Molecular and Cellular Mechanisms Underlying the Cardiac Hypertrophic and Pro-Remodelling Effects of Leptin
Abstract
1. Introduction
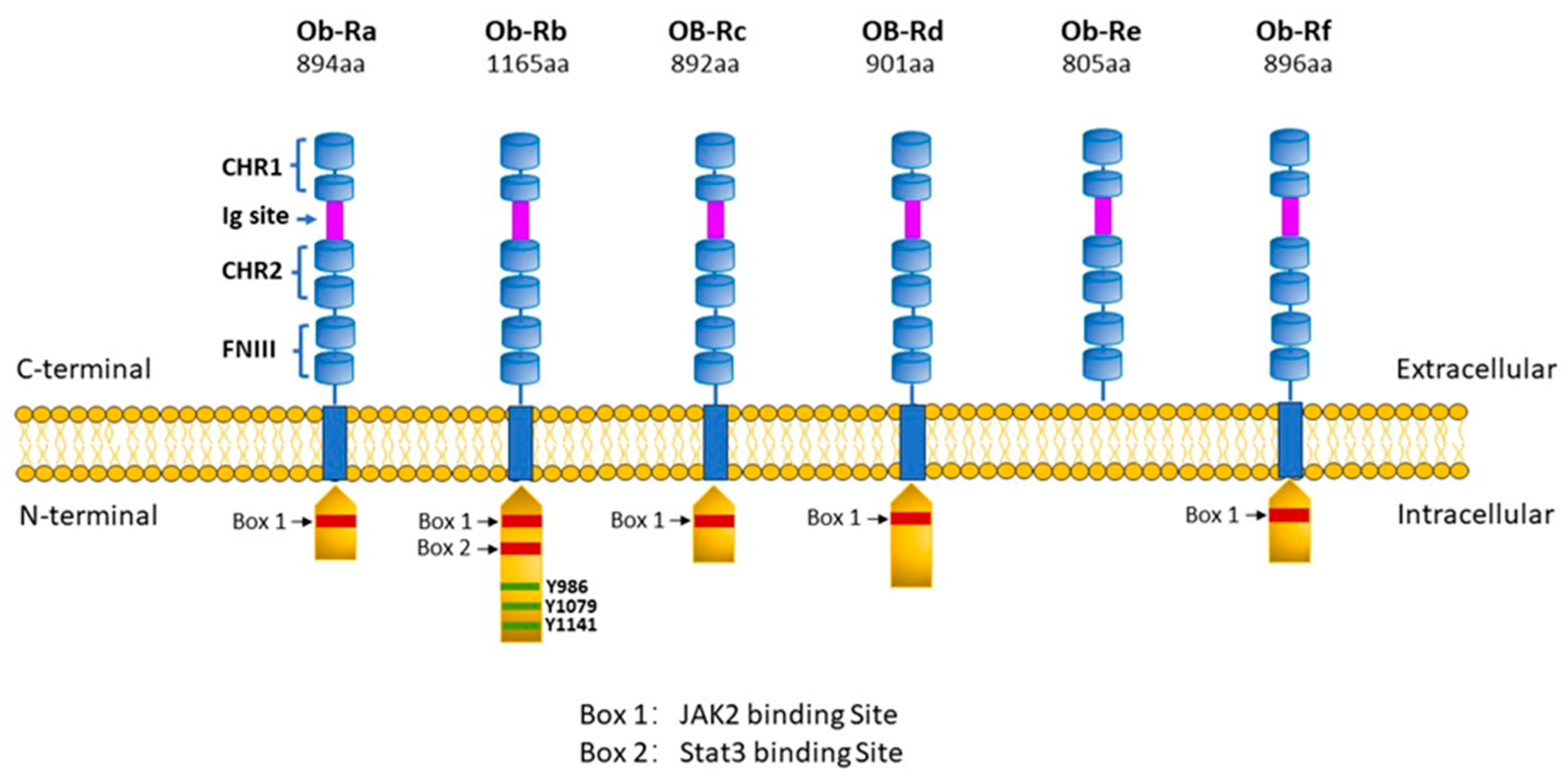
2. Leptin Receptors and Underlying Cell Signalling Processes: General Perspectives
3. ObR Antagonists
4. Leptin and Cardiovascular Disease in General
5. Leptin and Cardiac Function and Dysfunction
5.1. Leptin and Cardiac Hypertrophy
5.2. Proposed Cardiac Beneficial Effects of Leptin
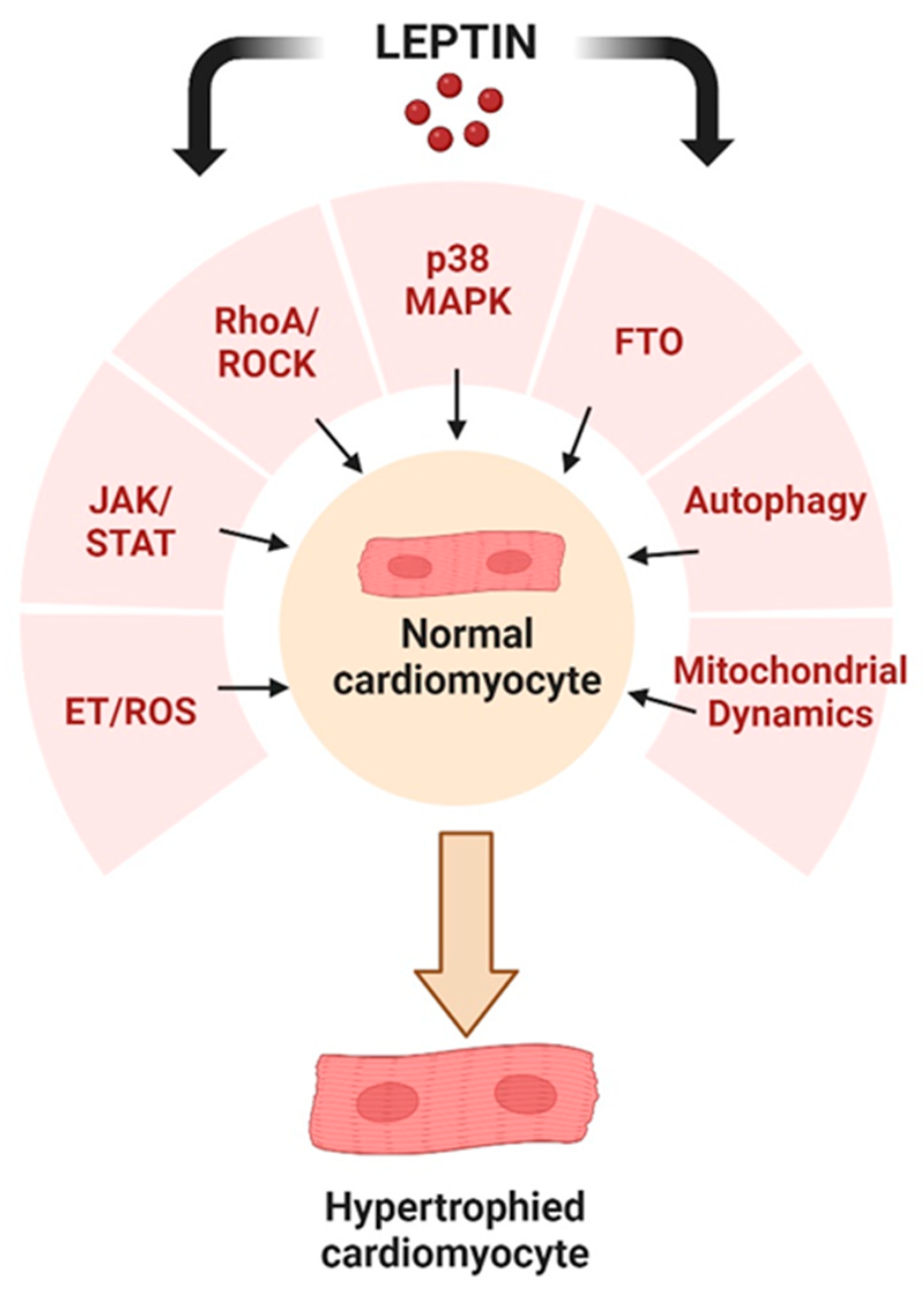
6. Intracellular Signalling Pathways Underlying the Pro-Hypertrophic Effects of Leptin
6.1. MAPK Activation
6.2. Endothelin/ROS Pathway Upregulation
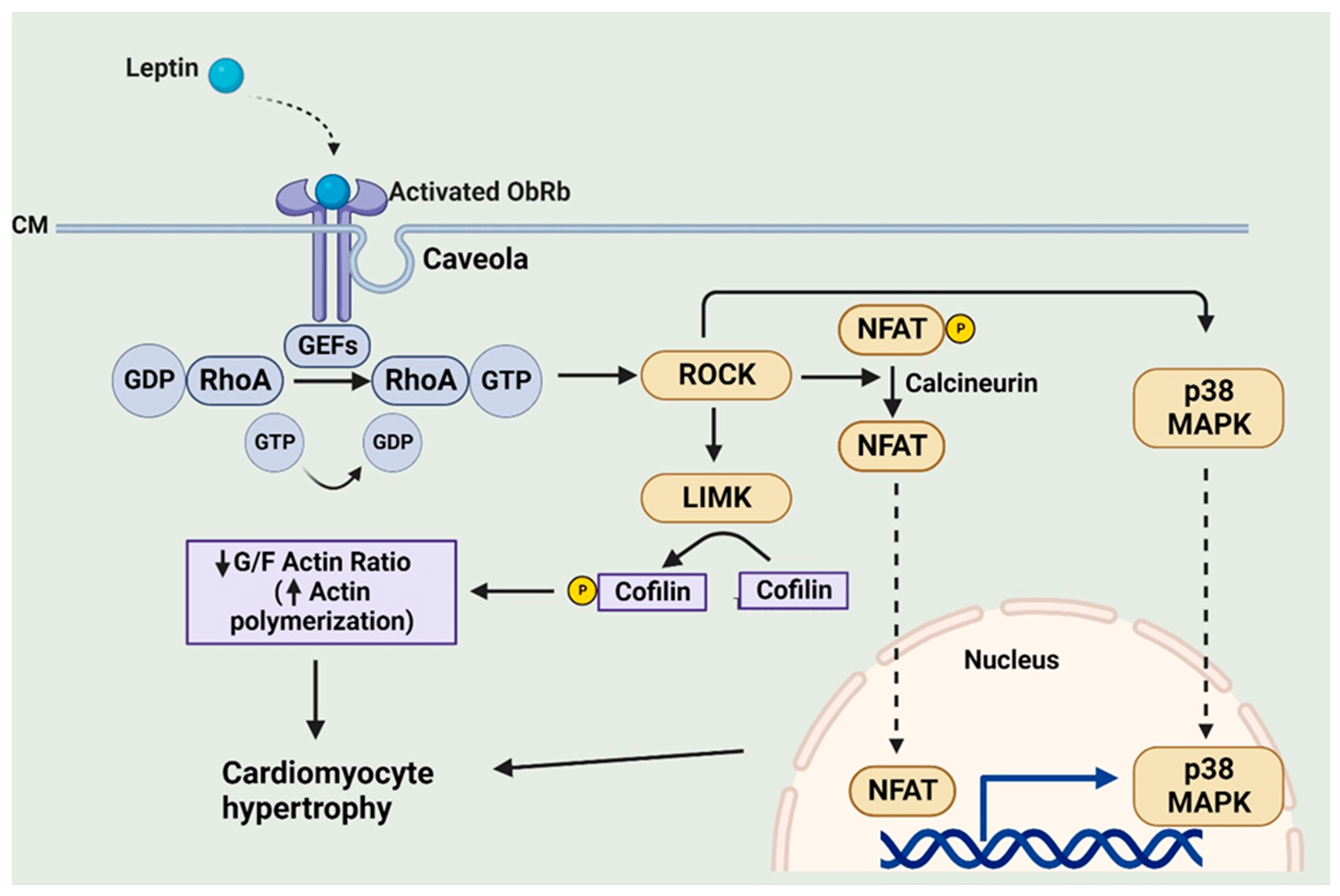
6.3. RhoA/ROCK Pathway
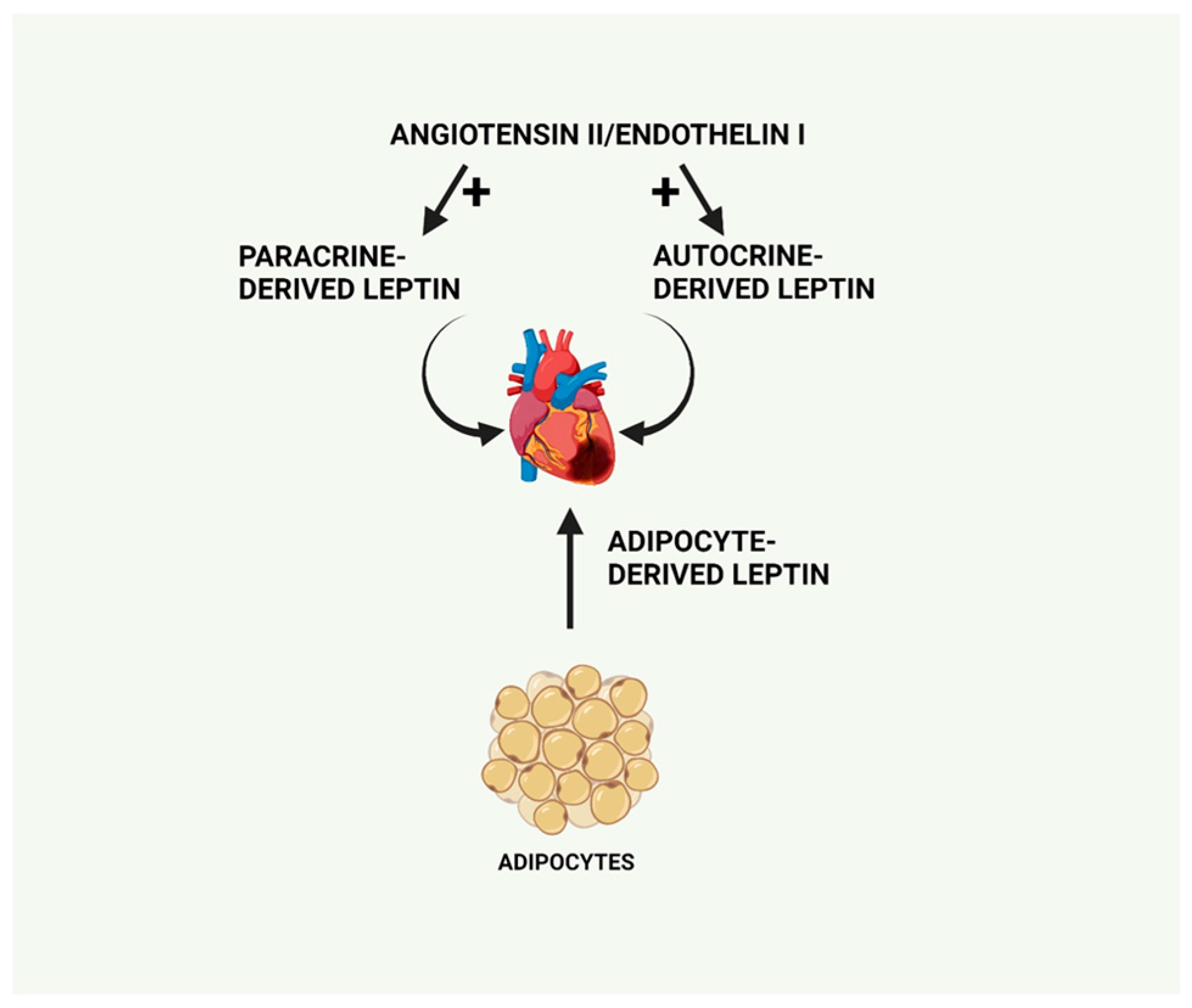
6.4. Upregulation of Cardiomyocyte Leptin Production by ET-1 and Angiotensin II May Mediate the Pro-Hypertrophic Effects of Both Factors through Activation of NF-κB and p38 MAPK
6.5. Leptin and FTO
6.6. Potential Role of the JAK/STAT Pathway
7. Autophagy as a Target for Leptin-Induced Cardiac Hypertrophy
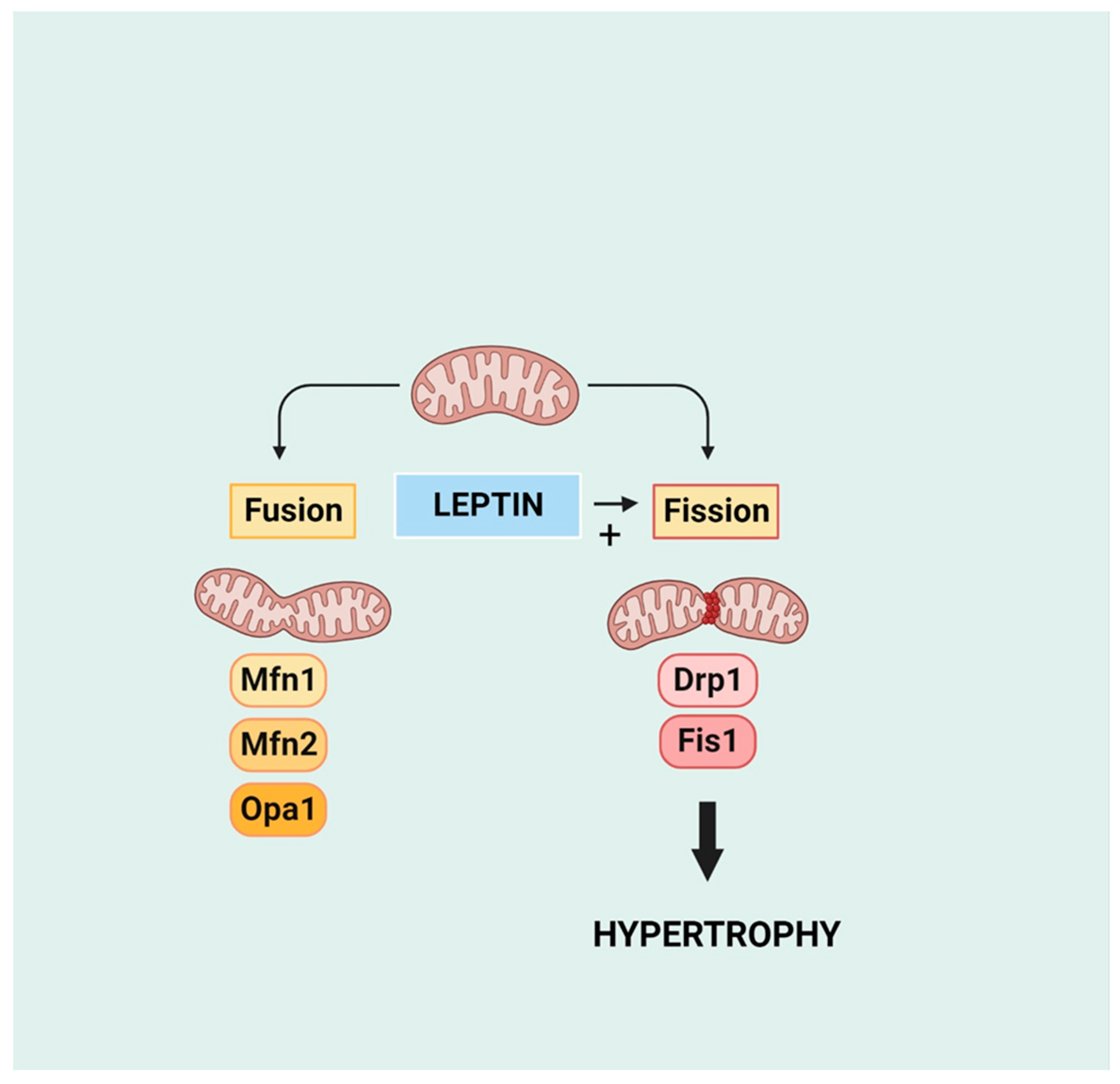
8. Mitochondrial Function and Dynamics in the Development of Cardiac Hypertrophy
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M. Leptin and the endocrine control of energy balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Maffei, M.; Fei, H.; Lee, G.H.; Dani, C.; Leroy, P.; Zhang, Y.; Proenca, R.; Negrel, R.; Ailhaud, G.; Friedman, J.M. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc. Natl. Acad. Sci. USA 1995, 92, 6957–6960. [Google Scholar] [CrossRef]
- Karmazyn, M.; Purdham, D.M.; Rajapurohitam, V.; Zeidan, A. Signalling mechanisms underlying the metabolic and other effects of adipokines on the heart. Cardiovasc. Res. 2008, 79, 279–286. [Google Scholar] [CrossRef]
- Conde, J.; Scotece, M.; Gómez, R.; López, V.; Gómez-Reino, J.J.; Lago, F.; Gualillo, O. Adipokines: Biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. Biofactors 2011, 37, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, physiological functions, role in diseases, and effects of nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef]
- Frey, N.; Katus, H.A.; Olson, E.N.; Hill, J.A. Hypertrophy of the heart: A new therapeutic target? Circulation 2004, 109, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Ritterhoff, J.; Tian, R. Metabolic mechanisms in physiological and pathological cardiac hypertrophy: New paradigms and challenges. Nat. Rev. Cardiol. 2023, 20, 812–829. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Myers, M.G., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog. Horm. Res. 2004, 59, 287–304. [Google Scholar] [CrossRef]
- Frühbeck, G. Intracellular signalling pathways activated by leptin. Biochem. J. 2006, 393 Pt 1, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Wauman, J.; Zabeau, L.; Tavernier, J. The leptin receptor complex: Heavier than expected? Front. Endocrinol. 2017, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Blüher, S.; Yannakouris, N.; Suchard, M.A.; Kratzsch, J.; Mantzoros, C.S. Regulation of circulating soluble leptin receptor levels by gender, adiposity, sex steroids, and leptin: Observational and interventional studies in humans. Diabetes 2002, 51, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Caveney, N.A.; Moya-Garzon, M.D.; Householder, K.D.; Rodriguez, G.E.; Burdsal, K.A.; Long, J.Z.; Garcia, K.C. Structural insights into the mechanism of leptin receptor activation. Nat. Commun. 2023, 14, 1797. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.A. The Jak/STAT pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011205. [Google Scholar] [CrossRef]
- Greco, M.; De Santo, M.; Comandè, A.; Belsito, E.L.; Andò, S.; Liguori, A.; Leggio, A. Leptin-activity modulators and their potential pharmaceutical applications. Biomolecules 2021, 11, 1045. [Google Scholar] [CrossRef]
- Leggio, A.; Catalano, S.; De Marco, R.; Barone, I.; Andò, S. Therapeutic potential of leptin receptor modulators. Eur. J. Med. Chem. 2014, 78, 97–105. [Google Scholar] [CrossRef]
- Zabeau, L.; Peelman, F.; Tavernier, J. Antagonizing leptin: Current status and future directions. Biol. Chem. 2014, 395, 499–514. [Google Scholar] [CrossRef]
- Raman, P.; Khanal, S. Leptin in atherosclerosis: Focus on macrophages, endothelial and smooth muscle cells. Int. J. Mol. Sci. 2021, 22, 5446. [Google Scholar] [CrossRef]
- Lieb, W.; Sullivan, L.M.; Harris, T.B.; Roubenoff, R.; Benjamin, E.J.; Levy, D.; Fox, C.S.; Wang, T.J.; Wilson, P.W.; Kannel, W.B.; et al. Plasma leptin levels and incidence of heart failure, cardiovascular disease, and total mortality in elderly individuals. Diabetes Care 2009, 32, 612–616. [Google Scholar] [CrossRef]
- Kang, K.W.; Ok, M.; Lee, S.K. Leptin as a key between obesity and cardiovascular disease. J. Obes. Metab. Syndr. 2020, 29, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Knudson, J.D.; Payne, G.A.; Borbouse, L.; Tune, J.D. Leptin and mechanisms of endothelial dysfunction and cardiovascular disease. Curr. Hypertens. Rep. 2008, 10, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.A.; Tune, J.D.; Knudson, J.D. Leptin-induced endothelial dysfunction: A target for therapeutic interventions. Curr. Pharm. Des. 2014, 20, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Knudson, J.D.; Dincer, U.D.; Zhang, C.; Swafford, A.N., Jr.; Koshida, R.; Picchi, A.; Focardi, M.; Dick, G.M.; Tune, J.D. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H48–H56. [Google Scholar] [CrossRef]
- Purdham, D.M.; Zou, M.X.; Rajapurohitam, V.; Karmazyn, M. Rat heart is a site of leptin production and action. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2877–H2884. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, X.; Ren, J. Leptin regulates cardiomyocyte contractile function through endothelin-1 receptor-NADPH oxidase pathway. Hypertension 2006, 47, 222–229. [Google Scholar] [CrossRef]
- Atkinson, L.L.; Fischer, M.A.; Lopaschuk, G.D. Leptin activates cardiac fatty acid oxidation independent of changes in the AMP-activated protein kinase-acetyl-CoA carboxylase-malonyl-CoA axis. J. Biol. Chem. 2002, 277, 29424–29430. [Google Scholar] [CrossRef]
- Pereira, S.; Cline, D.L.; Glavas, M.M.; Covey, S.D.; Kieffer, T.J. Tissue-specific effects of leptin on glucose and lipid metabolism. Endocr. Rev. 2021, 42, 1–28. [Google Scholar] [CrossRef]
- Sharma, V.; Mustafa, S.; Patel, N.; Wambolt, R.; Allard, M.F.; McNeill, J.H. Stimulation of cardiac fatty acid oxidation by leptin is mediated by a nitric oxide-p38 MAPK-dependent mechanism. Eur. J. Pharmacol. 2009, 617, 113–117. [Google Scholar] [CrossRef]
- Palanivel, R.; Eguchi, M.; Shuralyova, I.; Coe, I.; Sweeney, G. Distinct effects of short- and long-term leptin treatment on glucose and fatty acid uptake and metabolism in HL-1 cardiomyocytes. Metabolism 2006, 55, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Momken, I.; Chabowski, A.; Dirkx, E.; Nabben, M.; Jain, S.S.; McFarlan, J.T.; Glatz, J.F.; Luiken, J.J.; Bonen, A. A new leptin-mediated mechanism for stimulating fatty acid oxidation: A pivotal role for sarcolemmal FAT/CD36. Biochem. J. 2017, 474, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.E.; Maready, M.W.; Hall, J.E.; Stec, D.E. Rescue of cardiac leptin receptors in db/db mice prevents myocardial triglyceride accumulation. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E316–E325. [Google Scholar] [CrossRef]
- Lee, Y.; Naseem, R.H.; Duplomb, L.; Park, B.H.; Garry, D.J.; Richardson, J.A.; Schaffer, J.E.; Unger, R.H. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc. Natl. Acad. Sci. USA 2004, 101, 13624–13629. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; Tagliamonte, M.R.; Galderisi, M.; Zito, G.A.; Petrocelli, A.; Carella, C.; de Divitiis, O.; Varricchio, M. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin-resistant men. Hypertension 1999, 34, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Perego, L.; Pizzocri, P.; Corradi, D.; Maisano, F.; Paganelli, M.; Fiorina, P.; Barbieri, M.; Morabito, A.; Paolisso, G.; Folli, F.; et al. Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: A potential role for leptin in mediating human left ventricular hypertrophy. J. Clin. Endocrinol. Metab. 2005, 90, 4087–4093. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Whincup, P.H.; Lennon, L.; Sattar, N. Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: Does leptin have a role? J. Am. Coll. Cardiol. 2011, 58, 1870–1877. [Google Scholar] [CrossRef]
- Pladevall, M.; Williams, K.; Guyer, H.; Sadurní, J.; Falces, C.; Ribes, A.; Paré, C.; Brotons, C.; Gabriel, R.; Serrano-Ríos, M.; et al. The association between leptin and left ventricular hypertrophy: A population-based cross-sectional study. J. Hypertens. 2003, 21, 1467–1473. [Google Scholar] [CrossRef]
- Lieb, W.; Sullivan, L.M.; Aragam, J.; Harris, T.B.; Roubenoff, R.; Benjamin, E.J.; Vasan, R.S. Relation of serum leptin with cardiac mass and left atrial dimension in individuals >70 years of age. Am. J. Cardiol. 2009, 104, 602–605. [Google Scholar] [CrossRef]
- Allison, M.A.; Bluemke, D.A.; McClelland, R.; Cushman, M.; Criqui, M.; Polak, J.F.; Lima, J.A. Relation of leptin to left ventricular hypertrophy (from the Multi-Ethnic Study of Atherosclerosis). Am. J. Cardiol. 2013, 112, 726–730. [Google Scholar] [CrossRef][Green Version]
- Rajapurohitam, V.; Gan, X.T.; Kirshenbaum, L.A.; Karmazyn, M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ. Res. 2003, 93, 277–279. [Google Scholar] [CrossRef]
- Xu, F.P.; Chen, M.S.; Wang, Y.Z.; Yi, Q.; Lin, S.B.; Chen, A.F.; Luo, J.D. Leptin induces hypertrophy via endothelin-1-reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation 2004, 110, 1269–1275. [Google Scholar] [CrossRef]
- Tajmir, P.; Ceddia, R.B.; Li, R.K.; Coe, I.; Sweeney, G. Leptin increases cardiomyocyte hyperplasia via extracellular signal-regulated kinase- and phosphatidylinositol 3-kinase-dependent signaling pathways. Endocrinology 2004, 145, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Madani, S.; De Girolamo, S.; Muñoz, D.M.; Li, R.K.; Sweeney, G. Direct effects of leptin on size and extracellular matrix components of human pediatric ventricular myocytes. Cardiovasc. Res. 2006, 69, 716–725. [Google Scholar] [CrossRef]
- Moey, M.; Rajapurohitam, V.; Zeidan, A.; Karmazyn, M. Ginseng (Panax quinquefolius) attenuates leptin-induced cardiac hypertrophy through inhibition of p115Rho guanine nucleotide exchange factor-RhoA/Rho-associated, coiled-coil containing protein kinase-dependent mitogen-activated protein kinase pathway activation. J. Pharmacol. Exp. Ther. 2011, 339, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.; Hunter, J.C.; Javadov, S.; Karmazyn, M. mTOR mediates RhoA-dependent leptin-induced cardiomyocyte hypertrophy. Mol. Cell. Biochem. 2011, 352, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Abundis, E.; Rajapurohitam, V.; Haist, J.V.; Gan, X.T.; Karmazyn, M. The obesity-related peptide leptin sensitizes cardiac mitochondria to calcium-induced permeability transition pore opening and apoptosis. PLoS ONE 2012, 7, e41612. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gan, X.T.; Zhao, G.; Huang, C.X.; Rowe, A.C.; Purdham, D.M.; Karmazyn, M. Identification of fat mass and obesity associated (FTO) protein expression in cardiomyocytes: Regulation by leptin and its contribution to leptin-induced hypertrophy. PLoS ONE 2013, 8, e74235. [Google Scholar] [CrossRef]
- Jong, C.J.; Yeung, J.; Tseung, E.; Karmazyn, M. Leptin-induced cardiomyocyte hypertrophy is associated with enhanced mitochondrial fission. Mol. Cell. Biochem. 2019, 454, 33–44. [Google Scholar] [CrossRef]
- Kain, D.; Simon, A.J.; Greenberg, A.; Ben Zvi, D.; Gilburd, B.; Schneiderman, J. Cardiac leptin overexpression in the context of acute MI and reperfusion potentiates myocardial remodeling and left ventricular dysfunction. PLoS ONE 2018, 13, e0203902. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, K.; Li, P.; Sheng, Y. The emerging role of leptin in obesity-associated cardiac fibrosis: Evidence and mechanism. Mol. Cell. Biochem. 2023, 478, 991–1011. [Google Scholar] [CrossRef] [PubMed]
- Rajapurohitam, V.; Javadov, S.; Purdham, D.; Kirshenbaum, L.A.; Karmazyn, M. An autocrine role for leptin in mediating the cardiomycyte hypertrophic effects of angiotensin II and endotherlin-1. J. Mol. Cell. Cardiol. 2006, 41, 265–274. [Google Scholar] [CrossRef]
- Chen, H.; Li, M.; Liu, L.; Zhu, D.; Tian, G. Telmisartan improves myocardial remodeling by inhibiting leptin autocrine activity and activating PPARγ. Exp. Biol. Med. 2020, 245, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.F.M.; De Keulenaer, G.W. Autocrine signaling in cardiac remodeling: A rich source of therapeutic targets. J. Am. Heart Assoc. 2021, 10, e019169. [Google Scholar] [CrossRef]
- Barouch, L.A.; Berkowitz, D.E.; Harrison, R.W.; O’Donnell, C.; Hare, J.M. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation 2003, 108, 754–759. [Google Scholar] [CrossRef]
- McGaffin, K.R.; Witham, W.G.; Yester, K.A.; Romano, L.C.; O’Doherty, R.M.; McTiernan, C.F.; O’Donnell, C.P. Cardiac-specific leptin receptor deletion exacerbates ischaemic heart failure in mice. Cardiovasc. Res. 2011, 89, 60–71. [Google Scholar] [CrossRef]
- McGaffin, K.R.; Sun, C.K.; Rager, J.J.; Romano, L.C.; Zou, B.; Mathier, M.A.; O’Doherty, R.M.; McTiernan, C.F.; O’Donnell, C.P. Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury. Cardiovasc. Res. 2008, 77, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Streicher, J.M.; Ren, S.; Herschman, H.; Wang, Y. MAPK-activated protein kinase-2 in cardiac hypertrophy and cyclooxygenase-2 regulation in heart. Circ. Res. 2010, 106, 1434–1443. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, S.; Sah, V.P.; Ross, J., Jr.; Brown, J.H.; Han, J.; Chien, K.R. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 1998, 273, 2161–2168. [Google Scholar] [CrossRef]
- Yokota, T.; Wang, Y. p38 MAP kinases in the heart. Gene 2016, 575, 369–376. [Google Scholar] [CrossRef]
- Arabacilar, P.; Marber, M. The case for inhibiting p38 mitogen-activated protein kinase in heart failure. Front. Pharmacol. 2015, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Seccia, T.M.; Rigato, M.; Ravarotto, V.; Calò, L.A. ROCK (RhoA/Rho Kinase) in cardiovascular-renal pathophysiology: A review of new advancements. J. Clin. Med. 2020, 9, 1328. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Liao, J.K. Rho Kinases and Cardiac Remodeling. Circ. J. 2016, 80, 1491–1498. [Google Scholar] [CrossRef]
- Zeidan, A.; Javadov, S.; Karmazyn, M. Essential role of Rho/ROCK-dependent processes and actin dynamics in mediating leptin-induced hypertrophy in rat neonatal ventricular myocytes. Cardiovasc. Res. 2006, 72, 101–111. [Google Scholar] [CrossRef]
- Zeidan, A.; Javadov, S.; Chakrabarti, S.; Karmazyn, M. Leptin-induced cardiomyocyte hypertrophy involves selective caveolae and RhoA/ROCK-dependent p38 MAPK translocation to nuclei. Cardiovasc. Res. 2008, 77, 64–72. [Google Scholar] [CrossRef]
- Shin, H.J.; Oh, J.; Kang, S.M.; Lee, J.H.; Shin, M.J.; Hwang, K.C.; Jang, Y.; Chung, J.H. Leptin induces hypertrophy via p38 mitogen-activated protein kinase in rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2005, 329, 18–24. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Lu, J.R.; Antos, C.L.; Markham, B.; Richardson, J.; Robbins, J.; Grant, S.R.; Olson, E.N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 1998, 93, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Rajapurohitam, V.; Izaddoustdar, F.; Martinez-Abundis, E.; Karmazyn, M. Leptin-induced cardiomyocyte hypertrophy reveals both calcium-dependent and calcium-independent/RhoA-dependent calcineurin activation and NFAT nuclear translocation. Cell. Signal. 2012, 24, 2283–2290. [Google Scholar] [CrossRef]
- Karmazyn, M.; Gan, X.T. Inhibition of myocardial remodeling and heart failure by traditional herbal medications: Evidence from ginseng and ginkgo biloba. Rev. Cardiovasc. Med. 2023, 24, 212. [Google Scholar] [CrossRef]
- Li, Z.; Liang, J.; Wu, W.K.; Yu, X.; Yu, J.; Weng, X.; Shen, J. Leptin activates RhoA/ROCK pathway to induce cytoskeleton remodeling in nucleus pulposus cells. Int. J. Mol. Sci. 2014, 15, 1176–1188. [Google Scholar] [CrossRef]
- Liang, J.; Feng, J.; Wu, W.K.; Xiao, J.; Wu, Z.; Han, D.; Zhu, Y.; Qiu, G. Leptin-mediated cytoskeletal remodeling in chondrocytes occurs via the RhoA/ROCK pathway. J. Orthop. Res. 2011, 29, 369–374. [Google Scholar] [CrossRef]
- Rajapurohitam, V.; Kilic, A.; Javadov, S.; Karmazyn, M. Role of NF-κB and p38 MAPK activation in mediating angiotensin II and endothelin-1-induced stimulation in leptin production and cardiomyocyte hypertrophy. Mol. Cell. Biochem. 2012, 366, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Church, C.; Moir, L.; McMurray, F.; Girard, C.; Banks, G.T.; Teboul, L.; Wells, S.; Brüning, J.C.; Nolan, P.M.; Ashcroft, F.M.; et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat. Genet. 2010, 42, 1086–1092. [Google Scholar] [CrossRef]
- Fischer, J.; Koch, L.; Emmerling, C.; Vierkotten, J.; Peters, T.; Brüning, J.C.; Rüther, U. Inactivation of the Fto gene protects from obesity. Nature 2009, 458, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. The ‘Fat Mass and Obesity Related’ (FTO) gene: Mechanisms of impact on obesity and energy balance. Curr. Obes. Rep. 2015, 4, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Benak, D.; Kolar, F.; Zhang, L.; Devaux, Y.; Hlavackova, M. RNA modification m6Am: The role in cardiac biology. Epigenetics 2023, 18, 2218771. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Jing, X.; Xiong, X.D. Emerging role and mechanism of the FTO gene in cardiovascular diseases. Biomolecules 2023, 13, 850. [Google Scholar] [CrossRef]
- Li, W.; Xing, C.; Bao, L.; Han, S.; Luo, T.; Wang, Z.; Fan, H. Comprehensive analysis of RNA m6A methylation in pressure overload-induced cardiac hypertrophy. BMC Genom. 2022, 23, 576. [Google Scholar] [CrossRef]
- Mathiyalagan, P.; Adamiak, M.; Mayourian, J.; Sassi, Y.; Liang, Y.; Agarwal, N.; Jha, D.; Zhang, S.; Kohlbrenner, E.; Chepurko, E.; et al. FTO-dependent N6-methyladenosine regulates cardiac function during remodeling and repair. Circulation 2019, 139, 518–532. [Google Scholar] [CrossRef]
- Yu, Y.; Pan, Y.; Fan, Z.; Xu, S.; Gao, Z.; Ren, Z.; Yu, J.; Li, W.; Liu, F.; Gu, J.; et al. LuHui derivative, a novel compound that inhibits the fat mass and obesity-associated (FTO), alleviates the inflammatory response and injury in hyperlipidemia-induced cardiomyopathy. Front. Cell. Dev. Biol. 2021, 9, 731365. [Google Scholar] [CrossRef]
- Kunisada, K.; Negoro, S.; Tone, E.; Funamoto, M.; Osugi, T.; Yamada, S.; Okabe, M.; Kishimoto, T.; Yamauchi-Takihara, K. Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy. Proc. Natl. Acad. Sci. USA 2000, 97, 315–319. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, H.; Huang, X.; Wang, S.; Ouyang, X.; Wang, Y.; Cao, Q.; Yang, L.; Tao, Y.; Lai, H. Pirfenidone attenuates cardiac hypertrophy against isoproterenol by inhibiting activation of the janus tyrosine kinase-2/signal transducer and activator of transcription 3 (JAK-2/STAT3) signaling pathway. Bioengineered 2022, 13, 12772–12782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wu, D.; Sang, M.; Xu, Y.; Liu, Z.; Wu, Q. Stachydrine ameliorates isoproterenol-induced cardiac hypertrophy and fibrosis by suppressing inflammation and oxidative stress through inhibiting NF-κB and JAK/STAT signaling pathways in rats. Int. Immunopharmacol. 2017, 48, 102–109. [Google Scholar] [CrossRef]
- Wang, C.; Pan, Z. Hydrogen-rich saline mitigates pressure overload-induced cardiac hypertrophy and atrial fibrillation in rats via the JAK-STAT signalling pathway. J. Int. Med. Res. 2020, 48, 300060520936415. [Google Scholar] [CrossRef]
- Gan, X.T.; Rajapurohitam, V.; Xue, J.; Huang, C.; Bairwa, S.; Tang, X.; Chow, J.T.; Liu, M.F.; Chiu, F.; Sakamoto, K.; et al. Myocardial hypertrophic remodeling and impaired left ventricular function in mice with a cardiac-specific deletion of Janus Kinase 2. Am. J. Pathol. 2015, 185, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, J.; Kalinowski, A.; Liu, M.G.; Zhang, S.S.; Gao, Q.; Chai, G.X.; Ji, L.; Iwamoto, Y.; Li, E.; Schneider, M.; et al. Cardiomyocyte-restricted knockout of STAT3 results in higher sensitivity to inflammation, cardiac fibrosis, and heart failure with advanced age. Proc. Natl. Acad. Sci. USA 2003, 100, 12929–12934. [Google Scholar] [CrossRef]
- Oba, T.; Yasukawa, H.; Hoshijima, M.; Sasaki, K.; Futamata, N.; Fukui, D.; Mawatari, K.; Nagata, T.; Kyogoku, S.; Ohshima, H.; et al. Cardiac-specific deletion of SOCS-3 prevents development of left ventricular remodeling after acute myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 838–852. [Google Scholar] [CrossRef]
- Abe, Y.; Ono, K.; Kawamura, T.; Wada, H.; Kita, T.; Shimatsu, A.; Hasegawa, K. Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2387–H2396. [Google Scholar] [CrossRef] [PubMed]
- Leifheit-Nestler, M.; Wagner, N.M.; Gogiraju, R.; Didié, M.; Konstantinides, S.; Hasenfuss, G.; Schäfer, K. Importance of leptin signaling and signal transducer and activator of transcription-3 activation in mediating the cardiac hypertrophy associated with obesity. J. Transl. Med. 2013, 11, 170. [Google Scholar] [CrossRef]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef]
- Boya, P.; Reggiori, F.; Codogno, P. Emerging regulation and functions of autophagy. Nat. Cell. Biol. 2013, 15, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xu, W.; Liu, Y.; Zhou, J.; Cui, K.; Chen, Y. Autophagy protects mitochondrial health in heart failure. Heart Fail. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Bielawska, M.; Warszyńska, M.; Stefańska, M.; Błyszczuk, P. Autophagy in heart failure: Insights into mechanisms and therapeutic implications. J. Cardiovasc. Dev. Dis. 2023, 10, 352. [Google Scholar] [CrossRef]
- Shi, S.; Jiang, P. Therapeutic potentials of modulating autophagy in pathological cardiac hypertrophy. Biomed. Pharmacother. 2022, 156, 113967. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Yang, X. Functions of autophagy in pathological cardiac hypertrophy. Int. J. Biol. Sci. 2015, 11, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, L.; Samanta, A.; Mahmoudi, S.M.; Buehler, T.; Cantilena, A.; Vincent, R.J.; Girgis, M.; Breeden, J.; Asante, S.; et al. STAT3 balances myocyte hypertrophy vis-à-vis autophagy in response to Angiotensin II by modulating the AMPKα/mTOR axis. PLoS ONE 2017, 12, e0179835. [Google Scholar] [CrossRef]
- Kobara, M.; Toba, H.; Nakata, T. Roles of autophagy in angiotensin II-induced cardiomyocyte apoptosis. Clin. Exp. Pharmacol. Physiol. 2022, 49, 1342–1351. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Song, Z.P.; Chen, Y.G.; Zhang, D.D.; Wang, C.Q. Rapamycin inhibits cardiac hypertrophy by promoting autophagy via the MEK/ERK/Beclin-1 pathway. Front. Physiol. 2016, 7, 104. [Google Scholar] [CrossRef]
- Park, P.H. Autophagy induction: A critical event for the modulation of cell death/survival and inflammatory responses by adipokines. Arch. Pharm. Res. 2018, 41, 1062–1073. [Google Scholar] [CrossRef]
- Malik, S.A.; Mariño, G.; BenYounès, A.; Shen, S.; Harper, F.; Maiuri, M.C.; Kroemer, G. Neuroendocrine regulation of autophagy by leptin. Cell Cycle 2011, 10, 2917–2923. [Google Scholar] [CrossRef]
- Gogiraju, R.; Hubert, A.; Fahrer, J.; Straub, B.K.; Brandt, M.; Wenzel, P.; Münzel, T.; Konstantinides, S.; Hasenfuss, G.; Schäfer, K. Endothelial leptin receptor deletion promotes cardiac autophagy and angiogenesis following pressure overload by suppressing Akt/mTOR signaling. Circ. Heart Fail. 2019, 12, e005622. [Google Scholar] [CrossRef] [PubMed]
- Kandadi, M.R.; Roe, N.D.; Ren, J. Autophagy inhibition rescues against leptin-induced cardiac contractile dysfunction. Curr. Pharm. Des. 2014, 20, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.J.; Liu, Y.P.; Yuan, X.; Zhang, G.P.; Hou, N.; Wu, X.Q.; Luo, J.D.; Zhang, G.S. Leptin attenuates the contractile function of adult rat cardiomyocytes involved in oxidative stress and autophagy. Acta Cardiol. Sin. 2016, 32, 723–730. [Google Scholar] [CrossRef]
- Chen, G.; Kroemer, G.; Kepp, O. Mitophagy: An emerging role in aging and age-associated diseases. Front. Cell Dev. Biol. 2020, 8, 200. [Google Scholar] [CrossRef]
- Chen, Y.; Dorn, G.W., 2nd. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 2013, 340, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Blanquer-Rosselló, M.M.; Santandreu, F.M.; Oliver, J.; Roca, P.; Valle, A. Leptin Modulates Mitochondrial Function, Dynamics and Biogenesis in MCF-7 Cells. J. Cell. Biochem. 2015, 116, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Wauman, J.; Tavernier, J. The intracellular domain of the leptin receptor prevents mitochondrial depolarization and mitophagy. Biochim. Biophys. Acta Mol. Cell. Res. 2018, 1865, 1312–1325. [Google Scholar] [CrossRef]
- Facundo, H.D.T.F.; Brainard, R.E.; Caldas, F.R.L.; Lucas, A.M.B. Mitochondria and cardiac hypertrophy. Adv. Exp. Med. Biol. 2017, 982, 203–226. [Google Scholar] [CrossRef]
- Torrealba, N.; Aranguiz, P.; Alonso, C.; Rothermel, B.A.; Lavandero, S. Mitochondria in structural and functional cardiac remodeling. Adv. Exp. Med. Biol. 2017, 982, 277–306. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.Q.; Liu, F.Y.; Guo, Z.; An, P.; Wang, M.Y.; Yang, Z.; Fan, D.; Tang, Q.Z. Mitochondria in pathological cardiac hypertrophy research and therapy. Front. Cardiovasc. Med. 2022, 8, 822969. [Google Scholar] [CrossRef]
- Tokuyama, T.; Yanagi, S. Role of mitochondrial dynamics in heart diseases. Genes 2023, 14, 1876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.; Houtkooper, R.H. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Fu, M.; Wang, T.; Qi, Y.; Zhong, X.; Li, D.; Liu, B. Down-regulation of the mitochondrial fusion protein Opa1/Mfn2 promotes cardiomyocyte hypertrophy in Su5416/hypoxia-induced pulmonary hypertension rats. Arch. Biochem. Biophys. 2023, 747, 109743. [Google Scholar] [CrossRef]
- Guan, X.; Wang, L.; Liu, Z.; Guo, X.; Jiang, Y.; Lu, Y.; Peng, Y.; Liu, T.; Yang, B.; Shan, H.; et al. miR-106a promotes cardiac hypertrophy by targeting mitofusin 2. J. Mol. Cell. Cardiol. 2016, 99, 207–217. [Google Scholar] [CrossRef]
- Ikeda, Y.; Shirakabe, A.; Maejima, Y.; Zhai, P.; Sciarretta, S.; Toli, J.; Nomura, M.; Mihara, K.; Egashira, K.; Ohishi, M.; et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 2015, 116, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Zablocki, D.; Sadoshima, J. The role of Drp1 in mitophagy and cell death in the heart. J. Mol. Cell. Cardiol. 2020, 142, 138–145. [Google Scholar] [CrossRef]
- Commins, S.P.; Watson, P.M.; Padgett, M.A.; Dudley, A.; Argyropoulos, G.; Gettys, T.W. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology 1999, 140, 292–300. [Google Scholar] [CrossRef]
- Martinez-Abundis, E.; Rajapurohitam, V.; Gertler, A.; Karmazyn, M. Identification of functional leptin receptors expressed in ventricular mitochondria. Mol. Cell. Biochem. 2015, 408, 155–162. [Google Scholar] [CrossRef]
- Berzabá-Evoli, E.; Zazueta, C.; Cruz Hernández, J.H.; Gómez-Crisóstomo, N.P.; Juárez-Rojop, I.E.; De la Cruz-Hernández, E.N.; Martínez-Abundis, E. Leptin modifies the rat heart performance associated with mitochondrial dysfunction independently of its prohypertrophic effects. Int. J. Endocrinol. 2018, 2018, 6081415. [Google Scholar] [CrossRef]

| Adipokine | Selected Sites of Synthesis |
|---|---|
| Leptin | Adipocytes, cardiomyocyte, vascular endothelial cells |
| Adiponectin | Adipocytes, skeletal muscle, cardiomyocytes |
| Apelin | Adipocytes, cardiomyocyte, pancreas, stomach |
| Chemerin | Adipocytes, liver, lung, placenta |
| Resistin | Adipocytes, blood monocytes, liver |
| Visfatin | Adipocytes, lymphocytes, macrophages |
| Omentin | Adipocytes (primarily visceral) |
| Vaspin | Adipocytes, hypothalamus, stomach, liver, pancreas |
| Progranulin | Adipocytes, CNS |
| Interleukin-6 | Adipocytes, t cells, macrophages, fibroblasts |
| MCP-1 | Adipocytes, macrophages, epithelial cells, endothelial cells |
| PAI-1 | Adipocytes, vascular endothelium, macrophages, cardiomyocyte |
| RBP-4 | Adipocytes, macrophages, liver |
| TNFα | Adipocytes, macrophages, endothelial cells, cardiomyocytes |
| CTRP-4 | Adipocytes, brain, skeletal muscle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmazyn, M.; Gan, X.T. Molecular and Cellular Mechanisms Underlying the Cardiac Hypertrophic and Pro-Remodelling Effects of Leptin. Int. J. Mol. Sci. 2024, 25, 1137. https://doi.org/10.3390/ijms25021137
Karmazyn M, Gan XT. Molecular and Cellular Mechanisms Underlying the Cardiac Hypertrophic and Pro-Remodelling Effects of Leptin. International Journal of Molecular Sciences. 2024; 25(2):1137. https://doi.org/10.3390/ijms25021137
Chicago/Turabian StyleKarmazyn, Morris, and Xiaohong Tracey Gan. 2024. "Molecular and Cellular Mechanisms Underlying the Cardiac Hypertrophic and Pro-Remodelling Effects of Leptin" International Journal of Molecular Sciences 25, no. 2: 1137. https://doi.org/10.3390/ijms25021137
APA StyleKarmazyn, M., & Gan, X. T. (2024). Molecular and Cellular Mechanisms Underlying the Cardiac Hypertrophic and Pro-Remodelling Effects of Leptin. International Journal of Molecular Sciences, 25(2), 1137. https://doi.org/10.3390/ijms25021137





