Circ-MBOAT2 Regulates Angiogenesis via the miR-495/NOTCH1 Axis and Associates with Myocardial Perfusion in Patients with Coronary Chronic Total Occlusion
Abstract
1. Introduction
2. Results
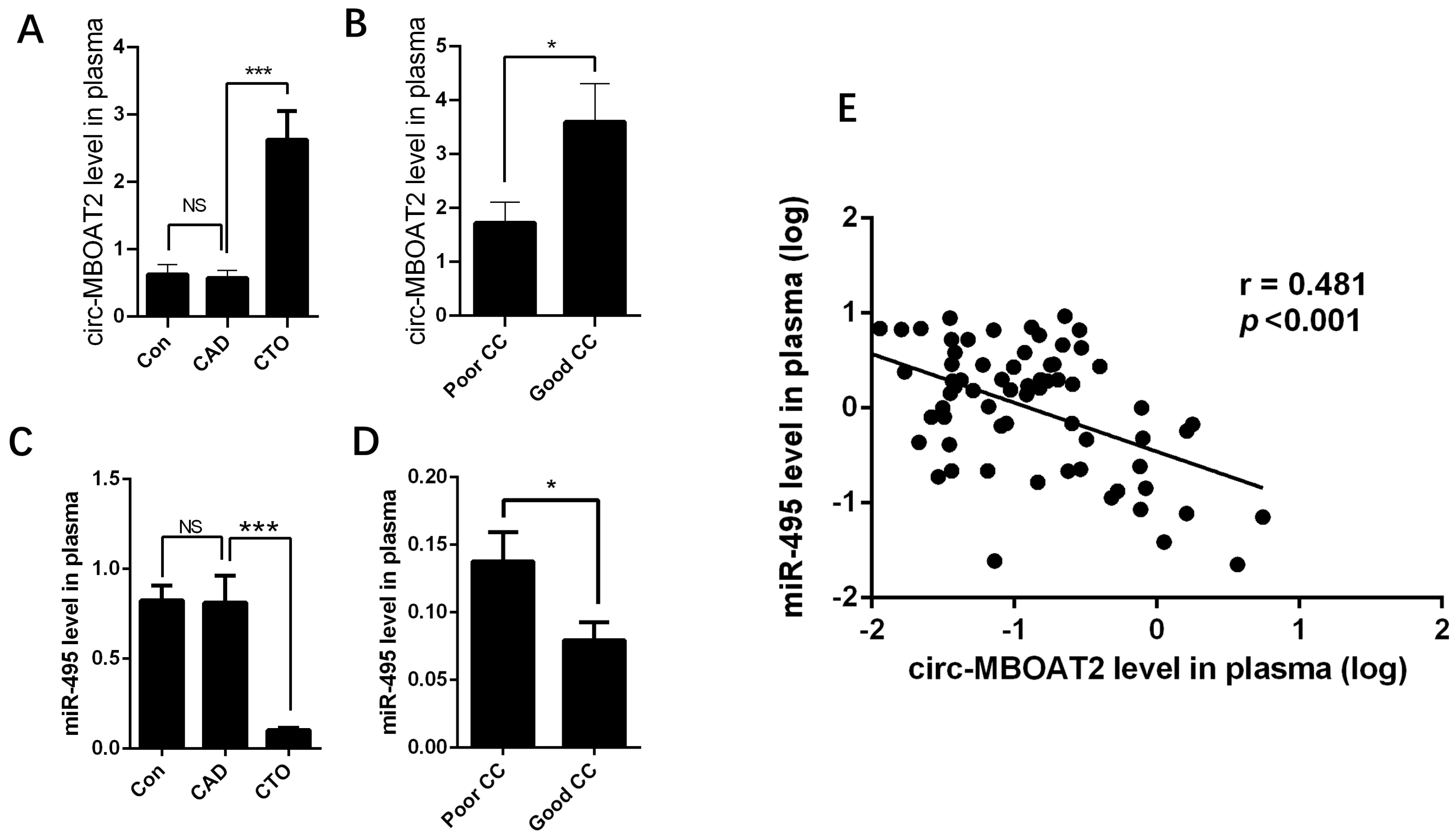
2.1. Expressions of Circ-MBOAT2 and miR-495 in Control, CAD and Patients with CTO
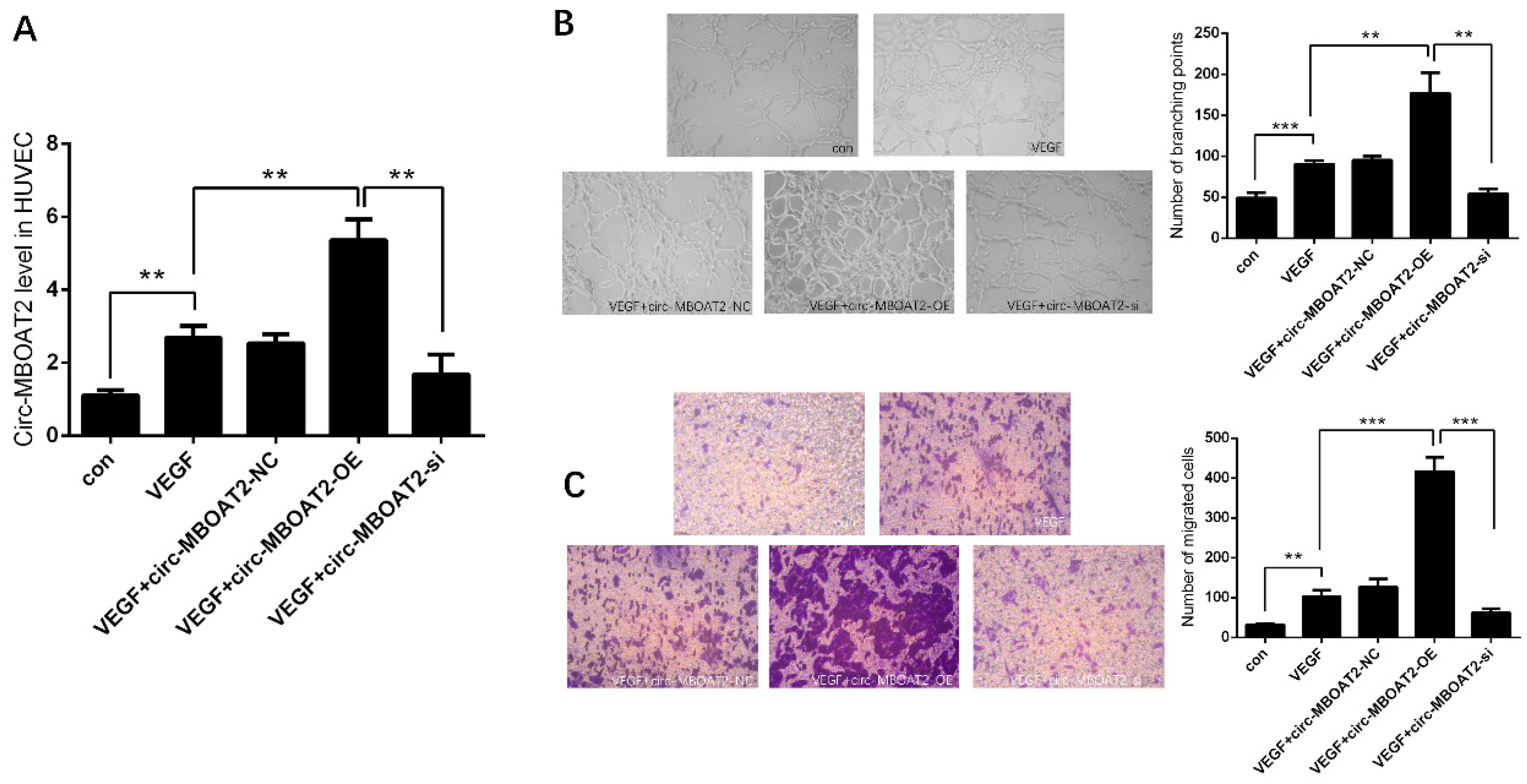
2.2. Circ-MBOAT2 Increases Tube Formation and Cell Migration in HUVEC
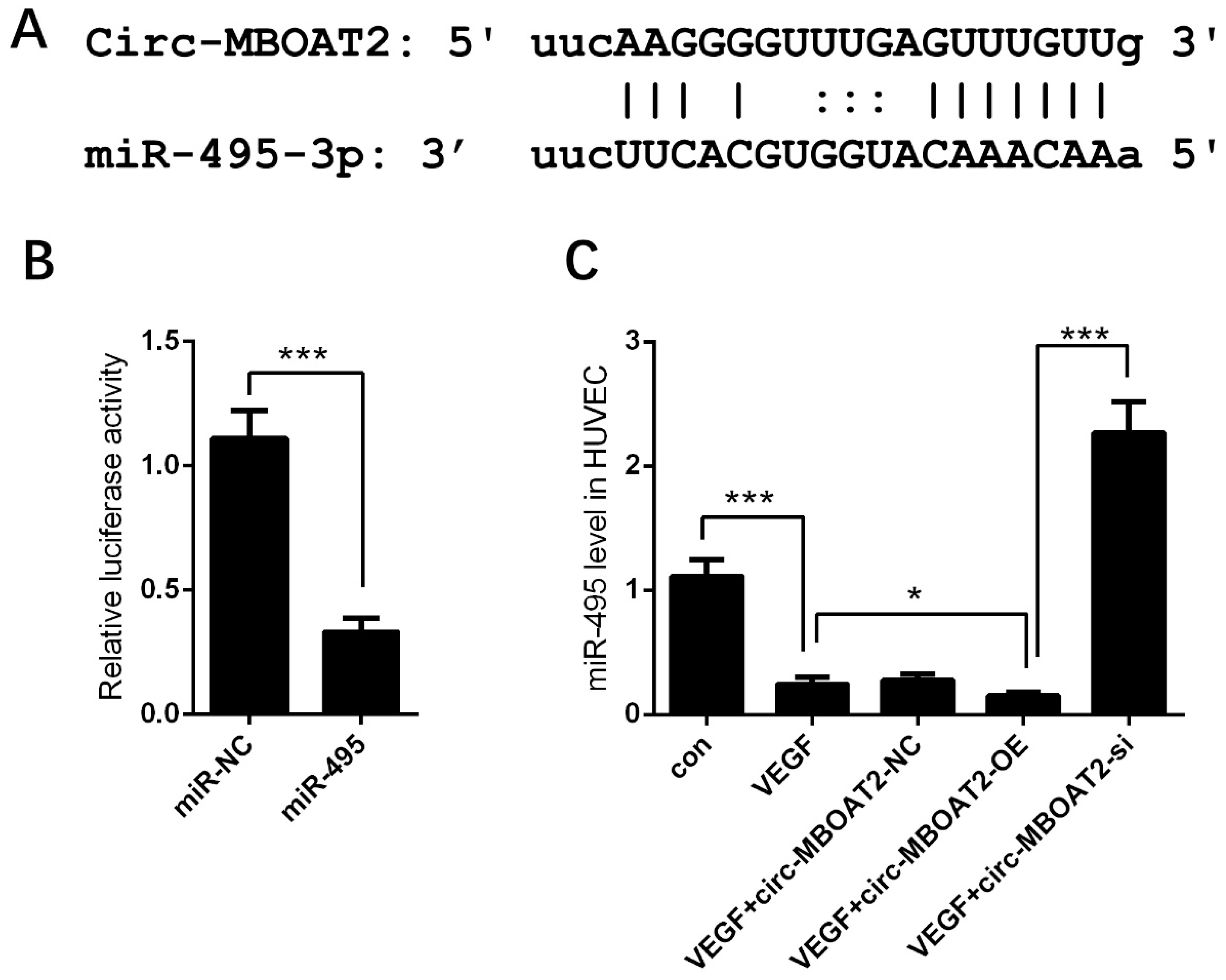
2.3. Circ-MBOAT2 Acted as a Sponge of miR-495
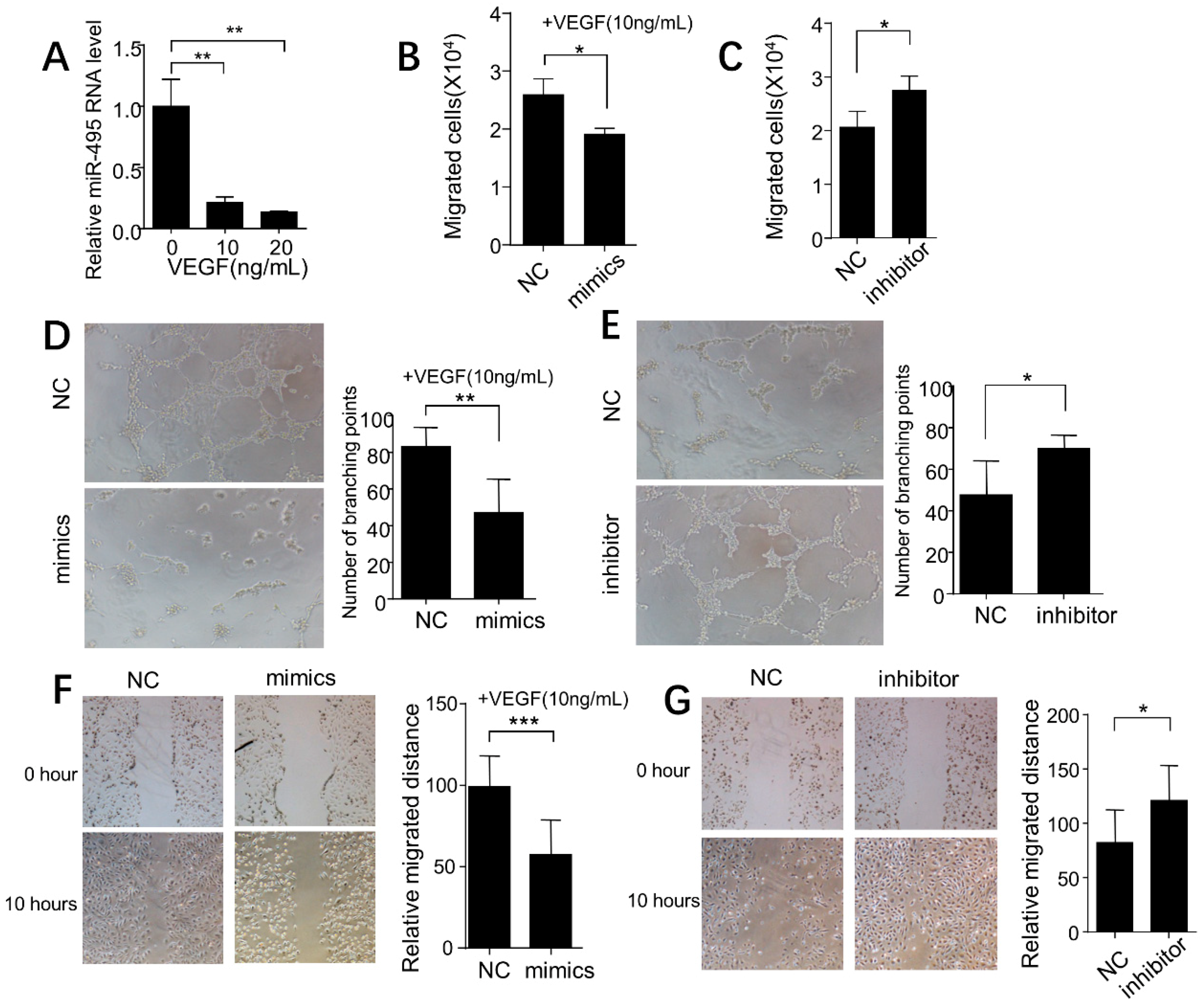
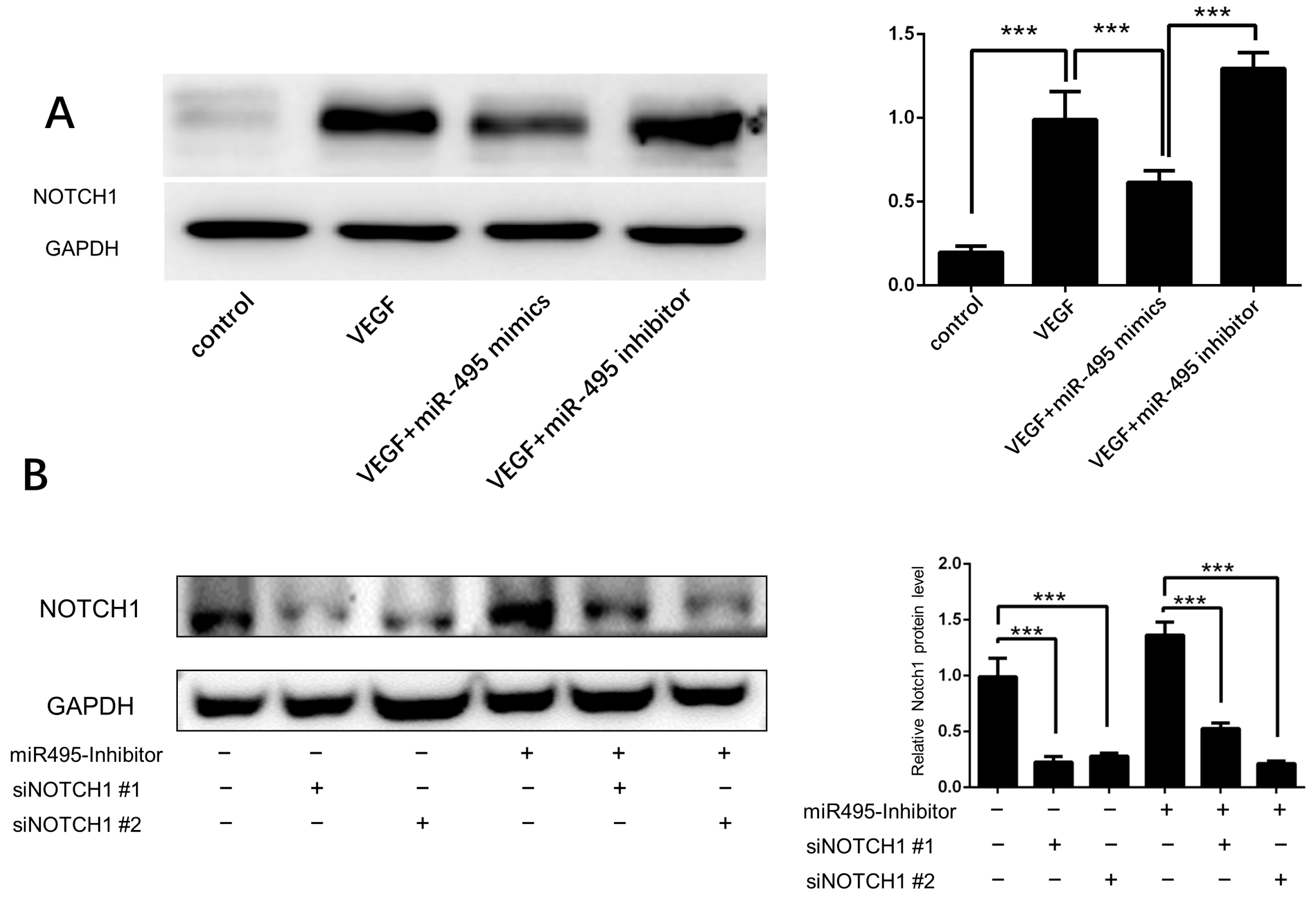
2.4. miR-495 Inhibits Angiogenesis through NOTCH1 Pathway in Endothelial Cells
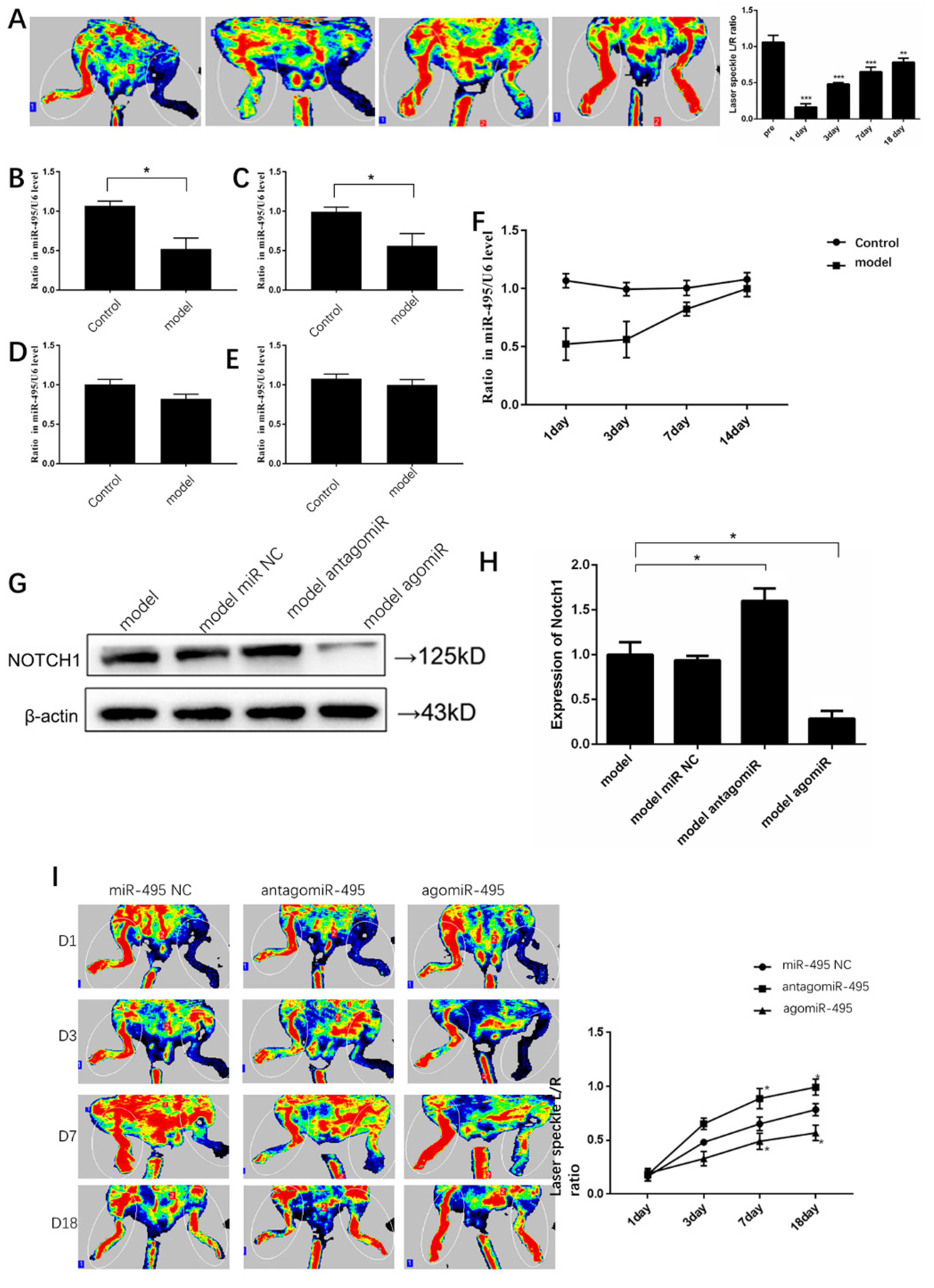
2.5. Inhibition of miR-495 Increases Collateral Formation in Mice after Hindlimb Ischemia
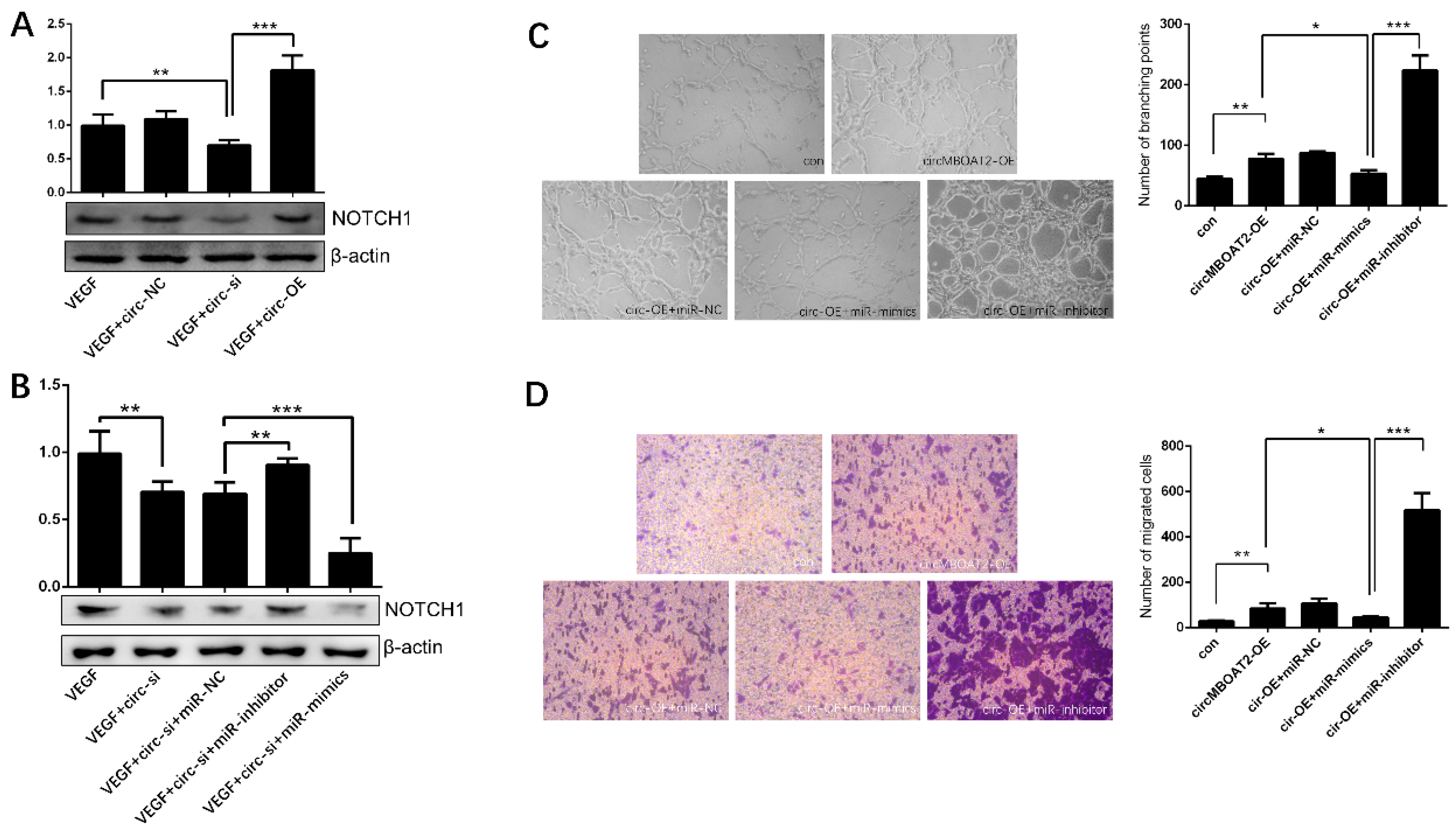
2.6. Circ-MBOAT2 Regulated Notch1 Expression and Angiogenesis by Absorbing miR-495
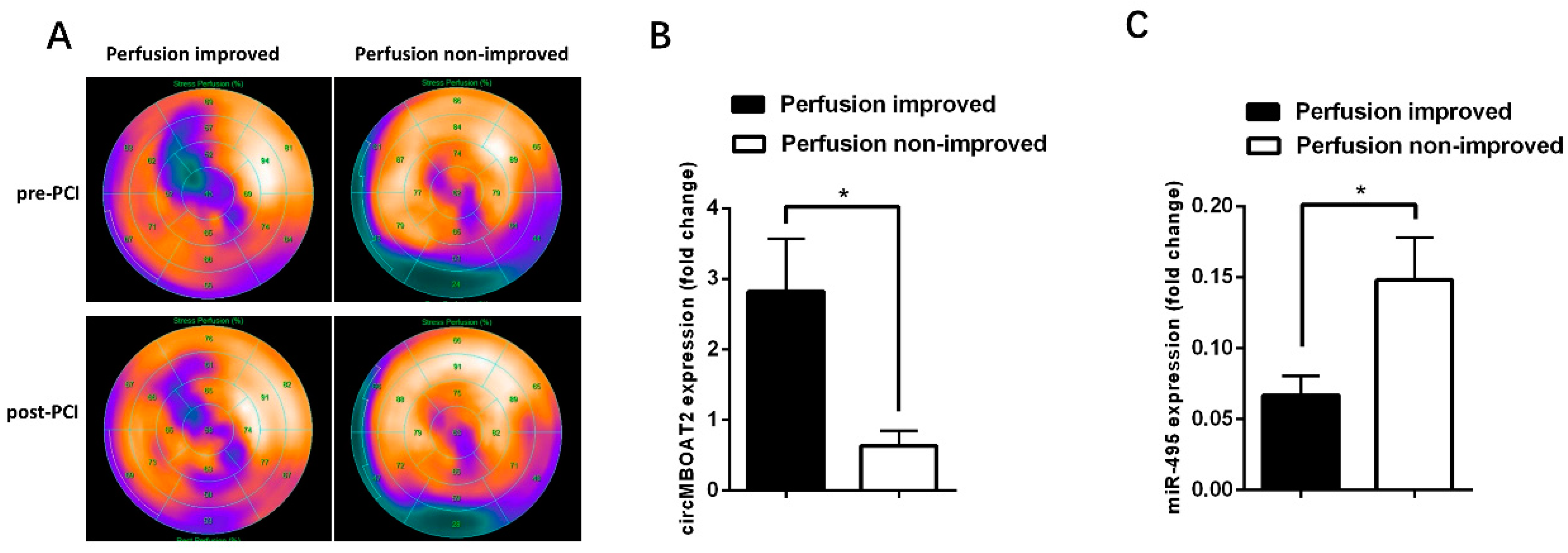
2.7. The Expressions of circ-MBOAT2 and miR-495 Are Associated with Myocardial Perfusion Improvement after Revascularization of CTO
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Collateral Grading
4.3. RNA Sequencing
4.4. Cell Culture and Transfection
4.5. Measurement of HUVEC Migration and Tube Formation
4.6. Reverse Transcription-PCR
4.7. Western Blot Analysis
4.8. Murine Hindlimb Ischemia Model
4.9. In Vivo Transfection
4.10. Hindlimb Blood Flow Measurement
4.11. SPECT among the Patients with CTO
4.12. Statistical Analyses
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christofferson, R.D.; Lehmann, K.G.; Martin, G.V.; Every, N.; Caldwell, J.H.; Kapadia, S.R. Effect of chronic total coronary occlusion on treatment strategy. Am. J. Cardiol. 2005, 95, 1088–1091. [Google Scholar] [CrossRef]
- Srinivas, V.S.; Brooks, M.M.; Detre, K.M.; King, S.B., 3rd; Jacobs, A.K.; Johnston, J.; Williams, D.O. Contemporary percutaneous coronary intervention versus balloon angioplasty for multivessel coronary artery disease: A comparison of the National Heart, Lung and Blood Institute Dynamic Registry and the Bypass Angioplasty Revascularization Investigation (BARI) study. Circulation 2002, 106, 1627–1633. [Google Scholar] [PubMed]
- Fefer, P.; Knudtson, M.L.; Cheema, A.N.; Galbraith, P.D.; Osherov, A.B.; Yalonetsky, S.; Gannot, S.; Samuel, M.; Weisbrod, M.; Bierstone, D.; et al. Current perspectives on coronary chronic total occlusions: The Canadian Multicenter Chronic Total Occlu-sions Registry. J. Am. Coll. Cardiol. 2012, 59, 991–997. [Google Scholar] [CrossRef]
- Authors/Task Force members; Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J. Mol. Cell. Cardiol. 2016, 94, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Economou, E.K.; Oikonomou, E.; Siasos, G.; Papageorgiou, N.; Tsalamandris, S.; Mourouzis, K.; Papaioanou, S.; Tousoulis, D. The role of microRNAs in coronary artery disease: From pathophysiology to diagnosis and treatment. Atherosclerosis 2015, 241, 624–633. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Zhou, Q.; Chen, C.; Yuan, W.; Liu, J.; Li, X.; Sun, Z. Roles of circRNAs in the tumour microenvironment. Mol. Cancer 2020, 19, 14. [Google Scholar] [CrossRef]
- Seitz, H.; Royo, H.; Bortolin, M.-L.; Lin, S.-P.; Ferguson-Smith, A.C.; Cavaillé, J. A large imprinted microRNA gene cluster at the mouse Dlk1-Gtl2 domain. Genome Res. 2004, 14, 1741–1748. [Google Scholar] [CrossRef]
- Welten, S.M.; Bastiaansen, A.J.; de Jong, R.C.; de Vries, M.R.; Peters, E.A.; Boonstra, M.C.; Sheikh, S.P.; La Monica, N.; Kandimalla, E.R.; Quax, P.H.; et al. Inhibition of 14q32 MicroRNAs miR-329, miR-487b, miR-494, and miR-495 increases neovascularization and blood flow recovery after ischemia. Circ Res. 2014, 115, 696–708. [Google Scholar] [CrossRef]
- Márquez, A.B.; van der Vorst, E.P.C.; Maas, S.L. Key Chemokine Pathways in Atherosclerosis and Their Therapeutic Potential. J. Clin. Med. 2021, 10, 3825. [Google Scholar] [CrossRef]
- Qin, Q.; Qian, J.; Ma, J.; Ge, L.; Ge, J. Relationship between thrombospondin-1, endostatin, angiopoietin-2, and coronary collateral development in patients with chronic total occlusion. Medicine 2016, 95, e4524. [Google Scholar] [CrossRef]
- Sahin, M.; Demir, S.; Kalkan, M.E.; Ozkan, B.; Alici, G.; Cakalagaoglu, K.C.; Yazicioglu, M.V.; Sarikaya, S.; Biteker, M.; Turkmen, M.M. The relationship between gamma-glutamyltransferase and coronary collateral circulation in patients with chronic total occlusion. Anadolu Kardiyol. Derg./Anatol. J. Cardiol. 2013, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Nacar, A.B.; Erayman, A.; Kurt, M.; Buyukkaya, E.; Karakaş, M.F.; Akcay, A.B.; Buyukkaya, S.; Sen, N. The relationship between coronary collateral circulation and neutrophil/lymphocyte ratio in patients with coronary chronic total occlusion. Med. Princ. Pract. 2014, 24, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, M.; Şahin, M.; Kalkan, A.; Güler, A.; Taş, M.; Bulut, M.; Demir, S.; Acar, R.; Arslantaş, U.; Öztürkeri, B.; et al. The relationship between the neutrophil–lymphocyte ratio and the coronary collateral circulation in patients with chronic total occlusion. Perfusion 2014, 29, 360–366. [Google Scholar] [CrossRef]
- Söğüt, E.; Kadı, H.; Karayakalı, M.; Mertoğlu, C. The association of plasma vitamin A and E levels with coronary collateral circulation. Atherosclerosis 2015, 239, 547–551. [Google Scholar] [CrossRef]
- Shen, Y.; Ding, F.H.; Zhang, R.Y.; Zhang, Q.; Lu, L.; Shen, W.F. Association of serum mimecan with angiographic coronary collateralization in patients with stable coronary artery disease and chronic total occlusion. Atherosclerosis 2016, 252, 75–81. [Google Scholar] [CrossRef]
- Gurses, K.M.; Yalcin, M.U.; Kocyigit, D.; Besler, M.S.; Canpinar, H.; Evranos, B.; Yorgun, H.; Şahiner, M.L.; Kaya, E.B.; Özer, N.; et al. The association between serum angiogenin and osteopontin levels and coronary collateral circulation in patients with chronic total occlusion. Anatol. J. Cardiol. 2019, 22, 77–84. [Google Scholar] [CrossRef]
- Kabłak-Ziembicka, A.; Badacz, R.; Okarski, M.; Wawak, M.; Przewłocki, T.; Podolec, J. Cardiac microRNAs: Diagnostic and therapeutic potential. Arch. Med. Sci. 2023, 19, 1360–1381. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Zacharia, E.; Tousoulis, D. Association between microRNAs and coronary collateral circulation: Is there a new role for the small non-coding RNAs? Ann. Transl. Med. 2016, 4, 223. [Google Scholar] [CrossRef]
- Hakimzadeh, N.; Nossent, A.Y.; van der Laan, A.M.; Schirmer, S.H.; de Ronde, M.W.J.; Pinto-Sietsma, S.-J.; van Royen, N.; Quax, P.H.A.; Hoefer, I.E.; Piek, J.J. Circulating MicroRNAs Characterizing Patients with Insufficient Coronary Collateral Artery Function. PLoS ONE 2015, 10, e0137035. [Google Scholar] [CrossRef][Green Version]
- Hou, L.-D.; Zhang, J. Circular RNAs: An emerging type of RNA in cancer. Int. J. Immunopathol. Pharmacol. 2017, 30, 1–6. [Google Scholar] [CrossRef]
- Najafi, S. Circular RNAs as emerging players in cervical cancer tumorigenesis; A review to roles and biomarker potentials. Int. J. Biol. Macromol. 2022, 206, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Ghaedrahmati, F.; Nasrolahi, A.; Najafi, S.; Mighani, M.; Anbiyaee, O.; Haybar, H.; Assareh, A.R.; Kempisty, B.; Dzięgiel, P.; Azizidoost, S.; et al. Circular RNAs-mediated angiogenesis in human cancers. Clin. Transl. Oncol. 2023, 25, 3101–3121. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, X.; Mo, C.; Li, L.; Nong, S.; Gui, C. The role of circRNAs in the regulation of myocardial angiogenesis in coronary heart disease. Microvasc. Res. 2022, 142, 104362. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, K.; Cui, J.; Xiong, J.; Wu, H.; Peng, T.; Guo, Y. Circ-MBOAT2 knockdown represses tumor progression and glutamine catabolism by miR-433-3p/GOT1 axis in pancreatic cancer. J. Exp. Clin. Cancer Res. 2021, 40, 124. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, C.; Chen, C.; Guo, K.; Tang, Z.; Luo, Y.; Chen, L.; Su, Y.; Xu, K. Circular RNA circMBOAT2 promotes prostate cancer progression via a miR-1271-5p/mTOR axis. Aging 2020, 12, 13255–13280. [Google Scholar] [CrossRef]
- Zhuang, Y.; Fan, W.P.; Yan, H.S. Overexpression of Circ_0005585 Alleviates Cerebral Ischemia Reperfusion Injury via Targeting MiR-16-5p. Bull. Exp. Biol. Med. 2023, 175, 304–310. [Google Scholar] [CrossRef]
- Sun, D.; Wang, J.; Tian, Y.; Narsinh, K.; Wang, H.; Li, C.; Ma, X.; Wang, Y.; Wang, D.; Li, C.; et al. Multimodality imaging evaluation of functional and clinical benefits of percutaneous coronary intervention in patients with chronic total occlusion lesion. Theranostics 2012, 2, 788–800. [Google Scholar] [CrossRef]
- Hachamovitch, R.; Hayes, S.W.; Friedman, J.D.; Cohen, I.; Berman, D.S. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003, 107, 2900–2907. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, J.; Wu, R.; Yuan, J.; Ge, J. Integrated Analysis of Angiogenesis Related lncRNA-miRNA-mRNA in Patients with Coronary Chronic Total Occlusion Disease. Front Genet. 2022, 13, 855549. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Mashayekhi, K.; Tsuchikane, E.; Rafeh, N.A.; Alaswad, K.; Araya, M.; Avran, A.; Azzalini, L.; Babunashvili, A.M.; Bayani, B.; et al. Guiding Principles for Chronic Total Occlusion Percutaneous Coronary Intervention. Circulation 2019, 140, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Meisel, S.R.; Frimerman, A.; Blondheim, D.S.; Shotan, A.; Asif, A.; Shani, J.; Shochat, M. Relation of the systemic blood pressure to the collateral pressure distal to an infarct-related coronary artery occlusion during acute myocardial infarction. Am. J. Cardiol. 2013, 111, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, H.; Yuan, J.; Wu, C.; Huang, D.; Ma, Y.; Zhu, J.; Ma, L.; Guo, J.; Shi, H.; et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-alpha mediated NF-kappaB pathway. J. Cell. Mol. Med. 2016, 20, 2318–2327. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, R.; Li, Z.; Zhang, Y.; Wang, X.; Wu, J.; Li, Z.; Qian, S.; Li, B.; Zhang, Z.; et al. VEGFR endocytosis regulates the angiogenesis in a mouse model of hindlimb ischemia. J. Thorac. Dis. 2019, 11, 1849–1859. [Google Scholar] [CrossRef]
- Li, C.; Xu, R.; Yao, K.; Zhang, J.; Chen, S.; Pang, L.; Lu, H.; Dai, Y.; Qian, J.; Shi, H.; et al. Functional significance of intermediate coronary stenosis in patients with single-vessel coronary artery disease: A comparison of dynamic SPECT coronary flow reserve with intracoronary pressure-derived fractional flow reserve (FFR). J. Nucl. Cardiol. 2020, 29, 622–629. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Li, C.; Yuan, J.; Zhang, Y.; Liu, G.; Zhang, J.; Shi, H.; Liu, H.; Ge, J. Circ-MBOAT2 Regulates Angiogenesis via the miR-495/NOTCH1 Axis and Associates with Myocardial Perfusion in Patients with Coronary Chronic Total Occlusion. Int. J. Mol. Sci. 2024, 25, 793. https://doi.org/10.3390/ijms25020793
Gao W, Li C, Yuan J, Zhang Y, Liu G, Zhang J, Shi H, Liu H, Ge J. Circ-MBOAT2 Regulates Angiogenesis via the miR-495/NOTCH1 Axis and Associates with Myocardial Perfusion in Patients with Coronary Chronic Total Occlusion. International Journal of Molecular Sciences. 2024; 25(2):793. https://doi.org/10.3390/ijms25020793
Chicago/Turabian StyleGao, Wei, Chenguang Li, Jie Yuan, Youming Zhang, Guobing Liu, Jianhui Zhang, Hongcheng Shi, Haibo Liu, and Junbo Ge. 2024. "Circ-MBOAT2 Regulates Angiogenesis via the miR-495/NOTCH1 Axis and Associates with Myocardial Perfusion in Patients with Coronary Chronic Total Occlusion" International Journal of Molecular Sciences 25, no. 2: 793. https://doi.org/10.3390/ijms25020793
APA StyleGao, W., Li, C., Yuan, J., Zhang, Y., Liu, G., Zhang, J., Shi, H., Liu, H., & Ge, J. (2024). Circ-MBOAT2 Regulates Angiogenesis via the miR-495/NOTCH1 Axis and Associates with Myocardial Perfusion in Patients with Coronary Chronic Total Occlusion. International Journal of Molecular Sciences, 25(2), 793. https://doi.org/10.3390/ijms25020793





