Extracellular Vesicles and Immunity: At the Crossroads of Cell Communication
Abstract
1. Introduction
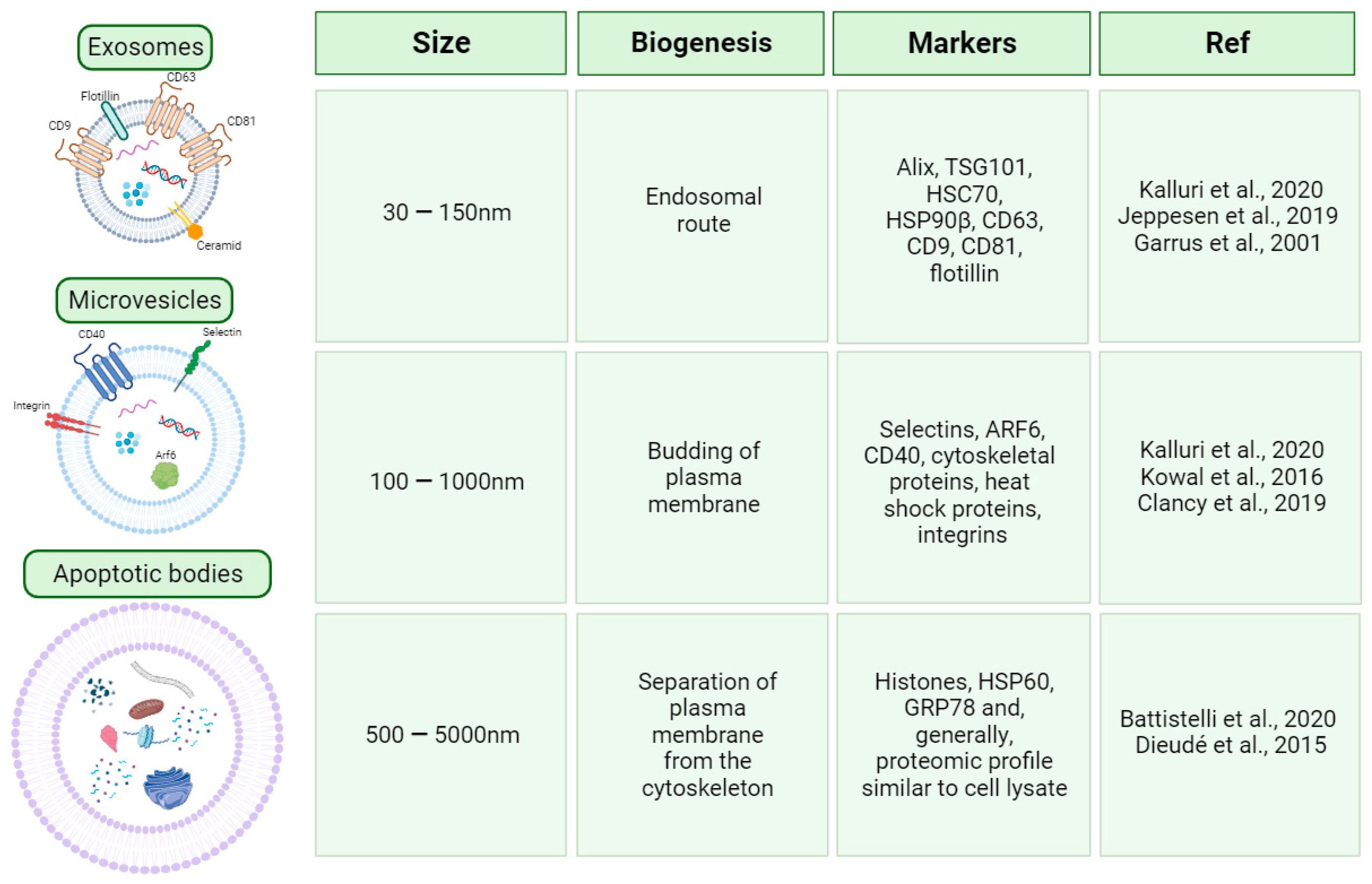
2. EV Classification and Biogenesis
2.1. Proteins Involved in Immune Cells’ EV Uptake: Integrins, Immunoglobulins, and Lectins
2.2. The New Mediators of Immunological Response: Immune Cell-Derived Extracellular Vesicles
2.2.1. Monocyte- and Macrophage-Derived EVs
2.2.2. Dendritic Cell-Derived EVs
2.2.3. T Cell-Derived EVs
2.2.4. B Cell-Derived EVs
2.2.5. Natural Killer (NK) Cell-Derived EVs
2.2.6. Red Blood Cell-Derived EVs
2.3. The Impact of Pollution on Extracellular Vesicle (EV) Biogenesis and Function
2.4. Extracellular Vesicles (EVs) in Pregnancy
2.5. Host–Pathogen EV-Mediated Interaction
2.6. EVs as Drug Delivery System
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Veerman, R.E.; Akpinar, G.G.; Eldh, M.; Gabrielsson, S. Immune Cell-Derived Extracellular Vesicles—Functions and Therapeutic Applications. Trends Mol. Med. 2019, 25, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Cerezo-Magana, M.; Bang-Rudenstam, A.; Belting, M. Proteoglycans: A common portal for SARS-CoV-2 and extracellular vesicle uptake. Am. J. Physiol. Cell Physiol. 2023, 324, C76–C84. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Garrus, J.E.; von Schwedler, U.K.; Pornillos, O.W.; Morham, S.G.; Zavitz, K.H.; Wang, H.E.; Wettstein, D.A.; Stray, K.M.; Côté, M.; Rich, R.L.; et al. Tsg101 and the Vacuolar Protein Sorting Pathway Are Essential for HIV-1 Budding. Cell 2001, 107, 55–65. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Clancy, J.W.; Zhang, Y.; Sheehan, C.; D’Souza-Schorey, C. An ARF6-Exportin-5 axis delivers pre-miRNA cargo to tumour microvesicles. Nat. Cell Biol. 2019, 21, 856–866. [Google Scholar] [CrossRef]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Dieudé, M.; Bell, C.; Turgeon, J.; Beillevaire, D.; Pomerleau, L.; Yang, B.; Hamelin, K.; Qi, S.; Pallet, N.; Béland, C.; et al. The 20 S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci. Transl. Med. 2015, 7, 318ra200. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Egea-Jimenez, A.L.; Zimmermann, P. Phospholipase D and phosphatidic acid in the biogenesis and cargo loading of extracellular vesicles. J. Lipid Res. 2018, 59, 1554–1560. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef]
- Rai, A.; Fang, H.; Claridge, B.; Simpson, R.J.; Greening, D.W. Proteomic dissection of large extracellular vesicle surfaceome unravels interactive surface platform. J. Extracell. Vesicles 2021, 10, e12164. [Google Scholar] [CrossRef]
- Liu, J.; Carmell, M.A.; Rivas, F.V.; Marsden, C.G.; Thomson, J.M.; Song, J.-J.; Hammond, S.M.; Joshua-Tor, L.; Hannon, G.J. Argonaute2 Is the Catalytic Engine of Mammalian RNAi. Science 2004, 305, 1437–1441. [Google Scholar] [CrossRef]
- Lv, Z.; Wei, Y.; Wang, D.; Zhang, C.-Y.; Zen, K.; Li, L. Argonaute 2 in Cell-Secreted Microvesicles Guides the Function of Secreted miRNAs in Recipient Cells. PLoS ONE 2014, 9, e103599. [Google Scholar] [CrossRef]
- Powell, B.H.; Turchinovich, A.; Wang, Y.; Gololobova, O.; Buschmann, D.; Zeiger, M.A.; Umbricht, C.B.; Witwer, K.W. miR-210 Expression Is Strongly Hypoxia-Induced in Anaplastic Thyroid Cancer Cell Lines and Is Associated with Extracellular Vesicles and Argonaute-2. Int. J. Mol. Sci. 2023, 24, 4507. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Azimi, I.; Monteith, G.; Bebawy, M. Ca2+ mediates extracellular vesicle biogenesis through alternate pathways in malignancy. J. Extracell. Vesicles 2020, 9, 1734326. [Google Scholar] [CrossRef] [PubMed]
- Poças, J.; Marques, C.; Gomes, C.; Otake, A.H.; Pinto, F.; Ferreira, M.; Silva, T.; Faria-Ramos, I.; Matos, R.; Ribeiro, A.R.; et al. Syndecan-4 is a maestro of gastric cancer cell invasion and communication that underscores poor survival. Proc. Natl. Acad. Sci. USA 2023, 120, e2214853120. [Google Scholar] [CrossRef]
- Pedrioli, G.; Paganetti, P. Hijacking Endocytosis and Autophagy in Extracellular Vesicle Communication: Where the Inside Meets the Outside. Front. Cell Dev. Biol. 2021, 8, 595515. [Google Scholar] [CrossRef]
- Tschuschke, M.; Kocherova, I.; Bryja, A.; Mozdziak, P.; Volponi, A.A.; Janowicz, K.; Sibiak, R.; Piotrowska-Kempisty, H.; Iżycki, D.; Bukowska, D.; et al. Inclusion Biogenesis, Methods of Isolation and Clinical Application of Human Cellular Exosomes. J. Clin. Med. 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Dasgupta, D.; Mauer, A.S.; Kakazu, E.; Nakao, K.; Malhi, H. StAR-related lipid transfer domain 11 (STARD11)-mediated ceramide transport mediates extracellular vesicle biogenesis. J. Biol. Chem. 2018, 293, 15277–15289. [Google Scholar] [CrossRef]
- Horbay, R.; Hamraghani, A.; Ermini, L.; Holcik, S.; Beug, S.T.; Yeganeh, B. Role of Ceramides and Lysosomes in Extracellular Vesicle Biogenesis, Cargo Sorting and Release. Int. J. Mol. Sci. 2022, 23, 15317. [Google Scholar] [CrossRef]
- Matsui, T.; Osaki, F.; Hiragi, S.; Sakamaki, Y.; Fukuda, M. ALIX and ceramide differentially control polarized small extracellular vesicle release from epithelial cells. Embo Rep. 2021, 22, e51475. [Google Scholar] [CrossRef]
- Menck, K.; Sönmezer, C.; Worst, T.S.; Schulz, M.; Dihazi, G.H.; Streit, F.; Erdmann, G.; Kling, S.; Boutros, M.; Binder, C.; et al. Neutral sphingomyelinases control extracellular vesicles budding from the plasma membrane. J. Extracell. Vesicles 2017, 6, 1378056. [Google Scholar] [CrossRef]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [PubMed]
- Morelli, A.E.; Larregina, A.T.; Shufesky, W.J.; Sullivan, M.L.G.; Stolz, D.B.; Papworth, G.D.; Zahorchak, A.F.; Logar, A.J.; Wang, Z.; Watkins, S.C.; et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004, 104, 3257–3266. [Google Scholar] [CrossRef] [PubMed]
- Marlin, S.D.; Springer, T.A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 1987, 51, 813–819. [Google Scholar] [CrossRef]
- Hao, S.; Bai, O.; Li, F.; Yuan, J.; Laferte, S.; Xiang, J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology 2007, 120, 90–102. [Google Scholar] [CrossRef]
- Hwang, I.; Shen, X.; Sprent, J. Direct stimulation of naïve T cells by membrane vesicles from antigen-presenting cells: Distinct roles for CD54 and B7 molecules. Proc. Natl. Acad. Sci. USA 2003, 100, 6670–6675. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Li, W.; Deng, Y.; Munegowda, M.A.; Chibbar, R.; Qureshi, M.; Xiang, J. Dendritic Cells Recruit T Cell Exosomes via Exosomal LFA-1 Leading to Inhibition of CD8+ CTL Responses through Downregulation of Peptide/MHC Class I and Fas Ligand-Mediated Cytotoxicity. J. Immunol. 2010, 185, 5268–5278. [Google Scholar] [CrossRef]
- Bhatia, P.K.; Mukhopadhyay, A. Protein glycosylation: Implications for in vivo functions and therapeutic applications. Adv. Biochem. Eng. Biotechnol. 1999, 64, 155–201. [Google Scholar]
- Dagenais, M.; Gerlach, J.Q.; Wendt, G.R.; Collins, J.J., 3rd; Atkinson, L.E.; Mousley, A.; Geary, T.G.; Long, T. Analysis of Schistosoma mansoni Extracellular Vesicles Surface Glycans Reveals Potential Immune Evasion Mechanism and New Insights on Their Origins of Biogenesis. Pathogens 2021, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Huet, G.; Gouyer, V.; Delacour, D.; Richet, C.; Zanetta, J.; Delannoy, P.; Degand, P. Involvement of glycosylation in the intracellular trafficking of glycoproteins in polarized epithelial cells. Biochimie 2003, 85, 323–330. [Google Scholar] [CrossRef]
- Schnaar, R.L. Glycans and glycan-binding proteins in immune regulation: A concise introduction to glycobiology for the allergist. J. Allergy Clin. Immunol. 2015, 135, 609–615. [Google Scholar] [CrossRef]
- Raposo, C.D.; Canelas, A.B.; Barros, M.T. Human Lectins, Their Carbohydrate Affinities and Where to Find Them. Biomolecules 2021, 11, 188. [Google Scholar] [CrossRef]
- Macedo-Da-Silva, J.; Santiago, V.F.; Rosa-Fernandes, L.; Marinho, C.R.; Palmisano, G. Protein glycosylation in extracellular vesicles: Structural characterization and biological functions. Mol. Immunol. 2021, 135, 226–246. [Google Scholar] [CrossRef]
- Gerlach, J.Q.; Griffin, M.D. Getting to know the extracellular vesicle glycome. Mol. Biosyst. 2016, 12, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Näslund, T.I.; Paquin-Proulx, D.; Paredes, P.T.; Vallhov, H.; Sandberg, J.K.; Gabrielsson, S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS 2014, 28, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Barrès, C.; Blanc, L.; Bette-Bobillo, P.; André, S.; Mamoun, R.; Gabius, H.-J.; Vidal, M. Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood 2010, 115, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, S.; Pucci, M.; Alessandro, R.; Fontana, S. Extracellular Vesicles and Tumor-Immune Escape: Biological Functions and Clinical Perspectives. Int. J. Mol. Sci. 2020, 21, 2286. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, L.N.; Alonso, M.A. Biogenesis and function of T cell-derived exosomes. Front. Cell Dev. Biol. 2016, 4, 84. [Google Scholar] [CrossRef]
- Clancy, J.W.; D’Souza-Schorey, C. Tumor-Derived Extracellular Vesicles: Multifunctional Entities in the Tumor Microenvironment. Annu. Rev. Pathol. 2023, 18, 205–229. [Google Scholar] [CrossRef]
- Dieterich, L.C. Mechanisms of extracellular vesicle-mediated immune evasion in melanoma. Front. Immunol. 2022, 13, 1002551. [Google Scholar] [CrossRef]
- Li, C.; Teixeira, A.F.; Zhu, H.-J.; Dijke, P.T. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol. Cancer 2021, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Khong, T.; Spencer, A. Extracellular Vesicles and Their Roles in the Tumor Immune Microenvironment. J. Clin. Med. 2022, 11, 6892. [Google Scholar] [CrossRef]
- Hu, B.; Chen, S.; Zou, M.; He, Z.; Shao, S.; Liu, B. Effect of Extracellular Vesicles on Neural Functional Recovery and Immunologic Suppression after Rat Cerebral Apoplexy. Cell. Physiol. Biochem. 2016, 40, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z.; Ratajczak, J. Innate Immunity Communicates Using the Language of Extracellular Microvesicles. Stem Cell Rev. Rep. 2021, 17, 502–510. [Google Scholar] [CrossRef]
- Li, Q.; Cai, S.; Li, M.; Salma, K.I.; Zhou, X.; Han, F.; Chen, J.; Huyan, T. Tumor-Derived Extracellular Vesicles: Their Role in Immune Cells and Immunotherapy. Int. J. Nanomed. 2021, 16, 5395–5409. [Google Scholar] [CrossRef] [PubMed]
- Shyu, K.-G.; Wang, B.-W.; Fang, W.-J.; Pan, C.-M.; Lin, C.-M. Exosomal MALAT1 Derived from High Glucose-Treated Macrophages Up-Regulates Resistin Expression via miR-150-5p Downregulation. Int. J. Mol. Sci. 2022, 23, 1095. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, Y.; Huang, L. Exosomes from M1-Polarized Macrophages Potentiate the Cancer Vaccine by Creating a Pro-inflammatory Microenvironment in the Lymph Node. Mol. Ther. 2017, 25, 1665–1675. [Google Scholar] [CrossRef]
- Hohensinner, P.J.; Mayer, J.; Kichbacher, J.; Kral-Pointner, J.; Thaler, B.; Kaun, C.; Hell, L.; Haider, P.; Mussbacher, M.; Schmid, J.A.; et al. Alternative activation of human macrophages enhances tissue factor expression and production of extracellular vesicles. Haematologica 2021, 106, 454–463. [Google Scholar] [CrossRef]
- Ji, G.; Feng, S.; Ren, H.; Chen, W.; Chen, R. Exosomes released from macrophages infected with Talaromyces marneffei activate the innate immune responses and decrease the replication. Immunity, Inflamm. Dis. 2023, 11, e881. [Google Scholar] [CrossRef]
- Ni, Z.; Kuang, L.; Chen, H.; Xie, Y.; Zhang, B.; Ouyang, J.; Wu, J.; Zhou, S.; Chen, L.; Su, N.; et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1beta production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019, 10, 522. [Google Scholar] [CrossRef]
- Singh, P.P.; Smith, V.L.; Karakousis, P.C.; Schorey, J.S. Exosomes Isolated from Mycobacteria-Infected Mice or Cultured Macrophages Can Recruit and Activate Immune Cells In Vitro and In Vivo. J. Immunol. 2012, 189, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Sun, X.; Dou, Y.; Wang, M.; Zhao, Y.; Yang, Q.; Zhao, Y. The Immuno-Modulation Effect of Macrophage-Derived Extracellular Vesicles in Chronic Inflammatory Diseases. Front. Immunol. 2021, 12, 785728. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, M.J.; Park, S.J.; Lee, M.S. Lipopolysaccharide-Preconditioned Periodontal Ligament Stem Cells Induce M1 Polarization of Macrophages through Extracellular Vesicles. Int. J. Mol. Sci. 2018, 19, 3843. [Google Scholar] [CrossRef]
- Shan, X.; Zhang, C.; Mai, C.; Hu, X.; Cheng, N.; Chen, W.; Peng, D.; Wang, L.; Ji, Z.; Xie, Y. The Biogenesis, Biological Functions, and Applications of Macrophage-Derived Exosomes. Front. Mol. Biosci. 2021, 8, 715461. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Niida, S.; Azuma, E.; Yanagibashi, T.; Muramatsu, M.; Huang, T.T.; Sagara, H.; Higaki, S.; Ikutani, M.; Nagai, Y.; et al. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci. Rep. 2015, 5, 8505. [Google Scholar] [CrossRef] [PubMed]
- Pieters, B.C.H.; Cappariello, A.; Bosch, M.H.J.v.D.; van Lent, P.L.E.M.; Teti, A.; van de Loo, F.A.J. Macrophage-Derived Extracellular Vesicles as Carriers of Alarmins and Their Potential Involvement in Bone Homeostasis. Front. Immunol. 2019, 10, 1901. [Google Scholar] [CrossRef] [PubMed]
- Segura, E.; Nicco, C.; Lombard, B.; Véron, P.; Raposo, G.; Batteux, F.; Amigorena, S.; Théry, C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 2005, 106, 216–223. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Théry, C.; Duban, L.; Segura, E.; Véron, P.; Lantz, O.; Amigorena, S. Indirect activation of naïve CD4+ T cells by dendritic cell–derived exosomes. Nat. Immunol. 2002, 3, 1156–1162. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Montecalvo, A.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.G.; Wang, Z.; DiVito, S.J.; Papworth, G.D.; Watkins, S.C.; Robbins, P.D.; Larregina, A.T.; et al. Exosomes as a Short-Range Mechanism to Spread Alloantigen between Dendritic Cells during T Cell Allorecognition. J. Immunol. 2008, 180, 3081–3090. [Google Scholar] [CrossRef]
- Chen, Z.; Larregina, A.T.; Morelli, A.E. Impact of extracellular vesicles on innate immunity. Curr. Opin. Organ Transplant. 2019, 24, 670–678. [Google Scholar] [CrossRef]
- Agarwal, A.; Fanelli, G.; Letizia, M.; Tung, S.L.; Boardman, D.; Lechler, R.; Lombardi, G.; Smyth, L.A. Regulatory T Cell-Derived Exosomes: Possible Therapeutic and Diagnostic Tools in Transplantation. Front. Immunol. 2014, 5, 555. [Google Scholar] [CrossRef]
- Asemani, Y.; Najafi, S.; Ezzatifar, F.; Zolbanin, N.M.; Jafari, R. Recent highlights in the immunomodulatory aspects of Treg cell-derived extracellular vesicles: Special emphasis on autoimmune diseases and transplantation. Cell Biosci. 2022, 12, 67. [Google Scholar] [CrossRef]
- Aiello, S.; Rocchetta, F.; Longaretti, L.; Faravelli, S.; Todeschini, M.; Cassis, L.; Pezzuto, F.; Tomasoni, S.; Azzollini, N.; Mister, M.; et al. Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival. Sci. Rep. 2017, 7, 11518. [Google Scholar] [CrossRef]
- Torri, A.; Carpi, D.; Bulgheroni, E.; Crosti, M.-C.; Moro, M.; Gruarin, P.; Rossi, R.L.; Rossetti, G.; Di Vizio, D.; Hoxha, M.; et al. Extracellular MicroRNA Signature of Human Helper T Cell Subsets in Health and Autoimmunity. J. Biol. Chem. 2017, 292, 2903–2915. [Google Scholar] [CrossRef]
- Thome, A.D.; Thonhoff, J.R.; Zhao, W.; Faridar, A.; Wang, J.; Beers, D.R.; Appel, S.H. Extracellular Vesicles Derived From Ex Vivo Expanded Regulatory T Cells Modulate In Vitro and In Vivo Inflammation. Front. Immunol. 2022, 13, 875825. [Google Scholar] [CrossRef]
- Yamada, N.; Kuranaga, Y.; Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Akao, Y. Colorectal cancer cell-derived extracellular vesicles induce phenotypic alteration of T cells into tumor-growth supporting cells with transforming growth factor-beta1-mediated suppression. Oncotarget 2016, 7, 27033–27043. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Tomita, Y.; Jankowska-Gan, E.; Lema, D.A.; Arvedson, M.P.; Nair, A.; Bracamonte-Baran, W.; Zhou, Y.; Meyer, K.K.; Zhong, W.; et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep. 2020, 30, 1039–1051.e5. [Google Scholar] [CrossRef]
- Sun, Y.-Z.; Ruan, J.-S.; Jiang, Z.-S.; Wang, L.; Wang, S.-M. Extracellular Vesicles: A New Perspective in Tumor Therapy. BioMed Res. Int. 2018, 2018, 2687954. [Google Scholar] [CrossRef] [PubMed]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen presentation by extracellular vesicles from professional antigen-presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar] [CrossRef]
- Fath, M.K.; Azami, J.; Jaafari, N.; Oryani, M.A.; Jafari, N.; Poor, A.K.; Azargoonjahromi, A.; Nabi-Afjadi, M.; Payandeh, Z.; Zalpoor, H.; et al. Exosome application in treatment and diagnosis of B-cell disorders: Leukemias, multiple sclerosis, and arthritis rheumatoid. Cell. Mol. Biol. Lett. 2022, 27, 74. [Google Scholar] [CrossRef]
- Xiong, J.; Chi, H.; Yang, G.; Zhao, S.; Zhang, J.; Tran, L.J.; Xia, Z.; Yang, F.; Tian, G. Revolutionizing anti-tumor therapy: Unleashing the potential of B cell-derived exosomes. Front. Immunol. 2023, 14, 1188760. [Google Scholar] [CrossRef]
- Saunderson, S.C.; McLellan, A.D. Role of lymphocyte subsets in the immune response to primary B cell–derived exosomes. J. Immunol. 2017, 199, 2225–2235. [Google Scholar] [CrossRef]
- Bauer, K.M.; Round, J.L.; O’Connell, R.M. No small matter: Emerging roles for exosomal miRNAs in the immune system. FEBS J. 2022, 289, 4021–4037. [Google Scholar] [CrossRef]
- Clayton, A.; Mitchell, J.P.; Court, J.; Mason, M.D.; Tabi, Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007, 67, 7458–7466. [Google Scholar] [CrossRef]
- Khare, D.; Or, R.; Resnick, I.; Barkatz, C.; Almogi-Hazan, O.; Avni, B. Mesenchymal Stromal Cell-Derived Exosomes Affect mRNA Expression and Function of B-Lymphocytes. Front. Immunol. 2018, 9, 3053. [Google Scholar] [CrossRef]
- Nazimek, K.; Ptak, W.; Nowak, B.; Ptak, M.; Askenase, P.W.; Bryniarski, K. Macrophages play an essential role in antigen-specific immune suppression mediated by T CD8(+) cell-derived exosomes. Immunology 2015, 146, 23–32. [Google Scholar] [CrossRef]
- Wen, C.; Seeger, R.C.; Fabbri, M.; Wang, L.; Wayne, A.S.; Jong, A.Y. Biological roles and potential applications of immune cell-derived extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1400370. [Google Scholar] [CrossRef] [PubMed]
- Vulpis, E.; Loconte, L.; Peri, A.; Molfetta, R.; Caracciolo, G.; Masuelli, L.; Tomaipitinca, L.; Peruzzi, G.; Petillo, S.; Petrucci, M.T.; et al. Impact on NK cell functions of acute versus chronic exposure to extracellular vesicle-associated MICA: Dual role in cancer immunosurveillance. J. Extracell. Vesicles 2022, 11, e12176. [Google Scholar] [CrossRef] [PubMed]
- Aarsund, M.; Nyman, T.A.; Stensland, M.E.; Wu, Y.; Inngjerdingen, M. Isolation of a cytolytic subpopulation of extracellular vesicles derived from NK cells containing NKG7 and cytolytic proteins. Front. Immunol. 2022, 13, 977353. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jin, H.; Tan, H.; Cai, X.; Sun, Y. Erythrocyte-derived extracellular vesicles aggravate inflammation by promoting the proinflammatory macrophage phenotype through TLR4-MyD88-NF-kappaB-MAPK pathway. J. Leukoc. Biol. 2022, 112, 693–706. [Google Scholar] [CrossRef]
- Norris, P.J.; Schechtman, K.; Inglis, H.C.; Adelman, A.; Heitman, J.W.; Vilardi, R.; Shah, A.; Roubinian, N.H.; Danesh, A.; Guiltinan, A.M.; et al. Influence of blood storage age on immune and coagulation parameters in critically ill transfused patients. Transfusion 2019, 59, 1223–1232. [Google Scholar] [CrossRef]
- Noubouossie, D.F.; Key, N.S. Red cell extracellular vesicles and coagulation activation pathways. Curr. Opin. Hematol. 2023, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jin, H.; Tan, H.; Wang, Y.; Wu, J.; Wang, Y.; Zhang, J.; Yang, Y.; Tian, W.; Hou, R. The role of extracellular vesicles from stored RBC units in B lymphocyte survival and plasma cell differentiation. J. Leukoc. Biol. 2020, 108, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, J.; Hao, X.; Zhang, W.; Zhong, J.; Zhu, T.; Liao, R. Exosomes From Packed Red Cells Induce Human Mast Cell Activation and the Production of Multiple Inflammatory Mediators. Front. Immunol. 2021, 12, 677905. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Profumo, E.; Riganò, R. Crosstalk between Red Blood Cells and the Immune System and Its Impact on Atherosclerosis. BioMed Res. Int. 2015, 2015, 616834. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Akbar, N.; Paget, D.; Choudhury, R.P. Extracellular Vesicles in Innate Immune Cell Programming. Biomedicines 2021, 9, 713. [Google Scholar] [CrossRef]
- Latifkar, A.; Hur, Y.H.; Sanchez, J.C.; Cerione, R.A.; Antonyak, M.A. New insights into extracellular vesicle biogenesis and function. J. Cell Sci. 2019, 132, jcs222406. [Google Scholar] [CrossRef]
- Gärtner, K.; Battke, C.; Dünzkofer, J.; Hüls, C.; von Neubeck, B.; Kellner, M.K.; Fiestas, E.; Fackler, S.; Lang, S.; Zeidler, R. Tumor-derived extracellular vesicles activate primary monocytes. Cancer Med. 2018, 7, 2013–2020. [Google Scholar] [CrossRef]
- Tohumeken, S.; Baur, R.; Bottcher, M.; Stoll, A.; Loschinski, R.; Panagiotidis, K.; Braun, M.; Saul, D.; Volkl, S.; Baur, A.S.; et al. Palmitoylated Proteins on AML-Derived Extracellular Vesicles Promote Myeloid-Derived Suppressor Cell Differentiation via TLR2/Akt/mTOR Signaling. Cancer Res. 2020, 80, 3663–3676. [Google Scholar] [CrossRef]
- Ramanathan, S.; Shenoda, B.B.; Lin, Z.; Alexander, G.M.; Huppert, A.; Sacan, A.; Ajit, S.K. Inflammation potentiates miR-939 expression and packaging into small extracellular vesicles. J. Extracell. Vesicles 2019, 8, 1650595. [Google Scholar] [CrossRef]
- Guo, X.-Y.D.; Cuillerot, J.-M.; Wang, T.; Wu, Y.; Arlinghaus, R.; Claxton, D.; Bachier, C.; Greenberger, J.; Colombowala, I.; Deisseroth, A.B. Peptide containing the BCR oligomerization domain (AA 1-160) reverses the transformed phenotype of p210bcr–abl positive 32D myeloid leukemia cells. Oncogene 1998, 17, 825–833. [Google Scholar] [CrossRef][Green Version]
- Reed, T.; Schorey, J.; D’souza-Schorey, C. Tumor-Derived Extracellular Vesicles: A Means of Co-opting Macrophage Polarization in the Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 746432. [Google Scholar] [CrossRef]
- Tung, S.L.; Boardman, D.A.; Sen, M.; Letizia, M.; Peng, Q.; Cianci, N.; Dioni, L.; Carlin, L.; Lechler, R.; Bollati, V.; et al. Regulatory T cell-derived extracellular vesicles modify dendritic cell function. Sci. Rep. 2018, 8, 6065. [Google Scholar] [CrossRef]
- Smyth, L.A.; Ratnasothy, K.; Tsang, J.Y.; Boardman, D.; Warley, A.; Lechler, R.; Lombardi, G. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur. J. Immunol. 2013, 43, 2430–2440. [Google Scholar] [CrossRef]
- Tung, S.L.; Fanelli, G.; Matthews, R.I.; Bazoer, J.; Letizia, M.; Vizcay-Barrena, G.; Faruqu, F.N.; Philippeos, C.; Hannen, R.; Al-Jamal, K.T.; et al. Regulatory T cell extracellular vesicles modify T-effector cell cytokine production and protect against human skin allograft damage. Front. Cell Dev. Biol. 2020, 8, 317. [Google Scholar] [CrossRef]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023739118. [Google Scholar] [CrossRef]
- Calvo, V.; Izquierdo, M. Inducible Polarized Secretion of Exosomes in T and B Lymphocytes. Int. J. Mol. Sci. 2020, 21, 2631. [Google Scholar] [CrossRef]
- Camussi, G.; Quesenberry, P.J. Perspectives on the Potential Therapeutic Uses of Vesicles. Exosomes Microvesicles 2013, 1, 6. [Google Scholar] [CrossRef]
- Phan, H.-D.; Longjohn, M.N.; Gormley, D.J.B.; Smith, R.H.; Dang-Lawson, M.; Matsuuchi, L.; Gold, M.R.; Christian, S.L. CD24 and IgM Stimulation of B Cells Triggers Transfer of Functional B Cell Receptor to B Cell Recipients Via Extracellular Vesicles. J. Immunol. 2021, 207, 3004–3015. [Google Scholar] [CrossRef]
- Gutknecht, M.F.; Holodick, N.E.; Rothstein, T.L. B cell extracellular vesicles contain monomeric IgM that binds antigen and enters target cells. iScience 2023, 26, 107526. [Google Scholar] [CrossRef]
- Saunderson, S.C.; Dunn, A.C.; Crocker, P.R.; McLellan, A.D. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 2014, 123, 208–216. [Google Scholar] [CrossRef]
- Ewen, C.L.; Kane, K.P.; Bleackley, R.C. A quarter century of granzymes. Cell Death Differ. 2012, 19, 28–35. [Google Scholar] [CrossRef]
- Federici, C.; Shahaj, E.; Cecchetti, S.; Camerini, S.; Casella, M.; Iessi, E.; Camisaschi, C.; Paolino, G.; Calvieri, S.; Ferro, S.; et al. Natural-killer-derived extracellular vesicles: Immune sensors and interactors. Front. Immunol. 2020, 11, 262. [Google Scholar] [CrossRef]
- Jong, A.Y.; Wu, C.; Li, J.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell. Vesicles 2017, 6, 1294368. [Google Scholar] [CrossRef]
- Keefe, D.; Shi, L.; Feske, S.; Massol, R.; Navarro, F.; Kirchhausen, T.; Lieberman, J. Perforin Triggers a Plasma Membrane-Repair Response that Facilitates CTL Induction of Apoptosis. Immunity 2005, 23, 249–262. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Lanier, L.L. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol. Res. 2015, 3, 575–582. [Google Scholar] [CrossRef]
- Soriani, A.; Vulpis, E.; Cuollo, L.; Santoni, A.; Zingoni, A. Cancer extracellular vesicles as novel regulators of NK cell response. Cytokine Growth Factor Rev. 2020, 51, 19–26. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Li, L.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C.; Jong, A.Y. Extracellular vesicles derived from natural killer cells use multiple cytotoxic proteins and killing mechanisms to target cancer cells. J. Extracell. Vesicles 2019, 8, 1588538. [Google Scholar] [CrossRef]
- Boyd-Gibbins, N.; Karagiannis, P.; Hwang, D.W.; Kim, S.-I. iPSCs in NK Cell Manufacturing and NKEV Development. Front. Immunol. 2022, 13, 890894. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Han, Q.; Han, Y.; Wang, J.; Zhang, H. Transfusion-related immunomodulation in patients with cancer: Focus on the impact of extracellular vesicles from stored red blood cells (Review). Int. J. Oncol. 2021, 59, 1–11. [Google Scholar] [CrossRef]
- Heo, J.; Kang, H. Exosome-Based Treatment for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1002. [Google Scholar] [CrossRef]
- Sudnitsyna, J.; Skverchinskaya, E.; Dobrylko, I.; Nikitina, E.; Gambaryan, S.; Mindukshev, I. Microvesicle Formation Induced by Oxidative Stress in Human Erythrocytes. Antioxidants 2020, 9, 929. [Google Scholar] [CrossRef]
- Huang, W.; Zhu, X.-Y.; Lerman, A.; Lerman, L.O. Extracellular Vesicles as Theranostic Tools in Kidney Disease. Clin. J. Am. Soc. Nephrol. 2022, 17, 1418–1429. [Google Scholar] [CrossRef]
- Chiangjong, W.; Netsirisawan, P.; Hongeng, S.; Chutipongtanate, S. Red Blood Cell Extracellular Vesicle-Based Drug Delivery: Challenges and Opportunities. Front. Med. 2021, 8, 761362. [Google Scholar] [CrossRef]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int. J. Mol. Sci. 2020, 22, 153. [Google Scholar] [CrossRef]
- Doncheva, A.I.; Romero, S.; Ramirez-Garrastacho, M.; Lee, S.; Kolnes, K.J.; Tangen, D.S.; Olsen, T.; Drevon, C.A.; Llorente, A.; Dalen, K.T.; et al. Extracellular vesicles and microRNAs are altered in response to exercise, insulin sensitivity and overweight. Acta Physiol. 2022, 236, e13862. [Google Scholar] [CrossRef]
- Gómez-Molina, C.; Sandoval, M.; Henzi, R.; Ramírez, J.P.; Varas-Godoy, M.; Luarte, A.; Lafourcade, C.A.; Lopez-Verrilli, A.; Smalla, K.-H.; Kaehne, T.; et al. Small Extracellular Vesicles in Rat Serum Contain Astrocyte-Derived Protein Biomarkers of Repetitive Stress. Int. J. Neuropsychopharmacol. 2019, 22, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Bussolati, B. Extracellular vesicles in kidney disease. Nat. Rev. Nephrol. 2022, 18, 499–513. [Google Scholar] [CrossRef]
- Longo, V.; Aloi, N.; Lo Presti, E.; Fiannaca, A.; Longo, A.; Adamo, G.; Urso, A.; Meraviglia, S.; Bongiovanni, A.; Cibella, F.; et al. Impact of the flame retardant 2,2′4,4′-tetrabromodiphenyl ether (PBDE-47) in THP-1 macrophage-like cell function via small extracellular vesicles. Front. Immunol. 2022, 13, 1069207. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Longo, A.; Adamo, G.; Fiannaca, A.; Picciotto, S.; La Paglia, L.; Romancino, D.; La Rosa, M.; Urso, A.; Cibella, F.; et al. 2,2’4,4’-Tetrabromodiphenyl Ether (PBDE-47) Modulates the Intracellular miRNA Profile, sEV Biogenesis and Their miRNA Cargo Exacerbating the LPS-Induced Pro-Inflammatory Response in THP-1 Macrophages. Front. Immunol. 2021, 12, 664534. [Google Scholar] [CrossRef]
- Hsu, P.; Nanan, R.K. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and pre-eclampsia. Front Immunol. 2014, 5, 125. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Zhao, Y.; Wang, H.; Pan, Q.; Shao, Q. Decidual Natural Killer Cells: A Good Nanny at the Maternal-Fetal Interface During Early Pregnancy. Front. Immunol. 2021, 12, 663660. [Google Scholar] [CrossRef] [PubMed]
- Than, N.G.; Hahn, S.; Rossi, S.W.; Szekeres-Bartho, J. Editorial: Fetal-Maternal Immune Interactions in Pregnancy. Front. Immunol. 2019, 10, 2729. [Google Scholar] [CrossRef]
- Beal, J.R.; Ma, Q.; Bagchi, I.C.; Bagchi, M.K. Role of Endometrial Extracellular Vesicles in Mediating Cell-to-Cell Communication in the Uterus: A Review. Cells 2023, 12, 2584. [Google Scholar] [CrossRef]
- Tannetta, D.; Dragovic, R.; Alyahyaei, Z.; Southcombe, J. Extracellular vesicles and reproduction–promotion of successful pregnancy. Cell. Mol. Immunol. 2014, 11, 548–563. [Google Scholar] [CrossRef]
- Sarker, S.; Scholz-Romero, K.; Perez, A.; Illanes, S.E.; Mitchell, M.D.; Rice, G.E.; Salomon, C. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J. Transl. Med. 2014, 12, 204. [Google Scholar] [CrossRef]
- Morelli, A.E.; Sadovsky, Y. Extracellular vesicles and immune response during pregnancy: A balancing act. Immunol. Rev. 2022, 308, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Sabapatha, A.; Gercel-Taylor, C.; Taylor, D.D. Specific Isolation of Placenta-Derived Exosomes from the Circulation of Pregnant Women and Their Immunoregulatory Consequences1. Am. J. Reprod. Immunol. 2006, 56, 345–355. [Google Scholar] [CrossRef]
- Yadava, S.M.; Feng, A.; Parobchak, N.; Wang, B.; Rosen, T. miR-15b-5p promotes expression of proinflammatory cytokines in human placenta by inhibiting Apelin signaling pathway. Placenta 2021, 104, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.D.; Peiris, H.N.; Kobayashi, M.; Koh, Y.Q.; Duncombe, G.; Illanes, S.E.; Rice, G.E.; Salomon, C. Placental exosomes in normal and complicated pregnancy. Am. J. Obstet. Gynecol. 2015, 213, S173–S181. [Google Scholar] [CrossRef]
- Salomon, C.; Scholz-Romero, K.; Sarker, S.; Sweeney, E.; Kobayashi, M.; Correa, P.; Longo, S.; Duncombe, G.; Mitchell, M.D.; Rice, G.E.; et al. Gestational Diabetes Mellitus Is Associated with Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation. Diabetes 2016, 65, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.; Tannetta, D.; Dragovic, R.; Gardiner, C.; Southcombe, J.; Collett, G.; Sargent, I. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012, 33, S48–S54. [Google Scholar] [CrossRef]
- Menon, R.; Debnath, C.; Lai, A.; Guanzon, D.; Bhatnagar, S.; Kshetrapal, P.K.; Sheller-Miller, S.; Salomon, C.; The Garbhini Study Team. Circulating Exosomal miRNA Profile During Term and Preterm Birth Pregnancies: A Longitudinal Study. Endocrinology 2019, 160, 249–275. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Martin, P.J.; Héliot, A.; Trémolet, G.; Landkocz, Y.; Dewaele, D.; Cazier, F.; Ledoux, F.; Courcot, D. Cellular response and extracellular vesicles characterization of human macrophages exposed to fine atmospheric particulate matter. Environ. Pollut. 2019, 254, 112933. [Google Scholar] [CrossRef]
- Ryu, A.-R.; Kim, D.H.; Kim, E.; Lee, M.Y. The Potential Roles of Extracellular Vesicles in Cigarette Smoke-Associated Diseases. Oxidative Med. Cell. Longev. 2018, 2018, 4692081. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nakayasu, E.S.; Oliveira, D.L.; Nimrichter, L.; Nosanchuk, J.D.; Almeida, I.C.; Casadevall, A. Extracellular Vesicles Produced by Cryptococcus neoformans Contain Protein Components Associated with Virulence. Eukaryot. Cell 2008, 7, 58–67. [Google Scholar] [CrossRef]
- Bayer-Santos, E.; Aguilar-Bonavides, C.; Rodrigues, S.P.; Cordero, E.M.; Marques, A.F.; Varela-Ramirez, A.; Choi, H.; Yoshida, N.; da Silveira, J.F.; Almeida, I.C. Proteomic Analysis of Trypanosoma cruzi Secretome: Characterization of Two Populations of Extracellular Vesicles and Soluble Proteins. J. Proteome Res. 2013, 12, 883–897. [Google Scholar] [CrossRef]
- Ribeiro, K.S.; Vasconcellos, C.I.; Soares, R.P.; Mendes, M.T.; Ellis, C.C.; Aguilera-Flores, M.; de Almeida, I.C.; Schenkman, S.; Iwai, L.K.; Torrecilhas, A.C. Proteomic analysis reveals different composition of extracellular vesicles released by two Trypanosoma cruzi strains associated with their distinct interaction with host cells. J. Extracell. Vesicles 2018, 7, 1463779. [Google Scholar] [CrossRef]
- Sampaio, N.G.; Emery, S.J.; Garnham, A.L.; Tan, Q.Y.; Sisquella, X.; Pimentel, M.A.; Jex, A.R.; Regev-Rudzki, N.; Schofield, L.; Eriksson, E.M. Extracellular vesicles from early stage Plasmodium falciparum-infected red blood cells contain PfEMP1 and induce transcriptional changes in human monocytes. Cell. Microbiol. 2018, 20, e12822. [Google Scholar] [CrossRef]
- Toda, H.; Diaz-Varela, M.; Segui-Barber, J.; Roobsoong, W.; Baro, B.; Garcia-Silva, S.; Galiano, A.; Gualdron-Lopez, M.; Almeida, A.C.G.; Brito, M.A.M.; et al. Plasma-derived extracellular vesicles from Plasmodium vivax patients signal spleen fibroblasts via NF-kB facilitating parasite cytoadherence. Nat. Commun. 2020, 11, 2761. [Google Scholar] [CrossRef]
- Rivera, J.; Cordero, R.J.B.; Nakouzi, A.S.; Frases, S.; Nicola, A.; Casadevall, A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. USA 2010, 107, 19002–19007. [Google Scholar] [CrossRef]
- Thay, B.; Wai, S.N.; Oscarsson, J. Staphylococcus aureus α-Toxin-Dependent Induction of Host Cell Death by Membrane-Derived Vesicles. PLoS ONE 2013, 8, e54661. [Google Scholar] [CrossRef]
- Coelho, C.; Brown, L.C.; Maryam, M.; Vij, R.; Smith, D.F.; Burnet, M.C.; Kyle, J.E.; Heyman, H.M.; Ramirez, J.; Prados-Rosales, R.; et al. Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles. J. Biol. Chem. 2019, 294, 1202–1217. [Google Scholar] [CrossRef]
- Marsollier, L.; Brodin, P.; Jackson, M.; Korduláková, J.; Tafelmeyer, P.; Carbonnelle, E.; Aubry, J.; Milon, G.; Legras, P.; André, J.-P.S.; et al. Impact of Mycobacterium ulcerans Biofilm on Transmissibility to Ecological Niches and Buruli Ulcer Pathogenesis. PLoS Pathog. 2007, 3, e62. [Google Scholar] [CrossRef]
- Wyllie, M.P.; Ramirez, M.I. Microvesicles released during the interaction between Trypanosoma cruzi TcI and TcII strains and host blood cells inhibit complement system and increase the infectivity of metacyclic forms of host cells in a strain-independent process. Pathog. Dis. 2017, 75, ftx077. [Google Scholar] [CrossRef]
- Baltazar, L.M.; Zamith-Miranda, D.; Burnet, M.C.; Choi, H.; Nimrichter, L.; Nakayasu, E.S.; Nosanchuk, J.D. Concentration-dependent protein loading of extracellular vesicles released by Histoplasma capsulatum after antibody treatment and its modulatory action upon macrophages. Sci. Rep. 2018, 8, 8065. [Google Scholar] [CrossRef]
- Kuipers, M.E.; Hoen, E.N.N.; van der Ham, A.J.; Ozir-Fazalalikhan, A.; Nguyen, D.L.; de Korne, C.M.; Koning, R.I.; Tomes, J.J.; Hoffmann, K.F.; Smits, H.H.; et al. DC-SIGN mediated internalisation of glycosylated extracellular vesicles from Schistosoma mansoni increases activation of monocyte-derived dendritic cells. J. Extracell. Vesicles 2020, 9, 1753420. [Google Scholar] [CrossRef]
- Murphy, A.; Cwiklinski, K.; Lalor, R.; O’connell, B.; Robinson, M.W.; Gerlach, J.; Joshi, L.; Kilcoyne, M.; Dalton, J.P.; O’neill, S.M. Fasciola hepatica Extracellular Vesicles isolated from excretory-secretory products using a gravity flow method modulate dendritic cell phenotype and activity. PLoS Neglected Trop. Dis. 2020, 14, e0008626. [Google Scholar] [CrossRef]
- Nogueira, P.M.; Ribeiro, K.; Silveira, A.C.O.; Campos, J.H.; Martins-Filho, O.A.; Bela, S.R.; Campos, M.A.; Pessoa, N.L.; Colli, W.; Alves, M.J.M.; et al. Vesicles from different Trypanosoma cruzi strains trigger differential innate and chronic immune responses. J. Extracell. Vesicles 2015, 4, 28734. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Ye, Z.; Xu, J. Engineering Extracellular Vesicles as Delivery Systems in Therapeutic Applications. Adv. Sci. 2023, 10, e2300552. [Google Scholar] [CrossRef]
- Danilushkina, A.A.; Emene, C.C.; Barlev, N.A.; Gomzikova, M.O. Strategies for Engineering of Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 13247. [Google Scholar] [CrossRef]
- Kumar, P.; Boyne, C.; Brown, S.; Qureshi, A.; Thorpe, P.; Synowsky, S.A.; Shirran, S.; Powis, S.J. Tumour-associated antigenic peptides are present in the HLA class I ligandome of cancer cell line derived extracellular vesicles. Immunology 2022, 166, 249–264. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W.; Feng, Y.; Xu, Z.; He, Y.; Xiong, Y.; Chen, L.; Li, X.; Liu, J.; Liu, G.; et al. Epithelial-derived exosomes promote M2 macrophage polarization via Notch2/SOCS1 during mechanical ventilation. Int. J. Mol. Med. 2022, 50, 96. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Kashanchi, F.; Jafari, R. Dexosomes as a cell-free vaccine for cancer immunotherapy. J. Exp. Clin. Cancer Res. 2020, 39, 258. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Q.; Lu, L.; Cui, S.; Ma, K.; Zhang, W.; Ma, F.; Li, H.; Fu, X.; Zhang, C. Immunomodulatory potential of mesenchymal stem cell-derived extracellular vesicles: Targeting immune cells. Front. Immunol. 2023, 14, 1094685. [Google Scholar] [CrossRef]
- Matheakakis, A.; Batsali, A.; Papadaki, H.A.; Pontikoglou, C.G. Therapeutic Implications of Mesenchymal Stromal Cells and Their Extracellular Vesicles in Autoimmune Diseases: From Biology to Clinical Applications. Int. J. Mol. Sci. 2021, 22, 10132. [Google Scholar] [CrossRef]
- Wu, F.; She, Z.; Li, C.; Mao, J.; Luo, S.; Chen, X.; Tian, J.; Wen, C. Therapeutic potential of MSCs and MSC-derived extracellular vesicles in immune thrombocytopenia. Stem Cell Res. Ther. 2023, 14, 79. [Google Scholar] [CrossRef]
- Yerneni, S.S.; Yalcintas, E.P.; Smith, J.D.; Averick, S.; Campbell, P.G.; Ozdoganlar, O.B. Skin-targeted delivery of extracellular vesicle-encapsulated curcumin using dissolvable microneedle arrays. Acta Biomater. 2022, 149, 198–212. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, Z.; Liu, F.; Wu, Q.; Sun, X.; Ma, H. The impact of exosomes derived from distinct sources on rheumatoid arthritis. Front. Immunol. 2023, 14, 1240747. [Google Scholar] [CrossRef]
- Wu, T.; Marakkath, B.; Ye, Y.; Khobahy, E.; Yan, M.; Hutcheson, J.; Zhu, J.; Zhou, X.; Mohan, C. Curcumin Attenuates Both Acute and Chronic Immune Nephritis. Int. J. Mol. Sci. 2020, 21, 1745. [Google Scholar] [CrossRef]
- Zinger, A.; Brozovich, A.; Pasto, A.; Sushnitha, M.; Martinez, J.O.; Evangelopoulos, M.; Boada, C.; Tasciotti, E.; Taraballi, F. Bioinspired Extracellular Vesicles: Lessons Learned from Nature for Biomedicine and Bioengineering. Nanomaterials 2020, 10, 2172. [Google Scholar] [CrossRef]
- Chu, C.; Wei, S.; Wang, Y.; Wang, Y.; Man, Y.; Qu, Y. Extracellular vesicle and mesenchymal stem cells in bone regeneration: Recent progress and perspectives. J. Biomed. Mater. Res. Part A 2019, 107, 243–250. [Google Scholar] [CrossRef]
- Jung, I.; Shin, S.; Baek, M.-C.; Yea, K. Modification of immune cell-derived exosomes for enhanced cancer immunotherapy: Current advances and therapeutic applications. Exp. Mol. Med. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | Effect of Cell Type-Derived EVs on Target Cells | Reference |
|---|---|---|
| Monocytes and Macrophages | - Involvement in the innate immune cell’s communication | [54] |
| - Regulation of inflammation, immune cell activation, and modulation of immune responses in various disease contexts | [55,56] | |
| - Immunomodulatory effects in cancer, acute kidney injury, and inflammatory disorders | [57,58,59] | |
| - M1-derived EVs induce macrophage activation, cytokine production, and immune cell recruitment | [60,61] | |
| - Implication in chronic inflammatory diseases (diabetes, cancer, cardiovascular disease, pulmonary disease, and gastrointestinal disease) | [62] | |
| - Activation of macrophage-mediated inflammation and effects on vascular diseases | [63,64] | |
| - Modulation the status of pericytes in response to inflammatory stimuli | [65] | |
| - Regulation of bone homeostasis | [66] | |
| Dendritic Cell | - T cell stimulation and antigen-specific T cell responses | [67,68,69] |
| - Modulation of T cells and NK cell function | [70] | |
| - Immunomodulatory activity mediated by miRNA cargo on immune target cells | [71] | |
| T Cell | - Modulation of leukocytes, parenchymal, or stromal cells functions | [72] |
| - Inhibition of effector T cell responses | [73] | |
| - Reduction of IL-6, iNOS, IL-1β, and IFN- γ transcripts in spleen-derived myeloid cells | [74] | |
| - Suppression of CD4+ and CD8+ T cell proliferation | [75] | |
| - Regulation of DCs function, highlighting their immunomodulatory effects | [76] | |
| - Treg-derived EVs ameliorate chronic prostatitis/chronic pelvic pain syndrome in rats | [77] | |
| - Involvement in modulation of autoimmune diseases and transplantation by inhibition of CD4+ T cell proliferation and relevant miR-146a-5p targets | [78] | |
| - Immunosuppressive effects of Treg EVs on target immune cells | [79] | |
| - Induction of tumour regression in tumour microenvironment | [80] | |
| B cell | - T cell interaction and immunomodulatory activity in T cell differentiation | [81,82,83] |
| - Activation of DCs, T CD4+ and NK to induce TCD8+ killing response | [83,84,85] | |
| - Inhibition of lymphocyte response to interleukin-2 | [86] | |
| - Modulation of gene expression in B-lymphocytes | [87] | |
| Natural Killer | - Involvement in immune tolerance and immunosuppression | [88] |
| - Antitumoral activity as effectors of NK cells | [88] | |
| - Activation of caspase-dependent or independent apoptosis pathways | [89] | |
| - Implication in immune surveillance | [90] | |
| - Regulation of cancer initiation, growth and metastasis as well as NK cells | [91] | |
| Red Blood Cell (RBC) | - Macrophage pro-inflammatory polarisation | [92] |
| - Activation of coagulation pathways | [93,94] | |
| - Impact on B lymphocyte survival and plasma cell differentiation | [95] | |
| - Human mast cell activation and induction of inflammatory mediators | [96] | |
| - T cell proliferation in peripheral blood mononuclear cell cultures | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloi, N.; Drago, G.; Ruggieri, S.; Cibella, F.; Colombo, P.; Longo, V. Extracellular Vesicles and Immunity: At the Crossroads of Cell Communication. Int. J. Mol. Sci. 2024, 25, 1205. https://doi.org/10.3390/ijms25021205
Aloi N, Drago G, Ruggieri S, Cibella F, Colombo P, Longo V. Extracellular Vesicles and Immunity: At the Crossroads of Cell Communication. International Journal of Molecular Sciences. 2024; 25(2):1205. https://doi.org/10.3390/ijms25021205
Chicago/Turabian StyleAloi, Noemi, Gaspare Drago, Silvia Ruggieri, Fabio Cibella, Paolo Colombo, and Valeria Longo. 2024. "Extracellular Vesicles and Immunity: At the Crossroads of Cell Communication" International Journal of Molecular Sciences 25, no. 2: 1205. https://doi.org/10.3390/ijms25021205
APA StyleAloi, N., Drago, G., Ruggieri, S., Cibella, F., Colombo, P., & Longo, V. (2024). Extracellular Vesicles and Immunity: At the Crossroads of Cell Communication. International Journal of Molecular Sciences, 25(2), 1205. https://doi.org/10.3390/ijms25021205







