Do Cuticular Gaps Make It Possible to Study the Composition of the Cell Walls in the Glands of Drosophyllum lusitanicum?
Abstract
1. Introduction
2. Results
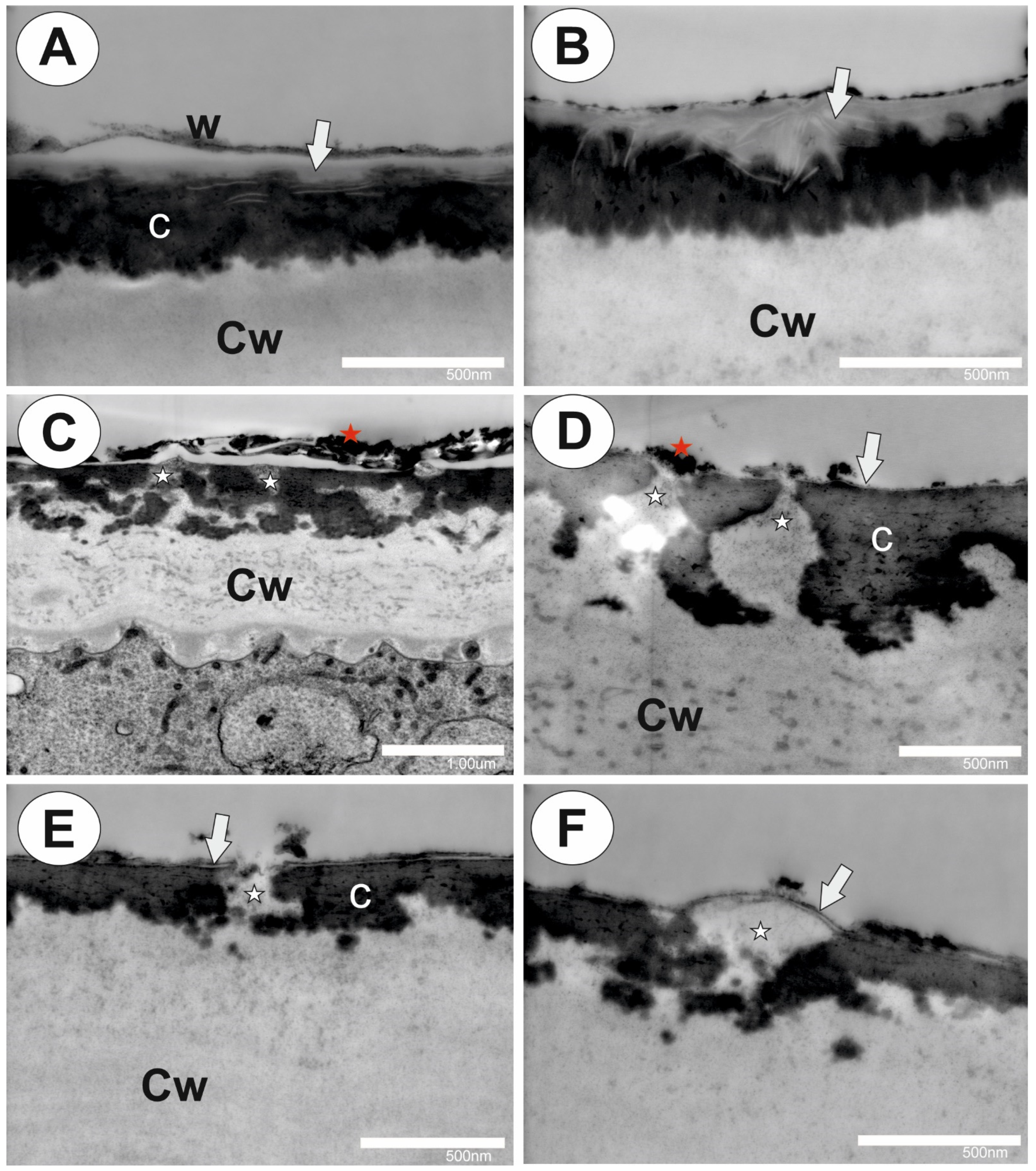
2.1. Cuticle and Cuticular Discontinuities
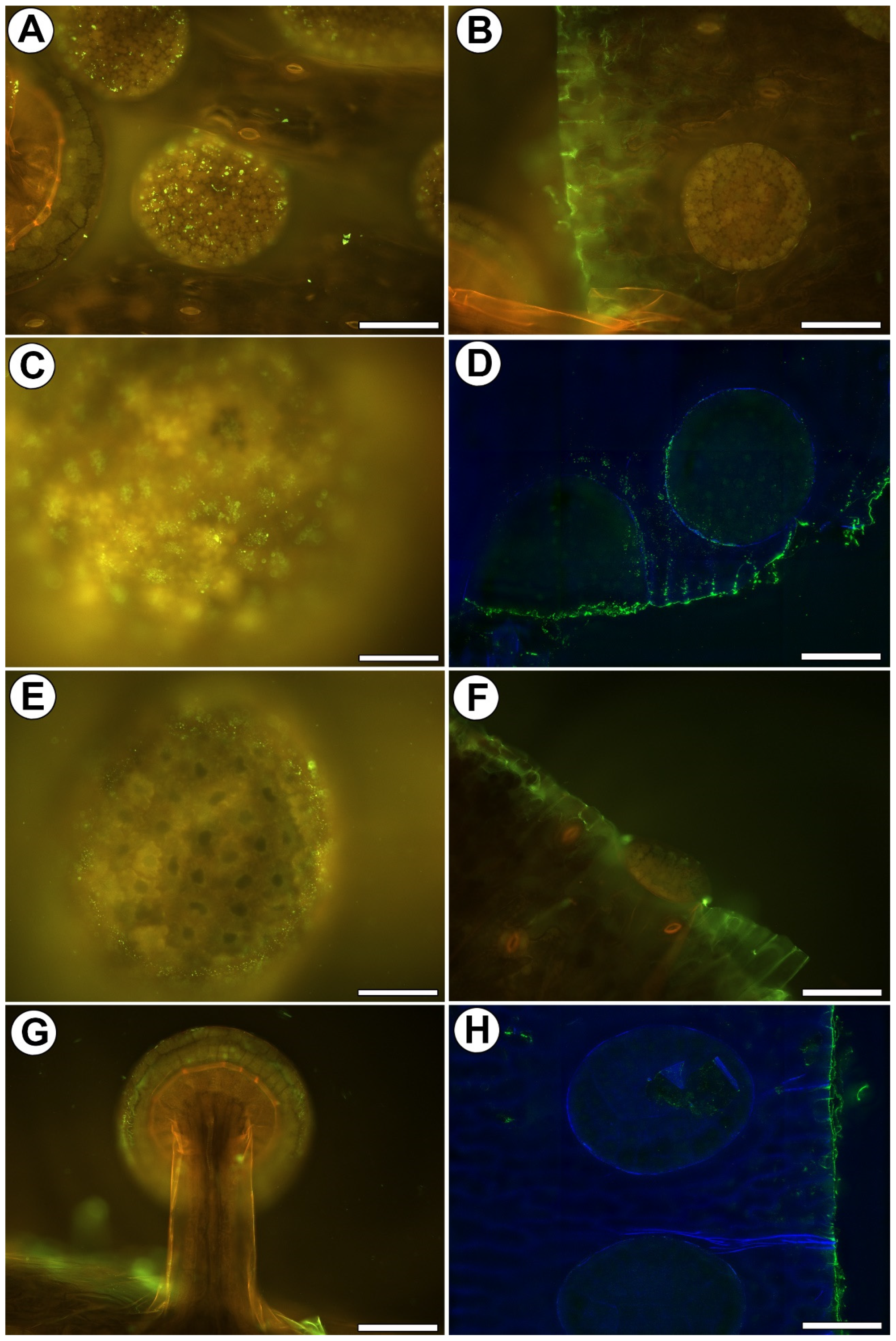
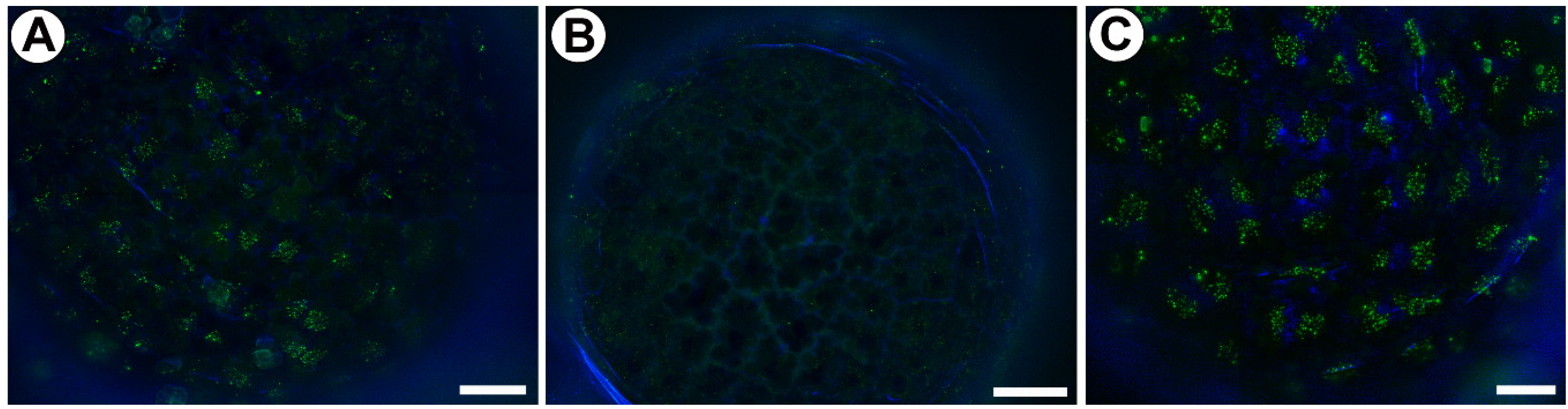
2.2. Distribution of Arabinogalactan Proteins (AGPs)
2.3. Distribution of Homogalacturonan
2.4. Distribution of Hemicellulose
3. Discussion
3.1. Cuticle Structure
3.2. Pros and Cons of the Whole-Mount Immunolabeled Gland Technique
4. Materials and Methods
4.1. Plant Material
4.2. Histological and Immunochemical Analysis
4.3. Light Microscopy (LM)
4.4. Scanning Transmission Electron Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Król, E.; Płachno, B.J.; Adamec, L.; Stolarz, M.; Dziubińska, H.; Trębacz, K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann. Bot. 2012, 109, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.M.; Adamec, L. Carnivorous Plants: Physiology, Ecology and Evolution; Oxford University Press: Oxford, UK, 2018; p. 510. [Google Scholar]
- Hedrich, R.; Fukushima, K. On the Origin of Carnivory: Molecular physiology and evolution of plants on an animal diet. Annu. Rev. Plant Biol. 2021, 72, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Juniper, B.E.; Robbins, R.J.; Joel, D.M. The Carnivorous Plants; Academic Press: London, UK, 1989. [Google Scholar]
- Barthlott, W.; Porembski, S.; Seine, R.; Theisen, I. Karnivoren: Biologie Und Kultur Fleischfressender Pflanzen; Ulmer: Stuttgart, Germany, 2004. [Google Scholar]
- McPherson, S. Pitcher Plants of the Americas; Redfern Natural History Productions: Poole, UK, 2006. [Google Scholar]
- McPherson, S. Glistening Carnivores—The Sticky-Leaved Insect-Eating Plants; Redfern Natural History Productions: Poole, UK, 2008. [Google Scholar]
- McPherson, S. Pitcher Plants of the Old World; Redfern Natural History Productions: Poole, UK, 2009. [Google Scholar]
- McPherson, S. Carnivorous Plants and Their Habitats; Fleischmann, A., Robinson, A., Eds.; Redfern Natural History Productions: Poole, UK, 2010; Volume 2, p. 1442. [Google Scholar]
- Taylor, P. The genus Utricularia: A taxonomic monograph. Kew Bull. 1989, 4, 1–724. [Google Scholar]
- Cross, A. Aldrovanda: The Waterwheel Plant; Redfern Natural History Productions Ltd.: Poole, UK, 2012. [Google Scholar]
- Adamec, L. Ecophysiology of aquatic carnivorous plants. In Carnivorous Plants: Physiology, Ecology and Evolution; Ellison, A., Adamec, L., Eds.; Oxford Academic: Oxford, UK, 2017. [Google Scholar]
- Adamec, L. Biological flora of Central Europe: Aldrovanda vesiculosa L. Perspect. Plant Ecol. Evol. Syst. 2018, 35, 8–21. [Google Scholar] [CrossRef]
- Givnish, T.J.; Burkhardt, E.L.; Happel, R.E.; Weintraub, J.D. Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. Am. Nat. 1984, 124, 479–497. [Google Scholar] [CrossRef]
- Givnish, T.J.; Sparks, K.W.; Hunter, S.J.; Pavlovič, A. Why are plants carnivorous? Cost/benefit analysis, whole-plant growth and the context-specific advantages of botanical carnivory. In Carnivorous Plants: Physiology, Ecology and Evolution; Ellison, A., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 232–255. [Google Scholar]
- Adlassnig, W.; Peroutka, M.; Eder, G.; Pois, W.; Lichtscheidl, I.K. Ecophysiological observations on Drosophyllum lusitanicum. Ecol. Res. 2006, 21, 255–262. [Google Scholar] [CrossRef]
- Paniw, M.; Gil-Cabeza, E.; Ojeda, F. Plant carnivory beyond bogs: Reliance on prey feeding in Drosophyllum lusitanicum (Drosophyllaceae) in dry Mediterranean heathland habitats. Ann. Bot. 2017, 119, 1035–1041. [Google Scholar] [CrossRef]
- Skates, L.M.; Paniw, M.; Cross, A.T.; Ojeda, F.; Dixon, K.W.; Stevens, J.C.; Gebauer, G. An ecological perspective on ‘plant carnivory beyond bogs’: Nutritional benefits of prey capture for the Mediterranean carnivorous plant Drosophyllum lusitanicum. Ann. Bot. 2019, 124, 65–76. [Google Scholar] [CrossRef]
- Adamec, L. Ecophysiological investigation on Drosophyllum lusitanicum: Why doesn’t the plant dry out? Carniv. Plant Newsl. 2009, 38, 71–74. [Google Scholar] [CrossRef]
- Carlquist, S.; Wilson, E.J. Wood anatomy of Drosophyllum (Droseraceae): Ecological and phylogenetic considerations. Bull. Torrey Bot. Club 1995, 122, 185–189. [Google Scholar] [CrossRef]
- Adlassnig, W.; Peroutka, M.; Lambers, H.; Lichtscheidl, I.K. The roots of carnivorous plants. Plant Soil 2005, 274, 127–140. [Google Scholar] [CrossRef]
- Heslop-Harrison, Y. Enzyme secretion and digest uptake in carnivorous plants. In Perspectives in Experimental Biology, Proceedings of the 50th Anniversary Meeting, Sydney, Australia, 6–8 March 2024; SEB symposium; Pergamon: Oxford, UK, 1976; Volume 2. [Google Scholar]
- Adlassnig, W.; Lendl, T.; Peroutka, M.; Lang, I. Deadly glue—Adhesive traps of carnivorous plants. In Biological Adhesive Systems; von Byern, J., Grunwald, I., Eds.; Springer: Vienna, Austria; New York, NY, USA, 2010; pp. 15–28. [Google Scholar]
- Lloyd, F.E. The Carnivorous Plants; Chronica Botanica Company: Waltham, MA, USA, 1942. [Google Scholar]
- Bertol, N.; Paniw, M.; Ojeda, F. Effective prey attraction in the rare Drosophyllum lusitanicum, a flypaper-trap carnivorous plant. Am. J. Bot. 2015, 102, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, F.; Carrera, C.; Paniw, M.; García-Moreno, L.; Barbero, G.F.; Palma, M. Volatile and semi-volatile organic compounds may help reduce pollinator-prey overlap in the carnivorous plant Drosophyllum lucitanicum (Drosophyllaceae). J. Chem. Ecol. 2021, 47, 73–86. [Google Scholar] [CrossRef]
- Płachno, B.J.; Adamec, L.; Huet, H. Mineral nutrient uptake from prey and glandular phosphatase activity as a dual test of carnivory in semi-desert plants with glandular leaves suspected of carnivory. Ann. Bot. 2009, 104, 649–654. [Google Scholar] [CrossRef]
- Adlassnig, W.; Peroutka, M.; Lang, I.; Lichtscheidl, I.K. Glands of carnivorous plants as a model system in cell biological research. Acta Bot. Gall. 2005, 152, 111–124. [Google Scholar] [CrossRef]
- Rottloff, S.; Müller, U.; Kilper, R.; Mithöfer, A. Micropreparation of single secretory glands from the carnivorous plant Nepenthes. Anal. Biochem. 2009, 394, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Rottloff, S.; Mithöfer, A.; Müller, U.; Kilper, R. Isolation of viable multicellular glands from tissue of the carnivorous plant, Nepenthes. J. Vis. Exp. 2013, 82, e50993. [Google Scholar]
- Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Koshkin, E.; Lendl, T.; Lichtscheidl, I.K. Endocytotic uptake of nutrients in carnivorous plants. Plant J. 2012, 71, 303–313. [Google Scholar] [CrossRef]
- Koller-Peroutka, M.; Krammer, S.; Pavlik, A.; Edlinger, M.; Lang, I.; Adlassnig, W. Endocytosis and Digestion in Carnivorous Pitcher Plants of the Family Sarraceniaceae. Plants 2019, 8, 367. [Google Scholar] [CrossRef]
- Ivesic, C.; Krammer, S.; Koller-Peroutka, M.; Laarouchi, A.; Gruber, D.; Lang, I.; Lichtscheidl, I.K.; Adlassnig, W. Quantification of Protein Uptake by Endocytosis in Carnivorous Nepenthales. Plants 2023, 12, 341. [Google Scholar] [CrossRef]
- Fineran, B.A.; Lee, M.S.L. Organization of quadrifid and bifid hairs in the trap of Utricularia monanthos. Protoplasma 1975, 84, 43–70. [Google Scholar] [CrossRef]
- Fineran, B.A. Glandular trichomes in Utricularia: A review of their structure and function. Isr. J. Bot. 1985, 34, 295–330. [Google Scholar]
- Joel, D.M.; Juniper, B.E. Cuticular gaps in carnivorous plant glands. In The Plant Cuticle; Cutler, D.F., Alvin, K.L., Price, C.E., Eds.; Academic Press: London, UK, 1982; pp. 121–130. [Google Scholar]
- Joel, D.M.; Rea, P.A.; Juniper, B.E. The cuticle of Dionaea muscipula Ellis (Venus’s Flytrap) in relation to stimulation, secretion and absorption. Protoplasma 1983, 114, 44–51. [Google Scholar] [CrossRef]
- Williams, S.E.; Pickard, B.G. Secretion, absorption and cuticular structure in Drosera tentacles. Plant Physiol. 1969, 44, 5. [Google Scholar]
- Williams, S.E.; Pickard, B.G. Connections and barriers between cells of Drosera tentacles in relation to their electrophysiology. Planta 1974, 116, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Faber, J.; Jankun, A. Cuticular discontinuities in glandular hairs of Genlisea St.-Hil. in relation to their functions. Acta Bot. Gall. 2005, 152, 125–130. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kozieradzka-Kiszkurno, M.; Świątek, P. Functional Ultrastructure of Genlisea (Lentibulariaceae) Digestive Hairs. Ann. Bot. 2007, 100, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B. Adaptations to foliar absorption of faeces: A pathway in plant carnivory. Ann. Bot. 2005, 95, 757–761. [Google Scholar] [CrossRef][Green Version]
- Schnepf, E. Zur feinstruktur der drüsen von Drosophyllum lusitanicum. Planta 1960, 54, 641–674. [Google Scholar] [CrossRef]
- Lichtscheidl, I.; Lancelle, S.; Weidinger, M.; Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Krammer, S.; Hepler, P.K. Gland cell responses to feeding in Drosera capensis, a carnivorous plant. Protoplasma 2021, 258, 1291–1306. [Google Scholar] [CrossRef]
- Li, Y.-X.; Chen, A.; Leu, W.-M. Sessile Trichomes Play Major Roles in Prey Digestion and Absorption, While Stalked Trichomes Function in Prey Predation in Byblis guehoi. Int. J. Mol. Sci. 2023, 24, 5305. [Google Scholar] [CrossRef] [PubMed]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, biosynthesis and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef] [PubMed]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Verhoef, R.; Schols, H.A.; McCleary, B.V.; McKee, L.; Gilbert, H.J.; Knox, P.J. Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J. 2009, 59, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Braybrook, S.; Höfte, H. Cell wall mechanics and growth control in plants: The role of pectins revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; Jönsson, H. Shifting foundations: The mechanical cell wall and development. Curr. Opin. Plant Biol. 2016, 29, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cornuault, V.; Buffetto, F.; Marcus, S.E.; Crépeau, M.J.; Guillon, F.; Ralet, M.C.; Knox, P. LM6-M: A high avidity rat monoclonal antibody to pectic α-1, 5-L-arabinan. BioRxiv 2017. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Chen, J.; Knox, J.P. Cell wall pectic arabinans influence the mechanical properties of Arabidopsis thaliana inflorescence stems and their response to mechanical stress. Plant Cell Physiol. 2013, 54, 1278–1288. [Google Scholar] [CrossRef]
- McCartney, L.; Steele-King, C.G.; Jordan, E.; Knox, J.P. Cell wall pectic (1→4)-β-d-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant J. 2003, 33, 447–454. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. Cell. Mol. Life Sci. 2001, 58, 1399–1417. [Google Scholar] [CrossRef]
- McCurdy, D.W.; Patrick, J.W.; Offler, C.E. Wall ingrowth formation in transfer cells: Novel examples of localized wall deposition in plant cells. Curr. Opin. Plant Biol. 2008, 11, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Nguema-Ona, E.; Coimbra, S.; Vicré-Gibouin, M.; Mollet, J.C.; Driouich, A. Arabinogalactan proteins in root and pollen-tube cells: Distribution and functional aspects. Ann. Bot. 2012, 110, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Miao, Y.; Huang, H.; Zhang, Y.; Huang, L.; Cao, J. Arabinogalactan Proteins: Focus on the Role in Cellulose Synthesis and Deposition during Plant Cell Wall Biogenesis. Int. J. Mol. Sci. 2022, 23, 6578. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, A.; Kalaitzis, P.; Kulik, J.; Zdunek, A. Review: Structure and modifications of arabinogalactan proteins (AGPs). BMC Plant Biol. 2023, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P.; Lichtscheidl, I. Differences in the Occurrence of Cell Wall Components between Distinct Cell Types in Glands of Drosophyllum lusitanicum. Int. J. Mol. Sci. 2023, 24, 15045. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Bruun, L.; Pierson, E.S.; Cresti, M. Periodic deposition of arabinogalactan epitopes in the cell wall of pollen tubes of Nicotiana tabacum L. Planta 1992, 188, 532–538. [Google Scholar] [CrossRef]
- Willats, W.G.; McCartney, L.; Knox, J.P. In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 2001, 213, 37–44. [Google Scholar] [CrossRef]
- Chebli, Y.; Kaneda, M.; Zerzour, R.; Geitmann, A. The cell wall of the Arabidopsis pollen tube--spatial distribution, recycling and network formation of polysaccharides. Plant Physiol. 2012, 160, 1940–1955. [Google Scholar] [CrossRef]
- Larson, E.R.; Tierney, M.L.; Tinaz, B.; Domozych, D.S. Using monoclonal antibodies to label living root hairs: A novel tool for studying cell wall microarchitecture and dynamics in Arabidopsis. Plant Methods 2014, 10, 30. [Google Scholar] [CrossRef]
- Marzec, M.; Szarejko, I.; Melzer, M. Arabinogalactan proteins are involved in root hair development in barley. J. Exp. Bot. 2015, 5, 1245–1257. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M. The Localization of Cell Wall Components in the Quadrifids of Whole-Mount Immunolabeled Utricularia dichotoma Traps. Int. J. Mol. Sci. 2024, 25, 56. [Google Scholar] [CrossRef]
- Vassilyev, A.E. Dynamics of ultrastructural characters of Drosophyllum lusitanicum Link (Droseraceae) digestive glands during maturation and after stimulation. Taiwania 2005, 50, 167–182. [Google Scholar]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P. Arabinogalactan proteins in the digestive glands of Dionaea muscipula J. Ellis Traps. Cells 2022, 11, 586. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P. Stellate trichomes in Dionaea muscipula Ellis (Venus Flytrap) Traps, Structure and Functions. Int. J. Mol. Sci. 2023, 24, 553. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P.; Strzemski, M.; Miranda, V.F.O. Immunocytochemical analysis of the wall ingrowths in the digestive gland transfer cells in Aldrovanda vesiculosa L. (Droseraceae). Cells 2022, 11, 2218. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Wójciak, M.; Świątek, P. Immunocytochemical analysis of bifid trichomes in Aldrovanda vesiculosa L. Traps. Int. J. Mol. Sci. 2023, 24, 3358. [Google Scholar] [CrossRef]
- Dahiya, P.; Brewin, N.J. Immunogold localization of callose and other cell wall components in pea nodule transfer cells. Protoplasma 2000, 214, 210–218. [Google Scholar] [CrossRef]
- Ligrone, R.; Vaughn, K.C.; Rascio, N. A cytochemical and immunocytochemical analysis of the wall labyrinth apparatus in leaf transfer cells in Elodea canadensis. Ann. Bot. 2011, 107, 717–722. [Google Scholar] [CrossRef]
- Henry, J.S.; Lopez, R.A.; Renzaglia, K.S. Differential localization of cell wall polymers across generations in the placenta of Marchantia polymorpha. J. Plant Res. 2020, 133, 911–924. [Google Scholar] [CrossRef]
- Henry, J.S.; Renzaglia, K.S. The placenta of Physcomitrium patens: Transfer cell wall polymers compared across the three bryophyte groups. Diversity 2021, 13, 378. [Google Scholar] [CrossRef]
- Henry, J.S.; Ligrone, R.; Vaughn, K.C.; Lopez, R.A.; Renzaglia, K.S. Cell wall polymers in the Phaeoceros placenta reflect developmental and functional differences across generations. Bryophyt. Divers. Evol. 2021, 43, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 8, 60. [Google Scholar] [CrossRef]
- Available online: https://www.kerafast.com/cat/799/paul-knox-phd (accessed on 13 November 2023).
- Knox, J.P.; Day, S.; Roberts, K. A set of cell surface glycoproteins forms an early position, but not cell type, in the root apical meristem of Daucus carota L. Development 1989, 106, 47–56. [Google Scholar] [CrossRef]
- Pennell, R.I.; Knox, J.P.; Scofield, G.N.; Selvendran, R.R.; Roberts, K. A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J. Cell Biol. 1989, 108, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Pennell, R.I.; Janniche, L.; Kjellbom, P.; Scofield, G.N.; Peart, J.M.; Roberts, K. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 1991, 3, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.P.; Linstead, P.J.; Cooper, J.P.C.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991, 1, 317–326. [Google Scholar] [CrossRef]
- McCartney, L.; Marcus, S.E.; Knox, J.P. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 2005, 53, 543–546. [Google Scholar] [CrossRef]
- Płachno, B.J.; Swiatek, P.; Jobson, R.W.; Malota, K.; Brutkowski, W. Serial block face SEM visualization of unusual plant nuclear tubular extensions in a carnivorous plant (Utricularia, Lentibulariaceae). Ann. Bot. 2017, 120, 673–680. [Google Scholar] [CrossRef]

| Antibody | Whole-Mount Immunolabeled Glands | Sectioned Glands |
|---|---|---|
| AGPs | ||
| JIM8 | Occurred | Occurred |
| JIM13 | Lack of or connection with secretion | Occurred |
| JIM14 | Lack | Weak signal |
| Homogalacturonan | ||
| JIM5 | Lack | Lack |
| LM19 | Occurred | Lack or weak signal |
| JIM7 | Occurred | Lack |
| LM5 | Weak signal | Weak signal |
| Hemicelluloses | ||
| LM15 | Occurred after being pre-treated with pectate lyase | Occurred |
| LM25 | Occurred | Occurred |
| Antibody | Epitope |
|---|---|
| AGPs | |
| JIM8 | Arabinogalactan |
| JIM13 | Arabinogalactan/arabinogalactan protein |
| JIM14 | Arabinogalactan/arabinogalactan protein |
| Homogalacturonan | |
| JIM5 | Homogalacturonan (HG) domain of c pectic polysaccharides recognizes partially methyl-esterified epitopes of HG and can also bind to unesterified HG |
| JIM7 | HG domain of the pectic polysaccharides recognizes partially methyl-esterified epitopes of HG but does not bind to unesterified HG |
| LM5 | Linear tetrasaccharide in (1–4)-β-D-galactans (RGI side chain) |
| LM19 | HG domain in pectic polysaccharides recognizes a range of HG with a preference to bind strongly to unesterified HG |
| Hemicelluloses | |
| LM15 | XXXG motif of xyloglucan |
| LM25 | XLLG, XXLG, and XXXG motifs of xyloglucan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P. Do Cuticular Gaps Make It Possible to Study the Composition of the Cell Walls in the Glands of Drosophyllum lusitanicum? Int. J. Mol. Sci. 2024, 25, 1320. https://doi.org/10.3390/ijms25021320
Płachno BJ, Kapusta M, Stolarczyk P, Świątek P. Do Cuticular Gaps Make It Possible to Study the Composition of the Cell Walls in the Glands of Drosophyllum lusitanicum? International Journal of Molecular Sciences. 2024; 25(2):1320. https://doi.org/10.3390/ijms25021320
Chicago/Turabian StylePłachno, Bartosz J., Małgorzata Kapusta, Piotr Stolarczyk, and Piotr Świątek. 2024. "Do Cuticular Gaps Make It Possible to Study the Composition of the Cell Walls in the Glands of Drosophyllum lusitanicum?" International Journal of Molecular Sciences 25, no. 2: 1320. https://doi.org/10.3390/ijms25021320
APA StylePłachno, B. J., Kapusta, M., Stolarczyk, P., & Świątek, P. (2024). Do Cuticular Gaps Make It Possible to Study the Composition of the Cell Walls in the Glands of Drosophyllum lusitanicum? International Journal of Molecular Sciences, 25(2), 1320. https://doi.org/10.3390/ijms25021320







