A Cross-Sectional Study of Alzheimer-Related Proteins in Women with Polycystic Ovary Syndrome
Abstract
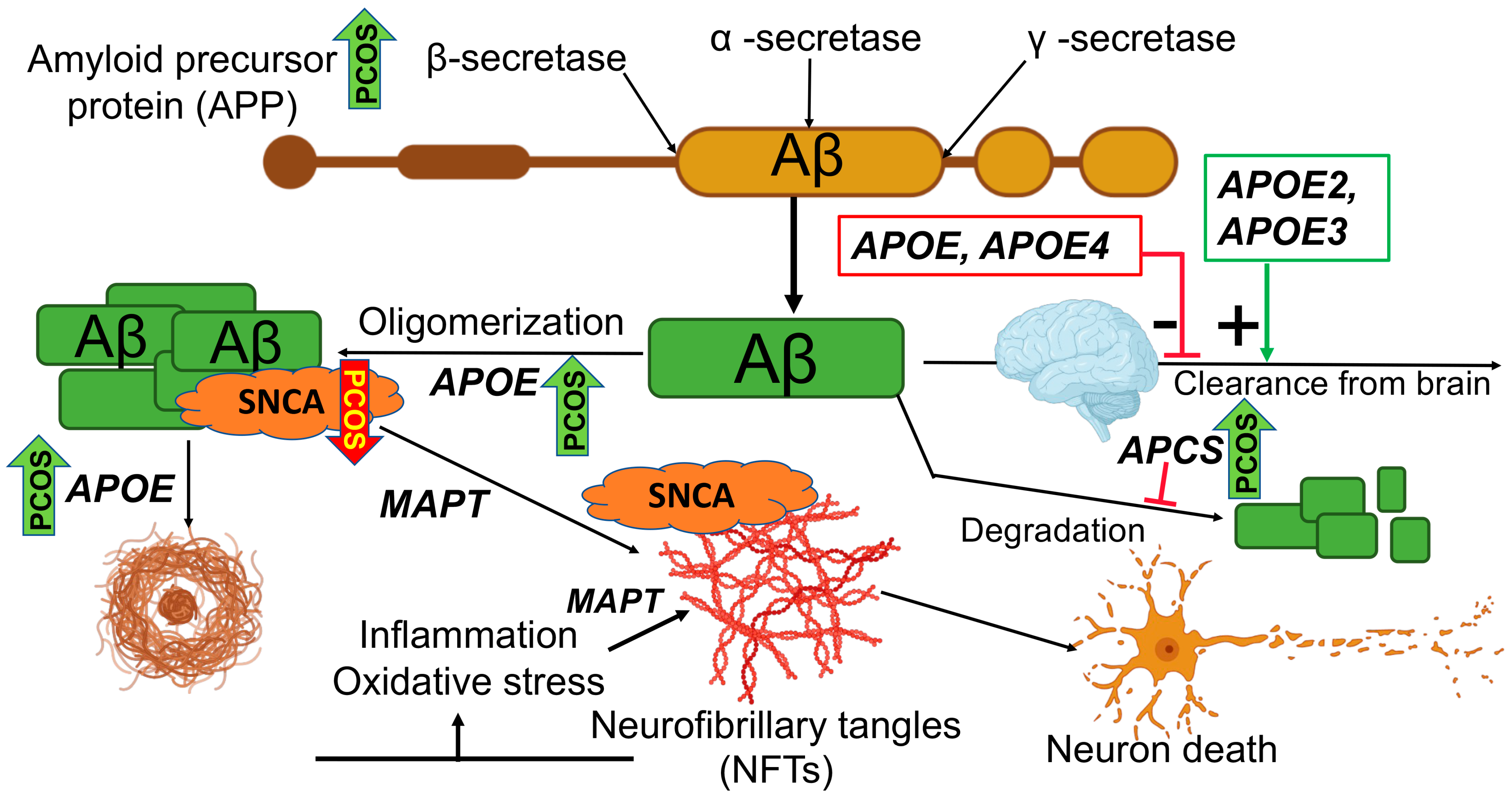
1. Introduction
2. Results
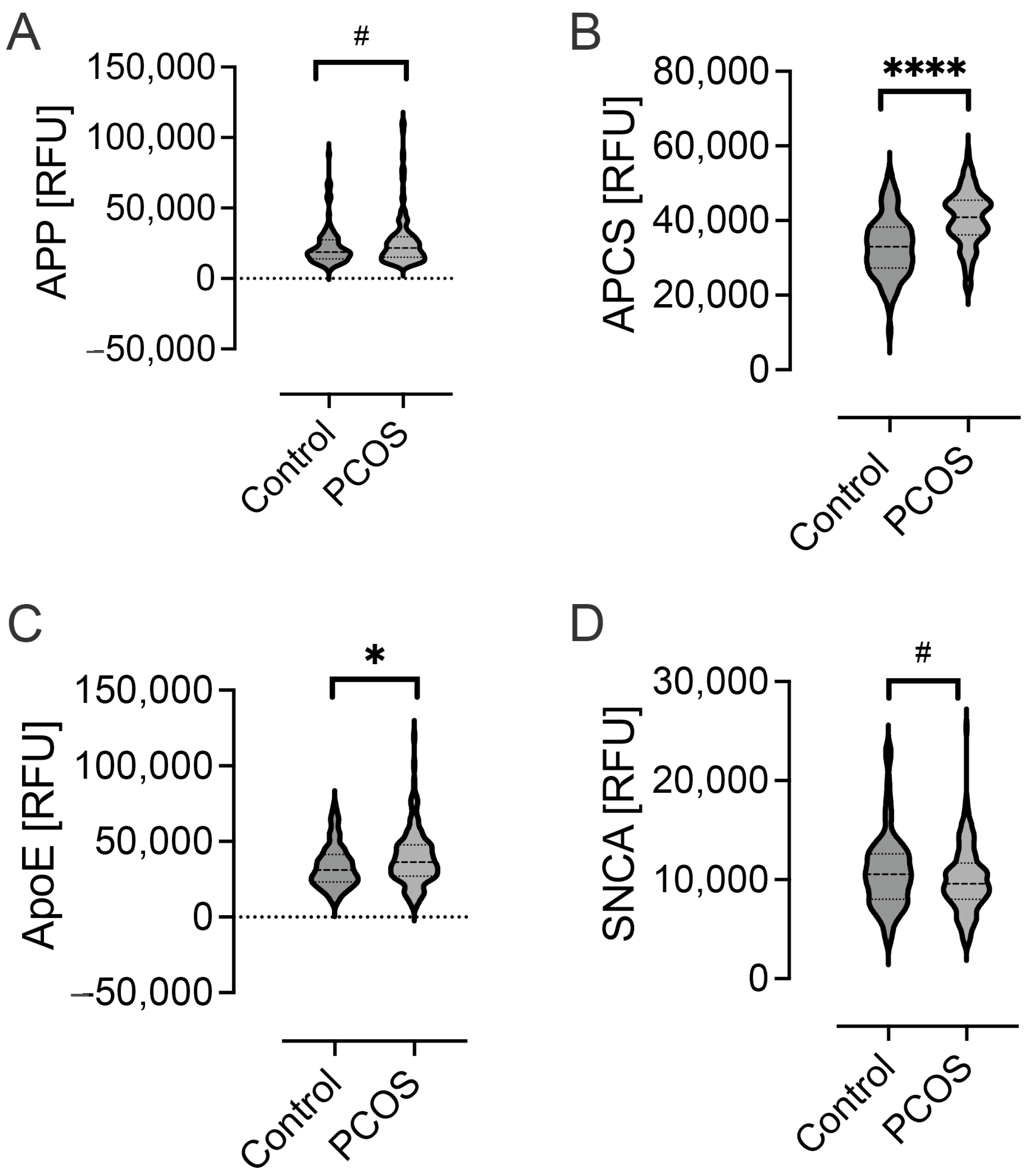
2.1. Levels of Alzheimer-Related Proteins in PCOS
2.2. Stratification of Hyperandrogenemia, Obesity, Insulin Resistance, Glucose and Age in the PCOS Population
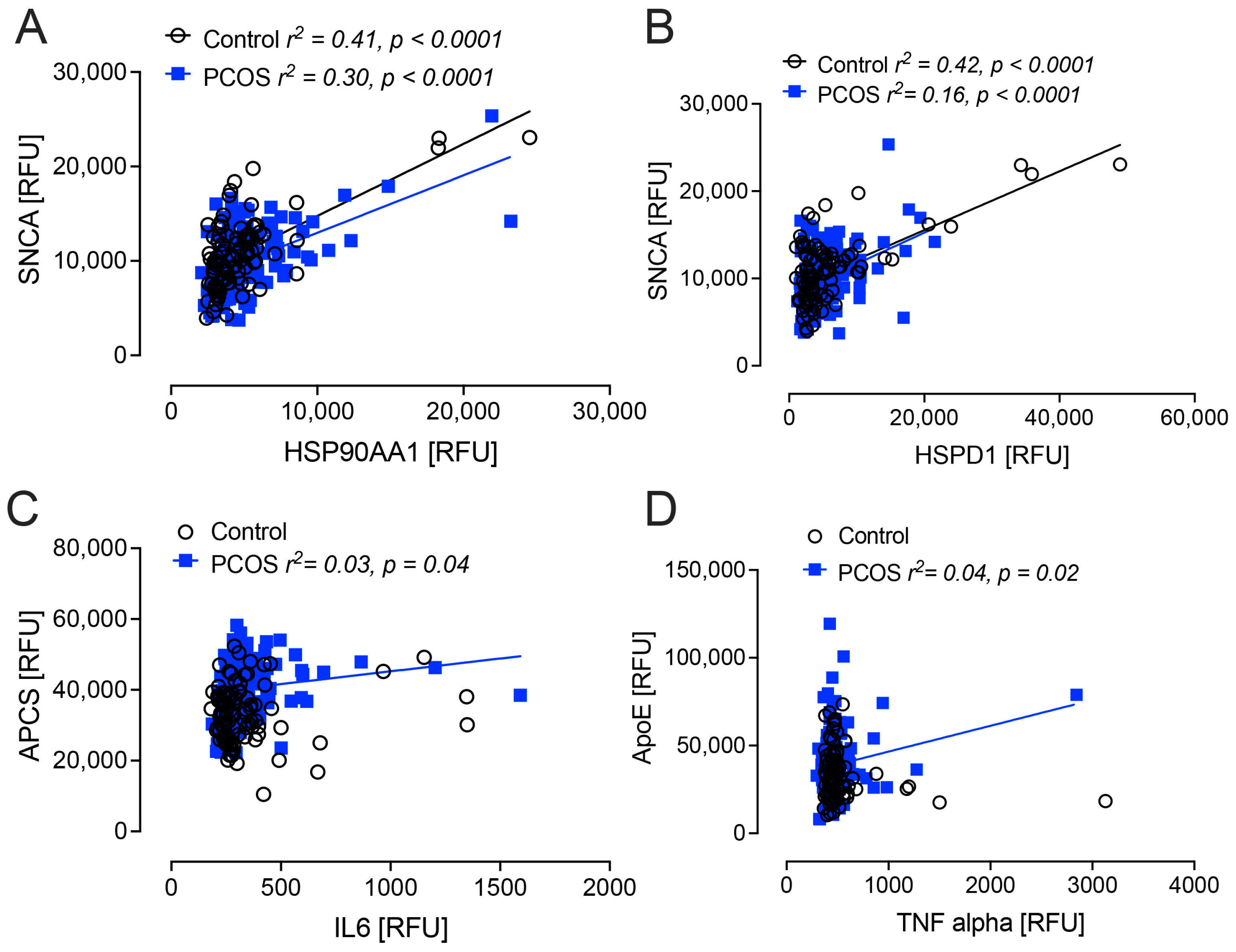
2.3. Correlation Analyses
3. Discussion
4. Materials and Methods
Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sathyapalan, T.; Atkin, S.L. Recent advances in cardiovascular aspects of polycystic ovary syndrome. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2012, 166, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Jabarpour, M.; Aleyasin, A.; Nashtaei, M.S.; Lotfi, S.; Amidi, F. Astaxanthin treatment ameliorates ER stress in polycystic ovary syndrome patients: A randomized clinical trial. Sci. Rep. 2023, 13, 3376. [Google Scholar] [CrossRef]
- Butler, A.E.; Moin, A.S.M.; Reiner, Ž.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A.; Atkin, S.L. HDL-Associated Proteins in Subjects with Polycystic Ovary Syndrome: A Proteomic Study. Cells 2023, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Boscaro, M.; Bordin, L.; Sabbadin, C. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. Int. J. Mol. Sci. 2022, 23, 4110. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutawa, J. Genetic contribution between APE1 variants in polycystic ovarian syndrome. Saudi J. Biol. Sci. 2023, 30, 103563. [Google Scholar] [CrossRef] [PubMed]
- Alshammary, A.F.; Alshammari, A.M.; Farzan, R.; Alsobaie, S.F.; Alageel, A.A.; Ali Khan, I. A study on the immunological vitality of an inflammatory biomarker explored with rs5743708 polymorphism in TLR2 gene among Saudi women confirmed with polycystic ovarian syndrome. Saudi J. Biol. Sci. 2023, 30, 103687. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Risal, S.; Jiang, H.; Lu, H.; Lindgren, E.; Stener-Victorin, E.; Deng, Q. Transcriptomic survey of key reproductive and metabolic tissues in mouse models of polycystic ovary syndrome. Commun. Biol. 2023, 6, 69. [Google Scholar] [CrossRef]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013, 9, 63–75 e62. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2014 Alzheimer’s disease facts and figures. Alzheimers Dement. 2014, 10, e47–e92. [Google Scholar]
- Ott, A.; Stolk, R.P.; van Harskamp, F.; Pols, H.A.; Hofman, A.; Breteler, M.M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999, 53, 1937–1942. [Google Scholar] [CrossRef]
- Sims-Robinson, C.; Kim, B.; Rosko, A.; Feldman, E.L. How does diabetes accelerate Alzheimer disease pathology? Nat. Rev. Neurol. 2010, 6, 551–559. [Google Scholar] [CrossRef]
- Zilkens, R.R.; Davis, W.A.; Spilsbury, K.; Semmens, J.B.; Bruce, D.G. Earlier age of dementia onset and shorter survival times in dementia patients with diabetes. Am. J. Epidemiol. 2013, 177, 1246–1254. [Google Scholar] [CrossRef]
- Kakoly, N.S.; Khomami, M.B.; Joham, A.E.; Cooray, S.D.; Misso, M.L.; Norman, R.J.; Harrison, C.L.; Ranasinha, S.; Teede, H.J.; Moran, L.J. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: A systematic review and meta-regression. Hum. Reprod. Update 2018, 24, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Twarowski, B.; Herbet, M. Inflammatory Processes in Alzheimer’s Disease-Pathomechanism, Diagnosis and Treatment: A Review. Int. J. Mol. Sci. 2023, 24, 6518. [Google Scholar] [CrossRef] [PubMed]
- Penke, B.; Bogár, F.; Fülöp, L. β-Amyloid and the Pathomechanisms of Alzheimer’s Disease: A Comprehensive View. Molecules 2017, 22, 1692. [Google Scholar] [CrossRef] [PubMed]
- Brutocao, C.; Zaiem, F.; Alsawas, M.; Morrow, A.S.; Murad, M.H.; Javed, A. Psychiatric disorders in women with polycystic ovary syndrome: A systematic review and meta-analysis. Endocrine 2018, 62, 318–325. [Google Scholar] [CrossRef]
- Sarahian, N.; Sarvazad, H.; Sajadi, E.; Rahnejat, N.; Eskandari Roozbahani, N. Investigation of common risk factors between polycystic ovary syndrome and Alzheimer’s disease: A narrative review. Reprod. Health 2021, 18, 156. [Google Scholar] [CrossRef]
- Barber, T.M.; Kyrou, I.; Randeva, H.S.; Weickert, M.O. Mechanisms of Insulin Resistance at the Crossroad of Obesity with Associated Metabolic Abnormalities and Cognitive Dysfunction. Int. J. Mol. Sci. 2021, 22, 546. [Google Scholar] [CrossRef]
- Li, G.; Hu, J.; Zhang, S.; Fan, W.; Wen, L.; Wang, G.; Zhang, D. Changes in Resting-State Cerebral Activity in Women with Polycystic Ovary Syndrome: A Functional MR Imaging Study. Front. Endocrinol. 2020, 11, 603279. [Google Scholar] [CrossRef]
- Lai, W.; Li, X.; Zhu, H.; Zhu, X.; Tan, H.; Feng, P.; Chen, L.; Luo, C. Plasma luteinizing hormone level affects the brain activity of patients with polycystic ovary syndrome. Psychoneuroendocrinology 2020, 112, 104535. [Google Scholar] [CrossRef]
- Schattmann, L.; Sherwin, B.B. Testosterone levels and cognitive functioning in women with polycystic ovary syndrome and in healthy young women. Horm. Behav. 2007, 51, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Macut, D.; Bjekić-Macut, J.; Rahelić, D.; Doknić, M. Insulin and the polycystic ovary syndrome. Diabetes Res. Clin. Pract. 2017, 130, 163–170. [Google Scholar] [CrossRef]
- Teunissen, C.; Del Campo, M.; Peeters, C.; Meeter, L.; Seelaar, H.; Koel-Simmelink, M.; Ramakers, I.; Middelkoop, H.; De Deyn, P.; Claessen, J. Blood-based protein biomarkers in definite frontotemporal dementia: A case-control study. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Moin, A.S.M.; Al-Qaissi, A.; Sathyapalan, T.; Atkin, S.L.; Butler, A.E. Hypoglycemia in Type 2 Diabetes exacerbates Amyloid-related proteins associated with dementia. Diabetes Obes. Metab. 2020, 23, 338–349. [Google Scholar] [CrossRef]
- Martins, R.N.; Muir, J.; Brooks, W.S.; Creasey, H.; Montgomery, P.; Sellers, P.; Broe, G.A. Plasma amyloid precursor protein is decreased in Alzheimer’s disease. Neuroreport 1993, 4, 757–759. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, F.; Kac, P.R.; Brum, W.S.; Zetterberg, H.; Blennow, K.; Karikari, T.K. Plasma phospho-tau in Alzheimer’s disease: Towards diagnostic and therapeutic trial applications. Mol. Neurodegener. 2023, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, E.; Iwamoto, N.; Kimura, M.; Arai, H. Serum amyloid P component level in Alzheimer’s disease. Dementia 1996, 7, 256–259. [Google Scholar] [CrossRef]
- Twohig, D.; Nielsen, H.M. α-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 23. [Google Scholar] [CrossRef]
- Jellinger, K.A. Alpha-synuclein pathology in Parkinson’s and Alzheimer’s disease brain: Incidence and topographic distribution—A pilot study. Acta Neuropathol. 2003, 106, 191–201. [Google Scholar] [CrossRef]
- Lv, S.; Zhou, X.; Li, Y.; Zhang, S.; Wang, Y.; Jia, S.; Niu, X.; Wang, L.; Peng, D. The Association between Plasma α-Synuclein (α-syn) Protein, Urinary Alzheimer-Associated Neuronal Thread Protein (AD7c-NTP), and Apolipoprotein Epsilon 4 (ApoE ε4) Alleles and Cognitive Decline in 60 Patients with Alzheimer’s Disease Compared with 28 Age-Matched Normal Individuals. Med. Sci. Monit. 2021, 27, e932998. [Google Scholar]
- Mihelcic, M.; Simic, G.; Babic Leko, M.; Lavrac, N.; Dzeroski, S.; Smuc, T.; Alzheimer’s Disease Neuroimaging Initiative. Using redescription mining to relate clinical and biological characteristics of cognitively impaired and Alzheimer’s disease patients. PLoS ONE 2017, 12, e0187364. [Google Scholar] [CrossRef]
- Irizarry, M.C. Biomarkers of Alzheimer disease in plasma. NeuroRx 2004, 1, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Taddei, K.; Clarnette, R.; Gandy, S.E.; Martins, R.N. Increased plasma apolipoprotein E (apoE) levels in Alzheimer’s disease. Neurosci. Lett. 1997, 223, 29–32. [Google Scholar] [CrossRef]
- Siest, G.; Bertrand, P.; Qin, B.; Herbeth, B.; Serot, J.M.; Masana, L.; Ribalta, J.; Passmore, A.P.; Evans, A.; Ferrari, M.; et al. Apolipoprotein E polymorphism and serum concentration in Alzheimer’s disease in nine European centres: The ApoEurope study. ApoEurope group. Clin. Chem. Lab. Med. 2000, 38, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Scacchi, R.; Gambina, G.; Ruggeri, M.; Martini, M.C.; Ferrari, G.; Silvestri, M.; Schiavon, R.; Corbo, R.M. Plasma levels of apolipoprotein E and genetic markers in elderly patients with Alzheimer’s disease. Neurosci. Lett. 1999, 259, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Urieli-Shoval, S.; Linke, R.P.; Matzner, Y. Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr. Opin. Hematol. 2000, 7, 64–69. [Google Scholar] [CrossRef]
- Do Carmo, S.; Kannel, B.; Cuello, A.C. The Nerve Growth Factor Metabolic Pathway Dysregulation as Cause of Alzheimer’s Cholinergic Atrophy. Cells 2021, 11, 16. [Google Scholar] [CrossRef]
- Shir, D.; Graff-Radford, J.; Hofrenning, E.I.; Lesnick, T.G.; Przybelski, S.A.; Lowe, V.J.; Knopman, D.S.; Petersen, R.C.; Jack, C.R., Jr.; Vemuri, P.; et al. Association of plasma glial fibrillary acidic protein (GFAP) with neuroimaging of Alzheimer’s disease and vascular pathology. Alzheimers Dement. 2022, 14, e12291. [Google Scholar] [CrossRef]
- Díaz-Moreno, M.; Armenteros, T.; Gradari, S.; Hortigüela, R.; García-Corzo, L.; Fontán-Lozano, Á.; Trejo, J.L.; Mira, H. Noggin rescues age-related stem cell loss in the brain of senescent mice with neurodegenerative pathology. Proc. Natl. Acad. Sci. USA 2018, 115, 11625–11630. [Google Scholar] [CrossRef]
- Baldacci, F.; Daniele, S.; Piccarducci, R.; Giampietri, L.; Pietrobono, D.; Giorgi, F.S.; Nicoletti, V.; Frosini, D.; Libertini, P.; Lo Gerfo, A.; et al. Potential Diagnostic Value of Red Blood Cells alpha-Synuclein Heteroaggregates in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6451–6459. [Google Scholar] [CrossRef]
- Yun, S.M.; Cho, S.J.; Jo, C.; Park, M.H.; Han, C.; Koh, Y.H. Elevation of plasma soluble amyloid precursor protein beta in Alzheimer’s disease. Arch. Gerontol. Geriatr. 2020, 87, 103995. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I.; Beyreuther, K.; Masters, C.L. Beta A4 amyloid protein and its precursor in Alzheimer’s disease. Pharmacol. Ther. 1992, 56, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Nakamura, T.; Kawarabayashi, T.; Hirohata, M.; Narita, S.; Wakasaya, Y.; Kaito, K.; Ueda, T.; Harigaya, Y.; Shoji, M. Cerebrospinal Fluid and Plasma Biomarkers in Neurodegenerative Diseases. J. Alzheimers Dis. 2019, 68, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Marriott, R.J.; Murray, K.; Flicker, L.; Hankey, G.J.; Matsumoto, A.M.; Dwivedi, G.; Antonio, L.; Almeida, O.P.; Bhasin, S.; Dobs, A.S.; et al. Lower serum testosterone concentrations are associated with a higher incidence of dementia in men: The UK Biobank prospective cohort study. Alzheimers Dement. 2022, 18, 1907–1918. [Google Scholar] [CrossRef]
- Yeung, C.H.C.; Au Yeung, S.L.; Kwok, M.K.; Zhao, J.V.; Schooling, C.M. The influence of growth and sex hormones on risk of alzheimer’s disease: A mendelian randomization study. Eur. J. Epidemiol. 2023, 38, 745–755. [Google Scholar] [CrossRef]
- Cho, L.W.; Jayagopal, V.; Kilpatrick, E.S.; Atkin, S.L. The mean and the biological variation of insulin resistance does not differ between polycystic ovary syndrome and type 2 diabetes. Ann. Clin. Biochem. 2009, 46, 218–221. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Szablewski, L. Insulin and Insulin Resistance in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9987. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Rahmani, F.; Wang, Q.; McKay, N.S.; Keefe, S.; Hantler, N.; Hornbeck, R.; Wang, Y.; Hassenstab, J.; Schindler, S.; Xiong, C.; et al. Sex-Specific Patterns of Body Mass Index Relationship with White Matter Connectivity. J. Alzheimers Dis. 2022, 86, 1831–1848. [Google Scholar] [CrossRef]
- Pennings, N.; Jaber, J.; Ahiawodzi, P. Ten-year weight gain is associated with elevated fasting insulin levels and precedes glucose elevation. Diabetes/Metab. Res. Rev. 2018, 34, e2986. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Tong, M.; Hang, S.; Deochand, C.; de la Monte, S. CSF and Brain Indices of Insulin Resistance, Oxidative Stress and Neuro-Inflammation in Early versus Late Alzheimer’s Disease. J. Alzheimers Dis. Park. 2013, 3, 128. [Google Scholar]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef] [PubMed]
- Bahmanyar, S.; Higgins, G.A.; Goldgaber, D.; Lewis, D.A.; Morrison, J.H.; Wilson, M.C.; Shankar, S.K.; Gajdusek, D.C. Localization of amyloid beta protein messenger RNA in brains from patients with Alzheimer’s disease. Science 1987, 237, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M. Neuronal localization of amyloid beta protein precursor mRNA in normal human brain and in Alzheimer’s disease. EMBO J. 1987, 6, 3627–3632. [Google Scholar] [CrossRef]
- Jayaraman, S.; Gantz, D.L.; Haupt, C.; Gursky, O. Serum amyloid A forms stable oligomers that disrupt vesicles at lysosomal pH and contribute to the pathogenesis of reactive amyloidosis. Proc. Natl. Acad. Sci. USA 2017, 114, E6507–E6515. [Google Scholar] [CrossRef]
- Campioni, S.; Mannini, B.; Zampagni, M.; Pensalfini, A.; Parrini, C.; Evangelisti, E.; Relini, A.; Stefani, M.; Dobson, C.M.; Cecchi, C.; et al. A causative link between the structure of aberrant protein oligomers and their toxicity. Nat. Chem. Biol. 2010, 6, 140–147. [Google Scholar] [CrossRef]
- Stefani, M. Structural features and cytotoxicity of amyloid oligomers: Implications in Alzheimer’s disease and other diseases with amyloid deposits. Prog. Neurobiol. 2012, 99, 226–245. [Google Scholar] [CrossRef]
- Guerrero-Munoz, M.J.; Castillo-Carranza, D.L.; Kayed, R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem. Pharmacol. 2014, 88, 468–478. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Verwey, N.A.; Schuitemaker, A.; van der Flier, W.M.; Mulder, S.D.; Mulder, C.; Hack, C.E.; Scheltens, P.; Blankenstein, M.A.; Veerhuis, R. Serum amyloid p component as a biomarker in mild cognitive impairment and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008, 26, 522–527. [Google Scholar] [CrossRef]
- Norman, R.J.; Teede, H.J. A new evidence-based guideline for assessment and management of polycystic ovary syndrome. Med. J. Aust. 2018, 209, 299–300. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, S.H. Effectiveness of Lifestyle Modification in Polycystic Ovary Syndrome Patients with Obesity: A Systematic Review and Meta-Analysis. Life 2022, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Ee, C.; Pirotta, S.; Mousa, A.; Moran, L.; Lim, S. Providing lifestyle advice to women with PCOS: An overview of practical issues affecting success. BMC Endocr. Disord. 2021, 21, 234. [Google Scholar] [CrossRef]
- Hu, L.; Ma, L.; Xia, X.; Ying, T.; Zhou, M.; Zou, S.; Yu, H.; Yin, J. Efficacy of Bariatric Surgery in the Treatment of Women with Obesity and Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, e3217–e3229. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, G.; Gainder, S.; Suri, V.; Sachdeva, N.; Chopra, S. Comparison of the Different PCOS Phenotypes Based on Clinical Metabolic, and Hormonal Profile, and their Response to Clomiphene. Indian J. Endocrinol. Metab. 2019, 23, 326–331. [Google Scholar] [PubMed]
- Sathyapalan, T.; Al-Qaissi, A.; Kilpatrick, E.S.; Dargham, S.R.; Atkin, S.L. Anti-Mullerian hormone measurement for the diagnosis of polycystic ovary syndrome. Clin. Endocrinol. 2018, 88, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- World Health Organization. Waist Circumference and Waist–Hip Ratio; Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Cunningham, T.K.; Allgar, V.; Dargham, S.R.; Kilpatrick, E.; Sathyapalan, T.; Maguiness, S.; Mokhtar Rudin, H.R.; Abdul Ghani, N.M.; Latiff, A.; Atkin, S.L. Association of Vitamin D Metabolites with Embryo Development and Fertilization in Women with and without PCOS Undergoing Subfertility Treatment. Front. Endocrinol. 2019, 10, 13. [Google Scholar] [CrossRef]
- Kahal, H.; Halama, A.; Aburima, A.; Bhagwat, A.M.; Butler, A.E.; Grauman, J.; Suhre, K.; Sathyapalan, T.; Atkin, S.L. Effect of induced hypoglycemia on inflammation and oxidative stress in type 2 diabetes and control subjects. Sci. Rep. 2020, 10, 4750. [Google Scholar] [CrossRef]
- Kraemer, S.; Vaught, J.D.; Bock, C.; Gold, L.; Katilius, E.; Keeney, T.R.; Kim, N.; Saccomano, N.A.; Wilcox, S.K.; Zichi, D.; et al. From SOMAmer-based biomarker discovery to diagnostic and clinical applications: A SOMAmer-based, streamlined multiplex proteomic assay. PLoS ONE 2011, 6, e26332. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K.; Arnold, M.; Bhagwat, A.M.; Cotton, R.J.; Engelke, R.; Raffler, J.; Sarwath, H.; Thareja, G.; Wahl, A.; DeLisle, R.K.; et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 2017, 8, 14357. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.D.; Narayanaswamy, A.K.; Farewell, D.; Rees, D.A. Complement activation in polycystic ovary syndrome occurs in the postprandial and fasted state and is influenced by obesity and insulin sensitivity. Clin. Endocrinol. 2021, 94, 74–84. [Google Scholar] [CrossRef]
| Baseline Demographics | PCOS (n = 137) | Controls (n = 97) | p-Value |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Age (years) | 27.9 (11.0) | 28.5 (11.0) | 0.09 |
| BMI (kg/m2) | 33.0 (9.9) | 25.0 (5.7) | <0.001 |
| Body weight (kg) | 93.2 (33.3) | 68.9 (20.9) | <0.001 |
| Waist Circumference (cm) | 101(21) | 78(14.9) | <0.001 |
| Insulin (IU/mL) | 9.0 (8.0) | 5.7 (4.1) | 0.001 |
| HOMA-IR | 2.6 (2.4) | 1.3 (1.1) | <0.005 |
| CRP (mg/L) | 3.1 (4.7) | 1.0 (1.7) | 0.001 |
| SHBG (nmol/L) | 21.0 (26.5) | 53.5 (37.0) | 0.001 |
| Testosterone (nmol/L) | 1.4 (0.9) | 1.0 (0.4) | <0.001 |
| FAI | 4.5 (5.3) | 2.2 (1.9) | <0.001 |
| FSH (IU/L) | 4.9 (2.8) | 5.6 (3.6) | 0.09 |
| LH (IU/L) | 6.1 (5.5) | 4.3 (5.4) | 0.009 |
| TSH (mU/L) | 1.9 (0.4) | 1.8 (0.4) | 0.09 |
| AMH (pmol/L) | 40 (42.7) | 18.1 (24.8) | <0.001 |
| Baseline Glucose (mmol/L) | 4.7 (0.5) | 4.5 (0.6) | 0.01 |
| 2 Hour Glucose (mmol/L) | 5.6 (1.8) | 4.9 (1.3) | 0.01 |
| Control (n = 97) Median (IQR) | PCOS (n = 137) Median (IQR) | p-Value | |
|---|---|---|---|
| APP | 18,763 (13,615) | 21,698 (14,260) | 0.04 |
| SNCA | 10,544 (4528) | 9589 (3468) | 0.04 |
| APCS | 32,981 (10,795) | 40,889 (9122) | <0.0001 |
| PAPPA | 10,809 (6030) | 11,597 (5196) | 0.49 |
| MAPT | 140 (45) | 157 (64) | 0.34 |
| ApoE | 31,169 (17,676) | 36,361 (20,187) | 0.01 |
| ApoE2 | 261,224 (55,647) | 265,481 (53,411) | 0.67 |
| ApoE3 | 195,313 (71,683) | 211,003 (74,881) | 0.06 |
| ApoE4 | 207,878 (67,027) | 218,393 (67,602) | 0.22 |
| SAA | 723 (1348) | 690 (1244) | 0.51 |
| NOG | 2534 (889) | 2307 (802) | 0.48 |
| ApoA1 | 14,679 (3003) | 14,562 (3837) | 0.33 |
| GFAP | 562 (312) | 564 (246) | 0.14 |
| NGF | 418 (94) | 418 (104) | 0.56 |
| vWF | 12,319 (5195) | 13,040 (9478) | 0.04 |
| FN | 14,898 (7663) | 17,380 (14,450) | 0.01 |
| FN1.3 | 3100 (947) | 3168 (1558) | 0.02 |
| FN1.4 | 66,449 (14,318) | 71,627 (25,002) | 0.007 |
| ECM1 | 20,403 (4377) | 19,721 (5860) | 0.99 |
| T2D (n = 23) | Controls (n = 23) | PCOS (n = 137) | Controls (n = 97) | |
|---|---|---|---|---|
| APP | 24,206 (38,065) | 14,004 (14,521) | 21,698 (14,260) | 18,763 (13,615) |
| APCS | 34,918 (6658) | 34,700 (8503) | 40,889 (9122) | 32,981 (10,795) |
| SNCA | 5519 * (1595) | 6644 (2606) | 9589 * (3468) | 10,544 (4528) |
| ApoE | 32,267 (19,636) | 33,762 (20,514) | 36,361 (20,187) | 31,169 (17,676) |
| GFAP | 328 * (45) | 354 (88) | 564 * (246) | 562 (312) |
| NGF | 399 (81) | 435 (134) | 418 (104) | 418 (94) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butler, A.E.; Moin, A.S.M.; Sathyapalan, T.; Atkin, S.L. A Cross-Sectional Study of Alzheimer-Related Proteins in Women with Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2024, 25, 1158. https://doi.org/10.3390/ijms25021158
Butler AE, Moin ASM, Sathyapalan T, Atkin SL. A Cross-Sectional Study of Alzheimer-Related Proteins in Women with Polycystic Ovary Syndrome. International Journal of Molecular Sciences. 2024; 25(2):1158. https://doi.org/10.3390/ijms25021158
Chicago/Turabian StyleButler, Alexandra E., Abu Saleh Md Moin, Thozhukat Sathyapalan, and Stephen L. Atkin. 2024. "A Cross-Sectional Study of Alzheimer-Related Proteins in Women with Polycystic Ovary Syndrome" International Journal of Molecular Sciences 25, no. 2: 1158. https://doi.org/10.3390/ijms25021158
APA StyleButler, A. E., Moin, A. S. M., Sathyapalan, T., & Atkin, S. L. (2024). A Cross-Sectional Study of Alzheimer-Related Proteins in Women with Polycystic Ovary Syndrome. International Journal of Molecular Sciences, 25(2), 1158. https://doi.org/10.3390/ijms25021158






