YB-1 Is a Novel Target for the Inhibition of α-Adrenergic-Induced Hypertrophy
Abstract
1. Introduction
2. Results
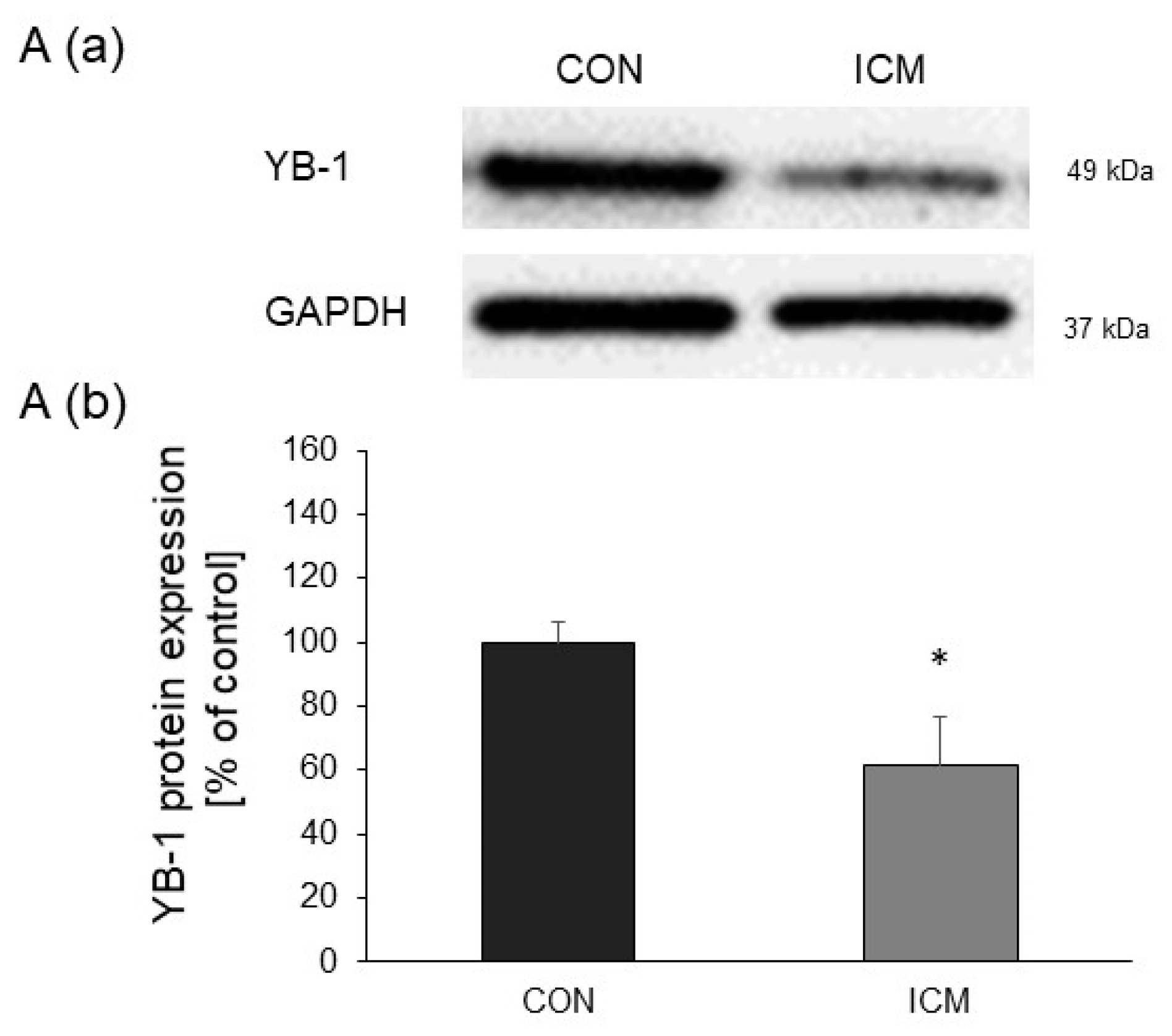
2.1. YB-1 Expression Is Downregulated in Human Heart Failure
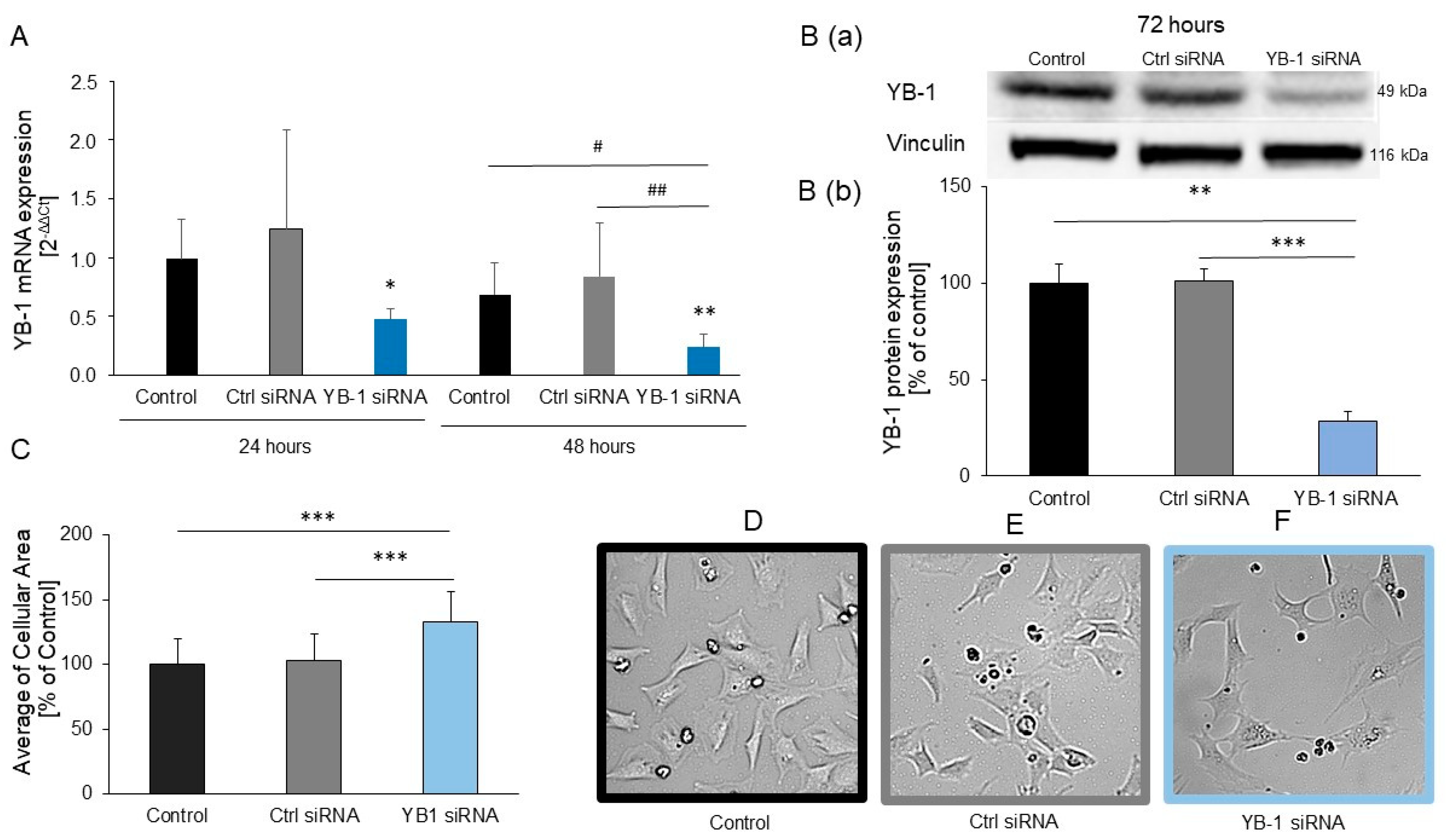
2.2. YB-1 Silencing in H9C2 Cells Promotes Hypertrophic Growth
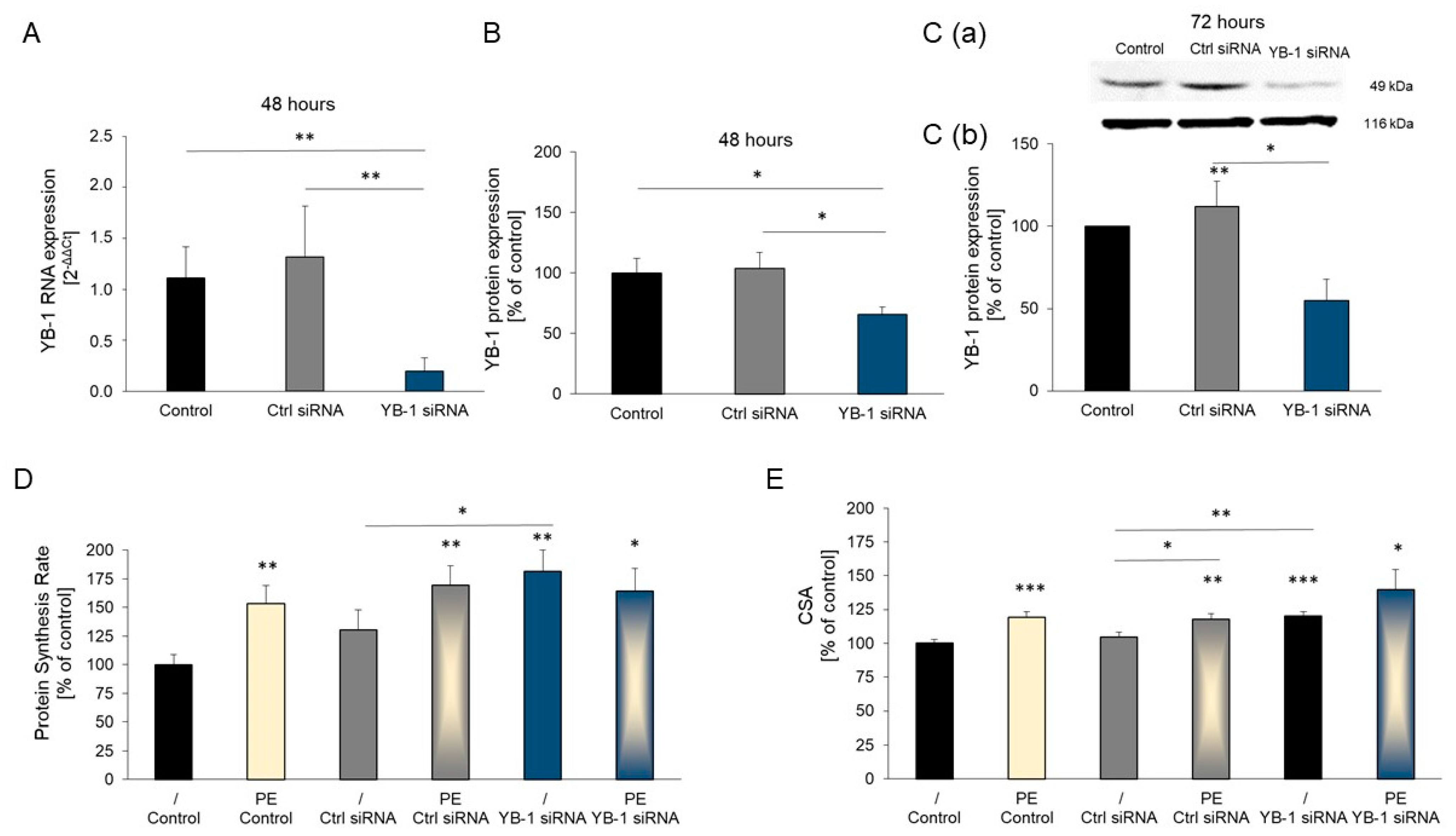
2.3. YB-1 Silencing in Adult Rat Cardiomyocytes Promotes Hypertrophic Growth
2.4. YB-1 siRNA Specifically Downregulates YB-1
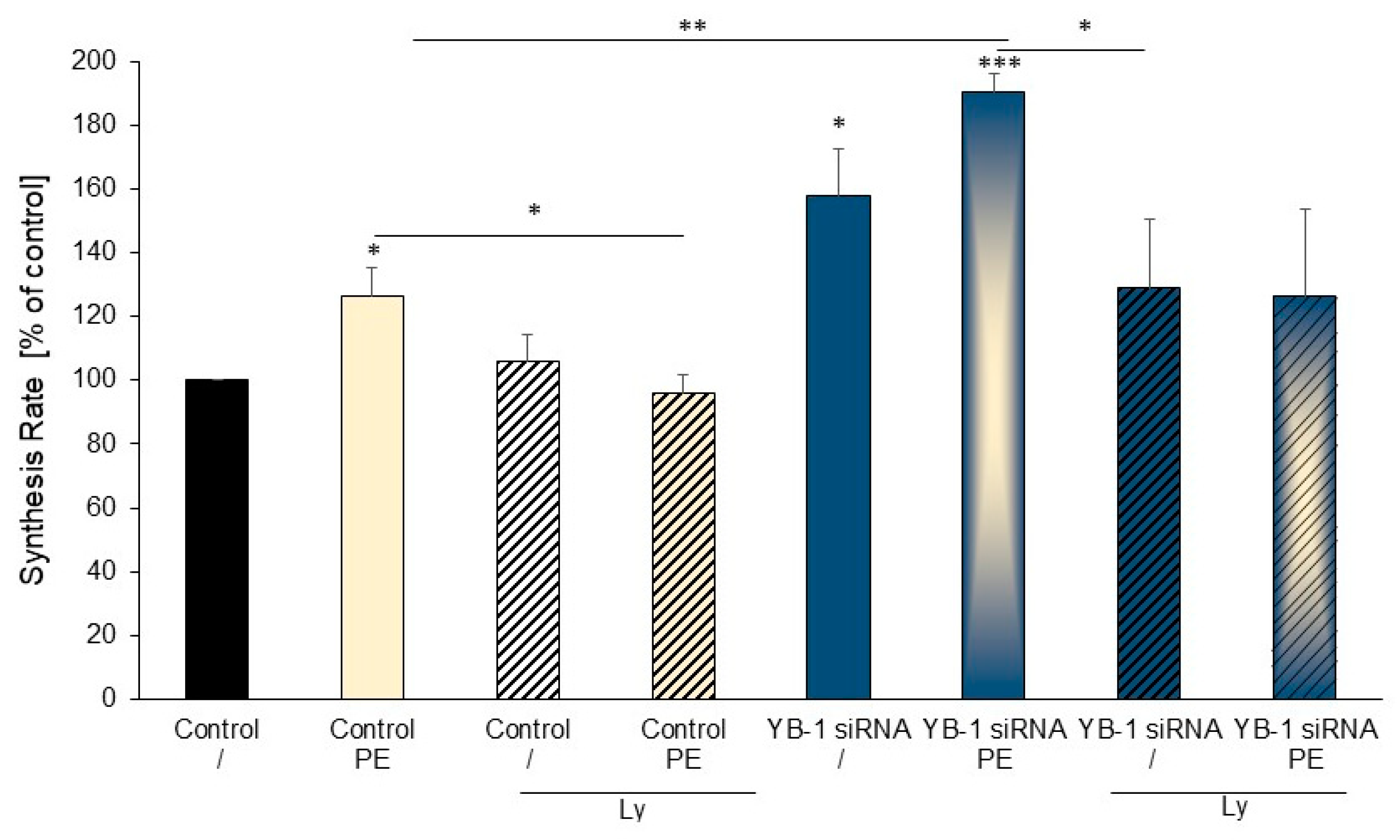
2.5. Treatment of Cardiomyocytes with the PI3K Inhibitor Ly294002 Reduced Hypertrophic Growth
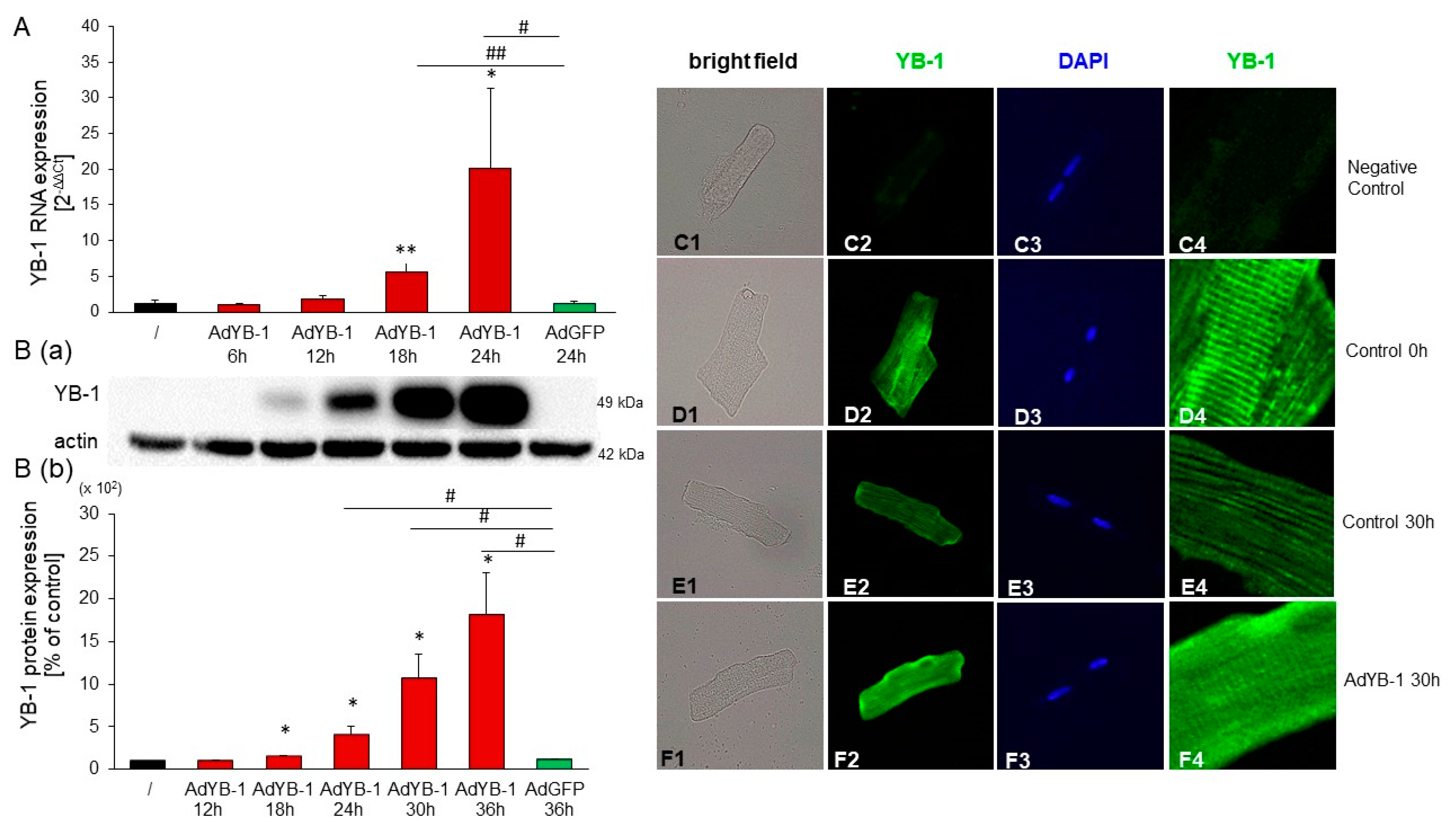
2.6. YB-1 Overexpression
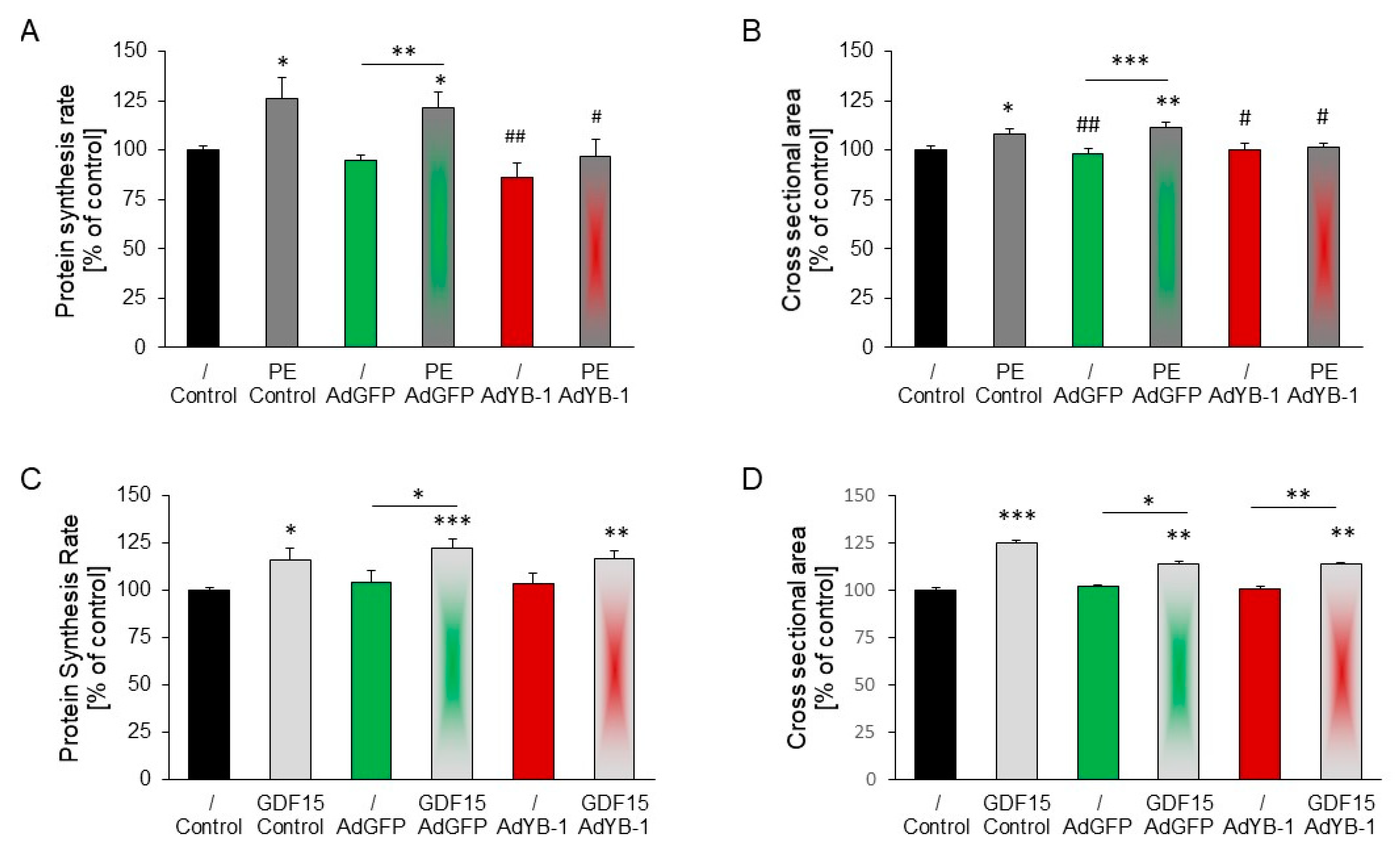
2.7. Inhibition of PE-Induced but Not GDF15-Induced Hypertrophy in YB-1-Overexpressing Cardiomyocytes
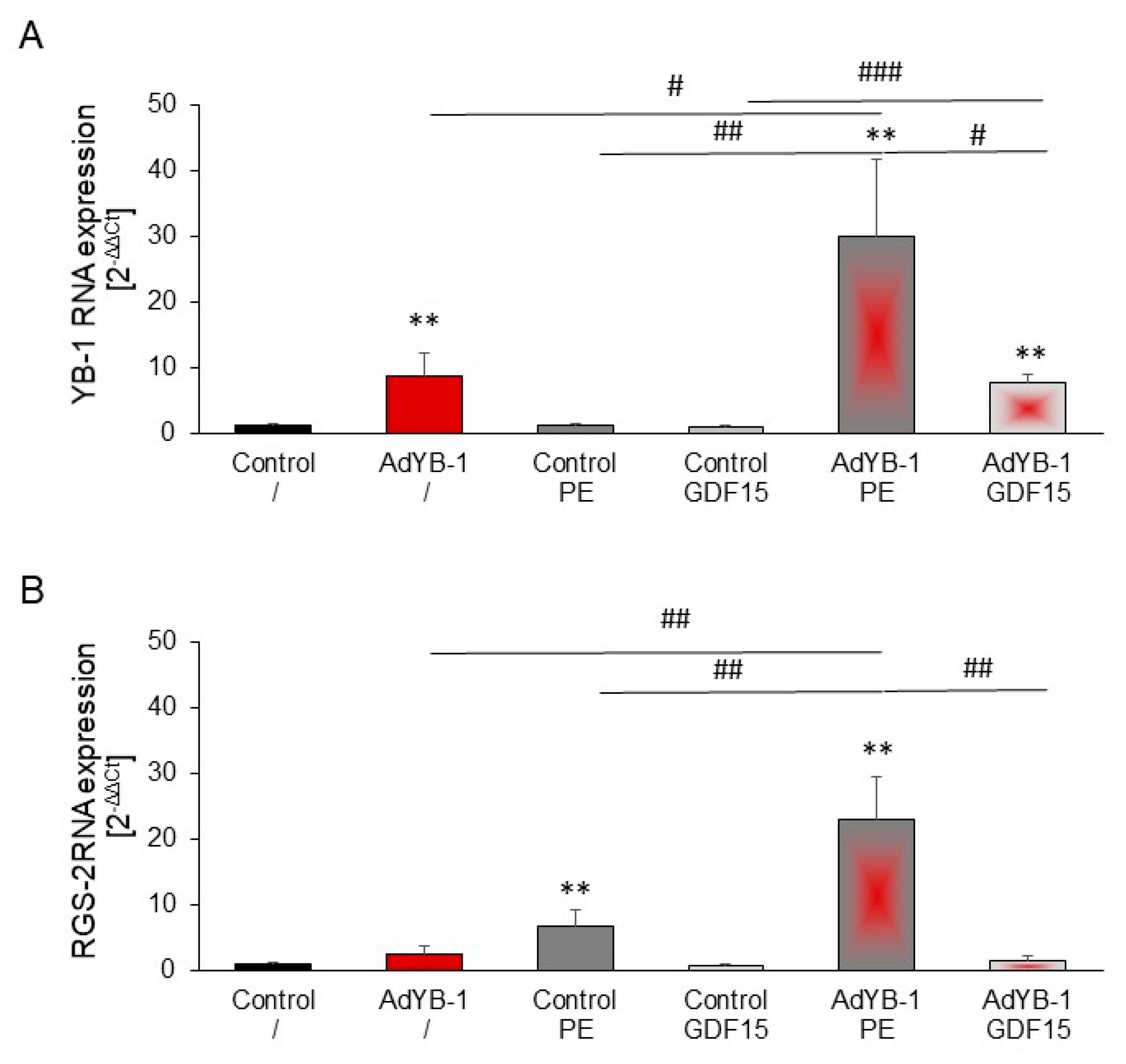
2.8. Induction of RGS2 under YB-1 Overexpression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Lines
4.3. Human Samples
4.4. Materials
4.5. Recombinant Adenovirus Construction
4.6. Cell Culture Analysis
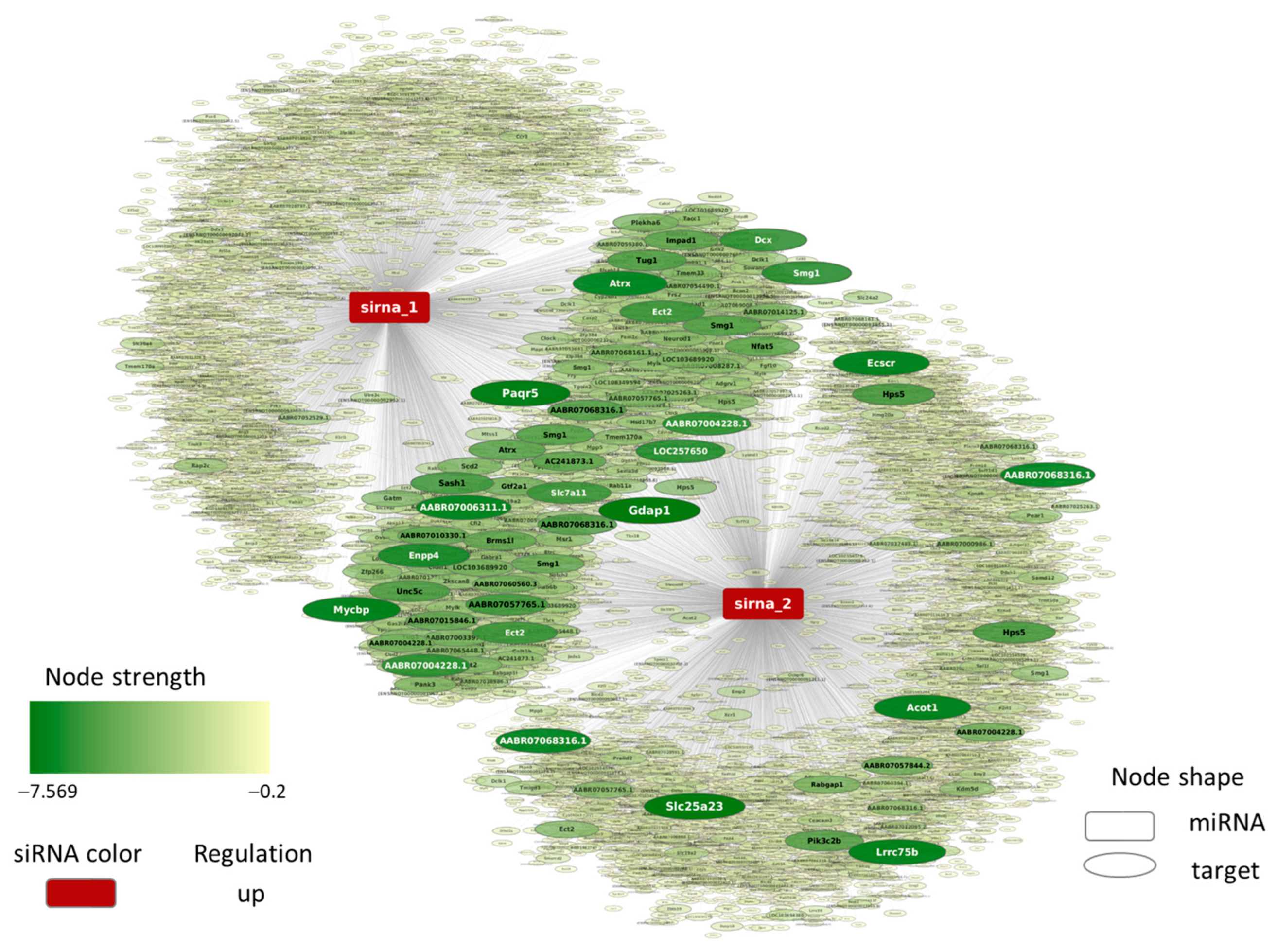
4.7. MicroRNA-like Off-Target Prediction for YB-1 siRNA
4.8. Real-Time qRT-PCR
4.9. Western Blots
4.10. Cardiomyocytes Monitoring and Cell Size Determination
4.11. Protein Synthesis Rate Determination
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICM | Ischemic cardiomyopathy |
| PE | Phenylephrine |
| YB-1 | Y-box binding protein 1 |
| GDF15 | Growth differentiation factor 15 |
| IFN-γ | Interferon-gamma |
| TGFβ | Transforming growth factor-beta |
| RGS | Regulator of G-protein signaling |
References
- Weil, J.; Schunkert, H. Pathophysiology of chronic heart failure. Clin. Res. Cardiol. 2006, 95 (Suppl. S4), 1–15. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Haider, N.; Virmani, R.; DiSalvo, T.G.; Kolodgie, F.D.; Hajjar, R.J.; Schmidt, U.; Semigran, M.J.; Dec, G.W.; Khaw, B.A. Apoptosis in myocytes in end-stage heart failure. N. Engl. J. Med. 1996, 335, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Kenchaiah, S.; Larson, M.G.; Benjamin, E.J.; Kupka, M.J.; Ho, K.K.; Murabito, J.M.; Vasan, R.S. Long-term trends in the incidence of and survival with heart failure. N. Engl. J. Med. 2002, 347, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Kamalov, G.; Varma, B.R.; Lu, L.; Sun, Y.; Weber, K.T.; Guntaka, R.V. Expression of the multifunctional Y-box protein, YB-1, in myofibroblasts of the infarcted rat heart. Biochem. Biophys. Res. Commun. 2005, 334, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Choong, O.K.; Chen, C.Y.; Zhang, J.; Lin, J.H.; Lin, P.J.; Ruan, S.C.; Kamp, T.J.; Hsieh, P.C.H. Hypoxia-induced H19/YB-1 cascade modulates cardiac remodeling after infarction. Theranostics 2019, 9, 6550–6567. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Izumi, H.; Uchiumi, T.; Ashizuka, M.; Kuwano, M. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays 2003, 25, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Stickeler, E.; Fraser, S.D.; Honig, A.; Chen, A.L.; Berget, S.M.; Cooper, T.A. The RNA binding protein YB-1 binds A/C-rich exon enhancers and stimulates splicing of the CD44 alternative exon v4. EMBO J. 2001, 20, 3821–3830. [Google Scholar] [CrossRef] [PubMed]
- Marenstein, D.R.; Ocampo, M.T.; Chan, M.K.; Altamirano, A.; Basu, A.K.; Boorstein, R.J.; Cunningham, R.P.; Teebor, G.W. Stimulation of human endonuclease III by Y box-binding protein 1 (DNA-binding protein B). Interaction between a base excision repair enzyme and a transcription factor. J. Biol. Chem. 2001, 276, 21242–21249. [Google Scholar] [CrossRef]
- Kolluri, R.; Torrey, T.A.; Kinniburgh, A.J. A CT promoter element binding protein: Definition of a double-strand and a novel single-strand DNA binding motif. Nucleic Acids Res. 1992, 20, 111–116. [Google Scholar] [CrossRef]
- Tafuri, S.R.; Wolffe, A.P. Selective recruitment of masked maternal mRNA from messenger ribonucleoprotein particles containing FRGY2 (mRNP4). J. Biol. Chem. 1993, 268, 24255–24261. [Google Scholar] [CrossRef]
- Wolffe, A.P. Structural and functional properties of the evolutionarily ancient Y-box family of nucleic acid binding proteins. Bioessays 1994, 16, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Inagaki, Y.; Fujimori, K.; Nakao, A.; Kaneko, H.; Nakatsuka, I. Interferon-gamma interferes with transforming growth factor-beta signaling through direct interaction of YB-1 with Smad3. J. Biol. Chem. 2003, 278, 43470–43479. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Inagaki, Y.; Suzuki, N.; Mitsui, S.; Mauviel, A.; Kaneko, H.; Nakatsuka, I. Y-box-binding protein YB-1 mediates transcriptional repression of human alpha 2(I) collagen gene expression by interferon-gamma. J. Biol. Chem. 2003, 278, 5156–5162. [Google Scholar] [CrossRef] [PubMed]
- Didier, D.K.; Schiffenbauer, J.; Woulfe, S.L.; Zacheis, M.; Schwartz, B.D. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc. Natl. Acad. Sci. USA 1988, 85, 7322–7326. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.H.; Itoh-Lindstrom, Y.; Ting, J.P. The transcriptional regulatory protein, YB-1, promotes single-stranded regions in the DRA promoter. J. Biol. Chem. 1995, 270, 3527–3533. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.P.; Painter, A.; Zeleznik-Le, N.J.; MacDonald, G.; Moore, T.M.; Brown, A.; Schwartz, B.D. YB-1 DNA-binding protein represses interferon gamma activation of class II major histocompatibility complex genes. J. Exp. Med. 1994, 179, 1605–1611. [Google Scholar] [CrossRef]
- Zou, M.-X.; Roy, A.A.; Zhao, Q.; Kirshenbaum, L.A.; Karmazyn, M.; Chidiac, M.P. RGS2 is upregulated by and attenuates the hypertrophic effect of alpha1-adrenergic activation in cultured ventricular myocytes. Cell Signal. 2006, 18, 1655–1663. [Google Scholar] [CrossRef]
- Cotecchia, S.; Del Vescovo, C.D.; Colella, M.; Caso, S.; Diviani, D. The alpha1-adrenergic receptors in cardiac hypertrophy: Signaling mechanisms and functional implications. Cell Signal. 2015, 27, 1984–1993. [Google Scholar] [CrossRef]
- Taimor, G.; Schlüter, K.D.; Best, P.; Helmig, S.; Piper, H.M. Transcription activator protein 1 mediates alpha- but not beta-adrenergic hypertrophic growth responses in adult cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2369–H2375. [Google Scholar] [CrossRef]
- Samuel, S.; Twizere, J.-C.; Bernstein, L.R. YB-1 represses AP1-dependent gene transactivation and interacts with an AP-1 DNA sequence. Biochem. J. 2005, 388 Pt 3, 921–928. [Google Scholar] [CrossRef][Green Version]
- Heger, J.; Schiegnitz, E.; von Waldthausen, D.; Anwar, M.M.; Piper, H.M.; Euler, G. Growth differentiation factor 15 acts anti-apoptotic and pro-hypertrophic in adult cardiomyocytes. J. Cell. Physiol. 2010, 224, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kempf, T.; von Haehling, S.; Peter, T.; Allhoff, T.; Cicoira, M.; Doehner, W.; Ponikowski, P.; Filippatos, G.S.; Rozentryt, P.; Drexler, H.; et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, L.; Hou, Y.; Li, Y.; Zhang, J.; Yang, N.; Zhang, Y.; Ji, W.; Yu, T.; Lv, L.; et al. Long non-coding RNA KCND1 protects hearts from hypertrophy by targeting YBX1. Cell Death Dis. 2023, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wang, T.; Xie, Y.; Wang, M.; Zhang, W.; Dai, L.; Lai, J.; Nie, X.; He, X.; Madhusudhan, T.; et al. Activated Protein C Ameliorates Diabetic Cardiomyopathy via Modulating OTUB1/YB-1/MEF2B Axis. Front. Cardiovasc. Med. 2021, 8, 758158. [Google Scholar] [CrossRef]
- Kojic, S.; . Medeot, E.; Guccione, E.; Krmac, H.; Zara, I.; Martinelli, V.; Valle, G.; Faulkner, G. The Ankrd2 protein, a link between the sarcomere and the nucleus in skeletal muscle. J. Mol. Biol. 2004, 339, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Lasham, A.; Samuel, W.; Cao, H.; Patel, R.; Mehta, R.; Stern, J.L.; Reid, G.; Woolley, A.G.; Miller, L.D.; Black, M.A.; et al. YB-1, the E2F pathway, and regulation of tumor cell growth. J. Natl. Cancer Inst. 2012, 104, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, R.; Gu, M.; Li, S.; Zhang, B.; Chi, Z.; Hao, L. shRNA-Mediated Silencing of Y-Box Binding Protein-1 (YB-1) Suppresses Growth of Neuroblastoma Cell SH-SY5Y In Vitro and In Vivo. PLoS ONE 2015, 10, e0127224. [Google Scholar] [CrossRef] [PubMed]
- Varma, E.; Burghaus, J.; Riechert, E.; Katus, H.A.; Völkers, M. Translational Control of Y-Box Binding Protein 1 (Yb-1) During Pathological Cardiac Hypertrophy. Circulation 2018, 138 (Suppl. S1), A13455. [Google Scholar]
- Sinnberg, T.; Sauer, B.; Holm, P.; Spangler, B.; Kuphal, S.; Bosserhoff, A.; Schittek, B. MAPK and PI3K/AKT mediated YB-1 activation promotes melanoma cell proliferation which is counteracted by an autoregulatory loop. Exp. Dermatol. 2012, 21, 265–270. [Google Scholar] [CrossRef]
- Schlüter, K.D.; Goldberg, Y.; Taimor, G.; Schäfer, M.; Piper, H.M. Role of phosphatidylinositol 3-kinase activation in the hypertrophic growth of adult ventricular cardiomyocytes. Cardiovasc. Res. 1998, 40, 174–181. [Google Scholar] [CrossRef]
- Evdokimova, V.; Ovchinnikov, L.P.; Sorensen, P.H. Y-box binding protein 1: Providing a new angle on translational regulation. Cell Cycle 2006, 5, 1143–1147. [Google Scholar] [CrossRef]
- Evdokimova, V.; Ruzanov, P.; Imataka, H.; Raught, B.; Svitkin, Y.; Ovchinnikov, L.P.; Sonenberg, N. The major mRNA-associated protein YB-1 is a potent 5' cap-dependent mRNA stabilizer. EMBO J. 2001, 20, 5491–5502. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Felts, K.A.; Jiang, N.; Chang, H.W.; PVogt, K. Y box-binding protein 1 induces resistance to oncogenic transformation by the phosphatidylinositol 3-kinase pathway. Proc. Natl. Acad. Sci. USA 2003, 100, 12384–12389. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Vogt, P.K. An essential role for protein synthesis in oncogenic cellular transformation. Oncogene 2004, 23, 3145–3150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schlüter, K.D.; Piper, H.M. Trophic effects of catecholamines and parathyroid hormone on adult ventricular cardiomyocytes. Am. J. Physiol. 1992, 263, H1739–H1746. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.; Woo, A.Y.; Zhu, W.; Xiao, R.P. Deregulation of RGS2 in cardiovascular diseases. Front. Biosci. (Schol. Ed.) 2010, 2, 547–557. [Google Scholar] [PubMed]
- Samuel, S.; Beifuss, K.K.; Bernstein, L.R. YB-1 binds to the MMP-13 promoter sequence and represses MMP-13 transactivation via the AP-1 site. Biochim. Biophys. Acta 2007, 1769, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Omura, T.; Yoshiyama, M.; Yoshida, K.; Nakamura, Y.; Kim, S.; Iwao, H.; Takeuchi, K.; Yoshikawa, J. Dominant negative mutant of c-Jun inhibits cardiomyocyte hypertrophy induced by endothelin 1 and phenylephrine. Hypertension 2002, 39, 81–86. [Google Scholar] [CrossRef]
- Wenzel, S.; Taimor, G.; Piper, H.M.; Schlüter, K.D. Redox-sensitive intermediates mediate angiotensin II-induced p38 MAP kinase activation, AP-1 binding activity, and TGF-beta expression in adult ventricular cardiomyocytes. FASEB J. 2001, 15, 2291–2293. [Google Scholar] [CrossRef]
- Said, H.M.; Gressner, A.M.; Floege, J.; En-Nia, A.; Mertens, P.R. Y-box protein-1 is the crucial mediator of antifibrotic interferon-gamma effects. J. Biol. Chem. 2006, 281, 1784–1795. [Google Scholar]
- Piper, H.M.; Probst, I.; Schwartz, P.; Hütter, F.J.; Spieckermann, P.G. Culturing of calcium stable adult cardiac myocytes. J. Mol. Cell. Cardiol. 1982, 14, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Barte, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4e, 05005. [Google Scholar] [CrossRef] [PubMed]
- Karolchik, D.; Hinrichs, A.S.; Furey, T.S.; Roskin, K.M.; Sugnet, C.W.; Haussler, D.; Kent, W.J. The UCSC Table Browser data retrieval tool. Nucleic Acids Res. 2004, 32, D493–D496. [Google Scholar] [CrossRef] [PubMed]
- Ágg, B.; Baranyai, T.; Makkos, A.; Vető, B.; Faragó, N.; Zvara, Á.; Giricz, Z.; Veres, D.V.; Csermely, P.; Arányi, T.; et al. Author Correction: MicroRNA interactome analysis predicts post-transcriptional regulation of ADRB2 and PPP3R1 in the hypercholesterolemic myocardium. Sci. Rep. 2018, 8, 12159, Erratum in Sci. Rep. 2018, 8, 10134. [Google Scholar] [CrossRef] [PubMed]
- Bencsik, P.; Kiss, K.; Ágg, B.; Baán, J.A.; Ágoston, G.; Varga, A.; Gömöri, K.; Mendler, L.; Faragó, N.; Zvara, Á.; et al. Sensory Neuropathy Affects Cardiac miRNA Expression Network Targeting IGF-1, SLC2a-12, EIF-4e, and ULK-2 mRNAs. Int. J. Mol. Sci. 2019, 20, 991. [Google Scholar] [CrossRef] [PubMed]
- Sághy, É.; Vörös, I.; Ágg, B.; Kiss, B.; Koncsos, G.; Varga, Z.V.; Görbe, A.; Giricz, Z.; Schulz, R.; Ferdinandy, P. Cardiac miRNA Expression and their mRNA Targets in a Rat Model of Prediabetes. Int. J. Mol. Sci. 2020, 21, 2128. [Google Scholar] [CrossRef] [PubMed]
- Schreckenberg, R.; Klein, J.; Kutsche, H.S.; Schulz, R.; Gömöri, K.; Bencsik, P.; Benczik, B.; Ágg, B.; Sághy, É.; Ferdinandy, P.; et al. Ischaemic post-conditioning in rats: Responder and non-responder differ in transcriptome of mitochondrial proteins. J. Cell. Mol. Med. 2020, 24, 5528–5541. [Google Scholar] [CrossRef] [PubMed]
- Makkos, A.; Ágg, B.; Petrovich, B.; Varga, Z.V.; Görbe, A.; Ferdinandy, P. Systematic review and network analysis of microRNAs involved in cardioprotection against myocardial ischemia/reperfusion injury and infarction: Involvement of redox signalling. Free Radic. Biol. Med. 2021, 172, 237–251. [Google Scholar] [CrossRef]
- Róka, B.; Tod, P.; Kaucsár, T.; Bukosza, É.N.; Vörös, I.; Varga, Z.V.; Petrovich, B.; Ágg, B.; Ferdinandy, P.; Szénási, G.; et al. Delayed Contralateral Nephrectomy Halted Post-Ischemic Renal Fibrosis Progression and Inhibited the Ischemia-Induced Fibromir Upregulation in Mice. Biomedicines 2021, 9, 815. [Google Scholar] [CrossRef]
- Makkos, A.; Ágg, B.; Varga, Z.V.; Giricz, Z.; Gyöngyösi, M.; Lukovic, D.; Schulz, R.; Barteková, M.; Görbe, A.; Ferdinandy, P. Molecular Network Approach Reveals Rictor as a Central Target of Cardiac ProtectomiRs. Int. J. Mol. Sci. 2021, 22, 9539. [Google Scholar] [CrossRef]
- Aczél, T.; Benczik, B.; Ágg, B.; Körtési, T.; Urbán, P.; Bauer, W.; Gyenesei, A.; Tuka, B.; Tajti, J.; Ferdinandy, P.; et al. Disease- and headache-specific microRNA signatures and their predicted mRNA targets in peripheral blood mononuclear cells in migraineurs: Role of inflammatory signalling and oxidative stress. J. Headache Pain 2022, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Ágg, B.; Császár, A.; Szalay-Bekő, M.; Veres, D.V.; Mizsei, R.; Ferdinandy, P.; Csermely, P.; Kovács, I.A. The EntOptLayout Cytoscape plug-in for the efficient visualization of major protein complexes in protein-protein interaction and signalling networks. Bioinformatics 2019, 35, 4490–4492. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; . Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Gene Ontology Consortium. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Schäfer, M.; Pönicke, K.; Heinroth-Hoffmann, I.; Brodde, O.E.; Piper, H.M.; Schlüter, K.D. β-Adrenoceptor stimulation attenuates the hypertrophic effect of α-adrenoceptor stimulation in adult rat ventricular cardiomyocytes. J. Am. Coll. Cardiol. 2001, 37, 300–307. [Google Scholar] [CrossRef]
- Pinson, A.; Schlüter, K.D.; Zhou, X.J.; Schwartz, P.; Kessler-Icekson, G.; Piper, H.M. α- and β-adrenergic stimulation of protein synthesis in cultured adult ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 1993, 25, 477–490. [Google Scholar] [CrossRef]
- Godfrey, K. Comparing the means of several groups. N. Engl. J. Med. 1985, 313, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heger, J.; Partsch, S.; Harjung, C.; Varga, Z.V.; Baranyai, T.; Weiß, J.; Kremer, L.; Locquet, F.; Leszek, P.; Ágg, B.; et al. YB-1 Is a Novel Target for the Inhibition of α-Adrenergic-Induced Hypertrophy. Int. J. Mol. Sci. 2024, 25, 401. https://doi.org/10.3390/ijms25010401
Heger J, Partsch S, Harjung C, Varga ZV, Baranyai T, Weiß J, Kremer L, Locquet F, Leszek P, Ágg B, et al. YB-1 Is a Novel Target for the Inhibition of α-Adrenergic-Induced Hypertrophy. International Journal of Molecular Sciences. 2024; 25(1):401. https://doi.org/10.3390/ijms25010401
Chicago/Turabian StyleHeger, Jacqueline, Stefan Partsch, Claudia Harjung, Zoltán V. Varga, Tamás Baranyai, Johannes Weiß, Lea Kremer, Fabian Locquet, Przemyslaw Leszek, Bence Ágg, and et al. 2024. "YB-1 Is a Novel Target for the Inhibition of α-Adrenergic-Induced Hypertrophy" International Journal of Molecular Sciences 25, no. 1: 401. https://doi.org/10.3390/ijms25010401
APA StyleHeger, J., Partsch, S., Harjung, C., Varga, Z. V., Baranyai, T., Weiß, J., Kremer, L., Locquet, F., Leszek, P., Ágg, B., Benczik, B., Ferdinandy, P., Schulz, R., & Euler, G. (2024). YB-1 Is a Novel Target for the Inhibition of α-Adrenergic-Induced Hypertrophy. International Journal of Molecular Sciences, 25(1), 401. https://doi.org/10.3390/ijms25010401







