Heart Failure with Preserved Ejection Fraction: The Pathophysiological Mechanisms behind the Clinical Phenotypes and the Therapeutic Approach
Abstract
1. Introduction
2. Definition of Heart Failure
2.1. The Role of Biomarkers in Defining Heart Failure
2.2. Universal Definition of Heart Failure
3. Prevalence and Demographics
4. Etiology
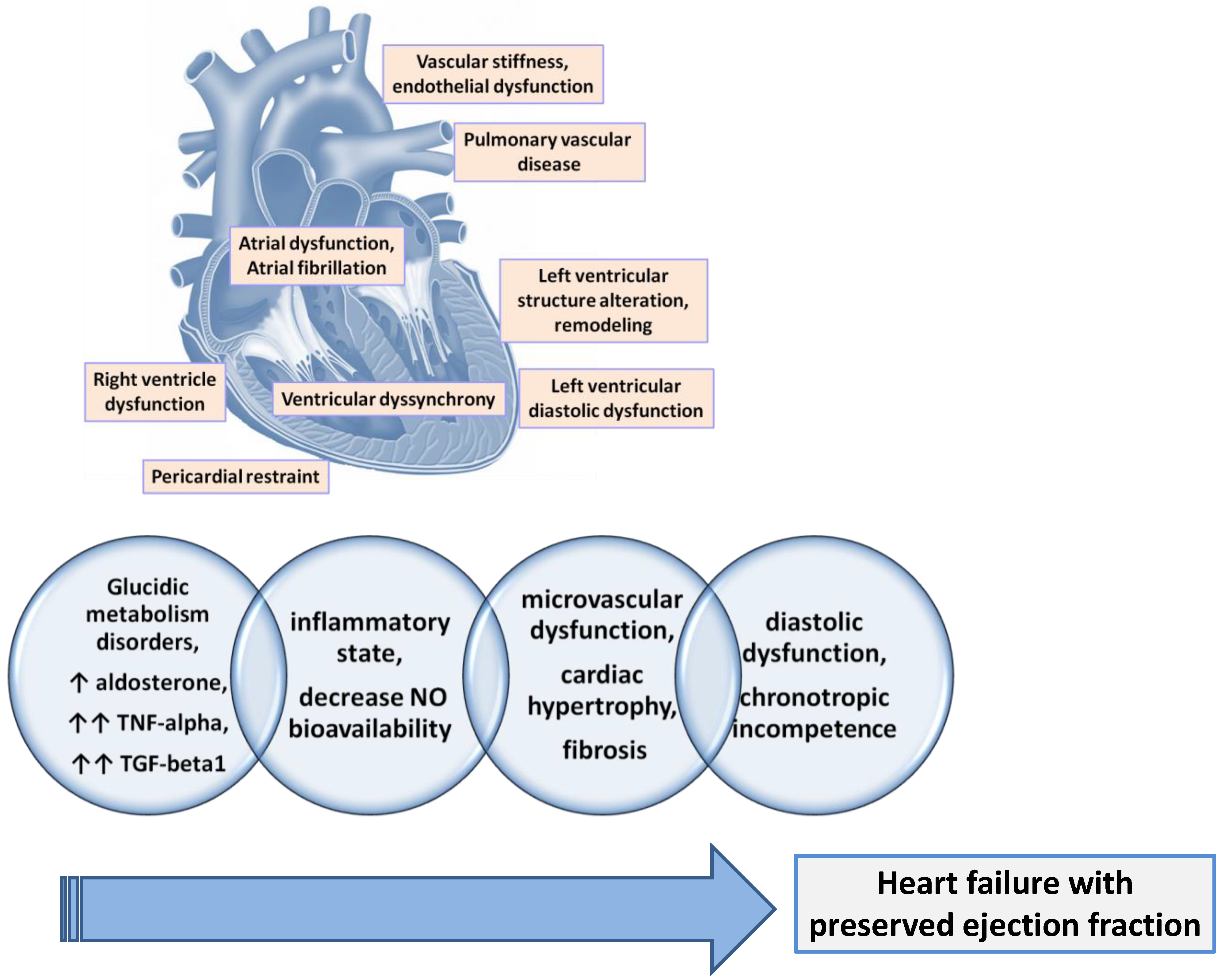
5. Pathophysiology of HFpEF
5.1. Left Ventricular Structure and Remodeling
5.2. Left Ventricular Diastolic Dysfunction
5.3. Ventricular Dyssynchrony
5.4. Atrial Dysfunction and Atrial Fibrillation
5.5. Right Ventricle Dysfunction (RVD) and Pulmonary Vascular Disease
5.6. Pericardial Restraint
5.7. Vascular Stiffness and Endothelial Dysfunction
5.8. Chronotropic Reserve
5.9. Cardiac Aging
5.10. Hypervolemia
6. Clinical Manifestations
7. Diagnosis
7.1. Clinical History and Physical Examination
7.2. Transthoracic Echocardiography
7.2.1. Speckle-Tracking Echocardiography (STE)
7.2.2. Automated Echocardiographic Detection of HFpEF
7.3. Cardiac Magnetic Resonance
7.4. Exercise Testing
8. HFpEF Phenotypes
- The phenotype characterized by the aging processes (aging phenotype);
- The phenotype characterized by the excess presence of adipose tissue (obesity or cardiometabolic phenotype);
- The phenotype associated with arterial hypertension;
- The phenotype associated with pulmonary arterial hypertension;
- The phenotype associated with ischemic coronary disease (the phenotype of coronary artery disease);
- The phenotype associated with left atrial myopathy.
8.1. Aging Phenotype
8.2. Obesity Phenotype (Cardiometabolic)
8.3. Hypertension Phenotype
8.4. Pulmonary Hypertension Phenotype
8.5. Coronary Artery Disease (CAD) Phenotype
8.6. Left Atrium (LA) Myopathy Phenotype
9. HFpEF Biological Phenotypes
9.1. Natriuretic Peptide Deficiency Syndrome
9.2. Excessive Activation of Plasminogen Activator Inhibitor (PAI)-1
9.3. Extreme Cardiometabolic Syndrome
9.4. Right Ventricle–Cardiac–Abdominal–Renal Syndrome
10. Phenotypes of HFpEF Identified Using Machine Learning Techniques
11. Differential Diagnosis
12. Comorbidities
13. Prognosis
14. Treatment
14.1. Pharmacological Therapy
14.2. Non-Pharmacological Therapy
15. Challenges, Findings and Limitations
16. Future Perspective and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Theresa, A.M.; Marco, M.; Marianna, A.; Roy, S.G.; Andreas, B.; Michael, B.; Haran, B.; Javed, B.; Jelena, Č.; Ovidiu, C.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the Special Contributio. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Romitan, D.M.; Rădulescu, D.; Berindan-Neagoe, I.; Stoicescu, L.; Grosu, A.; Rădulescu, L.; Gulei, D.; Ciuleanu, T.E. Cardiomyopathies and Arrhythmias Induced by Cancer Therapies. Biomedicines 2020, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. Evaluation and Management of Heart Failure with Preserved Ejection Fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef]
- Redfield, M.M. Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2016, 375, 1868–1877. [Google Scholar] [CrossRef]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; Van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-Specific Treatment of Heart Failure with Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef]
- Luchi, R.J.; Snow, E.; Luchi, J.M.; Nelson, C.L.; Pircher, F.J. Left Ventricular Function in Hospitalized Geriatric Patients. J. Am. Geriatr. Soc. 1982, 30, 700–705. [Google Scholar] [CrossRef]
- Obokata, M.; Kane, G.C.; Reddy, Y.N.V.; Olson, T.P.; Melenovsky, V.; Borlaug, B.A. Role of Diastolic Stress Testing in the Evaluation for Heart Failure With Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation 2017, 135, 825–838. [Google Scholar] [CrossRef]
- Adamczak, D.M.; Oduah, M.T.; Kiebalo, T.; Nartowicz, S.; Bęben, M.; Pochylski, M.; Ciepłucha, A.; Gwizdała, A.; Lesiak, M.; Straburzyńska-Migaj, E. Heart Failure with Preserved Ejection Fraction-a Concise Review. Curr. Cardiol. Rep. 2020, 22, 82. [Google Scholar] [CrossRef]
- Pop, C.; Ștefan, M.G.; Muntean, D.M.; Stoicescu, L.; Gal, A.F.; Kiss, B.; Morgovan, C.; Loghin, F.; Rochette, L.; Lauzier, B.; et al. Protective Effects of a Discontinuous Treatment with Alpha-Lipoic Acid in Obesity-Related Heart Failure with Preserved Ejection Fraction, in Rats. Antioxidants 2020, 9, 1073. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Pellicori, P.; Clark, A.L. Prevention or Procrastination for Heart Failure?: Why We Need a Universal Definition of Heart Failure. J. Am. Coll. Cardiol. 2019, 73, 2398–2400. [Google Scholar] [CrossRef]
- Tsutsui, H.; Goto, Y.; Higo, T.; Hirayama, A.; Isobe, M.; Ito, H.; Kihara, Y.; Kinugawa, K.; Kitakaze, M.; Komuro, I.; et al. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure-Digest Version. Circ. J. 2019, 83, 2084–2184. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Heart Failure. JACC. Heart Fail. 2013, 1, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.B.; Williams, S.G.; Tan, D.K.H.; Cohen-Solal, A. So Many Definitions of Heart Failure: Are They All Universally Valid? A Critical Appraisal. Expert. Rev. Cardiovasc. Ther. 2010, 8, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 2020, 141, E139–E596. [Google Scholar] [CrossRef] [PubMed]
- Pinney, S.P. Disparities in Heart Failure Care: Now Is the Time to Focus on Health Care Delivery. J. Am. Coll. Cardiol. 2014, 64, 808–810. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sabbah, H.N. Silent Disease Progression in Clinically Stable Heart Failure. Eur. J. Heart Fail. 2017, 19, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.H.; Lin, Y.Y.; Chu, S.J.; Hsu, C.W.; Cheng, S.M. Interpretation and Use of Natriuretic Peptides in Non-Congestive Heart Failure Settings. Yonsei Med. J. 2010, 51, 151–163. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef]
- Hicks, K.A.; Mahaffey, K.W.; Mehran, R.; Nissen, S.E.; Wiviott, S.D.; Dunn, B.; Solomon, S.D.; Marler, J.R.; Teerlink, J.R.; Farb, A.; et al. 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials. J. Am. Coll. Cardiol. 2018, 71, 1021–1034. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, K.; Teng, T.H.K.; Chandramouli, C.; Tromp, J.; Sakata, Y.; Lam, C.S.P. Epidemiology and Clinical Features of Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2022, 8, e27. [Google Scholar] [CrossRef]
- Vasan, R.S.; Xanthakis, V.; Lyass, A.; Andersson, C.; Tsao, C.; Cheng, S.; Aragam, J.; Benjamin, E.J.; Larson, M.G. Epidemiology of Left Ventricular Systolic Dysfunction and Heart Failure in the Framingham Study: An Echocardiographic Study Over 3 Decades. JACC Cardiovasc. Imaging 2018, 11, 1–11. [Google Scholar] [CrossRef]
- Heinzel, F.R.; Shah, S.J. The Future of Heart Failure with Preserved Ejection Fraction: Deep Phenotyping for Targeted Therapeutics. Herz 2022, 47, 308–323. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in Prevalence and Outcome of Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef]
- Abebe, T.B.; Gebreyohannes, E.A.; Tefera, Y.G.; Abegaz, T.M. Patients with HFpEF and HFrEF Have Different Clinical Characteristics but Similar Prognosis: A Retrospective Cohort Study. BMC Cardiovasc. Disord. 2016, 16, 232. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Shah, A.M.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction in Perspective. Circ. Res. 2019, 124, 1598–1617. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–577. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Paulus, W.J. Heart Failure with Preserved Ejection Fraction: Pathophysiology, Diagnosis, and Treatment. Eur. Heart J. 2011, 32, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.; Zotter-Tufaro, C.; Kammerlander, A.A.; Aschauer, S.; Binder, C.; Mascherbauer, J.; Bonderman, D. Gender-Related Differences in Heart Failure with Preserved Ejection Fraction. Sci. Rep. 2018, 8, 1080. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of Heart Failure with Preserved Ejection Fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.G.; Patel, B.; Blankstein, R.; Lima, J.A.C.; Blumenthal, R.S.; Nasir, K.; Blaha, M.J. Impact of Race, Ethnicity, and Multimodality Biomarkers on the Incidence of New-Onset Heart Failure with Preserved Ejection Fraction (from the Multi-Ethnic Study of Atherosclerosis). Am. J. Cardiol. 2016, 117, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Doughty, R.N.; Cubbon, R.; Ezekowitz, J.; Gonzalez-Juanatey, J.; Gorini, M.; Gotsman, I.; GrigorianShamagian, L.; Guazzi, M.; Kearney, M.; Køber, L.; et al. The Survival of Patients with Heart Failure with Preserved or Reduced Left Ventricular Ejection Fraction: An Individual Patient Data Meta-Analysis. Eur. Heart J. 2012, 33, 1750–1757. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; McClelland, R.L.; Marshall, R.; Shemanski, L.; Furberg, C.D.; Kitzman, D.W.; Cushman, M.; Polak, J.; Gardin, J.M.; Gersh, B.J.; et al. Outcome of Congestive Heart Failure in Elderly Persons: Influence of Left Ventricular Systolic Function. The Cardiovascular Health Study. Ann. Intern. Med. 2002, 137, 631–639. [Google Scholar] [CrossRef]
- Andersen, M.J.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction: Current Understandings and Challenges. Curr. Cardiol. Rep. 2014, 16, 501. [Google Scholar] [CrossRef]
- Mentz, R.J.; Kelly, J.P.; Von Lueder, T.G.; Voors, A.A.; Lam, C.S.P.; Cowie, M.R.; Kjeldsen, K.; Jankowska, E.A.; Atar, D.; Butler, J.; et al. Noncardiac Comorbidities in Heart Failure with Reduced versus Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2014, 64, 2281–2293. [Google Scholar] [CrossRef] [PubMed]
- Zafrir, B.; Lund, L.H.; Laroche, C.; Ruschitzka, F.; Crespo-Leiro, M.G.; Coats, A.J.S.; Anker, S.D.; Filippatos, G.; Seferovic, P.M.; Maggioni, A.P.; et al. Prognostic Implications of Atrial Fibrillation in Heart Failure with Reduced, Mid-Range, and Preserved Ejection Fraction: A Report from 14 964 Patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur. Heart J. 2018, 39, 4277–4284. [Google Scholar] [CrossRef]
- Redfield, M.M.; Jacobsen, S.J.; Burnett, J.C.; Mahoney, D.W.; Bailey, K.R.; Rodeheffer, R.J. Burden of Systolic and Diastolic Ventricular Dysfunction in the Community: Appreciating the Scope of the Heart Failure Epidemic. JAMA 2003, 289, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. Circulation 2005, 112, e154–e235. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Dávila-Román, V.G.; Mann, D.L.; McNulty, S.; Semigran, M.J.; Lewis, G.D.; De Las Fuentes, L.; Joseph, S.M.; Vader, J.; Hernandez, A.F.; et al. Cardiovascular Phenotype in HFpEF Patients with or without Diabetes: A RELAX Trial Ancillary Study. J. Am. Coll. Cardiol. 2014, 64, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Mogensen, U.M.; Jhund, P.S.; Petrie, M.C.; Preiss, D.; Win, S.; Køber, L.; McKelvie, R.S.; Zile, M.R.; Anand, I.S.; et al. Clinical and Echocardiographic Characteristics and Cardiovascular Outcomes according to Diabetes Status in Patients with Heart Failure and Preserved Ejection Fraction: A Report from the I-Preserve Trial (Irbesartan in Heart Failure with Preserved Ejection. Circulation 2017, 135, 724–735. [Google Scholar] [CrossRef]
- Unger, E.D.; Dubin, R.F.; Deo, R.; Daruwalla, V.; Friedman, J.L.; Medina, C.; Beussink, L.; Freed, B.H.; Shah, S.J. Association of Chronic Kidney Disease with Abnormal Cardiac Mechanics and Adverse Outcomes in Patients with Heart Failure and Preserved Ejection Fraction. Eur. J. Heart Fail. 2016, 18, 103–112. [Google Scholar] [CrossRef]
- Hegi, B.; Hernandez, J.M.; Ko, C.Y.; Hong, J.; Shen, E.Y.; Spencer, E.R.; Smoliarchuk, D.; Navedo, M.F.; Bers, D.M.; Bossuyt, J. Diabetes and Excess Aldosterone Promote Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2022, 11, 27164. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Gorter, T.M.; van Empel, V.P.M.; Hemels, M.E.W.; Hazebroek, E.J.; van Veldhuisen, S.L.; Willems, T.P.; Rienstra, M.; Westenbrink, B.D. Importance of Epicardial Adipose Tissue Localization Using Cardiac Magnetic Resonance Imaging in Patients with Heart Failure with Mid-Range and Preserved Ejection Fraction. Clin. Cardiol. 2021, 44, 987–993. [Google Scholar] [CrossRef]
- Mishra, S.; Kass, D.A. Publisher Correction: Cellular and Molecular Pathobiology of Heart Failure with Preserved Ejection Fraction. Nat. Rev. Cardiol. 2021, 18, 735. [Google Scholar] [CrossRef]
- Van Empel, V.; Brunner-La Rocca, H.P. Inflammation in HFpEF: Key or Circumstantial? Int. J. Cardiol. 2015, 189, 259–263. [Google Scholar] [CrossRef]
- Rech, M.; Barandiarán Aizpurua, A.; Van Empel, V.; Van Bilsen, M.; Schroen, B. Pathophysiological Understanding of HFpEF: MicroRNAs as Part of the Puzzle. Cardiovasc. Res. 2018, 114, 782–793. [Google Scholar] [CrossRef]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Iwamoto, A.; Kajikawa, M.; Matsumoto, T.; Oda, N.; et al. Endothelial Function Is Impaired in Patients Receiving Antihypertensive Drug Treatment Regardless of Blood Pressure Level FMD-J Study (Flow-Mediated Dilation Japan). Hypertension 2017, 70, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Su, M.Y.M.; Lin, L.Y.; Tseng, Y.H.E.; Chang, C.C.; Wu, C.K.; Lin, J.L.; Tseng, W.Y.I. CMR-Verified Diffuse Myocardial Fibrosis Is Associated with Diastolic Dysfunction in HFpEF. JACC Cardiovasc. Imaging 2014, 7, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Curl, C.L.; Danes, V.R.; Bell, J.R.; Raaijmakers, A.J.A.; Ip, W.T.K.; Chandramouli, C.; Harding, T.W.; Porrello, E.R.; Erickson, J.R.; Charchar, F.J.; et al. Cardiomyocyte Functional Etiology in Heart Failure with Preserved Ejection Fraction Is Distinctive-A New Preclinical Model. J. Am. Heart Assoc. 2018, 7, e007451. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Nayor, M.; DeFilippi, C.R.; Enserro, D.; Bhambhani, V.; Kizer, J.R.; Blaha, M.J.; Brouwers, F.P.; Cushman, M.; Lima, J.A.C.; et al. Association of Cardiovascular Biomarkers with Incident Heart Failure with Preserved and Reduced Ejection Fraction. JAMA Cardiol. 2018, 3, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. The Pathophysiology of Heart Failure with Preserved Ejection Fraction. Nat. Rev. Cardiol. 2014, 11, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.; Stoicescu, L.; Buzdugan, E.; Donca, V. Patterns of Left Ventricular Remodeling among Patients with Essential and Secondary Hypertension. Rev. Med. Chil. 2013, 141, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Roger, V.L.; Rodeheffer, R.J.; Bursi, F.; Borlaug, B.A.; Ommen, S.R.; Kass, D.A.; Redfield, M.M. Cardiac Structure and Ventricular-Vascular Function in Persons with Heart Failure and Preserved Ejection Fraction from Olmsted County, Minnesota. Circulation 2007, 115, 1982–1990. [Google Scholar] [CrossRef]
- Dai, D.F.; Chen, T.; Johnson, S.C.; Szeto, H.; Rabinovitch, P.S. Cardiac Aging: From Molecular Mechanisms to Significance in Human Health and Disease. Antioxid. Redox Signal. 2012, 16, 1492–1536. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Redfield, M.M.; Melenovsky, V.; Kane, G.C.; Karon, B.L.; Jacobsen, S.J.; Rodeheffer, R.J. Longitudinal Changes in Left Ventricular Stiffness: A Community-Based Study. Circ. Heart Fail. 2013, 6, 944–952. [Google Scholar] [CrossRef]
- Liu, S.; Guan, Z.; Jin, X.; Meng, P.; Wang, Y.; Zheng, X.; Jia, D.; Ma, C.; Yang, J. Left Ventricular Diastolic and Systolic Dyssynchrony and Dysfunction in Heart Failure with Preserved Ejection Fraction and a Narrow QRS Complex. Int. J. Med. Sci. 2018, 15, 108–114. [Google Scholar] [CrossRef]
- Shin, S.H.; Hung, C.L.; Uno, H.; Hassanein, A.H.; Verma, A.; Bourgoun, M.; Køber, L.; Ghali, J.K.; Velazquez, E.J.; Califf, R.M.; et al. Mechanical Dyssynchrony after Myocardial Infarction in Patients with Left Ventricular Dysfunction, Heart Failure, or Both. Circulation 2010, 121, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.B.S.; Kraigher-Krainer, E.; Bello, N.; Claggett, B.; Zile, M.R.; Pieske, B.; Voors, A.A.; McMurray, J.J.V.; Packer, M.; Bransford, T.; et al. Left Ventricular Dyssynchrony in Patients with Heart Failure and Preserved Ejection Fraction. Eur. Heart J. 2014, 35, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Pouleur, A.C.; Knappe, D.; Shah, A.M.; Uno, H.; Bourgoun, M.; Foster, E.; McNitt, S.; Hall, W.J.; Zareba, W.; Goldenberg, I.; et al. Relationship between Improvement in Left Ventricular Dyssynchrony and Contractile Function and Clinical Outcome with Cardiac Resynchronization Therapy: The MADIT-CRT Trial. Eur. Heart J. 2011, 32, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Savarese, G.; Dahlström, U.; Lund, L.H.; Fu, M. Age-Dependent Differences in Clinical Phenotype and Prognosis in Heart Failure with Mid-Range Ejection Compared with Heart Failure with Reduced or Preserved Ejection Fraction. Clin. Res. Cardiol. 2019, 108, 1394–1405. [Google Scholar] [CrossRef]
- Santos, A.B.S.; Roca, G.Q.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Fang, J.C.; Zile, M.R.; Pitt, B.; Solomon, S.D.; et al. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2016, 9, e002763. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.; Fovargue, L.; Tøndel, K.; Porter, B.; Sieniewicz, B.; Gould, J.; Rinaldi, C.A.; Ismail, T.; Chiribiri, A.; Carr-White, G. The Emerging Role of Cardiac Magnetic Resonance Imaging in the Evaluation of Patients with HFpEF. Curr. Heart Fail. Rep. 2018, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.F.; Hussain, I.; Abou Ezzeddine, O.F.; Takahama, H.; Kwon, S.H.; Forfia, P.; Roger, V.L.; Redfield, M.M. Right Ventricular Function in Heart Failure with Preserved Ejection Fraction: A Community-Based Study. Circulation 2014, 130, 2310–2320. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.J.; Lin, G.; Redfield, M.M.; Borlaug, B.A. Right Heart Dysfunction in Heart Failure with Preserved Ejection Fraction. Eur. Heart J. 2014, 35, 3452–3462. [Google Scholar] [CrossRef]
- Vanderpool, R.R.; Saul, M.; Nouraie, M.; Gladwin, M.T.; Simon, M.A. Association Between Hemodynamic Markers of Pulmonary Hypertension and Outcomes in Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2018, 3, 298–306. [Google Scholar] [CrossRef]
- Gorter, T.M.; Hoendermis, E.S.; van Veldhuisen, D.J.; Voors, A.A.; Lam, C.S.P.; Geelhoed, B.; Willems, T.P.; van Melle, J.P. Right Ventricular Dysfunction in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Eur. J. Heart Fail. 2016, 18, 1472–1487. [Google Scholar] [CrossRef]
- Berglund, F.; Piña, P.; Herrera, C.J. Right Ventricle in Heart Failure with Preserved Ejection Fraction. Heart 2020, 106, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Pislaru, S.; Borlaug, B.A. Deterioration in Right Ventricular Structure and Function over Time in Patients with Heart Failure and Preserved Ejection Fraction. Eur. Heart J. 2019, 40, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Jaber, W.A.; Ommen, S.R.; Lam, C.S.P.; Redfield, M.M.; Nishimura, R.A. Diastolic Relaxation and Compliance Reserve during Dynamic Exercise in Heart Failure with Preserved Ejection Fraction. Heart 2011, 97, 964–969. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Reddy, Y.N.V. The Role of the Pericardium in Heart Failure: Implications for Pathophysiology and Treatment. JACC Heart Fail. 2019, 7, 574–585. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Carter, R.E.; Melenovsky, V.; Desimone, C.V.; Gaba, P.; Killu, A.; Naksuk, N.; Lerman, L.; Asirvatham, S.J. Percutaneous Pericardial Resection: A Novel Potential Treatment for Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2017, 10, e003612. [Google Scholar] [CrossRef]
- Gorter, T.M.; Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Borlaug, B.A. Exercise Unmasks Distinct Pathophysiologic Features in Heart Failure with Preserved Ejection Fraction and Pulmonary Vascular Disease. Eur. Heart J. 2018, 39, 2825–2835. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Olson, T.P.; Lam, C.S.P.; Flood, K.S.; Lerman, A.; Johnson, B.D.; Redfield, M.M. Global Cardiovascular Reserve Dysfunction in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2010, 56, 845–854. [Google Scholar] [CrossRef]
- Chow, B.; Rabkin, S.W. The Relationship between Arterial Stiffness and Heart Failure with Preserved Ejection Fraction: A Systemic Meta-Analysis. Heart Fail. Rev. 2015, 20, 291–303. [Google Scholar] [CrossRef]
- Sucato, V.; Evola, S.; Novo, G.; Sansone, A.; Quagliana, A.; Andolina, G.; Assennato, P.; Novo, S. Angiographic Evaluation of Coronary Microvascular Dysfunction in Patients with Heart Failure and Preserved Ejection Fraction. Microcirculation 2015, 22, 528–533. [Google Scholar] [CrossRef]
- Akiyama, E.; Sugiyama, S.; Matsuzawa, Y.; Konishi, M.; Suzuki, H.; Nozaki, T.; Ohba, K.; Matsubara, J.; Maeda, H.; Horibata, Y.; et al. Incremental Prognostic Significance of Peripheral Endothelial Dysfunction in Patients with Heart Failure with Normal Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2012, 60, 1778–1786. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A Novel Paradigm for Heart Failure with Preserved Ejection Fraction: Comorbidities Drive Myocardial Dysfunction and Remodeling through Coronary Microvascular Endothelial Inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; D’Adda Di Fagagna, F. Cellular Senescence: When Bad Things Happen to Good Cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Fyhrquist, F.; Saijonmaa, O.; Strandberg, T. The Roles of Senescence and Telomere Shortening in Cardiovascular Disease. Nat. Rev. Cardiol. 2013, 10, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Voghel, G.; Thorin-Trescases, N.; Farhat, N.; Nguyen, A.; Villeneuve, L.; Mamarbachi, A.M.; Fortier, A.; Perrault, L.P.; Carrier, M.; Thorin, E. Cellular Senescence in Endothelial Cells from Atherosclerotic Patients Is Accelerated by Oxidative Stress Associated with Cardiovascular Risk Factors. Mech. Ageing Dev. 2007, 128, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, A.B.; Shakeri, H.; Leloup, A.J.; Van Hove, C.E.; De Meyer, G.R.Y.; Vrints, C.J.; Lemmens, K.; Van Craenenbroeck, E.M. Endothelial Senescence Contributes to Heart Failure with Preserved Ejection Fraction in an Aging Mouse Model. Circ. Heart Fail. 2017, 10, e003806. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Kass, D.A. Ventricular–Vascular Interaction in Heart Failure. Cardiol. Clin. 2011, 29, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Abudiab, M.M.; Redfield, M.M.; Melenovsky, V.; Olson, T.P.; Kass, D.A.; Johnson, B.D.; Borlaug, B.A. Cardiac Output Response to Exercise in Relation to Metabolic Demand in Heart Failure with Preserved Ejection Fraction. Eur. J. Heart Fail. 2013, 15, 776–785. [Google Scholar] [CrossRef]
- Cao, N.; Wong, Y.G.; Rosli, S.; Kiriazis, H.; Huynh, K.; Qin, C.; Du, X.J.; Kemp-Harper, B.K.; Ritchie, R.H. Chronic Administration of the Nitroxyl Donor 1-Nitrosocyclo Hexyl Acetate Limits Left Ventricular Diastolic Dysfunction in a Mouse Model of Diabetes Mellitus in Vivo. Circ. Heart Fail. 2015, 8, 572–581. [Google Scholar] [CrossRef]
- Hage, C.; Löfgren, L.; Michopoulos, F.; Nilsson, R.; Davidsson, P.; Kumar, C.; Ekström, M.; Eriksson, M.J.; Lyngå, P.; Persson, B.; et al. Metabolomic Profile in HFpEF vs HFrEF Patients. J. Card. Fail. 2020, 26, 1050–1059. [Google Scholar] [CrossRef]
- Katoh, S.; Shishido, T.; Kutsuzawa, D.; Arimoto, T.; Netsu, S.; Funayama, A.; Ishino, M.; Niizeki, T.; Nishiyama, S.; Takahashi, H.; et al. Iodine-123-Metaiodobenzylguanidine Imaging Can Predict Future Cardiac Events in Heart Failure Patients with Preserved Ejection Fraction. Ann. Nucl. Med. 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Kass, D.A.; Kitzman, D.W.; Alvarez, G.E. The Restoration of Chronotropic Competence in Heart Failure Patients with Normal Ejection Fraction (RESET) Study: Rationale and Design. J. Card. Fail. 2010, 16, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sarma, S.; Stoller, D.; Hendrix, J.; Howden, E.; Lawley, J.; Livingston, S.; Adams-Huet, B.; Holmes, C.; Goldstein, D.S.; Levine, B.D. Mechanisms of Chronotropic Incompetence in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2020, 13, E006331. [Google Scholar] [CrossRef]
- Baek, Y.S.; Lee, D.H.; Jo, Y.; Lee, S.C.; Choi, W.; Kim, D.H. Artificial Intelligence-Estimated Biological Heart Age Using a 12-Lead Electrocardiogram Predicts Mortality and Cardiovascular Outcomes. Front. Cardiovasc. Med. 2023, 10, 1137892. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Reddy, Y.N.V.; Attia, Z.I.; Friedman, P.A.; Noseworthy, P.A.; Lopez-Jimenez, F.; Kapa, S.; Borlaug, B.A. Obesity Accelerates Cardiac Senescence in Heart Failure with Preserved Ejection Fraction. J. Card. Fail. 2020, 26, S34–S35. [Google Scholar] [CrossRef]
- Li, C.; Qin, D.; Hu, J.; Yang, Y.; Hu, D.; Yu, B. Inflamed Adipose Tissue: A Culprit Underlying Obesity and Heart Failure with Preserved Ejection Fraction. Front. Immunol. 2022, 13, 947147. [Google Scholar] [CrossRef] [PubMed]
- Lafuse, W.P.; Wozniak, D.J.; Rajaram, M.V.S. Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair. Cells 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Oldham, W.M.; Priolo, C.; Pandey, A.K.; Loscalzo, J. Immunometabolic Endothelial Phenotypes: Integrating Inflammation and Glucose Metabolism. Circ. Res. 2021, 129, 9–29. [Google Scholar] [CrossRef]
- Bistola, V.; Polyzogopoulou, E.; Ikonomidis, I.; Parissis, J. Congestion in Acute Heart Failure with Reduced vs. Preserved Left Ventricular Ejection Fraction: Differences, Similarities and Remaining Gaps. Eur. J. Heart Fail. 2018, 20, 748–750. [Google Scholar] [CrossRef]
- Van Aelst, L.N.L.; Arrigo, M.; Placido, R.; Akiyama, E.; Girerd, N.; Zannad, F.; Manivet, P.; Rossignol, P.; Badoz, M.; Sadoune, M.; et al. Acutely Decompensated Heart Failure with Preserved and Reduced Ejection Fraction Present with Comparable Haemodynamic Congestion. Eur. J. Heart Fail. 2018, 20, 738–747. [Google Scholar] [CrossRef]
- Cogliati, C.; Ceriani, E.; Gambassi, G.; De Matteis, G.; Perlini, S.; Perrone, T.; Muiesan, M.L.; Salvetti, M.; Leidi, F.; Ferrara, F.; et al. Phenotyping Congestion in Patients with Acutely Decompensated Heart Failure with Preserved and Reduced Ejection Fraction: The Decongestion duRing TherapY for Acute DecOmpensated Heart Failure in HFpEF vs HFrEF- DRY-OFF Study. Eur. J. Intern. Med. 2022, 97, 69–77. [Google Scholar] [CrossRef]
- Fallick, C.; Sobotka, P.A.; Dunlap, M.E. Sympathetically Mediated Changes in Capacitance Redistribution of the Venous Reservoir as a Cause of Decompensation. Circ. Heart Fail. 2011, 4, 669–675. [Google Scholar] [CrossRef]
- Nagueh, S.F. Heart Failure with Preserved Ejection Fraction: Insights into Diagnosis and Pathophysiology. Cardiovasc. Res. 2021, 117, 999–1014. [Google Scholar] [CrossRef]
- Vaughan, D.E.; Rai, R.; Khan, S.S.; Eren, M.; Ghosh, A.K. PAI-1 Is a Marker and a Mediator of Senescence. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1446. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.S.; Sharma, K.; Fiuzat, M.; Surks, H.K.; George, J.T.; Honarpour, N.; Depre, C.; Desvigne-Nickens, P.; Nkulikiyinka, R.; Lewis, G.D.; et al. Heart Failure with Preserved Ejection Fraction Expert Panel Report: Current Controversies and Implications for Clinical Trials. JACC. Heart Fail. 2018, 6, 619–632. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Nishimura, R.A.; Sorajja, P.; Lam, C.S.P.; Redfield, M.M. Exercise Hemodynamics Enhance Diagnosis of Early Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2010, 3, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, Y.; Nishi, I.; Furuichi, S.; Noguchi, T.; Sase, K.; Kihara, Y.; Goto, Y.; Nonogi, H. B-Type Natriuretic Peptide Strongly Reflects Diastolic Wall Stress in Patients with Chronic Heart Failure: Comparison between Systolic and Diastolic Heart Failure. J. Am. Coll. Cardiol. 2006, 47, 742–748. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Faxén, U.L.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and Correlates of Coronary Microvascular Dysfunction in Heart Failure with Preserved Ejection Fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Kane, G.C.; Olson, T.P.; Jarolim, P.; Borlaug, B.A. Myocardial Injury and Cardiac Reserve in Patients with Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2018, 72, 29–40. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef]
- From, A.M.; Lam, C.S.P.; Pitta, S.R.; Kumar, P.V.; Balbissi, K.A.; Booker, J.D.; Singh, I.M.; Sorajja, P.; Reeder, G.S.; Borlaug, B.A. Bedside Assessment of Cardiac Hemodynamics: The Impact of Noninvasive Testing and Examiner Experience. Am. J. Med. 2011, 124, 1051–1057. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Memon, M.M.; Murad, M.H.; Vaduganathan, M.; Greene, S.J.; Hall, M.; Triposkiadis, F.; Lam, C.S.P.; Shah, A.M.; Butler, J.; et al. Left Atrial Function in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Eur. J. Heart Fail. 2020, 22, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, G.E.; Sisti, N.; Mondillo, S.; Cameli, M. Left Atrial Strain in Left Ventricular Diastolic Dysfunction: Have We Finally Found the Missing Piece of the Puzzle? Heart Fail. Rev. 2020, 25, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Aistrup, G.L.; Gupta, D.K.; O’Toole, M.J.; Nahhas, A.F.; Schuster, D.; Chirayil, N.; Bassi, N.; Ramakrishna, S.; Beussink, L.; et al. Ultrastructural and Cellular Basis for the Development of Abnormal Myocardial Mechanics during the Transition from Hypertension to Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H88–H100. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J. 20th Annual Feigenbaum Lecture: Echocardiography for Precision Medicine-Digital Biopsy to Deconstruct Biology. J. Am. Soc. Echocardiogr. 2019, 32, 1379–1395.e2. [Google Scholar] [CrossRef] [PubMed]
- Akerman, A.P.; Porumb, M.; Scott, C.G.; Beqiri, A.; Chartsias, A.; Ryu, A.J.; Hawkes, W.; Huntley, G.D.; Arystan, A.Z.; Kane, G.C.; et al. Automated Echocardiographic Detection of Heart Failure with Preserved Ejection Fraction Using Artificial Intelligence. JACC Adv. 2023, 2, 100452. [Google Scholar] [CrossRef]
- Chao, C.J.; Kato, N.; Scott, C.G.; Lopez-Jimenez, F.; Lin, G.; Kane, G.C.; Pellikka, P.A. Unsupervised Machine Learning for Assessment of Left Ventricular Diastolic Function and Risk Stratification. J. Am. Soc. Echocardiogr. 2022, 35, 1214–1225.e8. [Google Scholar] [CrossRef]
- Ho, J.E.; Zern, E.K.; Wooster, L.; Bailey, C.S.; Cunningham, T.; Eisman, A.S.; Hardin, K.M.; Zampierollo, G.A.; Jarolim, P.; Pappagianopoulos, P.P.; et al. Differential Clinical Profiles, Exercise Responses, and Outcomes Associated with Existing HFpEF Definitions. Circulation 2019, 140, 353–365. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Kramer, C.M. Role of Imaging Techniques for Diagnosis, Prognosis and Management of Heart Failure Patients: Cardiac Magnetic Resonance. Curr. Heart Fail. Rep. 2015, 12, 276–283. [Google Scholar] [CrossRef]
- Leng, S.; Tan, R.S.; Zhao, X.; Allen, J.C.; Koh, A.S.; Zhong, L. Fast Long-Axis Strain: A Simple, Automatic Approach for Assessing Left Ventricular Longitudinal Function with Cine Cardiovascular Magnetic Resonance. Eur. Radiol. 2020, 30, 3672–3683. [Google Scholar] [CrossRef]
- Maron, B.A.; Cockrill, B.A.; Waxman, A.B.; Systrom, D.M. The Invasive Cardiopulmonary Exercise Test. Circulation 2013, 127, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.W.; Kane, G.C.; et al. The Clinical Use of Stress Echocardiography in Non-Ischaemic Heart Disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Nauta, J.F.; Hummel, Y.M.; van der Meer, P.; Lam, C.S.P.; Voors, A.A.; van Melle, J.P. Correlation with Invasive Left Ventricular Filling Pressures and Prognostic Relevance of the Echocardiographic Diastolic Parameters Used in the 2016 ESC Heart Failure Guidelines and in the 2016 ASE/EACVI Recommendations: A Systematic Review in Patients with Heart Failure with Preserved Ejection Fraction. Eur. J. Heart Fail. 2018, 20, 1303–1311. [Google Scholar] [CrossRef]
- Holland, D.J.; Prasad, S.B.; Marwick, T.H. Contribution of Exercise Echocardiography to the Diagnosis of Heart Failure with Preserved Ejection Fraction (HFpEF). Heart 2010, 96, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J. H2FPEF Score: At Last, a Properly Validated Diagnostic Algorithm for Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 871–873. [Google Scholar] [CrossRef]
- Campbell, R.T.; McMurray, J.J.V. Comorbidities and Differential Diagnosis in Heart Failure with Preserved Ejection Fraction. Heart Fail. Clin. 2014, 10, 481–501. [Google Scholar] [CrossRef]
- Samson, R.; Jaiswal, A.; Ennezat, P.V.; Cassidy, M.; Jemtel, T.H.L. Clinical Phenotypes in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2016, 5, e002477. [Google Scholar] [CrossRef]
- Ho, J.E.; Enserro, D.; Brouwers, F.P.; Kizer, J.R.; Shah, S.J.; Psaty, B.M.; Bartz, T.M.; Santhanakrishnan, R.; Lee, D.S.; Chan, C.; et al. Predicting Heart Failure with Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ. Heart Fail. 2016, 9, e003116. [Google Scholar] [CrossRef]
- Salamanca-Bautista, P.; Conde-Martel, A.; Aramburu-Bodas, Ó.; Formiga, F.; Trullàs, J.C.; Quesada-Simón, M.A.; Casado-Cerrada, J.; Ruiz-Laiglesia, F.; Manzano, L.; Montero-Pérez-Barquero, M. Precipitating Factors of Heart Failure Admission: Differences Related to Age and Left Ventricular Ejection Fraction. Int. J. Cardiol. 2016, 219, 150–155. [Google Scholar] [CrossRef]
- Vasan, R.S.; Sullivan, L.M.; D’Agostino, R.B.; Roubenoff, R.; Harris, T.; Sawyer, D.B.; Levy, D.; Wilson, P.W.F. Serum Insulin-like Growth Factor I and Risk for Heart Failure in Elderly Individuals without a Previous Myocardial Infarction: The Framingham Heart Study. Ann. Intern. Med. 2003, 139, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Mammucari, C.; Rizzuto, R. Signaling Pathways in Mitochondrial Dysfunction and Aging. Mech. Ageing Dev. 2010, 131, 536–543. [Google Scholar] [CrossRef] [PubMed]
- DeQuach, J.A.; Mezzano, V.; Miglani, A.; Lange, S.; Keller, G.M.; Sheikh, F.; Christman, K.L. Simple and High Yielding Method for Preparing Tissue Specific Extracellular Matrix Coatings for Cell Culture. PLoS ONE 2010, 5, e13039. [Google Scholar] [CrossRef] [PubMed]
- Ouzounian, M.; Lee, D.S.; Liu, P.P. Diastolic Heart Failure: Mechanisms and Controversies. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Kass, D.A.; Bronzwaer, J.G.F.; Paulus, W.J. What Mechanisms Underlie Diastolic Dysfunction in Heart Failure? Circ. Res. 2004, 94, 1533–1542. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Kass, D.A. Mechanisms of Diastolic Dysfunction in Heart Failure. Trends Cardiovasc. Med. 2006, 16, 273–279. [Google Scholar] [CrossRef]
- Thalyana, S.V.; Slack, F.J. MicroRNAs and Their Roles in Aging. J. Cell Sci. 2012, 125, 7–17. [Google Scholar] [CrossRef]
- Chiao, Y.A.; Rabinovitch, P.S. The Aging Heart. Cold Spring Harb. Perspect. Med. 2015, 5, A025148. [Google Scholar] [CrossRef]
- Quiat, D.; Olson, E.N. MicroRNAs in Cardiovascular Disease: From Pathogenesis to Prevention and Treatment. J. Clin. Investig. 2013, 123, 11. [Google Scholar] [CrossRef]
- Banerjee, P.; Clark, A.L.; Cleland, J.G.F. Diastolic Heart Failure: A Difficult Problem in the Elderly. Am. J. Geriatr. Cardiol. 2004, 13, 16–21. [Google Scholar] [CrossRef]
- Waller, B.F. The Old-Age Heart: Normal Aging Changes Which Can Produce or Mimic Cardiac Disease. Clin. Cardiol. 1988, 11, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, F.S.; Nikolova, A.P.; Pancoast, J.R.; Lee, R.T. Heart Failure with Preserved Ejection Fraction: Molecular Pathways of the Aging Myocardium. Circ. Res. 2014, 115, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Lena, A.; Anker, M.S.; Springer, J. Muscle Wasting and Sarcopenia in Heart Failure-The Current State of Science. Int. J. Mol. Sci. 2020, 21, 6549. [Google Scholar] [CrossRef] [PubMed]
- Domenighetti, A.A.; Wang, Q.; Egger, M.; Richards, S.M.; Pedrazzini, T.; Delbridge, L.M.D. Angiotensin II-Mediated Phenotypic Cardiomyocyte Remodeling Leads to Age-Dependent Cardiac Dysfunction and Failure. Hypertension 2005, 46, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Tomomatsu, T. Age and the Cardiovascular System. N. Engl. J. Med. 1992, 327, 775–779. [Google Scholar] [CrossRef]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, Pathophysiology, and Therapy of Arterial Stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M. Hemodynamic Aging as the Consequence of Structural Changes Associated with Early Vascular Aging (EVA). Aging Dis. 2014, 5, 109. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Hay, I.; Fetics, B.; Kass, D.A. Combined Ventricular Systolic and Arterial Stiffening in Patients with Heart Failure and Preserved Ejection Fraction: Implications for Systolic and Diastolic Reserve Limitations. Circulation 2003, 107, 714–720. [Google Scholar] [CrossRef]
- Donato, A.J.; Eskurza, I.; Silver, A.E.; Levy, A.S.; Pierce, G.L.; Gates, P.E.; Seals, D.R. Direct Evidence of Endothelial Oxidative Stress with Aging in Humans: Relation to Impaired Endothelium-Dependent Dilation and Upregulation of Nuclear Factor-KappaB. Circ. Res. 2007, 100, 1659–1666. [Google Scholar] [CrossRef]
- Bursi, F.; Weston, S.A.; Redfield, M.M.; Jacobsen, S.J.; Pakhomov, S.; Nkomo, V.T.; Meverden, R.A.; Roger, V.L. Systolic and Diastolic Heart Failure in the Community. JAMA 2006, 296, 2209–2216. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Coresh, J.; Lazo, M.; Hoogeveen, R.C.; Blumenthal, R.S.; Folsom, A.R.; Selvin, E.; Ballantyne, C.M.; Nambi, V. Obesity, Subclinical Myocardial Injury, and Incident Heart Failure. JACC. Heart Fail. 2014, 2, 600–607. [Google Scholar] [CrossRef]
- Sutton-Tyrrell, K.; Newman, A.; Simonsick, E.M.; Havlik, R.; Pahor, M.; Lakatta, E.; Spurgeon, H.; Vaitkevicius, P. Aortic Stiffness Is Associated with Visceral Adiposity in Older Adults Enrolled in the Study of Health, Aging, and Body Composition. Hypertension 2001, 38, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Ghibu, S.; Ilie, I.; Mureșan, A.; Mogoșan, C. Perspectives in the Experimental Study of the Metabolic Syndrome. Farmacia 2015, 63, 4. [Google Scholar]
- Goldberger, J.J.; Cain, M.E.; Hohnloser, S.H.; Kadish, A.H.; Knight, B.P.; Lauer, M.S.; Maron, B.J.; Page, R.L.; Passman, R.S.; Siscovick, D.; et al. Sleep Apnea and Cardiovascular Disease: An American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In Collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008, 118, 1497–1518. [Google Scholar] [CrossRef] [PubMed]
- van Woerden, G.; van Veldhuisen, D.J.; Westenbrink, B.D.; de Boer, R.A.; Rienstra, M.; Gorter, T.M. Connecting Epicardial Adipose Tissue and Heart Failure with Preserved Ejection Fraction: Mechanisms, Management and Modern Perspectives. Eur. J. Heart Fail. 2022, 24, 2238–2250. [Google Scholar] [CrossRef]
- Otto, M.E.; Belohlavek, M.; Romero-Corral, A.; Gami, A.S.; Gilman, G.; Svatikova, A.; Amin, R.S.; Lopez-Jimenez, F.; Khandheria, B.K.; Somers, V.K. Comparison of Cardiac Structural and Functional Changes in Obese Otherwise Healthy Adults with versus without Obstructive Sleep Apnea. Am. J. Cardiol. 2007, 99, 1298–1302. [Google Scholar] [CrossRef]
- Lee, D.S.; Gona, P.; Vasan, R.S.; Larson, M.G.; Benjamin, E.J.; Wang, T.J.; Tu, J.V.; Levy, D. Relation of Disease Pathogenesis and Risk Factors to Heart Failure with Preserved or Reduced Ejection Fraction: Insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation 2009, 119, 3070–3077. [Google Scholar] [CrossRef]
- Rich, S.; Rabinovitch, M. Diagnosis and Treatment of Secondary (Non-Category 1) Pulmonary Hypertension. Circulation 2008, 118, 2190–2199. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Barberà, J.A.; Channick, R.N.; Hassoun, P.M.; Lang, I.M.; Manes, A.; Martinez, F.J.; Naeije, R.; Olschewski, H.; Pepke-Zaba, J.; et al. Diagnosis, Assessment, and Treatment of Non-Pulmonary Arterial Hypertension Pulmonary Hypertension. J. Am. Coll. Cardiol. 2009, 54, S85–S96. [Google Scholar] [CrossRef]
- Haddad, F.; Kudelko, K.; Mercier, O.; Vrtovec, B.; Zamanian, R.T.; De Jesus Perez, V. Pulmonary Hypertension Associated with Left Heart Disease: Characteristics, Emerging Concepts, and Treatment Strategies. Prog. Cardiovasc. Dis. 2011, 54, 154–167. [Google Scholar] [CrossRef]
- Rusinaru, D.; Houpe, D.; Szymanski, C.; Lévy, F.; Maréchaux, S.; Tribouilloy, C. Coronary Artery Disease and 10-Year Outcome after Hospital Admission for Heart Failure with Preserved and with Reduced Ejection Fraction. Eur. J. Heart Fail. 2014, 16, 967–976. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary Microvascular Rarefaction and Myocardial Fibrosis in Heart Failure with Preserved Ejection Fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.B.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Tromp, J.; Sanchez, C.; Njoroge, J.; et al. Disproportionate Left Atrial Myopathy in Heart Failure with Preserved Ejection Fraction among Participants of the PROMIS-HFpEF Study. Sci. Rep. 2021, 11, 4885. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, Z.; Goette, A.; Mera, F.; Honeycutt, C.; Feterik, K.; Wilcox, J.N.; Dudley, S.C.; Harrison, D.G.; Langberg, J.J. Downregulation of Endocardial Nitric Oxide Synthase Expression and Nitric Oxide Production in Atrial Fibrillation: Potential Mechanisms for Atrial Thrombosis and Stroke. Circulation 2002, 106, 2854–2858. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Lewis, G.A.; Schelbert, E.B.; Williams, S.G.; Cunnington, C.; Ahmed, F.; McDonagh, T.A.; Miller, C.A. Biological Phenotypes of Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2017, 70, 2186–2200. [Google Scholar] [CrossRef]
- Tromp, J.; Teng, T.H.; Tay, W.T.; Hung, C.L.; Narasimhan, C.; Shimizu, W.; Park, S.W.; Liew, H.B.; Ngarmukos, T.; Reyes, E.B.; et al. Heart Failure with Preserved Ejection Fraction in Asia. Eur. J. Heart Fail. 2019, 21, 23–36. [Google Scholar] [CrossRef]
- Shah, S.J.; Katz, D.H.; Selvaraj, S.; Burke, M.A.; Yancy, C.W.; Gheorghiade, M.; Bonow, R.O.; Huang, C.C.; Deo, R.C. Phenomapping for Novel Classification of Heart Failure with Preserved Ejection Fraction. Circulation 2015, 131, 269–279. [Google Scholar] [CrossRef]
- von Bibra, H.; Paulus, W.; St. John Sutton, M. Cardiometabolic Syndrome and Increased Risk of Heart Failure. Curr. Heart Fail. Rep. 2016, 13, 219–229. [Google Scholar] [CrossRef]
- Agrawal, A.; Naranjo, M.; Kanjanahattakij, N.; Rangaswami, J.; Gupta, S. Cardiorenal Syndrome in Heart Failure with Preserved Ejection Fraction—An under-Recognized Clinical Entity. Heart Fail. Rev. 2019, 24, 421–437. [Google Scholar] [CrossRef]
- Hedman, Å.K.; Hage, C.; Sharma, A.; Brosnan, M.J.; Buckbinder, L.; Gan, L.M.; Shah, S.J.; Linde, C.M.; Donal, E.; Daubert, J.C.; et al. Identification of Novel Pheno-Groups in Heart Failure with Preserved Ejection Fraction Using Machine Learning. Heart 2020, 106, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Segar, M.W.; Patel, K.V.; Ayers, C.; Basit, M.; Tang, W.H.W.; Willett, D.; Berry, J.; Grodin, J.L.; Pandey, A. Phenomapping of Patients with Heart Failure with Preserved Ejection Fraction Using Machine Learning-Based Unsupervised Cluster Analysis. Eur. J. Heart Fail. 2020, 22, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Woolley, R.J.; Ceelen, D.; Ouwerkerk, W.; Tromp, J.; Figarska, S.M.; Anker, S.D.; Dickstein, K.; Filippatos, G.; Zannad, F.; Marco, M.; et al. Machine Learning Based on Biomarker Profiles Identifies Distinct Subgroups of Heart Failure with Preserved Ejection Fraction. Eur. J. Heart Fail. 2021, 23, 983–991. [Google Scholar] [CrossRef]
- Xu, X.Y.; Nie, Y.; Wang, F.F.; Bai, Y.; Lv, Z.Z.; Zhang, Y.Y.; Li, Z.J.; Gao, W. Growth Differentiation Factor (GDF)-15 Blocks Norepinephrine-Induced Myocardial Hypertrophy via a Novel Pathway Involving Inhibition of Epidermal Growth Factor Receptor Transactivation. J. Biol. Chem. 2014, 289, 10084–10094. [Google Scholar] [CrossRef]
- Whitfield, J.B. Gamma Glutamyl Transferase. Crit. Rev. Clin. Lab. Sci. 2001, 38, 263–355. [Google Scholar] [CrossRef]
- Sala, V.; Margaria, J.P.; Murabito, A.; Morello, F.; Ghigo, A.; Hirsch, E. Therapeutic Targeting of PDEs and PI3K in Heart Failure with Preserved Ejection Fraction (HFpEF). Curr. Heart Fail. Rep. 2017, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Roger, V.L.; Rodeheffer, R.J.; Borlaug, B.A.; Enders, F.T.; Redfield, M.M. Pulmonary Hypertension in Heart Failure with Preserved Ejection Fraction: A Community-Based Study. J. Am. Coll. Cardiol. 2009, 53, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Anjan, V.Y.; Loftus, T.M.; Burke, M.A.; Akhter, N.; Fonarow, G.C.; Gheorghiade, M.; Shah, S.J. Prevalence, Clinical Phenotype, and Outcomes Associated with Normal B-Type Natriuretic Peptide Levels in Heart Failure with Preserved Ejection Fraction. Am. J. Cardiol. 2012, 110, 870–876. [Google Scholar] [CrossRef]
- Wachter, R.; Edelmann, F. Diagnosis of Heart Failure with Preserved Ejection Fraction. Heart Fail. Clin. 2014, 10, 399–406. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Baumbach, A.; Böhm, M.; Burri, H.; Čelutkiene, J.; Chioncel, O.; Cleland, J.G.F.; Coats, A.J.S.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- McHugh, K.; DeVore, A.D.; Wu, J.; Matsouaka, R.A.; Fonarow, G.C.; Heidenreich, P.A.; Yancy, C.W.; Green, J.B.; Altman, N.; Hernandez, A.F. Heart Failure with Preserved Ejection Fraction and Diabetes: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 602–611. [Google Scholar] [CrossRef]
- Gevaert, A.B.; Boen, J.R.A.; Segers, V.F.; Van Craenenbroeck, E.M. Heart Failure with Preserved Ejection Fraction: A Review of Cardiac and Noncardiac Pathophysiology. Front. Physiol. 2019, 10, e638. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, S.; Roy, C.; Slimani, A.; Pasquet, A.; Vancraeynest, D.; Vanoverschelde, J.L.; Gerber, B.L.; Beauloye, C.; Pouleur, A.C. Diabetic Phenotype and Prognosis of Patients with Heart Failure and Preserved Ejection Fraction in a Real Life Cohort. Cardiovasc. Diabetol. 2021, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Van Heerebeek, L.; Hamdani, N.; Handoko, M.L.; Falcao-Pires, I.; Musters, R.J.; Kupreishvili, K.; Ijsselmuiden, A.J.J.; Schalkwijk, C.G.; Bronzwaer, J.G.F.; Diamant, M.; et al. Diastolic Stiffness of the Failing Diabetic Heart: Importance of Fibrosis, Advanced Glycation End Products, and Myocyte Resting Tension. Circulation 2008, 117, 43–51. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Maack, C.; Lehrke, M.; Backs, J.; Heinzel, F.R.; Hulot, J.S.; Marx, N.; Paulus, W.J.; Rossignol, P.; Taegtmeyer, H.; Bauersachs, J.; et al. Heart Failure and Diabetes: Metabolic Alterations and Therapeutic Interventions: A State-of-the-Art Review from the Translational Research Committee of the Heart Failure Association-European Society of Cardiology. Eur. Heart J. 2018, 39, 4243–4254. [Google Scholar] [CrossRef] [PubMed]
- Yeo, T.J.; Yeo, P.S.D.; Ching-Chiew Wong, R.; Ong, H.Y.; Leong, K.T.G.; Jaufeerally, F.; Sim, D.; Santhanakrishnan, R.; Lim, S.L.; Chan, M.M.Y.; et al. Iron Deficiency in a Multi-Ethnic Asian Population with and without Heart Failure: Prevalence, Clinical Correlates, Functional Significance and Prognosis. Eur. J. Heart Fail. 2014, 16, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Kalra, R.; Rajapreyar, I.; Joly, J.M.; Pate, M.; Cribbs, M.G.; Ather, S.; Prabhu, S.D.; Bajaj, N.S. Anemia, Mortality, and Hospitalizations in Heart Failure with a Preserved Ejection Fraction (from the TOPCAT Trial). Am. J. Cardiol. 2020, 125, 1347–1354. [Google Scholar] [CrossRef]
- Edelmann, F. Epidemiology and Prognosis of Heart Failure. Herz 2015, 40, 176–184. [Google Scholar] [CrossRef]
- Bhatia, R.S.; Tu, J.V.; Lee, D.S.; Austin, P.C.; Fang, J.; Haouzi, A.; Gong, Y.; Liu, P.P. Outcome of Heart Failure with Preserved Ejection Fraction in a Population-Based Study. N. Engl. J. Med. 2006, 355, 260–269. [Google Scholar] [CrossRef]
- Lupón, J.; Gavidia-Bovadilla, G.; Ferrer, E.; De Antonio, M.; Perera-Lluna, A.; López-Ayerbe, J.; Domingo, M.; Núñez, J.; Zamora, E.; Moliner, P.; et al. Heart Failure with Preserved Ejection Fraction Infrequently Evolves toward a Reduced Phenotype in Long-Term Survivors. Circ. Heart Fail. 2019, 12, e5652. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, S.; Thunström, E.; Johansson, M.C.; Fu, M. Heart Failure with Preserved Ejection Fraction Has a Better Long-Term Prognosis than Heart Failure with Reduced Ejection Fraction in Old Patients in a 5-Year Follow-up Retrospective Study. Int. J. Cardiol. 2017, 232, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.A.; Rich, J.D.; Shah, S.J. The Emerging Epidemic of Heart Failure with Preserved Ejection Fraction. Curr. Heart Fail. Rep. 2013, 10, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.A.; Katz, D.A.; Beussink, L.; Selvaraj, S.; Gupta, D.K.; Fox, J.; Chakrabarti, S.; Sauer, A.J.; Rich, J.D.; Freed, B.H.; et al. Prognostic Importance of Pathophysiologic Markers in Patients with Heart Failure and Preserved Ejection Fraction. Circ. Heart Fail. 2014, 7, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Park, J.J.; Choi, D.J.; Yoon, C.H.; Oh, I.Y.; Kang, S.M.; Yoo, B.S.; Jeon, E.S.; Kim, J.J.; Cho, M.C.; et al. Prognostic Value of NT-ProBNP in Heart Failure with Preserved versus Reduced EF. Heart 2015, 101, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Van Veldhuisen, D.J.; Linssen, G.C.M.; Jaarsma, T.; Van Gilst, W.H.; Hoes, A.W.; Tijssen, J.G.P.; Paulus, W.J.; Voors, A.A.; Hillege, H.L. B-Type Natriuretic Peptide and Prognosis in Heart Failure Patients with Preserved and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2013, 61, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, Y.; Hanatani, S.; Kimura, Y.; Takashio, S.; Yamamoto, E.; Kusaka, H.; Tokitsu, T.; Rokutanda, T.; Araki, S.; Tsujita, K.; et al. Growth Differentiation Factor-15 Is a Useful Prognostic Marker in Patients with Heart Failure with Preserved Ejection Fraction. Can. J. Cardiol. 2014, 30, 338–344. [Google Scholar] [CrossRef]
- Tribouilloy, C.; Rusinaru, D.; Mahjoub, H.; Tartière, J.M.; Kesri-Tartière, L.; Godard, S.; Peltier, M. Prognostic Impact of Diabetes Mellitus in Patients with Heart Failure and Preserved Ejection Fraction: A Prospective Five-Year Study. Heart 2008, 94, 1450–1455. [Google Scholar] [CrossRef]
- Carrasco-Sánchez, F.J.; Galisteo-Almeda, L.; Páez-Rubio, I.; Martínez-Marcos, F.J.; Camacho-Vázquez, C.; Ruiz-Frutos, C.; Pujol-De La Llave, E. Prognostic Value of Cystatin C on Admission in Heart Failure with Preserved Ejection Fraction. J. Card. Fail. 2011, 17, 31–38. [Google Scholar] [CrossRef]
- Sandesara, P.B.; O’Neal, W.T.; Kelli, H.M.; Samman-Tahhan, A.; Hammadah, M.; Quyyumi, A.A.; Sperling, L.S. The Prognostic Significance of Diabetes and Microvascular Complications in Patients with Heart Failure with Preserved Ejection Fraction. Diabetes Care 2018, 41, 150–155. [Google Scholar] [CrossRef]
- Salah, K.; Stienen, S.; Pinto, Y.M.; Eurlings, L.W.; Metra, M.; Bayes-Genis, A.; Verdiani, V.; Tijssen, J.G.P.; Kok, W.E. Prognosis and NT-ProBNP in Heart Failure Patients with Preserved versus Reduced Ejection Fraction. Heart 2019, 105, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, S.; Sakata, Y.; Nochioka, K.; Yamauchi, T.; Onose, T.; Tsuji, K.; Abe, R.; Oikawa, T.; Sato, M.; Aoyanagi, H.; et al. Comparable Prognostic Impact of BNP Levels among HFpEF, Borderline HFpEF and HFrEF: A Report from the CHART-2 Study. Heart Vessels 2018, 33, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, Y.; Zhou, X. Cystatin C for Predicting All-Cause Mortality and Rehospitalization in Patients with Heart Failure: A Meta-Analysis. Biosci. Rep. 2019, 39, BSR20181761. [Google Scholar] [CrossRef] [PubMed]
- Somaratne, J.B.; Berry, C.; McMurray, J.J.V.; Poppe, K.K.; Doughty, R.N.; Whalley, G.A. The Prognostic Significance of Heart Failure with Preserved Left Ventricular Ejection Fraction: A Literature-Based Meta-Analysis. Eur. J. Heart Fail. 2009, 11, 855–862. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A Contemporary Appraisal of the Heart Failure Epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef]
- Quirós López, R.; García Alegría, J.; Martín Escalante, M.D.; Trujillo Santos, J.; Villena Ruiz, M.Á.; Perea Milla, E. Factores Pronósticos y Supervivencia a Largo Plazo Tras El Diagnóstico Inicial de Insuficiencia Cardiaca. Med. Clin. 2012, 138, 602–608. [Google Scholar] [CrossRef]
- Chan, M.M.Y.; Lam, C.S.P. How Do Patients with Heart Failure with Preserved Ejection Fraction Die? Eur. J. Heart Fail. 2013, 15, 604–613. [Google Scholar] [CrossRef]
- Henkel, D.M.; Redfield, M.M.; Weston, S.A.; Gerber, Y.; Roger, V.L. Death in Heart Failure: A Community Perspective. Circ. Heart Fail. 2008, 1, 91–97. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Claggett, B.L.; Chatterjee, N.A.; Anand, I.S.; Sweitzer, N.K.; Fang, J.C.; O’Meara, E.; Shah, S.J.; Hegde, S.M.; Desai, A.S.; et al. Sudden Death in Heart Failure with Preserved Ejection Fraction: A Competing Risks Analysis from the TOPCAT Trial. JACC. Heart Fail. 2018, 6, 653–661. [Google Scholar] [CrossRef]
- Zile, M.R.; Gaasch, W.H.; Anand, I.S.; Haass, M.; Little, W.C.; Miller, A.B.; Lopez-Sendon, J.; Teerlink, J.R.; White, M.; McMurray, J.J.; et al. Mode of Death in Patients with Heart Failure and a Preserved Ejection Fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-Preserve) Trial. Circulation 2010, 121, 1393–1405. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Cleland, J.G.; Girerd, N.; Bozec, E.; Rossignol, P.; Pellicori, P.; Cosmi, F.; Mariottoni, B.; Solomon, S.D.; Pitt, B.; et al. Spironolactone Effect on Cardiac Structure and Function of Patients with Heart Failure and Preserved Ejection Fraction: A Pooled Analysis of Three Randomized Trials. Eur. J. Heart Fail. 2023, 25, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, A.B.; Kataria, R.; Zannad, F.; Sauer, A.J.; Damman, K.; Sharma, K.; Shah, S.J.; Van Spall, H.G.C. Heart Failure with Preserved Ejection Fraction: Recent Concepts in Diagnosis, Mechanisms and Management. Heart 2022, 108, 319605. [Google Scholar] [CrossRef]
- Paulus, W.J. Phenotypic Persistence in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2019, 12, e5956. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Mentz, R.J.; Claggett, B.L.; Miao, Z.M.; Kulac, I.J.; Ward, J.H.; Hernandez, A.F.; Morrow, D.A.; Starling, R.C.; Velazquez, E.J.; et al. Sacubitril/Valsartan in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: A Pre-Specified Participant-Level Pooled Analysis of PARAGLIDE-HF and PARAGON-HF. Eur. Heart J. 2023, 44, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Nochioka, K.; Sakata, Y.; Miyata, S.; Miura, M.; Takada, T.; Tadaki, S.; Ushigome, R.; Yamauchi, T.; Takahashi, J.; Shimokawa, H. Prognostic Impact of Statin Use in Patients with Heart Failure and Preserved Ejection Fraction. Circ. J. 2015, 79, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.R.; Kostis, J.B.; Simpson, L.M.; Black, H.R.; Cushman, W.C.; Einhorn, P.T.; Farber, M.A.; Ford, C.E.; Levy, D.; Massie, B.M.; et al. Heart Failure with Preserved and Reduced Left Ventricular Ejection Fraction in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Circulation 2008, 118, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef]
- Kaplinsky, E.; Mallarkey, G. Cardiac Myosin Activators for Heart Failure Therapy: Focus on Omecamtiv Mecarbil. Drugs Context 2018, 7, 212518. [Google Scholar] [CrossRef]
- Bond, L.M.; Tumbarello, D.A.; Kendrick-Jones, J.; Buss, F. Small-Molecule Inhibitors of Myosin Proteins. Future Med. Chem. 2013, 5, 41–52. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Shah, S.J.; Kaye, D.M.; Gustafsson, F.; Hasenfuβ, G.; Hoendermis, E.; Litwin, S.E.; Komtebedde, J.; Lam, C.; et al. Effects of Interatrial Shunt on Pulmonary Vascular Function in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2019, 74, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Berry, N.; Mauri, L.; Feldman, T.; Komtebedde, J.; van Veldhuisen, D.J.; Solomon, S.D.; Massaro, J.M.; Shah, S.J. Transcatheter InterAtrial Shunt Device for the Treatment of Heart Failure: Rationale and Design of the Pivotal Randomized Trial to REDUCE Elevated Left Atrial Pressure in Patients with Heart Failure II (REDUCE LAP-HF II). Am. Heart J. 2020, 226, 222–231. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Greene, S.J.; Butler, J.; Filippatos, G.; Lam, C.S.P.; Maggioni, A.P.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Kraigher-Krainer, E.; et al. Effect of Vericiguat, a Soluble Guanylate Cyclase Stimulator, on Natriuretic Peptide Levels in Patients with Worsening Chronic Heart Failure and Reduced Ejection Fraction: The SOCRATES-REDUCED Randomized Trial. JAMA 2015, 314, 2251–2262. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.-A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.-C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Maggioni, A.P.; Lam, C.S.P.; Pieske-Kraigher, E.; Filippatos, G.; Butler, J.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Scalise, A.V.; et al. Vericiguat in Patients with Worsening Chronic Heart Failure and Preserved Ejection Fraction: Results of the SOluble Guanylate Cyclase StimulatoR in HeArT FailurE PatientS with PRESERVED EF (SOCRATES-PRESERVED) Study. Eur. Heart J. 2017, 38, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Lam, C.S.P.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; O’Connor, C.M.; Pieske, B.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; et al. Effect of Vericiguat vs Placebo on Quality of Life in Patients with Heart Failure and Preserved Ejection Fraction: The VITALITY-HFpEF Randomized Clinical Trial. JAMA 2020, 324, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.L.; Smyth, L.R.; Riche, D.M.; Bourret, E.M.; Chamberlin, K.W.; White, W.B. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Blood Pressure: A Systematic Review and Meta-Analysis. J. Am. Soc. Hypertens. 2014, 8, 262–275.e9. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Tsimihodimos, V.; Elisaf, M.S. Mechanisms of Blood Pressure Reduction with Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitors. Expert. Opin. Pharmacother. 2016, 17, 1581–1583. [Google Scholar] [CrossRef]
- Li, M.; Yi, T.; Fan, F.; Qiu, L.; Wang, Z.; Weng, H.; Ma, W.; Zhang, Y.; Huo, Y. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Blood Pressure in Patients with Heart Failure: A Systematic Review and Meta-Analysis. Cardiovasc. Diabetol. 2022, 21, 139. [Google Scholar] [CrossRef]
- Lambers Heerspink, H.J.; De Zeeuw, D.; Wie, L.; Leslie, B.; List, J. Dapagliflozin a Glucose-Regulating Drug with Diuretic Properties in Subjects with Type 2 Diabetes. Diabetes Obes. Metab. 2013, 15, 853–862. [Google Scholar] [CrossRef]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why Do SGLT2 Inhibitors Reduce Heart Failure Hospitalization? A Differential Volume Regulation Hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef]
- Nakamura, T.Y.; Iwata, Y.; Arai, Y.; Komamura, K.; Wakabayashi, S. Activation of Na+/H+ Exchanger 1 Is Sufficient to Generate Ca2+ Signals That Induce Cardiac Hypertrophy and Heart Failure. Circ. Res. 2008, 103, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Mark, M.; Mayoux, E. CV Protection in the EMPA-REG OUTCOME Trial: A Thrifty Substrate Hypothesis. Diabetes Care 2016, 39, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of SGLT2 Selective Inhibitor Ipragliflozin on Hyperglycemia, Hyperlipidemia, Hepatic Steatosis, Oxidative Stress, Inflammation, and Obesity in Type 2 Diabetic Mice. Eur. J. Pharmacol. 2013, 715, 246–255. [Google Scholar] [CrossRef]
- Withaar, C.; Meems, L.M.G.; Markousis-Mavrogenis, G.; Boogerd, C.J.; Silljé, H.H.W.; Schouten, E.M.; Dokter, M.M.; Voors, A.A.; Westenbrink, B.D.; Lam, C.S.P.; et al. The Effects of Liraglutide and Dapagliflozin on Cardiac Function and Structure in a Multi-Hit Mouse Model of Heart Failure with Preserved Ejection Fraction. Cardiovasc. Res. 2021, 117, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Nakamura, K.; Miyoshi, T.; Yoshida, M.; Akazawa, K.; Saito, Y.; Akagi, S.; Ohno, Y.; Kondo, M.; Miura, D.; et al. Inhibitory Effects of Tofogliflozin on Cardiac Hypertrophy in Dahl Salt-Sensitive and Salt-Resistant Rats Fed a High-Fat Diet. Int. Heart J. 2019, 60, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Peikert, A.; Martinez, F.A.; Vaduganathan, M.; Claggett, B.L.; Kulac, I.J.; Desai, A.S.; Jhund, P.S.; De Boer, R.A.; Demets, D.; Hernandez, A.F.; et al. Efficacy and Safety of Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction According to Age: The DELIVER Trial. Circ. Heart Fail. 2022, 15, E010080. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Jhund, P.S.; Kondo, T.; Butt, J.H.; Docherty, K.F.; Claggett, B.L.; Desai, A.S.; Vaduganathan, M.; Gasparyan, S.B.; Bengtsson, O.; Lindholm, D.; et al. Dapagliflozin across the Range of Ejection Fraction in Patients with Heart Failure: A Patient-Level, Pooled Meta-Analysis of DAPA-HF and DELIVER. Nat. Med. 2022, 28, 1956–1964. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Tamargo, J. Sodium–Glucose Cotransporter 2 Inhibitors in Heart Failure: Potential Mechanisms of Action, Adverse Effects and Future Developments. Eur. Cardiol. Rev. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Van Linthout, S.; Kherad, B. Heart Failure with Preserved Ejection Fraction and Future Pharmacological Strategies: A Glance in the Crystal Ball. Curr. Cardiol. Rep. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Durik, M.; Baker, D.J.; Van Deursen, J.M. Cellular Senescence in Aging and Age-Related Disease: From Mechanisms to Therapy. Nat. Med. 2015, 21, 1424–1435. [Google Scholar] [CrossRef]
- Suda, M.; Paul, K.H.; Minamino, T.; Miller, J.D.; Lerman, A.; Ellison-Hughes, G.M.; Tchkonia, T.; Kirkland, J.L. Senescent Cells: A Therapeutic Target in Cardiovascular Diseases. Cells 2023, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.; Lagnado, A.; Maggiorani, D.; Walaszczyk, A.; Dookun, E.; Chapman, J.; Birch, J.; Salmonowicz, H.; Ogrodnik, M.; Jurk, D.; et al. Length-Independent Telomere Damage Drives Post-Mitotic Cardiomyocyte Senescence. EMBO J. 2019, 38, e100492. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.E.; Tome, M.I.; Vasques-Novoa, D.F.; Silva, D.A.; Conceicao, D.G.; Miranda-Silva, D.D.; Pitrez, D.P.; Professor Barros, A.A.; Professor Leite-Moreira, A.A.; Pinto-Do-O, D.P.; et al. Pharmacological Targeting of Senescence with ABT-263 in Experimental Heart Failure with Preserved Ejection Fraction. Cardiovasc. Res. 2022, 118, cvac066-107. [Google Scholar] [CrossRef]
- Triana-Martínez, F.; Picallos-Rabina, P.; Da Silva-Álvarez, S.; Pietrocola, F.; Llanos, S.; Rodilla, V.; Soprano, E.; Pedrosa, P.; Ferreirós, A.; Barradas, M.; et al. Identification and Characterization of Cardiac Glycosides as Senolytic Compounds. Nat. Commun. 2019, 10, 4731. [Google Scholar] [CrossRef]
- Lazaro, I.; Oguiza, A.; Recio, C.; Mallavia, B.; Madrigal-Matute, J.; Blanco, J.; Egido, J.; Martin-Ventura, J.L.; Gomez-Guerrero, C. Targeting HSP90 Ameliorates Nephropathy and Atherosclerosis through Suppression of NF-ΚB and STAT Signaling Pathways in Diabetic Mice. Diabetes 2015, 64, 3600–3613. [Google Scholar] [CrossRef]
- Suda, M.; Shimizu, I.; Katsuumi, G.; Hsiao, C.L.; Yoshida, Y.; Matsumoto, N.; Yoshida, Y.; Katayama, A.; Wada, J.; Seki, M.; et al. Glycoprotein Nonmetastatic Melanoma Protein B Regulates Lysosomal Integrity and Lifespan of Senescent Cells. Sci. Rep. 2022, 12, 6522. [Google Scholar] [CrossRef]
- Rattka, M.; Pott, A.; Kühberger, A.; Weinmann, K.; Scharnbeck, D.; Stephan, T.; Baumhardt, M.; Bothner, C.; Orbe, M.I.; Rottbauer, W.; et al. Restoration of Sinus Rhythm by Pulmonary Vein Isolation Improves Heart Failure with Preserved Ejection Fraction in Atrial Fibrillation Patients. Europace 2020, 22, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Hasenfuß, G.; Hayward, C.; Burkhoff, D.; Silvestry, F.E.; McKenzie, S.; Gustafsson, F.; Malek, F.; Van Der Heyden, J.; Lang, I.; Petrie, M.C.; et al. A Transcatheter Intracardiac Shunt Device for Heart Failure with Preserved Ejection Fraction (REDUCE LAP-HF): A Multicentre, Open-Label, Single-Arm, Phase 1 Trial. Lancet 2016, 387, 1298–1304. [Google Scholar] [CrossRef] [PubMed]




| Drug Class | ESC Indication | ACC/AHA Indication | Purpose | Observations |
|---|---|---|---|---|
| Diuretics | I | 1 | alleviate symptoms | fluid retention |
| iSGLT2 | I | 2a | decreasing HF hospitalizations and cardiovascular mortality | |
| MRAs | NA | 2b | decrease hospitalizations | patients with LVEF on the lower end of this spectrum |
| ARNi | NA | 2b | decrease hospitalizations | patients with LVEF on the lower end of this spectrum |
| Possible Future Therapies | |||
|---|---|---|---|
| Type | Mechanism | Effect | References |
| Myosin modulators | binding to β-cardiac myosin | modulate cardiac contractility | [163,219,220,221,222] |
| Soluble guanylate cyclase stimulators | guanylate cyclase stimulator | vasodilator effect | [223,224] |
| Senolytic therapy | induce senescent cell apoptosis | improves cardiac function | [244,245,246,247,248,249,250] |
| PAI-1 inhibitor | TGF-β and plasminogen-mediated pathways | reduces cardiac fibrosis | [243] |
| Endothelin receptor A and B antagonists | improves hemodynamics | cardiac remodeling | [243] |
| Mitochondrial-targeted drugs | increases mitochondrial energy | improvement of LV systolic function | [243] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoicescu, L.; Crişan, D.; Morgovan, C.; Avram, L.; Ghibu, S. Heart Failure with Preserved Ejection Fraction: The Pathophysiological Mechanisms behind the Clinical Phenotypes and the Therapeutic Approach. Int. J. Mol. Sci. 2024, 25, 794. https://doi.org/10.3390/ijms25020794
Stoicescu L, Crişan D, Morgovan C, Avram L, Ghibu S. Heart Failure with Preserved Ejection Fraction: The Pathophysiological Mechanisms behind the Clinical Phenotypes and the Therapeutic Approach. International Journal of Molecular Sciences. 2024; 25(2):794. https://doi.org/10.3390/ijms25020794
Chicago/Turabian StyleStoicescu, Laurențiu, Dana Crişan, Claudiu Morgovan, Lucreţia Avram, and Steliana Ghibu. 2024. "Heart Failure with Preserved Ejection Fraction: The Pathophysiological Mechanisms behind the Clinical Phenotypes and the Therapeutic Approach" International Journal of Molecular Sciences 25, no. 2: 794. https://doi.org/10.3390/ijms25020794
APA StyleStoicescu, L., Crişan, D., Morgovan, C., Avram, L., & Ghibu, S. (2024). Heart Failure with Preserved Ejection Fraction: The Pathophysiological Mechanisms behind the Clinical Phenotypes and the Therapeutic Approach. International Journal of Molecular Sciences, 25(2), 794. https://doi.org/10.3390/ijms25020794






