of tumor cells within an endothelium lined space |
| 8 |
| Brooks et al., 2006 [28] | USA | Cancer | retrospective, multi-center | 1991–2001 | 160 | 18 | 11.3 | 142 | unequivocal presence of tumor cells within a vascular or lymphatic, endothelial-lined space |
| 7 |
| Cheng et al., 2005 [12] | USA | The Journal of Urology | retrospective, single-center | 1990–1998 | 504 | 106 | 21.0 | 398 | unequivocal presence of tumor cells in an endothelium lined space |
| 8 |
| D’Andrea et al., 2018 [29] | multi-center | Urologic Oncology | retrospective, multi-center | 2000–2011 | 6041 | 693 | 11.5 | 5348 | unequivocal presence of tumor cells within an endothelium lined space without underlying muscular walls |
| 7 |
| De La Taille et al., 2000 [20] | USA | European Urology | retrospective, single-center | 1993–1998 | 241 | 30 | 12.4 | 211 | unequivocal presence of tumor cells within simple endothelial-lined tissue spaces without underlying muscular walls |
| 8 |
| Elharram et al., 2012 [40] | Canada | The Canadian Journal of Urology | prospective, single-center | 2008–2010 | 138 | 5 | 3.6 | 133 | none |
| 5 |
| Fajkovic et al., 2016 [32] | multi-center | Urologic Oncology | retrospective, multi-center | 2000–2011 | 6678 | 767 | 11.5 | 5911 | presence of tumor cells within an endothelium lined space |
| 8 |
| Fawzy et al., 2015 [41] | Egypt | Medical Oncology | prospective, single-center | not specified | 112 | 52 | 46.4 | 60 | none |
| 6 |
| Ferrari et al., 2004 [21] | USA | Adult Urology | retrospective, single-center | 1984–1999 | 620 | 110 | 17.7 | 510 | LVI was in small channels consisting of only an endothelial layer, identified by its smooth luminal surface, and lined with one or two narrow endothelial cells |
| 8 |
| Galiabovitch et al., 2016 [22] | Australia | BJUI International | retrospective, single-center | 2004–2012 | 1267 | 82 | 6.5 | 1185 | unequivocal presence of tumor cells in an endothelial-lined space |
| 8 |
| Gesztes et al., 2022 [23] | USA | Scientific Reports | retrospective, single-center | 1993–2013 | 188 | 50 | 26.6 | 138 | tumor cells’ presence within spaces lined by lymphovascular endothelium with characteristic podoplanin staining |
| 8 |
| Jamil et al., 2021 [48] | multi-center | Clinical Genitourinary Cancer | retrospective, database registry | 2010–2015 | 232,704 | 17,758 | 7.6 | 214,946 | presence of tumor cells in lymphatic channels or blood vessels within the primary tumor |
| 8 |
| Jeon et al., 2009 [24] | Korea | International Journal of Urology | retrospective, single-center | 1995–2004 | 237 | 41 | 17.3 | 196 | unequivocal presence of tumor cells within an endothelium lined space without muscular walls |
| 8 |
| Jiménez Vacas et al., 2021 [10] | Spain | The Journal of Clinical Endocrinology & Metabolism | prospective, single-center | 2013–2015 | 79 | not specified | - | not specified | none |
| 4 |
| Jung et al., 2011 [25] | Korea | Annals of Surgical Oncology | retrospective, single-center | 2005–2009 | 407 | 27 | 6.6 | 380 | unequivocal presence of tumor cells in an endothelium lined space |
| 8 |
| Kang et al., 2016 [26] | Korea | Annals of Surgical Oncology | retrospective, single-center | 2003–2014 | 2034 | 252 | 12.4 | 1782 | presence of tumor emboli in intraprostatic vessels, particularly within the lumen of the endothelium |
| 8 |
| Kim et al., 2021 [27] | Korea | International Journal of Clinical Oncology | retrospective, single-center | 1997–2019 | 389 | 59 | 15.2 | 330 | none |
| 6 |
| Kızılay et al., 2020 [33] | Turkey | Prostate International | retrospective, multi-center | not specified | 177 | 10 | 5.6 | 167 | none |
| 5 |
| Lin et al., 2019 [42] | Taiwan | Urologic Oncology | prospective, single-center | 2012–2017 | 579 | 97 | 16.8 | 482 | none |
| 7 |
| Loeb et al., 2006 [34] | USA | Urology | retrospective, multi-center | 1989–2004 | 1709 | 118 | 6.9 | 1591 | presence of tumor emboli in small intraprostatic vessels |
| 8 |
| Lotan et al., 2004 [35] | USA | The Journal of Urology | retrospective, multi-center | 1994–2002 | 605 | 32 | 5.3 | 573 | none |
| 6 |
| Luo et al., 2012 [13] | Taiwan | Kaohsiung Journal of Medical Sciences | retrospective, single-center | 1998–2010 | 87 | 18 | 20.7 | 69 | none |
| 6 |
| Malaeb et al., 2007 [36] | USA | Urologic Oncology | retrospective, multi-center | 1994–2002 | 628 | 34 | 5.4 | 594 | none |
| 6 |
| May et al., 2006 [37] | Germany | BJUI International | retrospective, multi-center | 1996–2003 | 412 | 42 | 10.2 | 370 | unequivocal existence of tumor cells in an endothelium lined space with no underlying muscular walls |
| 8 |
| Milanese et al., 2009 [43] | Italy | The Journal of Urology | prospective, single-center | 2005–2006 | 30 | 4 | 13.3 | 26 | none |
| 6 |
| Mitsuzuka et al., 2015 [38] | Japan | Prostate Cancer and Prostatic Disease | retrospective, multi-center | 2000–2009 | 1160 | 121 | 10.4 | 1039 | unequivocal presence of tumor cells within endothelial-lined channels on routine light microscopic examination |
| 8 |
| Ohno et al., 2016 [14] | Japan | Molecular and Clinical Oncology | retrospective, single-center | 2002–2010 | 562 | 148 | 26.3 | 414 | none |
| 6 |
| Park et al., 2016 [39] | South Korea | Scientific Reports | retrospective, multi-center | 2001–2012 | 1210 | 260 | 21.5 | 950 | presence of cancer cells within an arterial, venous, or lymphatic lumen on routine hematoxylin and eosin sections |
| 8 |
| Rakic et al., 2021 [47] | USA | Urologic Oncology | retrospective, database registry | 2010–2015 | 126,682 | 12,632 | 10.0 | 114,050 | presence of tumor cells in lymphatic channels or blood vessels within the primary tumor, but not the lymph nodes |
| 8 |
| Sato et al., 2020 [15] | Japan | The Tohoku Journal of Experimental Medicine | retrospective, single-center | 2005–2011 | 116 | 12 | 10.3 | 104 | none |
| 5 |
| Shariat et al., 2004 [30] | USA | The Journal of Urology | retrospective, multi-center | 1994–2004 | 630 | 32 | 5.1 | 598 | unequivocal presence of tumor cells within an endothelium lined space without underlying muscular walls |
| 8 |
| Shariat et al., 2006 [44] | USA | European Urology | prospective, single-center | 1994–2002 | 351 | 13 | 3.7 | 338 | none |
| 6 |
| Shariat et al., 2007 [31] | USA | Journal of Clinical Oncology | retrospective, multi-center | 1994–2004 | 255 | 29 | 11.4 | 226 | none |
| 7 |
| Shin et al., 2020 [16] | Korea | Prostate International | retrospective, single-center | 2009–2016 | 216 | 14 | 6.5 | 202 | none |
| 5 |
| Van den Ouden et al., 1998 [45] | The Netherlands | Urologia Internationalis | prospective, single-center | 1977–1994 | 273 | 20 | 7.3 | 253 | unequivocal presence of tumor cells within endothelial-lined spaces |
| 7 |
| Wang et al., 2022 [17] | China | Frontiers in Endocrinology | retrospective, single-center | 2018–2021 | 348 | 53 | 15.2 | 295 | presence of tumor cells within an endothelial-lined space that is usually devoid of a muscular wall |
| 8 |
| Wang et al., 2022 [18] | China | Frontiers in Oncology | retrospective, single-center | 2018–2021 | 348 | 54 | 15.5 | 294 | none |
| 6 |
| Yamamoto et al., 2008 [19] | Japan | International Journal of Urology | retrospective, single-center | 1994–2005 | 94 | 26 | 27.7 | 68 | unequivocal presence of tumor cells in an endothelium lined space |
| 8 |
| Yee et al., 2010 [46] | USA | BJUI International | prospective, single-center | 2004–2007 | 1298 | 129 | 9.9 | 1169 | unequivocal presence of tumor cells within an endothelium lined space |
| 8 |
| Predictor | Number of Studies | Patients with LVI/All Patients (%) |
|---|---|---|
| PSA | 19 | 31,958/371,858 (8.6) |
| age | 4 | 30,538/360,953 (8.5) |
| GS | 4 | 695/5272 (13.2) |
| cT stage | 4 | 241/2454 (9.8) |
| PPBC | 3 | 121/1470 (8.2) |
| PSAD | 2 | 83/649 (12.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwacki, J.; Stodolak, M.; Nowak, Ł.; Kiełb, P.; Krajewski, W.; Lemiński, A.; Szydełko, T.; Małkiewicz, B. Preoperative Factors for Lymphovascular Invasion in Prostate Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 856. https://doi.org/10.3390/ijms25020856
Karwacki J, Stodolak M, Nowak Ł, Kiełb P, Krajewski W, Lemiński A, Szydełko T, Małkiewicz B. Preoperative Factors for Lymphovascular Invasion in Prostate Cancer: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2024; 25(2):856. https://doi.org/10.3390/ijms25020856
Chicago/Turabian StyleKarwacki, Jakub, Marcel Stodolak, Łukasz Nowak, Paweł Kiełb, Wojciech Krajewski, Artur Lemiński, Tomasz Szydełko, and Bartosz Małkiewicz. 2024. "Preoperative Factors for Lymphovascular Invasion in Prostate Cancer: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 25, no. 2: 856. https://doi.org/10.3390/ijms25020856
APA StyleKarwacki, J., Stodolak, M., Nowak, Ł., Kiełb, P., Krajewski, W., Lemiński, A., Szydełko, T., & Małkiewicz, B. (2024). Preoperative Factors for Lymphovascular Invasion in Prostate Cancer: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 25(2), 856. https://doi.org/10.3390/ijms25020856










