Hyperoxia: Effective Mechanism of Hyperbaric Treatment at Mild-Pressure
Abstract
1. Introduction
2. Hyperoxia
3. Oxygen Homeostasis
4. HBOT (Hyperbaric Oxygen Therapy)
4.1. Fundamentals of Hyperoxic Treatment
4.2. Physiological Effects of Hyperoxia Generated by HBOT
4.2.1. Reversal of Hypoxia
4.2.2. Non-Hypoxemic Vasoconstriction
4.2.3. Angiogenesis
4.2.4. Proliferation and Stimulation of Stem Cells
4.2.5. Collagen Synthesis
4.2.6. Osteogenesis
4.2.7. Antimicrobial Effect
4.2.8. Neural Function and Neuroprotection
4.2.9. Mitochondrial Function, Oxidative Stress, and Inflammatory Response
4.2.10. Regeneration
5. Safety
6. Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Wolde, S.D.; Hulskes, R.H.; Weenink, R.P.; Hollmann, M.W.; Van Hulst, R.A. The Effects of Hyperbaric Oxygenation on Oxidative Stress, Inflammation and Angiogenesis. Biomolecules 2021, 11, 1210. [Google Scholar] [CrossRef] [PubMed]
- Hadanny, A.; Efrati, S. The Hyperoxic-Hypoxic Paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef] [PubMed]
- Salvagno, M.; Coppalini, G.; Taccone, F.S.; Strapazzon, G.; Mrakic-Sposta, S.; Rocco, M.; Khalife, M.; Balestra, C. The Normobaric Oxygen Paradox—Hyperoxic Hypoxic Paradox: A Novel Expedient Strategy in Hematopoiesis Clinical Issues. Int. J. Mol. Sci. 2022, 24, 82. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.E. Hyperbaric Oxygen Therapy Indications, 14th ed.; Best Pub. Co.: North Palm Beach, FL, USA, 2019; ISBN 978-1-947239-16-6. [Google Scholar]
- Leveque, C.; Mrakic Sposta, S.; Theunissen, S.; Germonpré, P.; Lambrechts, K.; Vezzoli, A.; Bosco, G.; Lévénez, M.; Lafère, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different (1.4 ATA and 2.5 ATA) Hyperbaric Hyperoxia Exposures. Int. J. Mol. Sci. 2023, 24, 12361. [Google Scholar] [CrossRef]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafère, P.; Germonpré, P.; Balestra, C. Increasing Oxygen Partial Pressures Induce a Distinct Transcriptional Response in Human PBMC: A Pilot Study on the “Normobaric Oxygen Paradox”. Int. J. Mol. Sci. 2021, 22, 458. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M.A. Role of Reactive Oxygen Species in Inflammation: A Minireview. Mosc. Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef]
- Imray, C.; Wright, A.; Subudhi, A.; Roach, R. Acute Mountain Sickness: Pathophysiology, Prevention, and Treatment. Prog. Cardiovasc. Dis. 2010, 52, 467–484. [Google Scholar] [CrossRef]
- Smedley, T.; Grocott, M.P. Acute High-Altitude Illness: A Clinically Orientated Review. Br. J. Pain 2013, 7, 85–94. [Google Scholar] [CrossRef]
- Hafner, S.; Beloncle, F.; Koch, A.; Radermacher, P.; Asfar, P. Hyperoxia in Intensive Care, Emergency, and Peri-Operative Medicine: Dr. Jekyll or Mr. Hyde? A 2015 Update. Ann. Intensive Care 2015, 5, 42. [Google Scholar] [CrossRef]
- Brugniaux, J.V.; Coombs, G.B.; Barak, O.F.; Dujic, Z.; Sekhon, M.S.; Ainslie, P.N. Highs and Lows of Hyperoxia: Physiological, Performance, and Clinical Aspects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1–R27. [Google Scholar] [CrossRef]
- Avishay, D.M.; Tenny, K.M. Henry’s Law. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jain, K.K. Textbook of Hyperbaric Medicine; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-47138-9. [Google Scholar]
- Ishihara, A. Mild Hyperbaric Oxygen: Mechanisms and Effects. J. Physiol. Sci. 2019, 69, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A. The Number and Distribution of Capillaries in Muscles with Calculations of the Oxygen Pressure Head Necessary for Supplying the Tissue. J. Physiol. 1919, 52, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Holbach, K.-H.; Wassmann, H.; Linke, D. The Use of Hyperbaric Oxygenation in the Treatment of Spinal Cord Lesions. Eur. Neurol. 1977, 16, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Young, P.J.; Laffey, J.G.; Asfar, P.; Taccone, F.S.; Skrifvars, M.B.; Meyhoff, C.S.; Radermacher, P. Dangers of Hyperoxia. Crit. Care 2021, 25, 440. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Takada, R.; Maeda, T.; Yoshii, T.; Okawa, A.; Yagishita, K. Microcirculation and Tissue Oxygenation in the Head and Limbs during Hyperbaric Oxygen Treatment. Diving Hyperb. Med. 2021, 51, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Daugherty, W.P.; Sun, D.; Levasseur, J.E.; Altememi, N.; Hamm, R.J.; Rockswold, G.L.; Bullock, M.R. Protection of Mitochondrial Function and Improvement in Cognitive Recovery in Rats Treated with Hyperbaric Oxygen Following Lateral Fluid-Percussion Injury. J. Neurosurg. 2007, 106, 687–694. [Google Scholar] [CrossRef]
- Rockswold, S.B.; Rockswold, G.L.; Zaun, D.A.; Zhang, X.; Cerra, C.E.; Bergman, T.A.; Liu, J. A Prospective, Randomized Clinical Trial to Compare the Effect of Hyperbaric to Normobaric Hyperoxia on Cerebral Metabolism, Intracranial Pressure, and Oxygen Toxicity in Severe Traumatic Brain Injury: Clinical Article. J. Neurosurg. 2010, 112, 1080–1094. [Google Scholar] [CrossRef]
- Rockswold, S.B.; Rockswold, G.L.; Zaun, D.A.; Liu, J. A Prospective, Randomized Phase II Clinical Trial to Evaluate the Effect of Combined Hyperbaric and Normobaric Hyperoxia on Cerebral Metabolism, Intracranial Pressure, Oxygen Toxicity, and Clinical Outcome in Severe Traumatic Brain Injury. J. Neurosurg. 2013, 118, 1317–1328. [Google Scholar] [CrossRef]
- un Nisa, B.; Kondo, H.; Ishihara, A.; Hidemi, F. Beneficial Effects Of Exposure to Mild Hyperbaric Oxygen on Microcirculation in Peripheral Tissues: 857 May 27 4:05 PM–4:15 PM. Med. Sci. Sports Exerc. 2020, 52, 208. [Google Scholar] [CrossRef]
- Bosco, G.; Paganini, M.; Giacon, T.A.; Oppio, A.; Vezzoli, A.; Dellanoce, C.; Moro, T.; Paoli, A.; Zanotti, F.; Zavan, B.; et al. Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial. Int. J. Environ. Res. Public Health 2021, 18, 9755. [Google Scholar] [CrossRef]
- Woo, J.; Min, J.-H.; Lee, Y.-H.; Roh, H.-T. Effects of Hyperbaric Oxygen Therapy on Inflammation, Oxidative/Antioxidant Balance, and Muscle Damage after Acute Exercise in Normobaric, Normoxic and Hypobaric, Hypoxic Environments: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7377. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.M.; Minter, L.M.; Osborne, B.A.; Granowitz, E.V. Hyperbaric Oxygen Inhibits Stimulus-Induced Proinflammatory Cytokine Synthesis by Human Blood-Derived Monocyte-Macrophages. Clin. Exp. Immunol. 2003, 134, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Balestra, C.; Germonpré, P.; Poortmans, J.R.; Marroni, A. Serum Erythropoietin Levels in Healthy Humans after a Short Period of Normobaric and Hyperbaric Oxygen Breathing: The “Normobaric Oxygen Paradox”. J. Appl. Physiol. 2006, 100, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Nisa, B.U.; Nakanishi, R.; Tanaka, M.; Lin, H.; Hirabayashi, T.; Maeshige, N.; Kondo, H.; Fujino, H. Mild Hyperbaric Oxygen Exposure Enhances Peripheral Circulatory Natural Killer Cells in Healthy Young Women. Life 2023, 13, 408. [Google Scholar] [CrossRef] [PubMed]
- LL TA—Hyperbaric Oxygen Therapy in Treatment of Hypoxic Wounds. Available online: https://www.cms.gov/medicare-coverage-database/view/technology-assessments.aspx?taid=12 (accessed on 20 September 2023).
- Choudhury, R. Hypoxia and Hyperbaric Oxygen Therapy: A Review. Int. J. Gen. Med. 2018, 11, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Oliaei, S.; SeyedAlinaghi, S.; Mehrtak, M.; Karimi, A.; Noori, T.; Mirzapour, P.; Shojaei, A.; MohsseniPour, M.; Mirghaderi, S.P.; Alilou, S.; et al. The Effects of Hyperbaric Oxygen Therapy (HBOT) on Coronavirus Disease-2019 (COVID-19): A Systematic Review. Eur. J. Med. Res. 2021, 26, 96. [Google Scholar] [CrossRef]
- Cannellotto, M.; Duarte, M.; Keller, G.; Larrea, R.; Cunto, E.; Chediack, V.; Mansur, M.; Brito, D.M.; García, E.; Di Salvo, H.E.; et al. Hyperbaric Oxygen as an Adjuvant Treatment for Patients with COVID-19 Severe Hypoxaemia: A Randomised Controlled Trial. Emerg. Med. J. 2022, 39, 88–93. [Google Scholar] [CrossRef]
- Schipke, J.D.; Muth, T.; Pepper, C.; Schneppendahl, J.; Hoffmanns, M.; Dreyer, S. Hyperoxia and the Cardiovascular System: Experiences with Hyperbaric Oxygen Therapy. Med. Gas Res. 2022, 12, 153–157. [Google Scholar] [CrossRef]
- Whalen, R.E.; Saltzman, H.A.; Holloway, D.H.; Mcintosh, H.D.; Sieker, H.O.; Brown, I.W. Cardiovascular and blood gas responses to hyperbaric oxygenation. Am. J. Cardiol. 1965, 15, 638–646. [Google Scholar] [CrossRef]
- Sunkari, V.G.; Lind, F.; Botusan, I.R.; Kashif, A.; Liu, Z.-J.; Ylä-Herttuala, S.; Brismar, K.; Velazquez, O.; Catrina, S.-B. Hyperbaric Oxygen Therapy Activates Hypoxia-Inducible Factor 1 (HIF-1), Which Contributes to Improved Wound Healing in Diabetic Mice. Wound Repair Regen. 2015, 23, 98–103. [Google Scholar] [CrossRef]
- Buckley, C.J.; Cooper, J.S. Hyperbaric Oxygen Effects On Angiogenesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Liu, Z.-J.; Velazquez, O.C. Hyperoxia, Endothelial Progenitor Cell Mobilization, and Diabetic Wound Healing. Antioxid. Redox Signal. 2008, 10, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Bhopale, V.M.; Velazquez, O.C.; Goldstein, L.J.; Thom, L.H.; Buerk, D.G. Stem Cell Mobilization by Hyperbaric Oxygen. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1378–H1386. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.J.; Gallagher, K.A.; Bauer, S.M.; Bauer, R.J.; Baireddy, V.; Liu, Z.-J.; Buerk, D.G.; Thom, S.R.; Velazquez, O.C. Endothelial Progenitor Cell Release into Circulation Is Triggered by Hyperoxia-Induced Increases in Bone Marrow Nitric Oxide. Stem Cells 2006, 24, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, Y.; Yao, Y. HBO Promotes the Differentiation of Neural Stem Cells via Interactions Between the Wnt3/β-Catenin and BMP2 Signaling Pathways. Cell Transpl. 2019, 28, 1686–1699. [Google Scholar] [CrossRef]
- Ishii, Y.; Miyanaga, Y.; Shimojo, H.; Ushida, T.; Tateishi, T. Effects of Hyperbaric Oxygen on Procollagen Messenger RNA Levels and Collagen Synthesis in the Healing of Rat Tendon Laceration. Tissue Eng. 1999, 5, 279–286. [Google Scholar] [CrossRef]
- Gajendrareddy, P.K.; Junges, R.; Cygan, G.; Zhao, Y.; Marucha, P.T.; Engeland, C.G. Increased Oxygen Exposure Alters Collagen Expression and Tissue Architecture during Ligature-Induced Periodontitis. J. Periodontal. Res. 2017, 52, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Malda, J.; Crawford, R.; Xiao, Y. Effects of Hyperbaric Oxygen on Proliferation and Differentiation of Osteoblasts from Human Alveolar Bone. Connect. Tissue Res. 2007, 48, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Al Hadi, H.; Smerdon, G.R.; Fox, S.W. Hyperbaric Oxygen Therapy Accelerates Osteoblast Differentiation and Promotes Bone Formation. J. Dent. 2015, 43, 382–388. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, B.; Che, J.; Shang, P. Hypoxia Pathway in Osteoporosis: Laboratory Data for Clinical Prospects. Int. J. Environ. Res. Public Health 2023, 20, 3129. [Google Scholar] [CrossRef]
- Lima, F.L.; Joazeiro, P.P.; Lancellotti, M.; de Hollanda, L.M.; de Araújo Lima, B.; Linares, E.; Augusto, O.; Brocchi, M.; Giorgio, S. Effects of Hyperbaric Oxygen on Pseudomonas Aeruginosa Susceptibility to Imipenem and Macrophages. Future Microbiol. 2015, 10, 179–189. [Google Scholar] [CrossRef]
- Memar, M.Y.; Yekani, M.; Alizadeh, N.; Baghi, H.B. Hyperbaric Oxygen Therapy: Antimicrobial Mechanisms and Clinical Application for Infections. Biomed. Pharmacother. 2019, 109, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Marti, H.H.; Veltkamp, R. Hyperbaric Oxygen Reduces Tissue Hypoxia and Hypoxia-Inducible Factor-1 Alpha Expression in Focal Cerebral Ischemia. Stroke 2008, 39, 1000–1006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gu, G.-J.; Li, Y.-P.; Peng, Z.-Y.; Xu, J.-J.; Kang, Z.-M.; Xu, W.-G.; Tao, H.-Y.; Ostrowski, R.P.; Zhang, J.H.; Sun, X.-J. Mechanism of Ischemic Tolerance Induced by Hyperbaric Oxygen Preconditioning Involves Upregulation of Hypoxia-Inducible Factor-1alpha and Erythropoietin in Rats. J. Appl. Physiol. 2008, 104, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, Q.; Xiang, Y.; Zeng, X.-R.; Xiao, J.; Le, W.-D. The Neuroprotective Effects of Oxygen Therapy in Alzheimer’s Disease: A Narrative Review. Neural Regen. Res. 2022, 18, 57–63. [Google Scholar] [CrossRef]
- Tezgin, D.; Giardina, C.; Perdrizet, G.A.; Hightower, L.E. The Effect of Hyperbaric Oxygen on Mitochondrial and Glycolytic Energy Metabolism: The Caloristasis Concept. Cell Stress Chaperones 2020, 25, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Liu, K.; Li, L.; Li, X.; Zhao, P. The Effects of Hyperbaric Oxygen Therapy on Neuropathic Pain via Mitophagy in Microglia. Mol. Pain 2017, 13, 1744806917710862. [Google Scholar] [CrossRef]
- Kun, L.; Lu, L.; Yongda, L.; Xingyue, L.; Guang, H. Hyperbaric Oxygen Promotes Mitophagy by Activating CaMKKβ/AMPK Signal Pathway in Rats of Neuropathic Pain. Mol. Pain 2019, 15, 1744806919871381. [Google Scholar] [CrossRef] [PubMed]
- Godman, C.A.; Joshi, R.; Giardina, C.; Perdrizet, G.; Hightower, L.E. Hyperbaric Oxygen Treatment Induces Antioxidant Gene Expression. Ann. N. Y. Acad. Sci. 2010, 1197, 178–183. [Google Scholar] [CrossRef]
- Schottlender, N.; Gottfried, I.; Ashery, U. Hyperbaric Oxygen Treatment: Effects on Mitochondrial Function and Oxidative Stress. Biomolecules 2021, 11, 1827. [Google Scholar] [CrossRef]
- de Wolde, S.D.; Hulskes, R.H.; de Jonge, S.W.; Hollmann, M.W.; van Hulst, R.A.; Weenink, R.P.; Kox, M. The Effect of Hyperbaric Oxygen Therapy on Markers of Oxidative Stress and the Immune Response in Healthy Volunteers. Front. Physiol. 2022, 13, 826163. [Google Scholar] [CrossRef]
- Capó, X.; Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Batle, J.M.; Tur, J.A.; Pons, A.; Sureda, A.; Tejada, S. Hyperbaric Oxygen Therapy Reduces Oxidative Stress and Inflammation, and Increases Growth Factors Favouring the Healing Process of Diabetic Wounds. Int. J. Mol. Sci. 2023, 24, 7040. [Google Scholar] [CrossRef] [PubMed]
- Lindenmann, J.; Kamolz, L.; Graier, W.; Smolle, J.; Smolle-Juettner, F.-M. Hyperbaric Oxygen Therapy and Tissue Regeneration: A Literature Survey. Biomedicines 2022, 10, 3145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Jia, Y.; Wang, T.; Meng, D. Adverse Effects of Hyperbaric Oxygen Therapy: A Systematic Review and Meta-Analysis. Front. Med. 2023, 10, 1160774. [Google Scholar] [CrossRef] [PubMed]
- Gawdi, R.; Cooper, J.S. Hyperbaric Contraindications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Monge, G.; Otto, M.; Norambuena, N.; Martínez, V.; Retamales, D.; Torres-Castro, R. Safety of Hyperbaric Oxygenation Treatment and Evaluation of Associated Clinical Parameters: A Single-Institutional Prospective Cohort Study. Int. J. Transl. Med. Res. Public Health 2023, 7. [Google Scholar] [CrossRef]
- Ilmi, M.I.; Yunus, F.; Guritno, M.; Damayanti, T.; Samoedro, E.; Nazaruddin, A.M.; Nurwidya, F. Comparison of Lung Function Values of Trained Divers in 1.5 ATA Hyperbaric Chamber after Inhaling 100% Oxygen and Regular Air: A Crossover Study. Adv. Respir. Med. 2017, 85, 233–238. [Google Scholar] [CrossRef]
- Daly, S.; Thorpe, M.; Rockswold, S.; Hubbard, M.; Bergman, T.; Samadani, U.; Rockswold, G. Hyperbaric Oxygen Therapy in the Treatment of Acute Severe Traumatic Brain Injury: A Systematic Review. J. Neurotrauma 2018, 35, 623–629. [Google Scholar] [CrossRef]
- Jordá-Vargas, L.; Cannellotto, M. Terapia de oxigenación hiperbárica en individuos sanos: Efecto en algunos parámetros bioquímicos. Acta Bioquím. Clín. Latinoam. 2019, 53, 15–23. [Google Scholar]
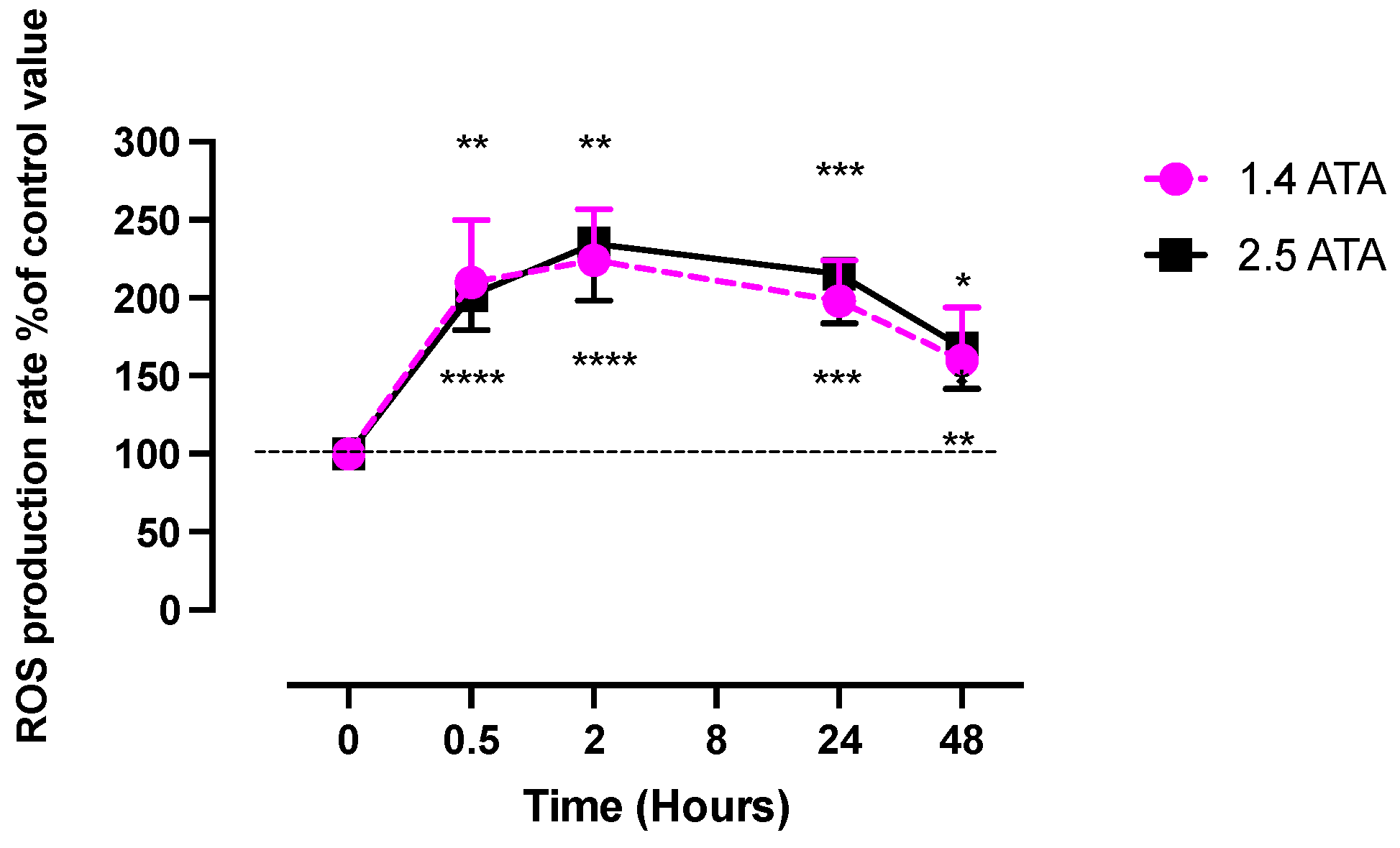
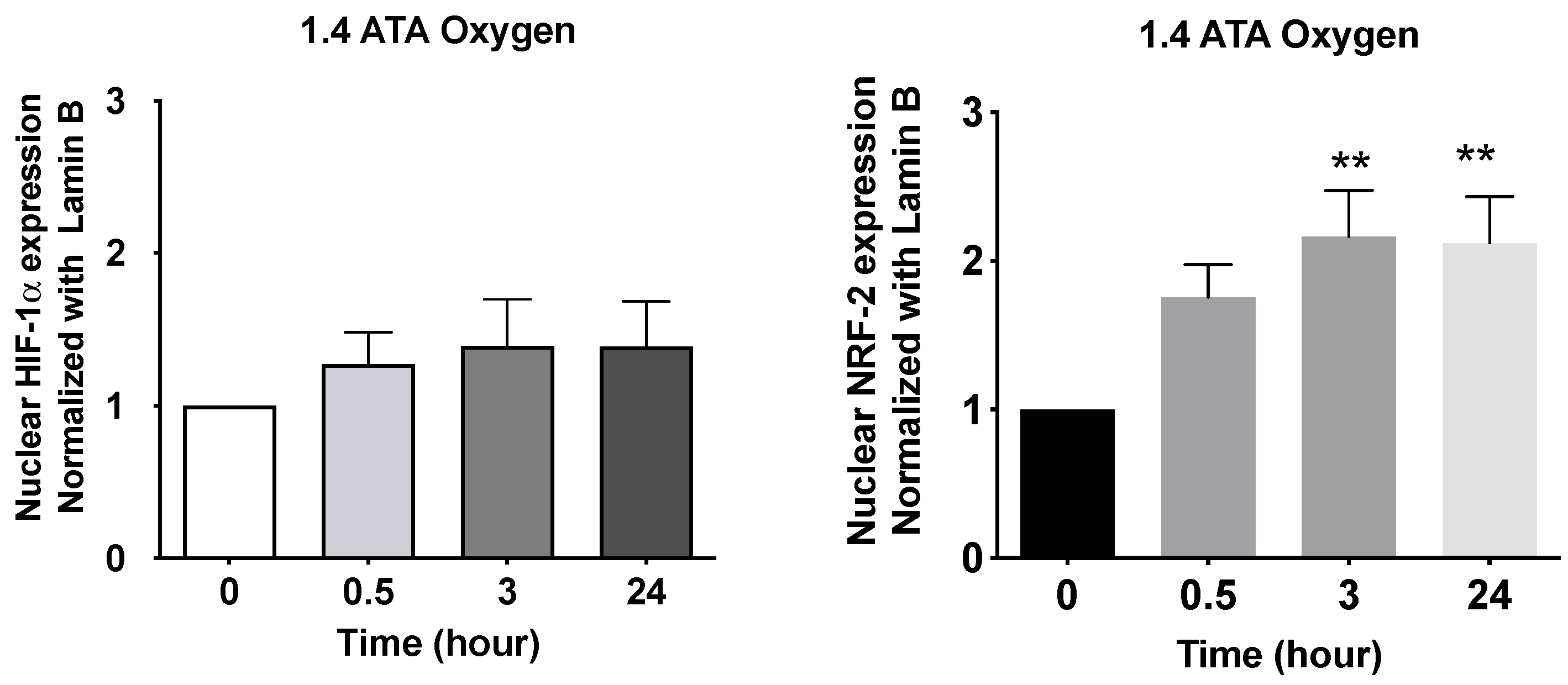
| Author | Type of Study | Sample Size | Intervention | Main Results |
|---|---|---|---|---|
| Bosco et al. (2021) [23] | Randomized, patient-blinded, controlled trial (NCT04366427) | 22 healthy humans. | 100% O2, HBOT 1.5 ATA, 60 min. 20 treatments | In plasmatic samples: Increase in ROS levels until day 14, followed by a decrease at the end of the treatment. Increase in total antioxidant capacity. Increase in glutathione and reduced cysteine. Decrease in IL-6 and IL-10. |
| Leveque et al. (2023) [5] | Human experimental study | 47 healthy non-smoking Caucasian subjects | 100% O2, HBOT 1.4 ATA, 60 min. 1 treatment. | Increase of plasmatic ROS production similar kinetic respect 2.5 ATA. Increase in cysteinylglycine. Plasmatic Nitric oxide metabolites decrease after 2 h and return normal level al 24 h |
| Fratantorio et al. (2021) [6] | Human experimental study | 20 healthy humans | 1 h exposure to normobaric oxygen FiO2 0.3 vs. normobaric oxygen FiO2 1.0 vs. hyperbaric oxygen 1.4 bar FiO2 1.4 | Increase in NRF2 nuclear level and not significative changes in HIF after the first session in peripheral blood mononuclear cells. Increase glutathione and matrix metalloproteinase (MMPs) in plasma. |
| Niza et al. (2023) [27] | Clinical Trial (crossover randomized experimental study) | 16 healthy women | 1.4 ATA, with 35.0–39.5% oxygen concentration and 18 L of oxygen per minute injected into the hyperbaric chamber for 70 min. | Parasympathetic activity (Electrocardiography Analysis) was significantly increased. NK cells were increased without changes IL-6 and IL-12p70 protein levels in blood samples. |
| Rockswold et al. (2010) [20] | Randomized clinical trial | 24 patients | 100% FiO2 (fraction of inspired oxygen) delivered for 60 min at 1.5 ATA. The NBH treatment consisted of 100% FiO2 given for 3 h at 1.0 ATA | Mean brain tissue PO2 levels were significantly increased after HBO2 therapy. |
| Rockswold et al. (2013) [21] | Prospective randomized Phase II clinical trial (NCT00170352) | 45 patients treated for severe TBI | 100% FiO2 delivered for 60 min at 1.5 ATA followed by 3 h at 1.0 ATA | Brain tissue PO2 in both the noninjured and pericontusional brain rose during the treatment sessions to approximately 600% of the control group value. Decrease of microdialysate lactate in the pericontusional brain without change in the noninjured brain. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannellotto, M.; Yasells García, A.; Landa, M.S. Hyperoxia: Effective Mechanism of Hyperbaric Treatment at Mild-Pressure. Int. J. Mol. Sci. 2024, 25, 777. https://doi.org/10.3390/ijms25020777
Cannellotto M, Yasells García A, Landa MS. Hyperoxia: Effective Mechanism of Hyperbaric Treatment at Mild-Pressure. International Journal of Molecular Sciences. 2024; 25(2):777. https://doi.org/10.3390/ijms25020777
Chicago/Turabian StyleCannellotto, Mariana, Ali Yasells García, and María Silvina Landa. 2024. "Hyperoxia: Effective Mechanism of Hyperbaric Treatment at Mild-Pressure" International Journal of Molecular Sciences 25, no. 2: 777. https://doi.org/10.3390/ijms25020777
APA StyleCannellotto, M., Yasells García, A., & Landa, M. S. (2024). Hyperoxia: Effective Mechanism of Hyperbaric Treatment at Mild-Pressure. International Journal of Molecular Sciences, 25(2), 777. https://doi.org/10.3390/ijms25020777







