Recreating Human Skin In Vitro: Should the Microbiota Be Taken into Account?
Abstract
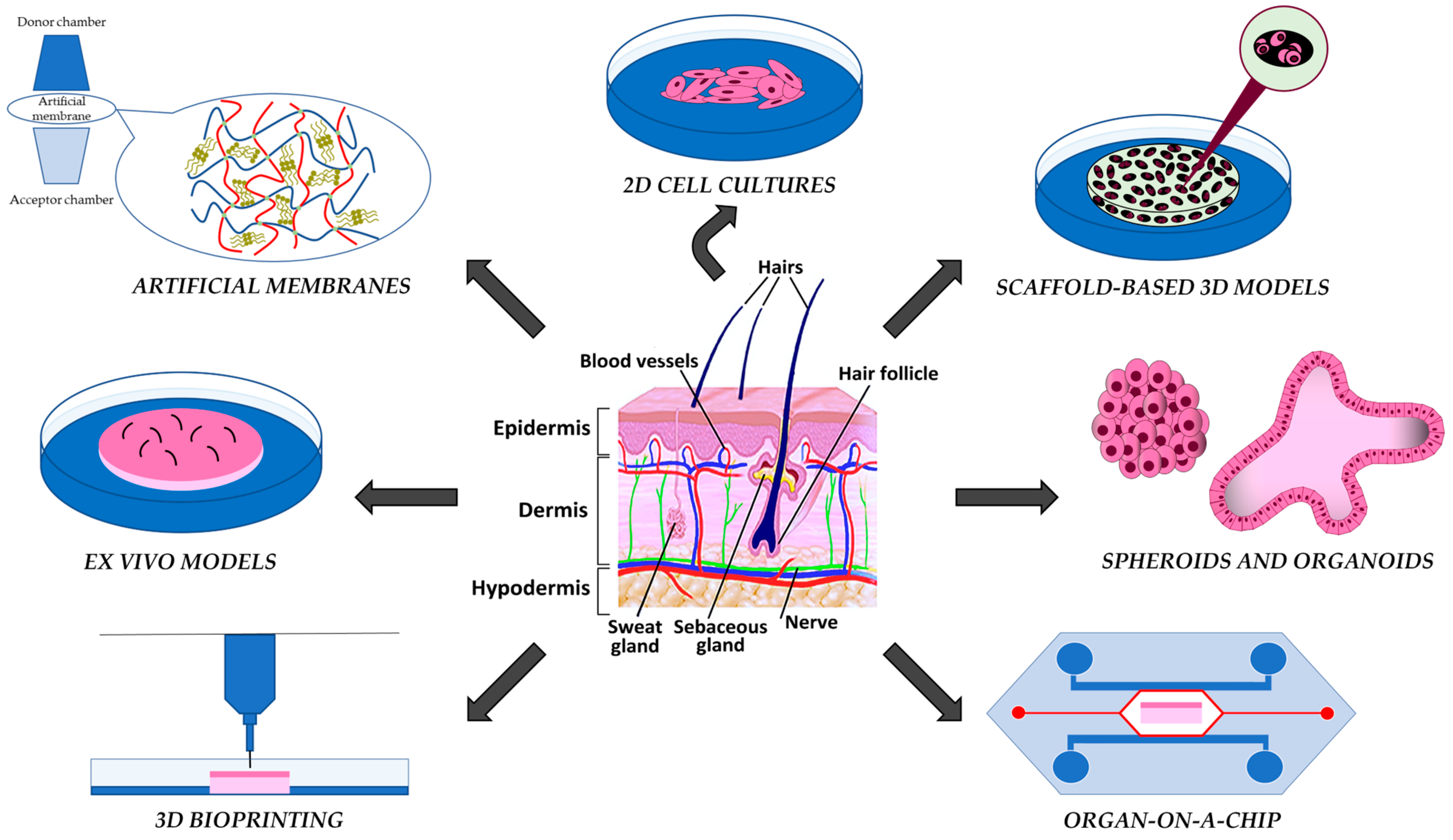
1. Introduction
2. Artificial Membranes
3. 2D and Scaffold-Based 3D Skin Models
4. Scaffold-Free Models: Spheroids and Organoids
5. Organ-on-a-Chip
6. 3D Bioprinting
7. Ex Vivo Models
8. The Four Levels of the Skin Barrier: A Focus on the Skin Microbiota
9. Human Skin Microbiota on In Vitro Human Skin Models
10. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dellambra, E.; Odorisio, T.; D’Arcangelo, D.; Failla, C.M.; Facchiano, A. Non-animal models in dermatological research. ALTEX Altern. Anim. Exp. 2019, 36, 177–202. [Google Scholar] [CrossRef]
- Filaire, E.; Nachat-Kappes, R.; Laporte, C.; Harmand, M.F.; Simon, M.; Poinsot, C. Alternative in vitro models used in the main safety tests of cosmetic products and new challenges. Int. J. Cosmet. Sci. 2022, 44, 604–613. [Google Scholar] [CrossRef]
- Planz, V.; Lehr, C.M.; Windbergs, M. In vitro models for evaluating safety and efficacy of novel technologies for skin drug delivery. J. Control Release 2016, 242, 89–104. [Google Scholar] [CrossRef]
- Fournière, M.; Latire, T.; Souak, D.; Feuilloley, M.G.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two major sentinels of skin microbiota and the influence of cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef]
- Capone, K.A.; Dowd, S.E.; Stamatas, G.N.; Nikolovski, J. Diversity of the human skin microbiome early in life. J. Investig. Dermatol. 2011, 131, 2026–2032. [Google Scholar] [CrossRef]
- Somerville, D.A. The normal flora of the skin in different age groups. Br. J. Dermatol. 1969, 81, 248–258. [Google Scholar]
- Leyden, J.J.; McGinley, K.J.; Mills, O.H.; Kligman, A.M. Age-related changes in the resident bacterial flora of the human face. J. Investig. Dermatol. 1975, 65, 379–381. [Google Scholar] [CrossRef]
- Hu, X.; Tang, M.; Dong, K.; Zhou, J.; Wang, D.; Song, L. Changes in the skin microbiome during male maturation from 0 to 25 years of age. Skin Res. Technol. 2023, 29, e13432. [Google Scholar] [CrossRef]
- Moniz, T.; Costa Lima, S.A.; Reis, S. Human skin models: From healthy to disease-mimetic systems; characteristics and applications. Br. J. Pharamcol. 2020, 177, 4314–4329. [Google Scholar]
- Haq, A.; Dorrani, M.; Goodyear, B.; Joshi, V.; Michniak-Kohn, B. Membrane properties for permeability testing: Skin versus synthetic membranes. Int. J. Pharm. 2018, 539, 58–64. [Google Scholar] [CrossRef]
- De Almeida Borges, V.R.; Simon, A.; Sena, A.R.C.; Cabral, L.M.; de Sousa, V.P. Nanoemulsion containing dapsone for topical administration: A study of in vitro release and epidermal permeation. Int. J. Nanomed. 2013, 8, 535–544. [Google Scholar]
- Köllmer, M.; Mossahebi, P.; Sacharow, E.; Gorissen, S.; Gräfe, N.; Evers, D.H.; Herbig, M.E. Investigation of the compatibility of the skin PAMPA model with topical formulation and acceptor media additives using different assay setups. AAPS PharmSciTech 2019, 20, 89. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Rahma, A.; Lane, M.E.; Sinkó, B. A comparative study of the in vitro permeation of 2-phenoxyethanol in the skin PAMPA model and mammalian skin. Int. J. Pharm. 2023, 635, 122692. [Google Scholar]
- Kumar, M.; Sharma, A.; Mahmood, S.; Thakur, A.; Mirza, M.A.; Bhatia, A. Franz diffusion cell and its implication in skin permeation studies. J. Dispers. Sci. Technol. 2023, 1–14. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 148. [Google Scholar]
- Gruber, J.V.; Terpak, N.; Massard, S.; Schwartz, A.; Bojanowski, K. Passive Enhancement of Retinol Skin Penetration by Jojoba Oil Measured Using the Skin Parallel Artificial Membrane Permeation Assay (Skin-PAMPA): A Pilot Study. Clin. Cosmet. Investig. Dermatol. 2023, 16, 317–324. [Google Scholar] [CrossRef]
- Luo, L.; Patel, A.; Sinko, B.; Bell, M.; Wibawa, J.; Hadgraft, J.; Lane, M.E. A comparative study of the in vitro permeation of ibuprofen in mammalian skin, the PAMPA model and silicone membrane. Int. J. Pharm. 2016, 505, 14–19. [Google Scholar] [CrossRef]
- Kirk, R.D.; Akanji, T.; Li, H.; Shen, J.; Allababidi, S.; Seeram, N.P.; Bertin, M.J.; Ma, H. Evaluations of Skin Permeability of Cannabidiol and Its Topical Formulations by Skin Membrane-Based Parallel Artificial Membrane Permeability Assay and Franz Cell Diffusion Assay. Med. Cannabis Cannabinoids 2022, 5, 129–137. [Google Scholar]
- Kittaneh, M.; Qurt, M.; Malkieh, N.; Naseef, H.; Muqedi, R. Preparation and Evaluation of Vitamin D3 Supplementation as Transdermal Film-Forming Solution. Pharmaceutics 2022, 15, 39. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szymańska, E.; Winnicka, K. Nanostructured Lipid Carriers (NLC)-Based Gel Formulations as Etodolac Delivery: From Gel Preparation to Permeation Study. Molecules 2022, 28, 235. [Google Scholar] [CrossRef]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using Strat-M® membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Green, H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 1977, 265, 421–424. [Google Scholar]
- Letsiou, S.; Ganopoulos, I.; Kapazoglou, A.; Xanthopoulou, A.; Sarrou, E.; Tanou, G.; Molassiotis, A. Probing the effects of sweet cherry (Prunus avium L.) extract on 2D and 3D human skin models. Mol. Biol. Rep. 2022, 49, 2687–2693. [Google Scholar] [CrossRef]
- Pérez-Salas, J.L.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; González-Laredo, R.F.; Medina-Torres, L.; Gallegos-Infante, J.A. In Vitro and Ex Vivo Models for Screening Topical Anti-Inflammatory Drugs. Sci. Pharm. 2023, 91, 20. [Google Scholar] [CrossRef]
- Teimouri, A.; Yeung, P.; Agu, R. 2D vs. 3D cell culture models for in vitro topical (dermatological) medication testing. In Cell Culture; Mehanna, R.E., Ed.; IntechOpen Limited: London, UK, 2019. [Google Scholar] [CrossRef]
- Jiang, M.; Min, Y.; DeBusk, L.; Fernandez, S.; Strand, D.W.; Hayward, S.W.; Lin, P.C. Spontaneous immortalization of human dermal microvascular endothelial cells. World J. Stem Cells 2010, 2, 114. [Google Scholar]
- Smolińska, E.; Moskot, M.; Jakóbkiewicz-Banecka, J.; Węgrzyn, G.; Banecki, B.; Szczerkowska-Dobosz, A.; Purzycka-Bohdan, D.; Gabig-Cimińska, M. Molecular action of isoflavone genistein in the human epithelial cell line HaCaT. PLoS ONE 2018, 13, e0192297. [Google Scholar] [CrossRef]
- Sun, T.; Jackson, S.; Haycock, J.W.; MacNeil, S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J. Biotechnol. 2006, 122, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, M.; Jiang, S.; Zhang, Y.; Li, R.; Lu, Y.; Liu, L.; Wu, G.; Liu, Y.; Xie, L.; et al. Skin toxicity assessment of silver nanoparticles in a 3D epidermal model compared to 2D keratinocytes. Int. J. Nanomed. 2019, 14, 9707–9719. [Google Scholar]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [PubMed]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol. Biol. 2011, 695, 17–39. [Google Scholar] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef]
- Pruniéras, M.; Régnier, M.; Woodley, D. Methods for cultivation of keratinocytes with an air-liquid interface. J. Investig. Dermatol. 1983, 81, S28–S33. [Google Scholar]
- Sun, T.; Norton, D.; Haycock, J.W.; Ryan, A.J.; MacNeil, S. Development of a closed bioreactor system for culture of tissue-engineered skin at an air–liquid interface. Tissue Eng. 2005, 11, 1824–1831. [Google Scholar]
- Frankart, A.; Malaisse, J.; De Vuyst, E.; Minner, F.; de Rouvroit, C.L.; Poumay, Y. Epidermal morphogenesis during progressive in vitro 3D reconstruction at the air–liquid interface. Exp. Dermatol. 2012, 21, 871–875. [Google Scholar] [CrossRef]
- Idrees, A.; Schmitz, I.; Zoso, A.; Gruhn, D.; Pacharra, S.; Shah, S.; Ciardelli, G.; Viebahn, R.; Chiono, V.; Salber, J. Fundamental in vitro 3D human skin equivalent tool development for assessing biological safety and biocompatibility–towards alternative for animal experiments. 4open 2021, 4, 1. [Google Scholar] [CrossRef]
- Zhang, Z.; Michniak-Kohn, B.B. Tissue engineered human skin equivalents. Pharmaceutics 2012, 4, 26–41. [Google Scholar] [CrossRef]
- Oualla-Bachiri, W.; Fernández-González, A.; Quiñones-Vico, M.I.; Arias-Santiago, S. From grafts to human bioengineered vascularized skin substitutes. Int. J. Mol. Sci. 2020, 21, 8197. [Google Scholar] [CrossRef] [PubMed]
- Schäfer-Korting, M.; Bock, U.; Diembeck, W.; Düsing, H.J.; Gamer, A.; Haltner-Ukomadu, E.; Hoffmann, C.; Kaca, M.; Kamp, H.; Kersen, S.; et al. The use of reconstructed human epidermis for skin absorption testing: Results of the validation study. Altern. Lab. Anim. 2008, 36, 161–187. [Google Scholar] [PubMed]
- Alépée, N.; Tornier, C.; Robert, C.; Amsellem, C.; Roux, M.H.; Doucet, O.; Pachot, J.; Méloni, M.; de Fraissinette, A.D.B. A catch-up validation study on reconstructed human epidermis (SkinEthic™ RHE) for full replacement of the Draize skin irritation test. Toxicol. Vitr. 2010, 24, 257–266. [Google Scholar] [CrossRef]
- Faller, C.; Bracher, M.; Dami, N.; Roguet, R. Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics. Toxicol. Vitr. 2002, 16, 557–572. [Google Scholar]
- Müller, G.; Langer, J.; Siebert, J.; Kramer, A. Residual antimicrobial effect of chlorhexidine digluconate and octenidine dihydrochloride on reconstructed human epidermis. Skin Pharmacol. Physiol. 2013, 27, 1–8. [Google Scholar] [PubMed]
- Chaudhuri, R.K.; Bojanowski, K. Bakuchiol: A retinol-like functional compound revealed by gene expression profiling and clinically proven to have anti-aging effects. Int. J. Cosmet. Sci. 2014, 36, 221–230. [Google Scholar] [CrossRef]
- Rohrbeck, A.; Bruhn, V.A.; Hussein, N.; Hagemann, S.; Just, I. Clostridium botulinum C3bot mediated effects on cytokine-induced psoriasis-like phenotype in full-thickness skin model. Naunyn Schmiedebergs Arch. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Mieremet, A.; van Dijk, R.; Boiten, W.; Gooris, G.; Bouwstra, J.A.; El Ghalbzouri, A. Characterization of human skin equivalents developed at body’s core and surface temperatures. J. Tissue Eng. Regen. Med. 2019, 13, 1122–1133. [Google Scholar] [CrossRef]
- Randall, M.J.; Jüngel, A.; Rimann, M.; Wuertz-Kozak, K. Advances in the Biofabrication of 3D Skin in vitro: Healthy and Pathological Models. Front. Bioeng. Biotechnol. 2018, 6, 154. [Google Scholar] [CrossRef]
- Zhuang, P.; Chiang, Y.H.; Fernanda, M.S.; He, M. Using spheroids as building blocks towards 3D bioprinting of tumor microenvironment. Int. J. Bioprint. 2021, 7, 444. [Google Scholar]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a type of three-dimensional cell cultures—Examples of methods of preparation and the most important application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef]
- Lewis, N.S.; Lewis, E.E.; Mullin, M.; Wheadon, H.; Dalby, M.J.; Berry, C.C. Magnetically levitated mesenchymal stem cell spheroids cultured with a collagen gel maintain phenotype and quiescence. J. Tissue Eng. 2017, 8, 1–11. [Google Scholar]
- Elliott, N.T.; Yuan, F.A.N. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J. Pharm. Sci. 2011, 100, 59–74. [Google Scholar] [CrossRef]
- Pampaloni, F.; Stelzer, E.H. Three-dimensional cell cultures in toxicology. Biotechnol. Genet. Eng. Rev. 2009, 26, 117–138. [Google Scholar] [CrossRef]
- Aiello, G.; Rescigno, F.; Meloni, M.; Zoanni, B.; Aldini, G.; Carini, M.; D’Amato, A. The Effect of Carnosine on UVA-Induced Changes in Intracellular Signaling of Human Skin Fibroblast Spheroids. Antioxidants 2023, 12, 300. [Google Scholar] [CrossRef]
- Lee, W.J.; Choi, I.K.; Lee, J.H.; Kim, Y.O.; Yun, C.O. A novel three-dimensional model system for keloid study: Organotypic multicellular scar model. Wound Repair Regen. 2013, 21, 155–165. [Google Scholar]
- Vörsmann, H.; Groeber, F.; Walles, H.; Busch, S.; Beissert, S.; Walczak, H.; Kulms, D. Development of a human three-dimensional organotypic skin-melanoma spheroid model for in vitro drug testing. Cell Death Dis. 2013, 4, e719. [Google Scholar]
- Velasco, V.; Shariati, S.A.; Esfandyarpour, R. Microtechnology-based methods for organoid models. Microsyst. Nanoeng. 2020, 6, 76. [Google Scholar]
- Sun, H.; Zhang, Y.X.; Li, Y.M. Generation of skin organoids: Potential opportunities and challenges. Front. Cell Dev. Biol. 2021, 9, 3176. [Google Scholar] [CrossRef]
- Prasad, M.; Kumar, R.; Buragohain, L.; Kumari, A.; Ghosh, M. Organoid technology: A reliable developmental biology tool for organ-specific nanotoxicity evaluation. Front. Cell Dev. Biol. 2021, 9, 696668. [Google Scholar] [CrossRef]
- Hong, Z.X.; Zhu, S.T.; Li, H.; Luo, J.Z.; Yang, Y.; An, Y.; Wang, X.; Wang, K. Bioengineered skin organoids: From development to applications. Mil. Med. Res. 2023, 10, 40. [Google Scholar] [CrossRef]
- Lee, J.; van der Valk, W.H.; Serdy, S.A.; Deakin, C.; Kim, J.; Le, A.P.; Koehler, K.R. Generation and characterization of hair-bearing skin organoids from human pluripotent stem cells. Nat. Protoc. 2022, 17, 1266–1305. [Google Scholar] [CrossRef]
- Lee, J.; Koehler, K.R. Skin organoids: A new human model for developmental and translational research. Exp. Dermatol. 2021, 30, 613–620. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef]
- Oceguera-Yanez, F.; Avila-Robinson, A.; Woltjen, K. Differentiation of pluripotent stem cells for modeling human skin development and potential applications. Front. Cell Dev. Biol. 2022, 10, 1030339. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Gao, D.; Li, X.; Zhang, Q.; Lv, L.; Wang, Y.; Li, J.; Zhu, Y.; Wu, Z.; et al. Establishment of human pluripotent stem cell-derived skin organoids enabled pathophysiological model of SARS-CoV-2 infection. Adv. Sci. 2022, 9, 2104192. [Google Scholar] [CrossRef]
- Jung, S.Y.; You, H.J.; Kim, M.J.; Ko, G.; Lee, S.; Kang, K.S. Wnt-activating human skin organoid model of atopic dermatitis induced by Staphylococcus aureus and its protective effects by Cutibacterium acnes. iScience 2022, 25, 105150. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.; Vu, K.P.; London, N.R., Jr. Organoid and spheroid tumor models: Techniques and applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Risueño, I.; Valencia, L.; Jorcano, J.L.; Velasco, D. Skin-on-a-chip models: General overview and future perspectives. APL Bioeng. 2021, 5, 030901. [Google Scholar] [CrossRef]
- Song, H.J.; Lim, H.Y.; Chun, W.; Choi, K.C.; Lee, T.Y.; Sung, J.H.; Sung, G.Y. Development of 3D skin-equivalent in a pump-less microfluidic chip. J. Ind. Eng. Chem. 2018, 60, 355–359. [Google Scholar] [CrossRef]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.; Feng, Z.; Ramasamy, S.; Bigliardi, P.L.; Bigliardi-Qi, M.; Wang, Z. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2018, 21, 326–340. [Google Scholar] [CrossRef]
- Lee, S.; Jin, S.P.; Kim, Y.K.; Sung, G.Y.; Chung, J.H.; Sung, J.H. Construction of 3D multicellular microfluidic chip for an in vitro skin model. Biomed. Microdevices 2017, 19, 22. [Google Scholar]
- Kim, K.; Kim, H.; Sung, G.Y. An interleukin-4 and interleukin-13 induced atopic dermatitis human skin equivalent model by a skin-on-a-chip. Int. J. Mol. Sci. 2022, 23, 2116. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.; Kim, H.; Sung, G.Y. Effect of α-lipoic acid on the development of human skin equivalents using a pumpless skin-on-a-chip model. Int. J. Mol. Sci. 2021, 22, 2160. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Sung, G.Y. Coenzyme Q10 efficacy test for human skin equivalents using a pumpless skin-on-a-chip system. Int. J. Mol. Sci. 2020, 21, 8475. [Google Scholar] [CrossRef]
- Sutterby, E.; Thurgood, P.; Baratchi, S.; Khoshmanesh, K.; Pirogova, E. Microfluidic skin-on-a-chip models: Toward biomimetic artificial skin. Small 2020, 16, 2002515. [Google Scholar]
- Abaci, H.E.; Gledhill, K.; Guo, Z.; Christiano, A.M.; Shuler, M.L. Pumpless microfluidic platform for drug testing on human skin equivalents. Lab Chip 2015, 15, 882–888. [Google Scholar] [CrossRef]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017, 116, 48–56. [Google Scholar]
- Vahav, I.; Thon, M.; van den Broek, L.J.; Spiekstra, S.W.; Atac, B.; Lindner, G.; Schimek, K.; Marx, U.; Gibbs, S. Proof-of-Concept Organ-on-Chip Study: Topical Cinnamaldehyde Exposure of Reconstructed Human Skin with Integrated Neopapillae Cultured under Dynamic Flow. Pharmaceutics 2022, 14, 1529. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Z.; Zhang, M.; Song, F.; Feng, C.; Liu, H. Simple and robust 3D bioprinting of full-thickness human skin tissue. Bioengineered 2022, 13, 10090–10100. [Google Scholar]
- Ng, W.L.; Qi, J.T.Z.; Yeong, W.Y.; Naing, M.W. Proof-of-concept: 3D bioprinting of pigmented human skin constructs. Biofabrication 2018, 10, 025005. [Google Scholar] [CrossRef]
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D.K. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080. [Google Scholar]
- Yang, Y.; Xu, R.; Wang, C.; Guo, Y.; Sun, W.; Ouyang, L. Recombinant human collagen-based bioinks for the 3D bioprinting of full-thickness human skin equivalent. Int. J. Bioprinting 2022, 8, 611. [Google Scholar] [CrossRef]
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C.; et al. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574. [Google Scholar] [CrossRef]
- Tarassoli, S.P.; Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Whitaker, S.; Doak, S.; Whitaker, I.S. Skin tissue engineering using 3D bioprinting: An evolving research field. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 615–623. [Google Scholar]
- Baltazar, T.; Jiang, B.; Moncayo, A.; Merola, J.; Albanna, M.Z.; Saltzman, W.M.; Pober, J.S. 3D bioprinting of an implantable xeno-free vascularized human skin graft. Bioeng. Transl. Med. 2023, 8, e10324. [Google Scholar]
- Liu, X.; Michael, S.; Bharti, K.; Ferrer, M.; Song, M.J. A biofabricated vascularized skin model of atopic dermatitis for preclinical studies. Biofabrication 2020, 12, 035002. [Google Scholar] [CrossRef]
- Olejnik, A.; Semba, J.A.; Kulpa, A.; Danczak-Pazdrowska, A.; Rybka, J.D.; Gornowicz-Porowska, J. 3D bioprinting in skin related research: Recent achievements and application perspectives. ACS Synth. Biol. 2021, 11, 26–38. [Google Scholar] [CrossRef]
- Millás, A.; Lago, J.; Vasquez-Pinto, L.; Massaguer, P.; Maria-Engler, S.S. Approaches to the development of 3d bioprinted skin models: The case of natura cosmetics. Int. J. Adv. Med. Biotechnol. 2019, 2, 3–13. [Google Scholar] [CrossRef]
- Itoh, T.; Xia, J.; Magavi, R.; Nishihata, T.; Rytting, J.H. Use of shed snake skin as a model membrane for in vitro percutaneous penetration studies: Comparison with human skin. Pharm. Res. 1990, 7, 1042–1047. [Google Scholar]
- Haigh, J.M.; Beyssac, E.; Aiache, J.-M. Can shed snake skin be considered to be a ‘model’ membrane for human stratum corneum? In Proceedings of the 2nd World Meeting APGI/APV, Paris, France, 25–28 May 1998; pp. 1041–1042. [Google Scholar]
- Rigg, P.C.; Barry, B.W. Shed snake skin and hairless mouse skin as model membranes for human skin during permeation studies. J. Investig. Dermatol. 1990, 94, 235–240. [Google Scholar] [CrossRef]
- Pongjanyakul, T.; Prakongpan, S.; Panomsuk, A.; Puttipipatkhachorn, S.; Priprem, A. Shed king cobra and cobra skins as model membranes for in-vitro nicotine permeation studies. J. Pharm. Pharmacol. 2002, 54, 1345–1350. [Google Scholar] [CrossRef]
- Corzo-León, D.E.; Munro, C.A.; MacCallum, D.M. An ex vivo human skin model to study superficial fungal infections. Front. Microbiol. 2019, 10, 1172. [Google Scholar]
- Cappellozza, E.; Zanzoni, S.; Malatesta, M.; Calderan, L. Integrated microscopy and metabolomics to test an innovative fluid dynamic system for skin explants in vitro. Microsc. Microanal. 2021, 27, 923–934. [Google Scholar] [CrossRef]
- Cappellozza, E.; Boschi, F.; Sguizzato, M.; Esposito, E.; Cortesi, R.; Malatesta, M.; Calderan, L. A spectrofluorometric analysis to evaluate transcutaneous biodistribution of fluorescent nanoparticulate gel formulations. Eur. J. Histochem. 2022, 66, 3321. [Google Scholar]
- Herbig, M.E.; Houdek, P.; Gorissen, S.; Zorn-Kruppa, M.; Wladykowski, E.; Volksdorf, T.; Grzybowski, S.; Kolios, G.; Willers, C.; Mallwitz, H.; et al. A custom tailored model to investigate skin penetration in porcine skin and its comparison with human skin. Eur. J. Pharm. Biopharm. 2015, 95, 99–109. [Google Scholar]
- Quiñones, R.; Moreno, S.; Smythers, A.L.; Sullins, C.; Pijor, H.; Brown, G.; Trouten, A.; Richards-Waugh, L.L.; Siddig, A. Quantification of Cannabis in Infused Consumer Products and Their Residues on Skin. ACS Pharmacol. Transl. Sci. 2022, 5, 642–651. [Google Scholar]
- Nasiri, M.I.; Vora, L.K.; Ershaid, J.A.; Peng, K.; Tekko, I.A.; Donnelly, R.F. Nanoemulsion-based dissolving microneedle arrays for enhanced intradermal and transdermal delivery. Drug Deliv. Transl. Res. 2022, 12, 881–896. [Google Scholar] [CrossRef]
- Xiang, H.; Xu, S.; Zhang, W.; Li, Y.; Zhou, Y.; Miao, X. Skin permeation of curcumin nanocrystals: Effect of particle size, delivery vehicles, and permeation enhancer. Colloids Surf. B Biointerfaces 2023, 224, 113203. [Google Scholar]
- Eberlin, S.; Silva, M.S.D.; Facchini, G.; Silva, G.H.D.; Pinheiro, A.L.T.A.; Eberlin, S.; Pinheiro, A.D.S. The ex vivo skin model as an alternative tool for the efficacy and safety evaluation of topical products. Altern. Lab. Anim. 2020, 48, 10–22. [Google Scholar] [CrossRef]
- Esposito, E.; Calderan, L.; Galvan, A.; Cappellozza, E.; Drechsler, M.; Mariani, P.; Pepe, A.; Sguizzato, M.; Vigato, E.; Dalla Pozza, E.; et al. Ex Vivo Evaluation of Ethosomes and Transethosomes Applied on Human Skin: A Comparative Study. Int. J. Mol. Sci. 2022, 23, 15112. [Google Scholar]
- Sidgwick, G.P.; McGeorge, D.; Bayat, A. Functional testing of topical skin formulations using an optimised ex vivo skin organ culture model. Arch. Dermatol. Res. 2016, 308, 297–308. [Google Scholar] [CrossRef]
- Ameri, M.; Lewis, H.; Lehman, P. Effect of Skin Model on In Vitro Performance of an Adhesive Dermally Applied Microarray Coated with Zolmitriptan. J. Pharm. 2018, 2018, 7459124. [Google Scholar] [CrossRef]
- Neil, J.E.; Brown, M.B.; Williams, A.C. Human skin explant model for the investigation of topical therapeutics. Sci. Rep. 2020, 10, 21192. [Google Scholar]
- Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In vitro human skin penetration, antioxidant and antimicrobial activity of ethanol-water extract of fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. [Google Scholar]
- Neil, M.J.E.; Lenn, J.D.; Brown, M.B.; Williams, A.C. A new ex vivo skin model for mechanistic understanding of putative anti-inflammatory topical therapeutics. Int. J. Pharm. 2022, 617, 121610. [Google Scholar] [CrossRef]
- Galvan, A.; Cappellozza, E.; Pellequer, Y.; Conti, A.; Pozza, E.D.; Vigato, E.; Malatesta, M.; Calderan, L. An Innovative Fluid Dynamic System to Model Inflammation in Human Skin Explants. Int. J. Mol. Sci. 2023, 24, 6284. [Google Scholar] [CrossRef]
- Tiirikainen, M.L.; Woetmann, A.; Norsgaard, H.; Santamaria-Babí, L.F.; Lovato, P. Ex vivo culture of lesional psoriasis skin for pharmacological testing. J. Dermatol. Sci. 2020, 97, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Fink, J.; Eberl, A.; Prugger, E.M.; Kolb, D.; Luze, H.; Schwingenschuh, S.; Birngruber, T.; Magnes, C.; Mautner, S.I.; et al. A novel human ex vivo skin model to study early local responses to burn injuries. Sci. Rep. 2021, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Yoshida, C.M.; Leonardi, G.R.; Cano, A.; Sanchez-Lopez, E.; Zielinska, A.; Viseras, C.; Severino, P.; Silva, C.F.D.; Barbosa, R.D.M. Lipid-polymeric films: Composition, production and applications in wound healing and skin repair. Pharmaceutics 2021, 13, 1199. [Google Scholar]
- De Luca, C.; Valacchi, G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediat. Inflamm. 2010, 2010, 321494. [Google Scholar] [CrossRef]
- Eyerich, S.; Eyerich, K.; Traidl-Hoffmann, C.; Biedermann, T. Cutaneous barriers and skin immunity: Differentiating a connected network. Trends Immunol. 2018, 39, 315–327. [Google Scholar]
- Niyonsaba, F.; Nagaoka, I.; Ogawa, H. Human defensins and cathelicidins in the skin: Beyond direct antimicrobial properties. Crit. Rev. Immunol. 2006, 26, 545–576. [Google Scholar]
- Niyonsaba, F.; Kiatsurayanon, C.; Chieosilapatham, P.; Ogawa, H. Friends or Foes? Host defense (antimicrobial) peptides and proteins in human skin diseases. Exp. Dermatol. 2017, 26, 989–998. [Google Scholar] [CrossRef]
- Van Smeden, J.; Bouwstra, J.A. Stratum corneum lipids: Their role for the skin barrier function in healthy subjects and atopic dermatitis patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Brandwein, M.; Bentwich, Z.; Steinberg, D. Endogenous antimicrobial peptide expression in response to bacterial epidermal colonization. Front. Immunol. 2017, 8, 1637. [Google Scholar] [PubMed]
- Ohnemus, U.; Kohrmeyer, K.; Houdek, P.; Rohde, H.; Wladykowski, E.; Vidal, S.; Horstkotte, M.A.; Aepfelbacher, M.; Kirschner, N.; Behne, M.J.; et al. Regulation of epidermal tight-junctions (TJ) during infection with exfoliative toxin-negative Staphylococcus strains. J. Investig. Dermatol. 2008, 128, 906–916. [Google Scholar]
- Lee, H.J.; Kim, M. Skin barrier function and the microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Belkaid, Y.; Segre, J.A. Dialogue between skin microbiota and immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Grice, E.A. The skin microbiome: A focus on pathogens and their association with skin disease. PLoS Pathog. 2014, 10, e1004436. [Google Scholar] [CrossRef]
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Sáez-de-Ocariz, M. The human skin microbiome in selected cutaneous diseases. Front. Cell Infect. Microbiol. 2022, 12, 834135. [Google Scholar]
- Niehues, H.; Bouwstra, J.A.; El Ghalbzouri, A.; Brandner, J.M.; Zeeuwen, P.L.; van den Bogaard, E.H. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp. Dermatol. 2018, 27, 501–511. [Google Scholar] [CrossRef]
- Rademacher, F.; Simanski, M.; Gläser, R.; Harder, J. Skin microbiota and human 3D skin models. Exp. Dermatol. 2018, 27, 489–494. [Google Scholar]
- Bojar, R.A. Studying the human skin microbiome using 3D in vitro skin models. Appl. Vitr. Toxicol. 2015, 1, 165–171. [Google Scholar]
- Larson, P.J.; Chong, D.; Fleming, E.; Oh, J. Challenges in developing a human model system for skin microbiome research. J. Investig. Dermatol. 2021, 141, 228–231. [Google Scholar] [CrossRef]
- Emmert, H.; Rademacher, F.; Gläser, R.; Harder, J. Skin microbiota analysis in human 3D skin models—“Free your mice”. Exp. Dermatol. 2020, 29, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Janvier, X.; Alexandre, S.; Boukerb, A.M.; Souak, D.; Maillot, O.; Barreau, M.; Gouriou, F.; Grillon, C.; Feuilloley, M.G.J.; Groboillot, A. Deleterious effects of an air pollutant (NO2) on a selection of commensal skin bacterial strains, potential contributor to dysbiosis? Front. Microbiol. 2020, 11, 591839. [Google Scholar] [CrossRef] [PubMed]
- Loomis, K.H.; Wu, S.K.; Ernlund, A.; Zudock, K.; Reno, A.; Blount, K.; Karig, D.K. A mixed community of skin microbiome representatives influences cutaneous processes more than individual members. Microbiome 2021, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Landemaine, L.; Cenizo, V.; Lemaire, G.; Portes, P. 961 Colonization of a 3D skin model with a complete microbiota is more beneficial to the skin barrier than with Staphylococcus epidermidis alone. J. Investig. Dermatol. 2018, 138, S163. [Google Scholar] [CrossRef]
- Fernandez-Carro, E.; Angenent, M.; Gracia-Cazaña, T.; Gilaberte, Y.; Alcaine, C.; Ciriza, J. Modeling an Optimal 3D Skin-on-Chip within Microfluidic Devices for Pharmacological Studies. Pharmaceutics 2022, 14, 1417. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Toh, Y.C. What can microfluidics do for human microbiome research? Biomicrofluidics 2020, 14, 051303. [Google Scholar]
- Van Der Krieken, D.A.; Ederveen, T.H.; van Hijum, S.A.; Jansen, P.A.; Melchers, W.J.; Scheepers, P.T.; Schalkwijk, J.; Zeeuwen, P.L. An in vitro model for bacterial growth on human stratum corneum. Acta Derm. Venereol. 2016, 96, 873–879. [Google Scholar]
- Van Belkum, A.; Lisotto, P.; Pirovano, W.; Mongiat, S.; Zorgani, A.; Gempeler, M.; Bongoni, R.; Klaassens, E. Being friendly to the skin microbiome: Experimental assessment. Front. Microbiomes 2023, 1, 1077151. [Google Scholar]
- Vitorino, C.; Sousa, J.; Pais, A. Overcoming the skin permeation barrier: Challenges and opportunities. Curr. Pharm. Des. 2015, 21, 2698–2712. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvan, A.; Pellicciari, C.; Calderan, L. Recreating Human Skin In Vitro: Should the Microbiota Be Taken into Account? Int. J. Mol. Sci. 2024, 25, 1165. https://doi.org/10.3390/ijms25021165
Galvan A, Pellicciari C, Calderan L. Recreating Human Skin In Vitro: Should the Microbiota Be Taken into Account? International Journal of Molecular Sciences. 2024; 25(2):1165. https://doi.org/10.3390/ijms25021165
Chicago/Turabian StyleGalvan, Andrea, Carlo Pellicciari, and Laura Calderan. 2024. "Recreating Human Skin In Vitro: Should the Microbiota Be Taken into Account?" International Journal of Molecular Sciences 25, no. 2: 1165. https://doi.org/10.3390/ijms25021165
APA StyleGalvan, A., Pellicciari, C., & Calderan, L. (2024). Recreating Human Skin In Vitro: Should the Microbiota Be Taken into Account? International Journal of Molecular Sciences, 25(2), 1165. https://doi.org/10.3390/ijms25021165







