Sophoridine Counteracts Obesity via Src-Mediated Inhibition of VEGFR Expression and PI3K/AKT Phosphorylation
Abstract
1. Introduction
2. Results
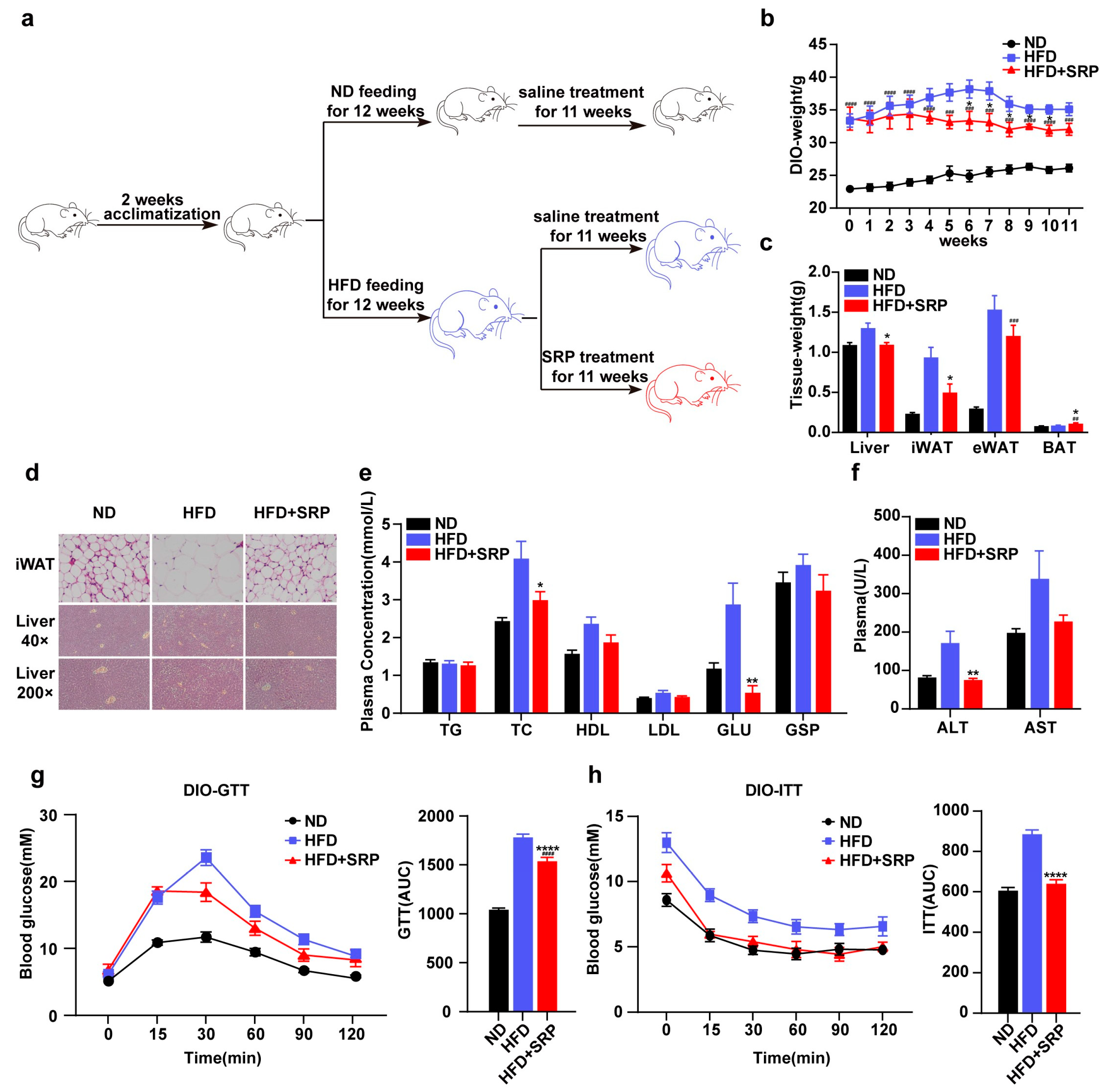
2.1. SRP Provides Resistance to Diet-Induced Obesity and Metabolic Disorders in HFD-Fed Mice
2.2. SRP Inhibits 3T3-L1 Cell Proliferation
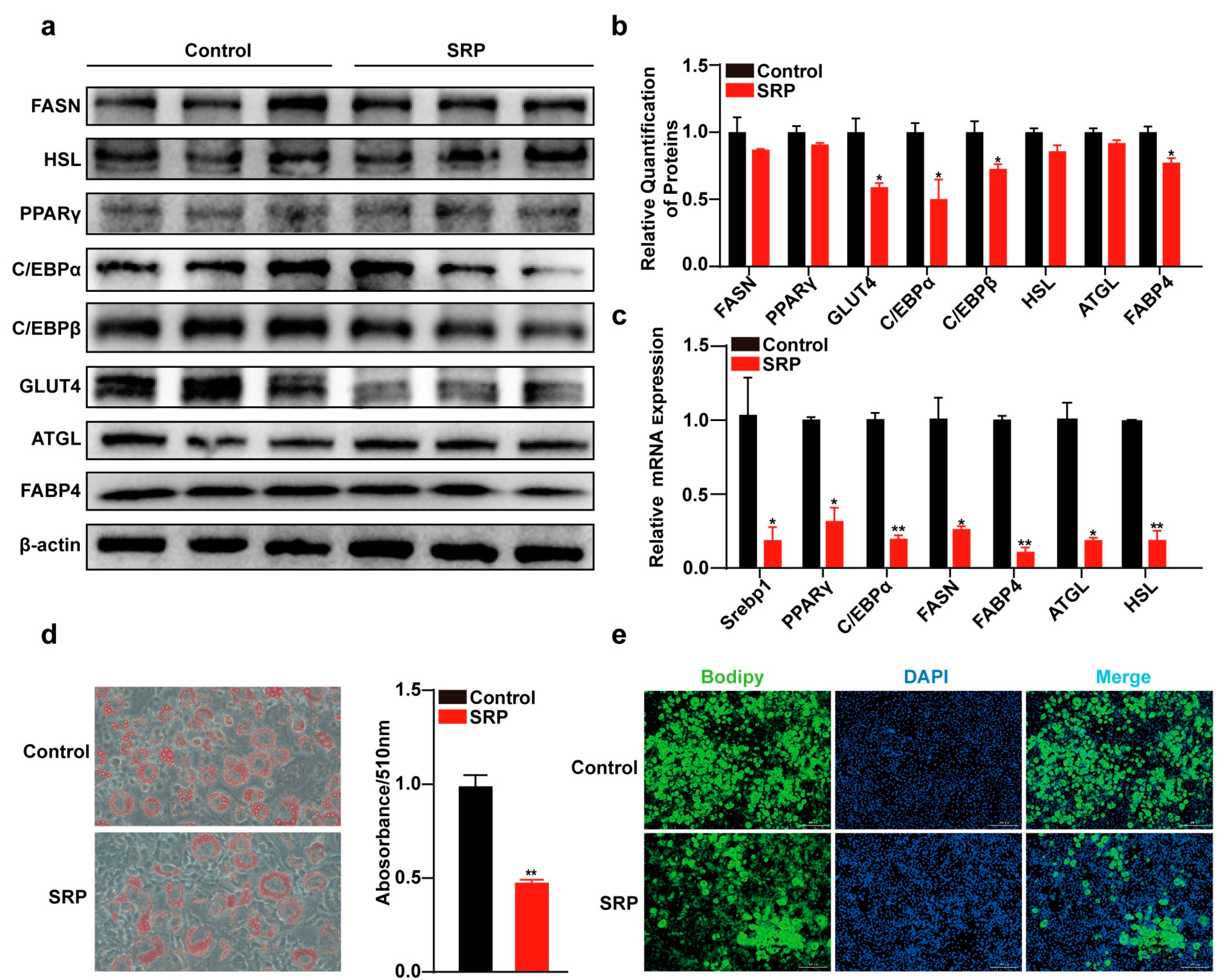
2.3. SRP Inhibits the Differentiation of 3T3-L1 Cells
2.4. Proteomic Analysis of Differentially Expressed Proteins
2.5. SRP Inhibits Lipogenic Differentiation of 3T3-L1 Cells by Targeting Src to Downregulate VEGFR2 Expression and Phosphorylation of PI3K/AKT
3. Discussion
4. Materials and Methods
4.1. Ethic Statement
4.2. Animal Experiments and Methods
4.3. Chemicals and Reagents
4.4. Cell Culture
4.5. Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
4.6. Hematoxylin and Eosin (H/E) Staining
4.7. Oil Red O Staining
4.8. Boron Dipyrromethene Fluorescent Dye Staining (Bodipy)
4.9. 5-Ethynyl-2deoxyuridine (EdU) Staining
4.10. Cell Counting Kit-8 Assay
4.11. Flow Cytometry
4.12. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.13. Western Blotting
4.14. Proteomics Sequencing Analysis
4.15. Molecular Docking
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 February 2023).
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef]
- Alamuddin, N.; Bakizada, Z.; Wadden, T.A. Management of Obesity. J. Clin. Oncol. 2016, 34, 4295–4305. [Google Scholar] [CrossRef] [PubMed]
- Acosta, A.; Abu Dayyeh, B.K.; Port, J.D.; Camilleri, M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut 2014, 63, 687–695. [Google Scholar] [CrossRef]
- Su, T.; Huang, C.H.; Yang, C.F.; Jiang, T.; Su, J.F.; Chen, M.T.; Fatima, S.; Gong, R.H.; Hu, X.J.; Bian, Z.X.; et al. Apigenin inhibits STAT3/CD36 signaling axis and reduces visceral obesity. Pharm. Res. 2020, 152, 104586. [Google Scholar] [CrossRef]
- Li, R.; Xiao, J.; Cao, Y.; Huang, Q.R.; Ho, C.T.; Lu, M.W. Capsaicin Attenuates Oleic Acid-Induced Lipid Accumulation via the Regulation of Circadian Clock Genes in HepG2 Cells. J. Agric. Food Chem. 2022, 70, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.F.; Zhu, M.Q.; Xie, B.C.; Shi, X.C.; Liu, H.; Zhang, R.X.; Xia, B.; Wu, J.W. Camptothecin effectively treats obesity in mice through GDF15 induction. PLoS Biol. 2022, 20, e3001517. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, F.; Bai, C.; Yao, C.; Zhong, H.; Zou, C.; Chen, X. Sophoridine induces apoptosis and S phase arrest via ROS-dependent JNK and ERK activation in human pancreatic cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 124. [Google Scholar] [CrossRef]
- Bi, C.W.; Ye, C.; Li, Y.H.; Zhao, W.L.; Shao, R.G.; Song, D.Q. Synthesis and biological evaluation of 12-N-p-chlorobenzyl sophoridinol derivatives as a novel family of anticancer agents. Acta Pharm. Sin. B 2016, 6, 222–228. [Google Scholar] [CrossRef]
- Li, J.C.; Dai, W.F.; Liu, D.; Zhang, Z.J.; Jiang, M.Y.; Rao, K.R.; Li, R.T.; Li, H.M. Quinolizidine alkaloids from Sophora alopecuroides with anti-inflammatory and anti-tumor properties. Bioorg. Chem. 2021, 110, 104781. [Google Scholar] [CrossRef]
- Zhuang, H.; Dai, X.; Zhang, X.; Mao, Z.; Huang, H. Sophoridine suppresses macrophage-mediated immunosuppression through TLR4/IRF3 pathway and subsequently upregulates CD8(+) T cytotoxic function against gastric cancer. Biomed. Pharmacother. 2020, 121, 109636. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.H.; Hu, Y.T.; Zhou, X.; Huang, Z.S.; Ye, J.M.; Rao, Y. Matrine counteracts obesity in mice via inducing adipose thermogenesis by activating HSF1/PGC-1alpha axis. Pharmacol. Res. 2022, 177, 106136. [Google Scholar] [CrossRef]
- Roskoski, R. Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharm. Res. 2015, 94, 9–25. [Google Scholar] [CrossRef]
- Imjeti, N.S.; Menck, K.; Egea-Jimenez, A.L.; Lecointre, C.; Lembo, F.; Bouguenina, H.; Badache, A.; Ghossoub, R.; David, G.; Roche, S.; et al. Syntenin mediates SRC function in exosomal cell-to-cell communication. Proc. Natl. Acad. Sci. USA 2017, 114, 12495–12500. [Google Scholar] [CrossRef]
- Martellucci, S.; Clementi, L.; Sabetta, S.; Mattei, V.; Botta, L.; Angelucci, A. Src Family Kinases as Therapeutic Targets in Advanced Solid Tumors: What We Have Learned So Far. Cancers 2020, 12, 1448. [Google Scholar] [CrossRef]
- Capurso, G.; Sette, C.; Delle Fave, G. The Role of Src Family Kinases in Neuroendocrine Tumors. Gastroenterology 2012, 142, E19–E21. [Google Scholar] [CrossRef][Green Version]
- Eliceiri, B.P.; Paul, R.; Schwartzberg, P.L.; Hood, J.D.; Leng, J.; Cheresh, D.A. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell. 1999, 4, 915–924. [Google Scholar] [CrossRef]
- Song, L.T.; Liu, Z.H.; Hu, H.H.; Yang, Y.; Li, T.Y.; Lin, Z.Z.; Ye, J.; Chen, J.N.; Huang, X.; Liu, D.T.; et al. Proto-oncogene Src links lipogenesis via lipin-1 to breast cancer malignancy. Nat. Commun. 2020, 11, 5842. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, W.L.; Fidler, T.; Wang, Y.; Tang, Y.; Woods, B.; Welch, C.; Cai, B.S.; Silvestre-Roig, C.; Ai, D.; et al. Macrophage Inflammation, Erythrophagocytosis, and Accelerated Atherosclerosis in Jak2(V617F) Mice. Circ. Res. 2018, 123, E35–E47. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Cabrera, D.; Sehrawat, T.S.; Jalan-Sakrikar, N.; Verma, V.K.; Simonetto, D.; Cao, S.; Yaqoob, U.; Leon, J.; Freire, M.; et al. Hepatic stellate cell activation promotes alcohol-induced steatohepatitis through Igfbp3 and SerpinA12. J. Hepatol. 2020, 73, 149–160. [Google Scholar] [CrossRef]
- Cheng, F.E.; Han, L.; Xiao, Y.; Pan, C.Y.; Li, Y.L.; Ge, X.H.; Zhang, Y.; Yan, S.Q.; Wang, M. D-chiro-Inositol Ameliorates High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance via PKC epsilon-PI3K/AKT Pathway. J. Agric. Food. Chem. 2019, 67, 5957–5967. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Bi, X.; Hou, G.; Liu, A.; Zhao, Y.; Wang, G.; Cao, X. Src acts as the target of matrine to inhibit the proliferation of cancer cells by regulating phosphorylation signaling pathways. Cell Death Dis. 2021, 12, 931. [Google Scholar] [CrossRef]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Liu, J.; Ouyang, L. Quinolizidine alkaloids derivatives from Sophora alopecuroides Linn: Bioactivities, structure-activity relationships and preliminary molecular mechanisms. Eur. J. Med. Chem. 2020, 188, 111972. [Google Scholar] [CrossRef]
- Mahzari, A.; Zeng, X.Y.; Zhou, X.; Li, S.; Xu, J.; Tan, W.; Vlahos, R.; Robinson, S.; Ye, J.M. Repurposing matrine for the treatment of hepatosteatosis and associated disorders in glucose homeostasis in mice. Acta Pharmacol. Sin. 2018, 39, 1753–1759. [Google Scholar] [CrossRef]
- Mahzari, A.; Li, S.; Zhou, X.; Li, D.; Fouda, S.; Alhomrani, M.; Alzahrani, W.; Robinson, S.R.; Ye, J.M. Matrine Protects Against MCD-Induced Development of NASH via Upregulating HSP72 and Downregulating mTOR in a Manner Distinctive from Metformin. Front. Pharmacol. 2019, 10, 405. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Wang, H.; Bai, F.; Zhou, X.; Li, S.P.; Ren, L.P.; Sun, R.Q.; Xue, C.C.; Jiang, H.L.; Hu, L.H.; et al. Identification of matrine as a promising novel drug for hepatic steatosis and glucose intolerance with HSP72 as an upstream target. Br. J. Pharmacol. 2015, 172, 4303–4318. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Li, K.W.; Zhou, C.Z. Sophoridine: A review of its pharmacology, pharmacokinetics and toxicity. Phytomedicine 2022, 95, 153756. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Ye, D.; Yang, Z.; Shen, Q.; Liu, X.; Chen, C.; Chen, X. Research progress of sophoridine’s pharmacological activities and its molecular mechanism: An updated review. Front. Pharmacol. 2023, 14, 1126636. [Google Scholar] [CrossRef]
- Ma, X.; Xu, L.; Alberobello, A.T.; Gavrilova, O.; Bagattin, A.; Skarulis, M.; Liu, J.; Finkel, T.; Mueller, E. Celastrol Protects against Obesity and Metabolic Dysfunction through Activation of a HSF1-PGC1alpha Transcriptional Axis. Cell Metab. 2015, 22, 695–708. [Google Scholar] [CrossRef]
- Luo, D.; Fan, N.; Zhang, X.; Ngo, F.Y.; Zhao, J.; Zhao, W.; Huang, M.; Li, D.; Wang, Y.; Rong, J. Covalent inhibition of endoplasmic reticulum chaperone GRP78 disconnects the transduction of ER stress signals to inflammation and lipid accumulation in diet-induced obese mice. eLife 2022, 11, e72182. [Google Scholar] [CrossRef]
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019, 10, 468. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef]
- Liu, H.; Liu, M.; Jin, Z.; Yaqoob, S.; Zheng, M.; Cai, D.; Liu, J.; Guo, S. Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food. Funct. 2019, 10, 3603–3614. [Google Scholar] [CrossRef]
- Park, S.J.; Park, M.; Sharma, A.; Kim, K.; Lee, H.J. Black Ginseng and Ginsenoside Rb1 Promote Browning by Inducing UCP1 Expression in 3T3-L1 and Primary White Adipocytes. Nutrients 2019, 11, 2747. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.; Rupérez, A.; Gomez-Llorente, C.; Gil, A.; Aguilera, C. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, D.; Wu, F.; Tu, J.; Song, J.; Xu, M.; Ji, J. Sophoridine suppresses lenvatinib-resistant hepatocellular carcinoma growth by inhibiting RAS/MEK/ERK axis via decreasing VEGFR2 expression. J. Cell. Mol. Med. 2021, 25, 549–560. [Google Scholar] [CrossRef]
- Xing, Y.; Yan, F.; Liu, Y.; Liu, Y.; Zhao, Y. Matrine inhibits 3T3-L1 preadipocyte differentiation associated with suppression of ERK1/2 phosphorylation. Biochem. Biophys. Res. Commun. 2010, 396, 691–695. [Google Scholar] [CrossRef]
- Sung, H.K.; Doh, K.O.; Son, J.E.; Park, J.G.; Bae, Y.; Choi, S.; Nelson, S.M.; Cowling, R.; Nagy, K.; Michael, I.P.; et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013, 17, 61–72. [Google Scholar] [CrossRef]
- Sun, K.; Wernstedt Asterholm, I.; Kusminski, C.M.; Bueno, A.C.; Wang, Z.V.; Pollard, J.W.; Brekken, R.A.; Scherer, P.E. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl. Acad. Sci. USA 2012, 109, 5874–5879. [Google Scholar] [CrossRef]
- Warren, C.M.; Ziyad, S.; Briot, A.; Der, A.; Iruela-Arispe, M.L. A ligand-independent VEGFR2 signaling pathway limits angiogenic responses in diabetes. Sci. Signal. 2014, 7, ra1. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yang, X.; Li, Q.; Wu, M.; Costyn, L.; Beharry, Z.; Bartlett, M.G.; Cai, H. Myristoylation of Src kinase mediates Src-induced and high-fat diet-accelerated prostate tumor progression in mice. J. Biol. Chem. 2017, 292, 18422–18433. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, X.; Shan, C.; Xia, J.; Zhang, Z.; Shi, H.; Leng, K.; Wu, Y.; Ji, C.; Zhong, T. Src family kinases involved in the differentiation of human preadipocytes. Mol. Cell Endocrinol. 2021, 533, 111323. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| SREBP1 | CGCTCTTGACCGACATCGA | GGCACGGACGGGTACATCTT |
| PPARγ | CCAAGAATACAAAGTGCGATCA | CCCACAGACTCGGCACTCAAT |
| FASN | AATGCAGACACCTTGGCACT | ACAGCTGTACTCTTGTTCTGGA |
| C/EBPα | TGGACAAGAACAGCAACGAG | TCACTGGTCAACTCCAGCAC |
| C/EBPβ | GAAGACGGTGGACAAGCTGA | TTGTGCTGCGTCTCCAGG |
| FABP4 | AAGAAGTGGGAGTGGGCTTTG | CTCTTCACCTTCCTGTCGTCTG |
| GLUT4 | GTGACTGGAACACTGGTCCTA | CCAGCCACGTTCATTGTAG |
| ATGL | TTCGCAATCTCTACCGCCTC | AAAGGGTTGGGTTGGTTCAG |
| HSL | GCTGGGCTGTCAAGCACTGT | GTAACTGGGTAGGCTGCCAT |
| cyclinD | TAGGCCCTCAGCCTCACTA | CCACCCCTGGGATAAAGCAC |
| cyclinE | GCTTGCTCCGGGGATGAAAT | GCGAGGACACCATAAGGAAATCTG |
| cyclinB | GCGTACCCTGACACCAATCTC | CTCCTCTTCGCACTTCTGCTC |
| CDK4 | ATGGCTGCCACTCGATATGAA | TCCTCCATTAGGAACTCTCACAC |
| P21 | AGTGTGCCGTTGTCTCTTCG | ACACCAGAGTGCAAGACAGC |
| P27 | AGAAGCACTGCCGGGATATG | GACCCAATTAAAGGCACCGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Wang, X.; He, Y.; Tian, X.; Yuan, T.; Yang, G.; Yu, T. Sophoridine Counteracts Obesity via Src-Mediated Inhibition of VEGFR Expression and PI3K/AKT Phosphorylation. Int. J. Mol. Sci. 2024, 25, 1206. https://doi.org/10.3390/ijms25021206
Sun J, Wang X, He Y, Tian X, Yuan T, Yang G, Yu T. Sophoridine Counteracts Obesity via Src-Mediated Inhibition of VEGFR Expression and PI3K/AKT Phosphorylation. International Journal of Molecular Sciences. 2024; 25(2):1206. https://doi.org/10.3390/ijms25021206
Chicago/Turabian StyleSun, Jingchun, Xiaoting Wang, Yulin He, Xuekai Tian, Tiantian Yuan, Gongshe Yang, and Taiyong Yu. 2024. "Sophoridine Counteracts Obesity via Src-Mediated Inhibition of VEGFR Expression and PI3K/AKT Phosphorylation" International Journal of Molecular Sciences 25, no. 2: 1206. https://doi.org/10.3390/ijms25021206
APA StyleSun, J., Wang, X., He, Y., Tian, X., Yuan, T., Yang, G., & Yu, T. (2024). Sophoridine Counteracts Obesity via Src-Mediated Inhibition of VEGFR Expression and PI3K/AKT Phosphorylation. International Journal of Molecular Sciences, 25(2), 1206. https://doi.org/10.3390/ijms25021206






