Transcriptional Inflammatory Signature in Healthy Donors and Different Radiotherapy Cancer Patients
Abstract
1. Introduction
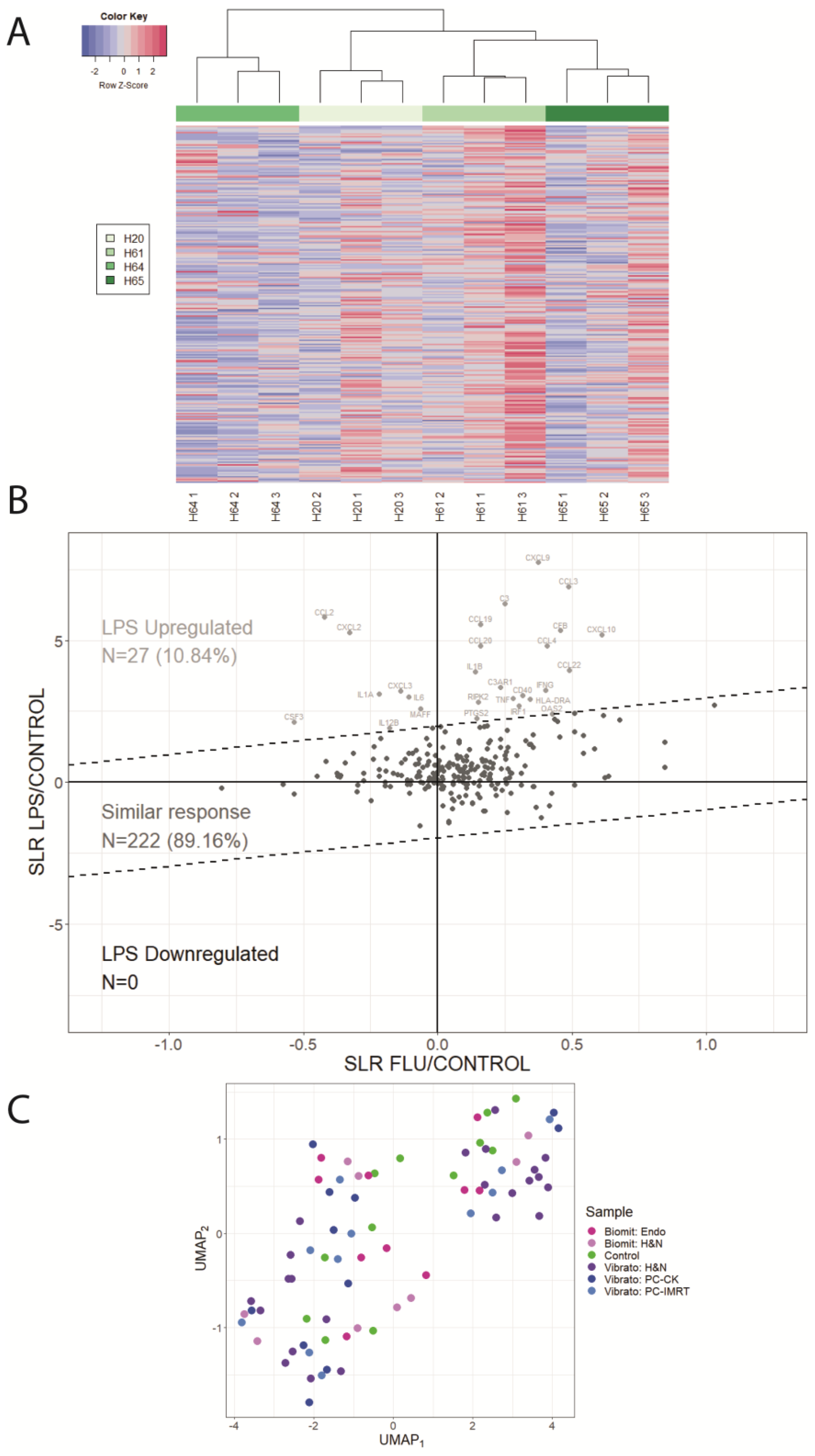
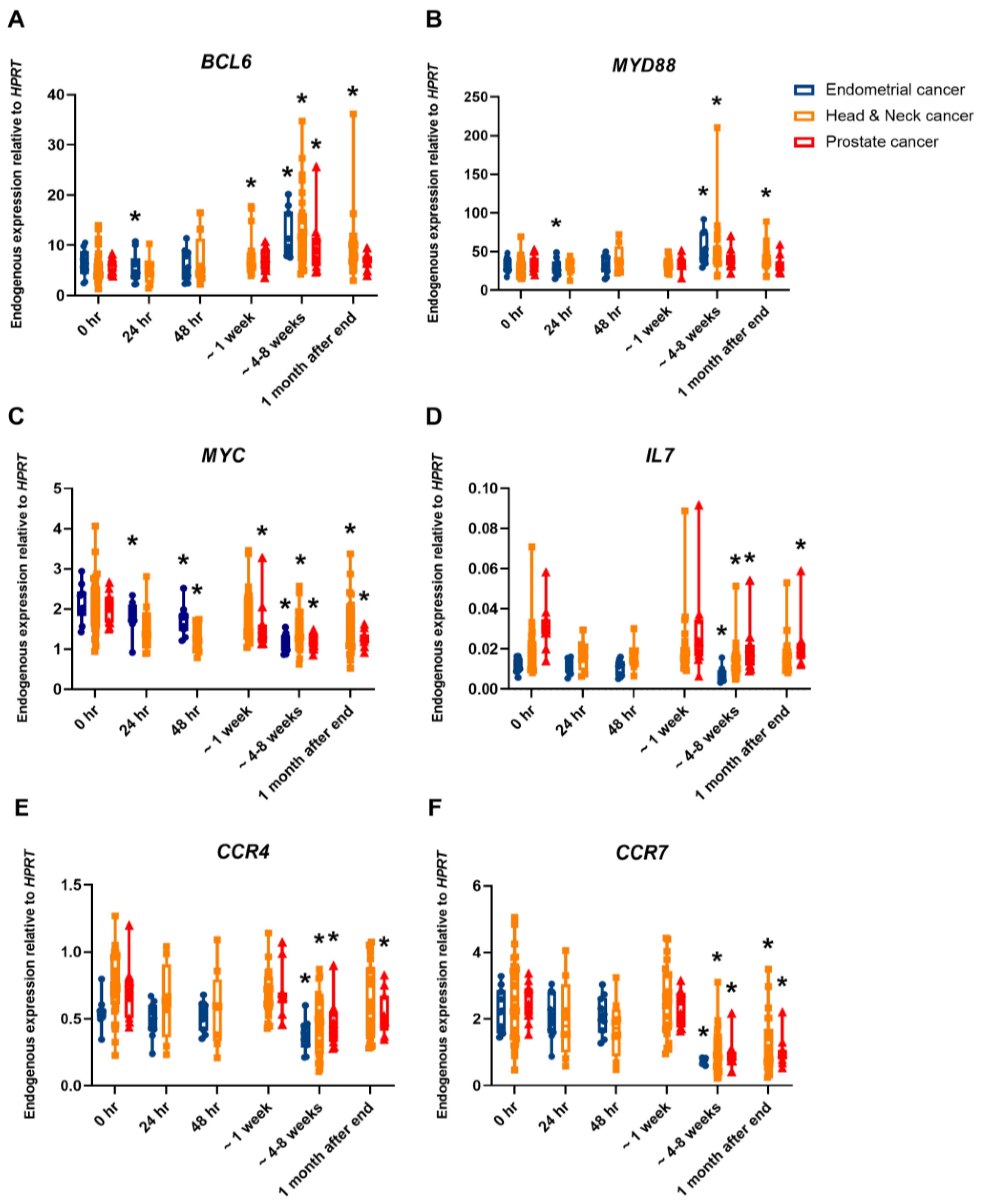
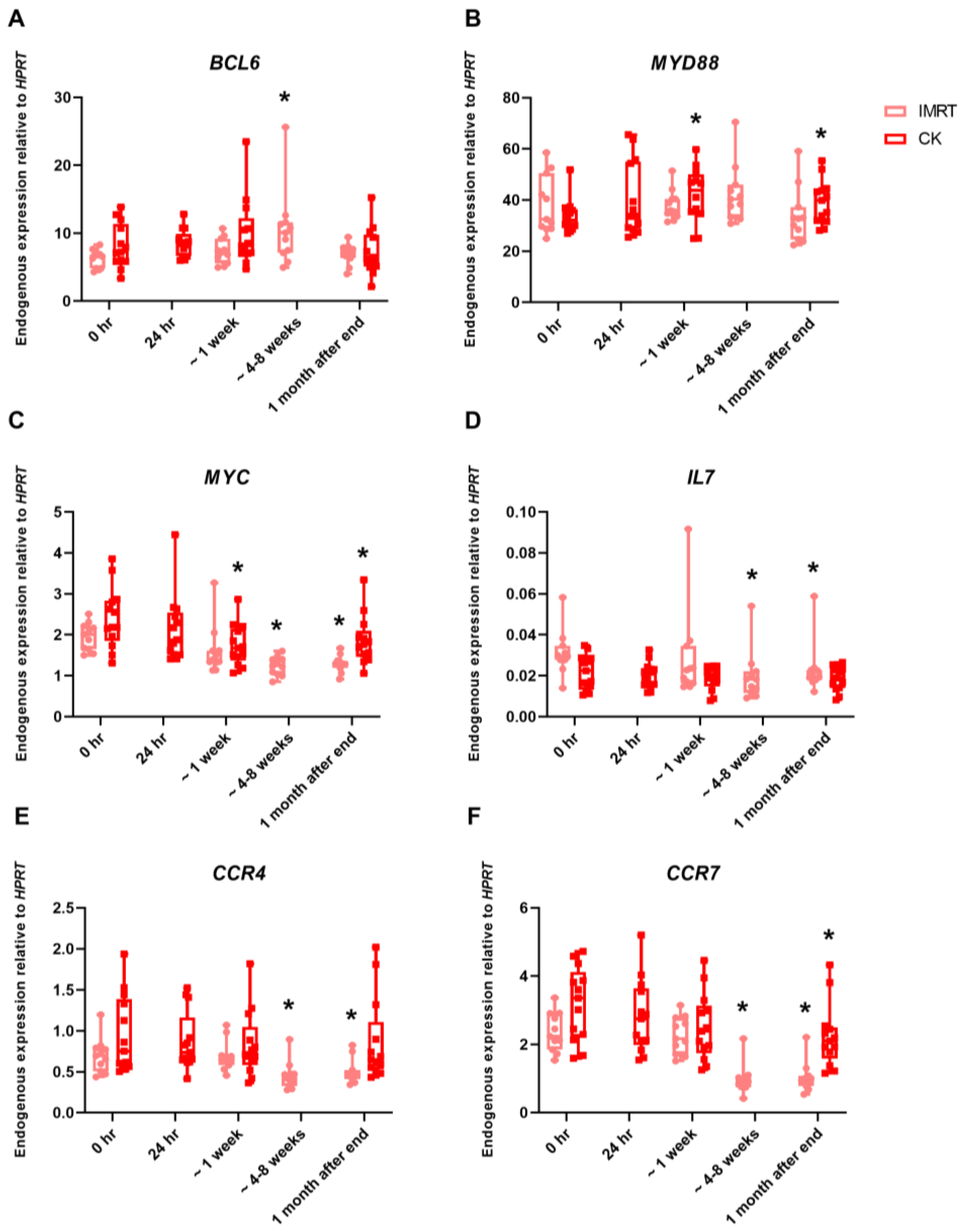
2. Results
3. Discussion
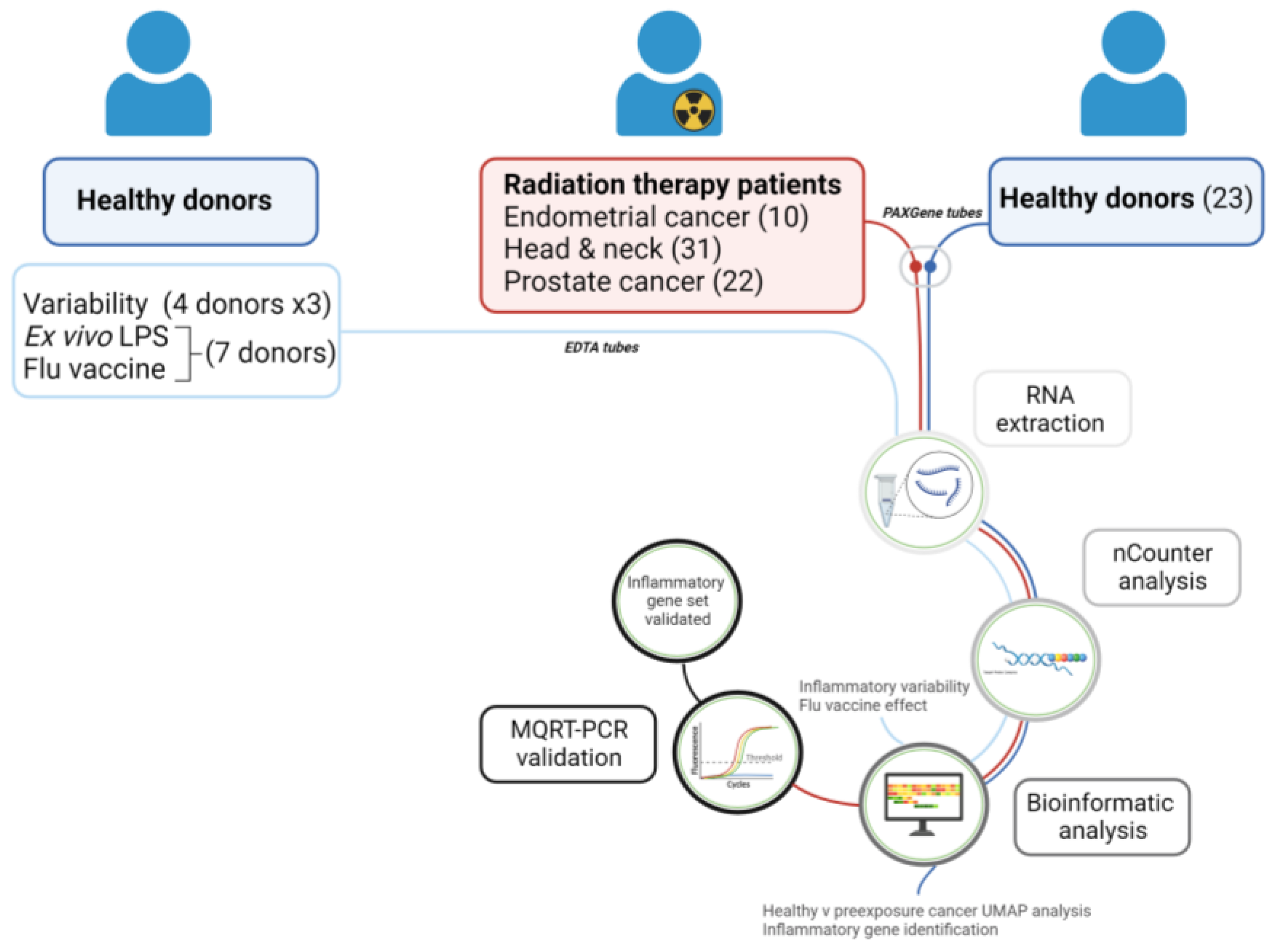
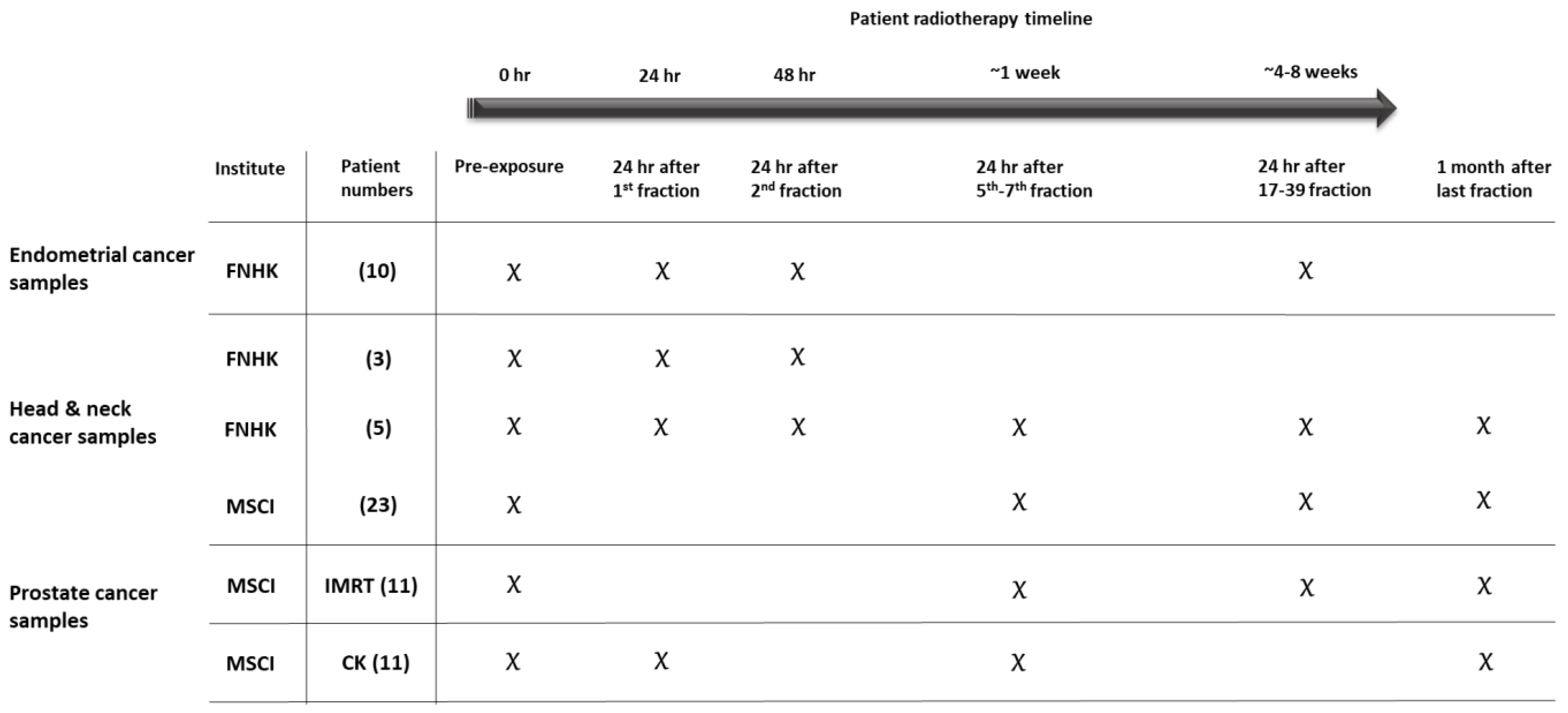
4. Materials and Methods
4.1. Bioethics
4.2. Blood Collection—Healthy Donors
4.3. Blood Collection—Cancer Patients
4.4. Blood Stimulated with LPS or Flu Vaccine
4.5. RNA Extraction
4.6. nCounter Analysis
4.7. Data Analysis
4.8. Gene Expression Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kabacik, S.; Mackay, A.; Tamber, N.; Manning, G.; Finnon, P.; Paillier, F.; Ashworth, A.; Bouffler, S.; Badie, C. Gene expression following ionising radiation: Identification of biomarkers for dose estimation and prediction of individual response. Int. J. Radiat. Biol. 2011, 87, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Kabacik, S.; Finnon, P.; Bouffler, S.; Badie, C. High and low dose responses of transcriptional biomarkers in ex vivo X-irradiated human blood. Int. J. Radiat. Biol. 2013, 89, 512–522. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Constanzo, J.; Faget, J.; Ursino, C.; Badie, C.; Pouget, J.P. Radiation-Induced Immunity and Toxicities: The Versatility of the cGAS-STING Pathway. Front. Immunol. 2021, 12, 680503. [Google Scholar] [CrossRef] [PubMed]
- El-Saghire, H.; Thierens, H.; Monsieurs, P.; Michaux, A.; Vandevoorde, C.; Baatout, S. Gene set enrichment analysis highlights different gene expression profiles in whole blood samples X-irradiated with low and high doses. Int. J. Radiat. Biol. 2013, 89, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Smilenov, L.B.; Amundson, S.A. Widespread decreased expression of immune function genes in human peripheral blood following radiation exposure. Radiat. Res. 2013, 180, 575–583. [Google Scholar] [CrossRef]
- Cruz-Garcia, L.; Badie, C.; Anbalagan, S.; Moquet, J.; Gothard, L.; O’Brien, G.; Somaiah, N.; Ainsbury, E.A. An ionising radiation-induced specific transcriptional signature of inflammation-associated genes in whole blood from radiotherapy patients: A pilot study. Radiat. Oncol. 2021, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Balázs, K.; Kis, E.; Badie, C.; Bogdándi, E.N.; Candéias, S.; Cruz Garcia, L.; Dominczyk, I.; Frey, B.; Gaipl, U.; Jurányi, Z.; et al. Radiotherapy-Induced Changes in the Systemic Immune and Inflammation Parameters of Head and Neck Cancer Patients. Cancers 2019, 11, 1324. [Google Scholar] [CrossRef]
- Manning, G.; Tichý, A.; Sirák, I.; Badie, C. Radiotherapy-Associated Long-term Modification of Expression of the Inflammatory Biomarker Genes ARG1, BCL2L1, and MYC. Front. Immunol. 2017, 8, 412. [Google Scholar] [CrossRef]
- Jardin, F.; Ruminy, P.; Bastard, C.; Tilly, H. The BCL6 proto-oncogene: A leading role during germinal center development and lymphomagenesis. Pathol. Biol. 2007, 55, 73–83. [Google Scholar] [CrossRef]
- Huang, C.; Hatzi, K.; Melnick, A. Lineage-specific functions of Bcl-6 in immunity and inflammation are mediated by distinct biochemical mechanisms. Nat. Immunol. 2013, 14, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Lin, H.; Wang, L.; Chen, X.; Liu, Q.; Zuo, Q.; Hu, J.; Wang, H.; Guo, J.; et al. Bcl6 Preserves the Suppressive Function of Regulatory T Cells During Tumorigenesis. Front. Immunol. 2020, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Barman, I.S.M. MyD88 and Cancer. Explor. Res. Hypothesis Med. 2016, 1, 29–33. [Google Scholar]
- Brackett, C.M.; Greene, K.F.; Aldrich, A.R.; Trageser, N.H.; Pal, S.; Molodtsov, I.; Kandar, B.M.; Burdelya, L.G.; Abrams, S.I.; Gudkov, A.V. Signaling through TLR5 mitigates lethal radiation damage by neutrophil-dependent release of MMP-9. Cell Death Discov. 2021, 7, 266. [Google Scholar] [CrossRef]
- Burdelya, L.G.; Krivokrysenko, V.I.; Tallant, T.C.; Strom, E.; Gleiberman, A.S.; Gupta, D.; Kurnasov, O.V.; Fort, F.L.; Osterman, A.L.; Didonato, J.A.; et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008, 320, 226–230. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, C.; Mitchel, R.E.; Cui, J.; Lin, J.; Yang, Y.; Liu, X.; Cai, J. A critical role of toll-like receptor 4 (TLR4) and its’ in vivo ligands in basal radio-resistance. Cell Death Dis. 2013, 4, e649. [Google Scholar] [CrossRef] [PubMed]
- Djureinovic, D.; Wang, M.; Kluger, H.M. Agonistic CD40 Antibodies in Cancer Treatment. Cancers 2021, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Bories, J.C.; Willerford, D.M.; Grevin, D.; Davidson, L.; Camus, A.; Martin, P.; Stehelin, D.; Alt, F.W. Increased T-cell apoptosis and terminal B-cell differentiation induced by inactivation of the Ets-1 proto-oncogene. Nature 1995, 377, 635–638. [Google Scholar] [CrossRef]
- Duddy, M.E.; Alter, A.; Bar-Or, A. Distinct profiles of human B cell effector cytokines: A role in immune regulation? J. Immunol. 2004, 172, 3422–3427. [Google Scholar] [CrossRef]
- Muthusamy, N.; Barton, K.; Leiden, J.M. Defective activation and survival of T cells lacking the Ets-1 transcription factor. Nature 1995, 377, 639–642. [Google Scholar] [CrossRef]
- Hipp, N.; Symington, H.; Pastoret, C.; Caron, G.; Monvoisin, C.; Tarte, K.; Fest, T.; Delaloy, C. IL-2 imprints human naive B cell fate towards plasma cell through ERK/ELK1-mediated BACH2 repression. Nat. Commun. 2017, 8, 1443. [Google Scholar] [CrossRef] [PubMed]
- Bonecchi, R.; Bianchi, G.; Bordignon, P.P.; D’Ambrosio, D.; Lang, R.; Borsatti, A.; Sozzani, S.; Allavena, P.; Gray, P.A.; Mantovani, A.; et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998, 187, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Kojder, K.; Siminska, D.; Bohatyrewicz, R.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. Int. J. Mol. Sci. 2020, 21, 8412. [Google Scholar] [CrossRef]
- Wiedemann, G.M.; Knott, M.M.; Vetter, V.K.; Rapp, M.; Haubner, S.; Fesseler, J.; Kuhnemuth, B.; Layritz, P.; Thaler, R.; Kruger, S.; et al. Cancer cell-derived IL-1alpha induces CCL22 and the recruitment of regulatory T cells. Oncoimmunology 2016, 5, e1175794. [Google Scholar] [CrossRef] [PubMed]
- Karasaki, T.; Qiang, G.; Anraku, M.; Sun, Y.; Shinozaki-Ushiku, A.; Sato, E.; Kashiwabara, K.; Nagayama, K.; Nitadori, J.I.; Sato, M.; et al. High CCR4 expression in the tumor microenvironment is a poor prognostic indicator in lung adenocarcinoma. J. Thorac. Dis. 2018, 10, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.J. Kidney cancer: CCR4: A new target for RCC. Nat. Rev. Nephrol. 2017, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, Y.S.; Lee, J.Y.; Kook, M.C.; Park, J.W.; Nam, B.H.; Bae, J.M. The chemokine receptor CCR4 is expressed and associated with a poor prognosis in patients with gastric cancer. Ann. Surg. 2009, 249, 933–941. [Google Scholar] [CrossRef]
- Li, J.Y.; Ou, Z.L.; Yu, S.J.; Gu, X.L.; Yang, C.; Chen, A.X.; Di, G.H.; Shen, Z.Z.; Shao, Z.M. The chemokine receptor CCR4 promotes tumor growth and lung metastasis in breast cancer. Breast Cancer Res. Treat. 2012, 131, 837–848. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Zhu, Y.; Zhang, X.; Yang, Y.; Wang, C. CCR4 Expression Is Associated with Poor Prognosis in Patients with Early Stage (pN0) Oral Tongue Cancer. J. Oral Maxillofac. Surg. 2019, 77, 426–432. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Li, L.; Mao, W.; Shen, D.; Yao, N.; Zhang, L. CCR4 is a prognostic biomarker and correlated with immune infiltrates in head and neck squamous cell carcinoma. Ann. Transl. Med. 2021, 9, 1443. [Google Scholar] [CrossRef]
- Meng, L.; He, X.; Hong, Q.; Qiao, B.; Zhang, X.; Wu, B.; Zhang, X.; Wei, Y.; Li, J.; Ye, Z.; et al. CCR4, CCR8, and P2RY14 as Prognostic Factors in Head and Neck Squamous Cell Carcinoma Are Involved in the Remodeling of the Tumor Microenvironment. Front. Oncol. 2021, 11, 618187. [Google Scholar] [CrossRef]
- Zhong, Y.; Lin, Z.; Lu, J.; Lin, X.; Xu, W.; Wang, N.; Huang, S.; Wang, Y.; Zhu, Y.; Chen, Z.; et al. CCL2-CCL5/CCR4 contributed to radiation-induced epithelial-mesenchymal transition of HPAEpiC cells via the ERK signaling pathways. Am. J. Transl. Res. 2019, 11, 733–743. [Google Scholar]
- Rizeq, B.; Malki, M.I. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers 2020, 12, 1036. [Google Scholar] [CrossRef]
- Brandum, E.P.; Jorgensen, A.S.; Rosenkilde, M.M.; Hjorto, G.M. Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. Int. J. Mol. Sci. 2021, 22, 8340. [Google Scholar] [CrossRef]
- Christensen, E.; Pintilie, M.; Evans, K.R.; Lenarduzzi, M.; Menard, C.; Catton, C.N.; Diamandis, E.P.; Bristow, R.G. Longitudinal cytokine expression during IMRT for prostate cancer and acute treatment toxicity. Clin. Cancer Res. 2009, 15, 5576–5583. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, L.; Wan, Y.Y.; Zhu, B. Mechanism of Action of IL-7 and Its Potential Applications and Limitations in Cancer Immunotherapy. Int. J. Mol. Sci. 2015, 16, 10267–10280. [Google Scholar] [CrossRef]
- Byun, H.K.; Kim, K.J.; Han, S.C.; Seong, J. Effect of Interleukin-7 on Radiation-Induced Lymphopenia and Its Antitumor Effects in a Mouse Model. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1559–1569. [Google Scholar] [CrossRef]
- Murn, J.; Mlinaric-Rascan, I.; Vaigot, P.; Alibert, O.; Frouin, V.; Gidrol, X. A Myc-regulated transcriptional network controls B-cell fate in response to BCR triggering. BMC Genom. 2009, 10, 323. [Google Scholar] [CrossRef]
- Preston, G.C.; Sinclair, L.V.; Kaskar, A.; Hukelmann, J.L.; Navarro, M.N.; Ferrero, I.; MacDonald, H.R.; Cowling, V.H.; Cantrell, D.A. Single cell tuning of Myc expression by antigen receptor signal strength and interleukin-2 in T lymphocytes. EMBO J. 2015, 34, 2008–2024. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, L.V.; Rolf, J.; Emslie, E.; Shi, Y.B.; Taylor, P.M.; Cantrell, D.A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013, 14, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Kabilan, U.; Graber, T.E.; Alain, T.; Klokov, D. Ionizing Radiation and Translation Control: A Link to Radiation Hormesis? Int. J. Mol. Sci. 2020, 21, 6650. [Google Scholar] [CrossRef]
- Wilkinson, S.E.; Nixon, J.S. T-cell signal transduction and the role of protein kinase C. Cell Mol. Life Sci. 1998, 54, 1122–1144. [Google Scholar] [CrossRef]
- Weigel, C.; Veldwijk, M.R.; Oakes, C.C.; Seibold, P.; Slynko, A.; Liesenfeld, D.B.; Rabionet, M.; Hanke, S.A.; Wenz, F.; Sperk, E.; et al. Epigenetic regulation of diacylglycerol kinase alpha promotes radiation-induced fibrosis. Nat. Commun. 2016, 7, 10893. [Google Scholar] [CrossRef]
- Yu, J.B.; Cramer, L.D.; Herrin, J.; Soulos, P.R.; Potosky, A.L.; Gross, C.P. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: Comparison of toxicity. J. Clin. Oncol. 2014, 32, 1195–1201. [Google Scholar] [CrossRef]
- Pan, H.Y.; Jiang, J.; Hoffman, K.E.; Tang, C.; Choi, S.L.; Nguyen, Q.N.; Frank, S.J.; Anscher, M.S.; Shih, Y.T.; Smith, B.D. Comparative Toxicities and Cost of Intensity-Modulated Radiotherapy, Proton Radiation, and Stereotactic Body Radiotherapy Among Younger Men with Prostate Cancer. J. Clin. Oncol. 2018, 36, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates Inc.: Hillsdale, NJ, USA, 1988. [Google Scholar]
- O’Brien, G.; Cruz-Garcia, L.; Majewski, M.; Grepl, J.; Abend, M.; Port, M.; Tichy, A.; Sirak, I.; Malkova, A.; Donovan, E.; et al. FDXR is a biomarker of radiation exposure in vivo. Sci. Rep. 2018, 8, 684. [Google Scholar] [CrossRef] [PubMed]
| Common across All Cancers | Up-Regulated | Down-Regulated | ||
|---|---|---|---|---|
| 15 | 16 | |||
| Genes | ALOX5 | NLRP3 | CCR4 | LTA |
| BCL6 | NOD2 | CCR7 | LTB | |
| CEBPB | TLR1 | CD40 | MAPKA | |
| CFD | TLR4 | CD40LG | MAPKAPK5 | |
| CXCL5 | TLR5 | ELK1 | MYC | |
| LIMK1 | TLR8 | HMGN1 | PRKCA | |
| MAPK14 | TYROBP | IL23A | TCF4 | |
| MYD88 | IL7 | TRADD | ||
| IL8 | ||||
| Cancer Type | No. of Patients | Total Dose (Gy) | Dose per Fraction (Gy) | Number of Fractions |
|---|---|---|---|---|
| Endometrium | 10 | 45 | 1.8 | 25 |
| Head and neck | 8 * | 50–70 | 2–2.1 | 25–33 |
| Head and neck | 23 | 51–64.8 | 1.8–3 | 17–36 |
| Prostate IMRT | 11 | 78 | 2 | 39 |
| Prostate CK | 11 | 36.25 | 7.25 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, G.; Kamuda, M.; Cruz-Garcia, L.; Polozova, M.; Tichy, A.; Markova, M.; Sirak, I.; Zahradnicek, O.; Widłak, P.; Ponge, L.; et al. Transcriptional Inflammatory Signature in Healthy Donors and Different Radiotherapy Cancer Patients. Int. J. Mol. Sci. 2024, 25, 1080. https://doi.org/10.3390/ijms25021080
O’Brien G, Kamuda M, Cruz-Garcia L, Polozova M, Tichy A, Markova M, Sirak I, Zahradnicek O, Widłak P, Ponge L, et al. Transcriptional Inflammatory Signature in Healthy Donors and Different Radiotherapy Cancer Patients. International Journal of Molecular Sciences. 2024; 25(2):1080. https://doi.org/10.3390/ijms25021080
Chicago/Turabian StyleO’Brien, Gráinne, Malgorzata Kamuda, Lourdes Cruz-Garcia, Mariia Polozova, Ales Tichy, Marketa Markova, Igor Sirak, Oldrich Zahradnicek, Piotr Widłak, Lucyna Ponge, and et al. 2024. "Transcriptional Inflammatory Signature in Healthy Donors and Different Radiotherapy Cancer Patients" International Journal of Molecular Sciences 25, no. 2: 1080. https://doi.org/10.3390/ijms25021080
APA StyleO’Brien, G., Kamuda, M., Cruz-Garcia, L., Polozova, M., Tichy, A., Markova, M., Sirak, I., Zahradnicek, O., Widłak, P., Ponge, L., Polanska, J., & Badie, C. (2024). Transcriptional Inflammatory Signature in Healthy Donors and Different Radiotherapy Cancer Patients. International Journal of Molecular Sciences, 25(2), 1080. https://doi.org/10.3390/ijms25021080








