Coatings for Cardiovascular Stents—An Up-to-Date Review
Abstract
1. Introduction
2. Cardiovascular Stent Evolution
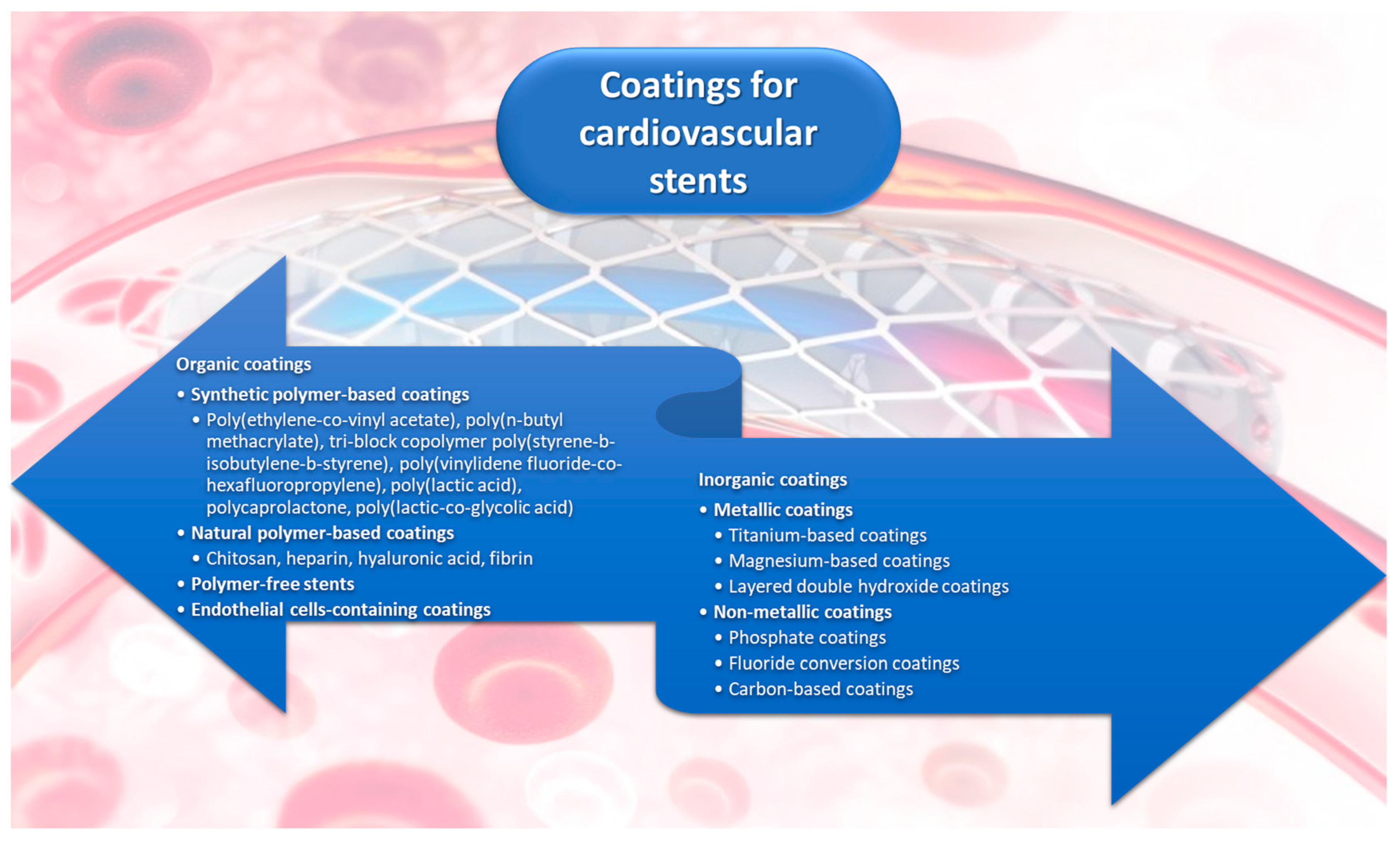
3. Organic Coatings for Stents
3.1. Synthetic Polymer-Based Coatings
3.2. Natural Polymer-Based Coatings
3.2.1. Chitosan-Based Coatings
3.2.2. Heparin-Based Coatings
3.2.3. Hyaluronic Acid-Based Coatings
3.2.4. Fibrin-Based Coatings
3.3. Polymer-Free Stents
3.4. Coatings Containing Endothelial Cells
4. Metallic Coatings for Stents
4.1. Titanium-Based Coatings
4.2. Magnesium-Based Coatings
4.3. Layered Double Hydroxide (LDH) Coatings
5. Inorganic, Nonmetallic Coatings
5.1. Phosphate Coatings
5.2. Fluoride Conversion Coatings
5.3. Carbon-Based Coatings
6. Summative Discussion and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahjehan, R.D.; Bhutta, B.S. Coronary Artery Disease; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Flora, D.G.; Nayak, K.M. A Brief Review of Cardiovascular Diseases, Associated Risk Factors and Current Treatment Regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Freedman, N.D.; Albert, P.S.; Huxley, R.R.; Shiels, M.S.; Withrow, D.R.; Spillane, S.; Powell-Wiley, T.M.; Berrington de González, A. Association of Cardiovascular Disease with Premature Mortality in the United States. JAMA Cardiol. 2019, 4, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S. Global epidemiology of ischemic heart disease: Results from the global burden of disease study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef]
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Scafa Udriște, A.; Niculescu, A.G.; Grumezescu, A.M.; Bădilă, E. Cardiovascular Stents: A Review of Past, Current, and Emerging Devices. Materials 2021, 14, 2498. [Google Scholar] [CrossRef]
- Ma, J.; Thompson, M.; Zhao, N.; Zhu, D. Similarities and differences in coatings for magnesium-based stents and orthopaedic implants. J. Orthop. Transl. 2014, 2, 118–130. [Google Scholar] [CrossRef]
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Bays, H.E.; Taub, P.R.; Epstein, E.; Michos, E.D.; Ferraro, R.A.; Bailey, A.L.; Kelli, H.M.; Ferdinand, K.C.; Echols, M.R.; Weintraub, H.; et al. Ten things to know about ten cardiovascular disease risk factors. Am. J. Prev. Cardiol. 2021, 5, 100149. [Google Scholar] [CrossRef]
- Adhikary, D.; Barman, S.; Ranjan, R.; Stone, H. A Systematic Review of Major Cardiovascular Risk Factors: A Growing Global Health Concern. Cureus 2022, 14, e30119. [Google Scholar] [CrossRef]
- Gaziano, T.A.; Opie, L.H.; Weinstein, M.C. Cardiovascular disease prevention with a multidrug regimen in the developing world: A cost-effectiveness analysis. Lancet 2006, 368, 679–686. [Google Scholar] [CrossRef]
- Tomberli, B.; Mattesini, A.; Baldereschi, G.I.; Di Mario, C. A Brief History of Coronary Artery Stents. Rev. Española Cardiol. 2018, 71, 312–319. [Google Scholar] [CrossRef]
- Carbonaro, D.; Mezzadri, F.; Ferro, N.; De Nisco, G.; Audenino, A.L.; Gallo, D.; Chiastra, C.; Morbiducci, U.; Perotto, S. Design of innovative self-expandable femoral stents using inverse homogenization topology optimization. Comput. Methods Appl. Mech. Eng. 2023, 416, 116288. [Google Scholar] [CrossRef]
- Beshchasna, N.; Ho, A.Y.K.; Saqib, M.; Kraśkiewicz, H.; Wasyluk, Ł.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Trusca, R.D.; Ficai, A. Surface evaluation of titanium oxynitride coatings used for developing layered cardiovascular stents. Mater. Sci. Eng. C 2019, 99, 405–416. [Google Scholar] [CrossRef]
- Choubey, R.K.; Pradhan, S.K. Prediction of strength and radial recoil of various stents using FE analysis. Mater. Today Proc. 2020, 27, 2254–2259. [Google Scholar] [CrossRef]
- Sandell, M.; Ericsson, A.; Al-Saadi, J.; Södervall, B.; Södergren, E.; Grass, S.; Sanchez, J.; Holmin, S. A novel noble metal stent coating reduces in vitro platelet activation and acute in vivo thrombosis formation: A blinded study. Sci. Rep. 2023, 13, 17225. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Abbott, J.D. Coronary Stents: History, Design, and Construction. J. Clin. Med. 2018, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.; Fouladian, P.; Blencowe, A.; Albrecht, H.; Song, Y.; Garg, S. Drug-eluting non-vascular stents for localised drug targeting in obstructive gastrointestinal cancers. J. Control. Release 2019, 309, 209–231. [Google Scholar] [CrossRef]
- Koźlik, M.; Harpula, J.; Chuchra, P.J.; Nowak, M.; Wojakowski, W.; Gąsior, P. Drug-Eluting Stents: Technical and Clinical Progress. Biomimetics 2023, 8, 72. [Google Scholar] [CrossRef]
- Fischell, T.A.; Balmuri, A.; Agarwal, S.; Verhye, S. Integrated stent delivery system: A next generation of stent delivery and drug-eluting stent. Cardiovasc. Revascular. Med. 2019, 21, 205–212. [Google Scholar] [CrossRef]
- Pilgrim, T.; Muller, O.; Heg, D.; Roffi, M.; Kurz, D.J.; Moarof, I.; Weilenmann, D.; Kaiser, C.; Tapponnier, M.; Losdat, S.; et al. Biodegradable- Versus Durable-Polymer Drug-Eluting Stents for STEMI: Final 2-Year Outcomes of the BIOSTEMI Trial. JACC Cardiovasc. Interv. 2021, 14, 639–648. [Google Scholar] [CrossRef]
- Partida, R.A.; Yeh, R.W. Contemporary drug-eluting stent platforms: Design, safety, and clinical efficacy. Cardiol. Clin. 2017, 35, 281–296. [Google Scholar] [CrossRef]
- Khan, W.; Farah, S.; Domb, A.J. Drug eluting stents: Developments and current status. J. Control. Release 2012, 161, 703–712. [Google Scholar] [CrossRef]
- Ako, J.; Bonneau, H.N.; Honda, Y.; Fitzgerald, P.J. Design criteria for the ideal drug-eluting stent. Am. J. Cardiol. 2007, 100, S3–S9. [Google Scholar] [CrossRef]
- Ranade, S.V.; Miller, K.M.; Richard, R.E.; Chan, A.K.; Allen, M.J.; Helmus, M.N. Physical characterization of controlled release of paclitaxel from the TAXUS™ Express2™ drug-eluting stent. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 71, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Veerubhotla, K.; Jeong, M.H.; Lee, C.H. Deep Learning in Personalization of Cardiovascular Stents. J. Cardiovasc. Pharmacol. Ther. 2019, 25, 110–120. [Google Scholar] [CrossRef]
- Oh, B.; Lee, C.H. Advanced Cardiovascular Stent Coated with Nanofiber. Mol. Pharm. 2013, 10, 4432–4442. [Google Scholar] [CrossRef] [PubMed]
- Beshchasna, N.; Saqib, M.; Kraskiewicz, H.; Wasyluk, Ł.; Kuzmin, O.; Duta, O.C.; Ficai, D.; Ghizdavet, Z.; Marin, A.; Ficai, A.; et al. Recent Advances in Manufacturing Innovative Stents. Pharmaceutics 2020, 12, 349. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Yang, Y.X.; Li, J.A.; Zeng, R.C.; Guan, S.K. Advances in coatings on magnesium alloys for cardiovascular stents—A review. Bioact. Mater. 2021, 6, 4729–4757. [Google Scholar] [CrossRef]
- Karoussos, I.A.; Wieneke, H.; Sawitowski, T.; Wnendt, S.; Fischer, A.; Dirsch, O.; Dahmen, U.; Erbel, R. Inorganic materials as drug delivery systems in coronary artery stenting. Mater. Und Werkst. 2002, 33, 738–746. [Google Scholar] [CrossRef]
- Canfield, J.; Totary-Jain, H. 40 Years of Percutaneous Coronary Intervention: History and Future Directions. J. Pers. Med. 2018, 8, 33. [Google Scholar] [CrossRef]
- Khatri, M.; Kumar, S.; Mahfooz, K.; Sugandh, F.N.U.; Dembra, D.; Mehak, F.N.U.; Panjwani, G.A.R.; Islam, H.; Islam, R.; Patel, T. Clinical Outcomes of Polymer-Free Versus Polymer-Coated Drug-Eluting Stents in Patients with Coronary Artery Disease: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e38215. [Google Scholar] [CrossRef]
- Zhu, J.-Z.; Xiong, X.-W.; Du, R.; Jing, Y.-J.; Ying, Y.; Fan, X.-M.; Zhu, T.-Q.; Zhang, R.-Y. Hemocompatibility of drug-eluting coronary stents coated with sulfonated poly (styrene-block-isobutylene-block-styrene). Biomaterials 2012, 33, 8204–8212. [Google Scholar] [CrossRef]
- Abbasnezhad, N.; Zirak, N.; Champmartin, S.; Shirinbayan, M.; Bakir, F. An Overview of In Vitro Drug Release Methods for Drug-Eluting Stents. Polymers 2022, 14, 2751. [Google Scholar] [CrossRef]
- Hou, D.; Huibregtse, B.; Dawkins, K.; Donnelly, J.; Roy, K.; Chen, P.J.; Akinapelli, A. Current State of Bioabsorbable Polymer-Coated Drug-Eluting Stents. Curr. Cardiol. Rev. 2017, 13, 139–154. [Google Scholar] [CrossRef]
- Ishaque, N.; Naseer, N.; Abbas, M.A.; Javed, F.; Mushtaq, S.; Ahmad, N.M.; Khan, M.F.; Ahmed, N.; Elaissari, A. Optimize PLA/EVA Polymers Blend Compositional Coating for Next Generation Biodegradable Drug-Eluting Stents. Polymers 2022, 14, 3547. [Google Scholar] [CrossRef]
- Borhani, S.; Hassanajili, S.; Ahmadi Tafti, S.H.; Rabbani, S. Cardiovascular stents: Overview, evolution, and next generation. Prog. Biomater. 2018, 7, 175–205. [Google Scholar] [CrossRef]
- Williams, P.D.; Awan, M. Stent selection for percutaneous coronary intervention. Contin. Cardiol. Educ. 2017, 3, 64–69. [Google Scholar] [CrossRef]
- Strohbach, A.; Busch, R. Polymers for Cardiovascular Stent Coatings. Int. J. Polym. Sci. 2015, 2015, 782653. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef]
- Piedade, A.P.; Nunes, J.; Vieira, M.T. Thin films with chemically graded functionality based on fluorine polymers and stainless steel. Acta Biomater. 2008, 4, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Jinnouchi, H.; Diljon, C.; Torii, S.; Sakamoto, A.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. A new category stent with novel polyphosphazene surface modification. Future Cardiol. 2018, 14, 225–235. [Google Scholar] [CrossRef]
- Shah, P.; Chandra, S. Review on emergence of nanomaterial coatings in bio-engineered cardiovascular stents. Journal of Drug Delivery Science and Technology 2022, 70, 103224. [Google Scholar] [CrossRef]
- Herrmann, R.; Schmidmaier, G.; Märkl, B.; Resch, A.; Hähnel, I.; Stemberger, A.; Alt, E. Antithrombogenic coating of stents using a biodegradable drug delivery technology. Thromb. Haemost. 1999, 82, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bakola, V.; Karagkiozaki, V.; Tsiapla, A.R.; Pappa, F.; Moutsios, I.; Pavlidou, E.; Logothetidis, S. Dipyridamole-loaded biodegradable PLA nanoplatforms as coatings for cardiovascular stents. Nanotechnology 2018, 29, 275101. [Google Scholar] [CrossRef]
- Szott, L.M.; Irvin, C.A.; Trollsas, M.; Hossainy, S.; Ratner, B.D. Blood compatibility assessment of polymers used in drug eluting stent coatings. Biointerphases 2016, 11, 029806. [Google Scholar] [CrossRef] [PubMed]
- Arab Shomali, A. Formulation and Testing of Biodegradable Polymeric coating on Zinc Wires in Cardiovascular Stent Application. Master’s Thesis, Michigan Technological University, Houghton, MI, USA, 2017. [Google Scholar]
- Sevostyanov, M.A.; Baikin, A.S.; Sergienko, K.V.; Shatova, L.A.; Kirsankin, A.A.; Baymler, I.V.; Shkirin, A.V.; Gudkov, S.V. Biodegradable stent coatings on the basis of PLGA polymers of different molecular mass, sustaining a steady release of the thrombolityc enzyme streptokinase. React. Funct. Polym. 2020, 150, 104550. [Google Scholar] [CrossRef]
- Puskas, J.E.; Muñoz-Robledo, L.G.; Hoerr, R.A.; Foley, J.; Schmidt, S.P.; Evancho-Chapman, M.; Dong, J.; Frethem, C.; Haugstad, G. Drug-eluting stent coatings. WIREs Nanomed. Nanobiotechnol. 2009, 1, 451–462. [Google Scholar] [CrossRef]
- Tsukada, Y.; Tsujimoto, H. Development of drug-eluting stent coated with PLGA nanoparticles as drug carriers. KONA Powder Part J. 2013, 30, 2. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A.u. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Jia, Z.; Ma, C.; Zhang, H. PLGA Coatings and PLGA Drug-Loading Coatings for Cardiac Stent Samples: Degradation Characteristics and Blood Compatibility. Coatings 2021, 11, 1427. [Google Scholar] [CrossRef]
- Rao, J.; Pan Bei, H.; Yang, Y.; Liu, Y.; Lin, H.; Zhao, X. Nitric Oxide-Producing Cardiovascular Stent Coatings for Prevention of Thrombosis and Restenosis. Front. Bioeng. Biotechnol. 2020, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, K.A.; Murashov, I.S.; Chernonosova, V.S.; Chelobanov, B.P.; Stepanova, A.O.; Sergeevichev, D.S.; Karpenko, A.A.; Laktionov, P.P. Vascular Stents Coated with Electrospun Drug-Eluting Material: Functioning in Rabbit Iliac Artery. Polymers 2020, 12, 1741. [Google Scholar] [CrossRef] [PubMed]
- Kariminejad, M.; Zibaei, R.; Kolahdouz-Nasiri, A.; Mohammadi, R.; Mortazavian, A.M.; Sohrabvandi, S.; Khanniri, E.; Khorshidian, N. Chitosan/polyvinyl alcohol/SiO2 nanocomposite films: Physicochemical and structural characterization. Biointerface Res. Appl. Chem. 2022, 12, 3725–3734. [Google Scholar]
- Hu, Q.; Fang, Z.; Ge, J.; Li, H. Nanotechnology for cardiovascular diseases. Innovation (Cambridge (Mass.)) 2022, 3, 100214. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.-S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Badry, M.D.; Wahba, M.A.; Khaled, R.K.; Moawad, M. Hydrothermally assisted synthesis of magnetic iron oxide-chitosan nanocomposites: Electrical and biological evaluation. Challenge 2021, 17, 20. [Google Scholar]
- Souza, M.P.C.d.; Sábio, R.M.; Ribeiro, T.d.C.; Santos, A.M.d.; Meneguin, A.B.; Chorilli, M. Highlighting the impact of chitosan on the development of gastroretentive drug delivery systems. Int. J. Biol. Macromol. 2020, 159, 804–822. [Google Scholar] [CrossRef]
- Mohebbi, S.; Nezhad, M.N.; Zarrintaj, P.; Jafari, S.H.; Gholizadeh, S.S.; Saeb, M.R.; Mozafari, M. Chitosan in Biomedical Engineering: A Critical Review. Curr. Stem Cell Res. Ther. 2019, 14, 93–116. [Google Scholar] [CrossRef]
- Papafaklis, M.I.; Chatzizisis, Y.S.; Naka, K.K.; Giannoglou, G.D.; Michalis, L.K. Drug-eluting stent restenosis: Effect of drug type, release kinetics, hemodynamics and coating strategy. Pharmacol. Ther. 2012, 134, 43–53. [Google Scholar] [CrossRef]
- Milewski, M.; Ng, C.K.; Gąsior, P.; Lian, S.S.; Qian, S.X.; Lu, S.; Foin, N.; Kedhi, E.; Wojakowski, W.; Ang, H.Y. Polymer Coating Integrity, Thrombogenicity and Computational Fluid Dynamics Analysis of Provisional Stenting Technique in the Left Main Bifurcation Setting: Insights from an In-Vitro Model. Polymers 2022, 14, 1715. [Google Scholar] [CrossRef]
- Gao, F.; Hu, Y.; Li, G.; Liu, S.; Quan, L.; Yang, Z.; Wei, Y.; Pan, C. Layer-by-layer deposition of bioactive layers on magnesium alloy stent materials to improve corrosion resistance and biocompatibility. Bioact. Mater. 2020, 5, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ye, P.; Guo, F.; Zhu, Y.; Nan, W.; Chang, Z. A heparin-functionalized covered stent prepared by plasma technology. J. Biomater. Appl. 2021, 36, 1243–1253. [Google Scholar] [CrossRef]
- Brockmann, M.A.; Gutensohn, K.; Bau, J.; Kuehnl, P.; Meinertz, T.; Nienaber, C.; Beythien, C. Influence of heparin coating of coronary stents and ex vivo efficacy of different doses of acetylsalicylic acid and ticlopidine in a pulsed floating model of recirculating human plasma. Platelets 2002, 13, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Nazarzadeh Zare, E.; Khorsandi, D.; Zarepour, A.; Yilmaz, H.; Agarwal, T.; Hooshmand, S.; Mohammadinejad, R.; Ozdemir, F.; Sahin, O.; Adiguzel, S.; et al. Biomedical applications of engineered heparin-based materials. Bioact. Mater. 2024, 31, 87–118. [Google Scholar] [CrossRef]
- Haude, M.; Konorza, T.F.M.; Kalnins, U.; Erglis, A.; Saunamäki, K.; Glogar, H.D.; Grube, E.; Gil, R.; Serra, A.; Richardt, H.G.; et al. Heparin-Coated Stent Placement for the Treatment of Stenoses in Small Coronary Arteries of Symptomatic Patients. Circulation 2003, 107, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Babapulle, M.N.; Eisenberg, M.J. Coated Stents for the Prevention of Restenosis: Part I. Circulation 2002, 106, 2734–2740. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Phoon, P.H.Y.; Hwang, N.C. Heparin resistance during cardiopulmonary bypass in adult cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2022, 36, 4150–4160. [Google Scholar] [CrossRef]
- Christensen, K.; Larsson, R.; Emanuelsson, H.; Elgue, G.; Larsson, A. Heparin coating of the stent graft—Effects on platelets, coagulation and complement activation. Biomaterials 2001, 22, 349–355. [Google Scholar] [CrossRef]
- Verheye, S.; Markou, C.P.; Salame, M.Y.; Wan, B.; King, S.B.; Robinson, K.A.; Chronos, N.A.F.; Hanson, S.R. Reduced Thrombus Formation by Hyaluronic Acid Coating of Endovascular Devices. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1168–1172. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Zhang, K.; He, Z.; Yang, P.; Zou, D.; Huang, N. Multifunctional Coating Based on Hyaluronic Acid and Dopamine Conjugate for Potential Application on Surface Modification of Cardiovascular Implanted Devices. ACS Appl. Mater. Interfaces 2016, 8, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. Sub-Cell. Biochem. 2017, 82, 405–456. [Google Scholar] [CrossRef]
- Deng, Y.; Wen, Y.; Yin, J.; Huang, J.; Zhang, R.; Zhang, G.; Qiu, D. Corroded iron stent increases fibrin deposition and promotes endothelialization after stenting. Bioeng. Transl. Med. 2023, 8, e10469. [Google Scholar] [CrossRef] [PubMed]
- Mühl-Benninghaus, R.; Fries, F.; Kießling, M.; Tomori, T.; Krajewski, S.; Simgen, A.; Bauer, S.; Hey, N.; Brynda, E.; Taborska, J.; et al. Vascular Response on a Novel Fibrin-Based Coated Flow Diverter. Cardiovasc. Interv. Radiol. 2022, 45, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Byer, A.; Peters, S.; Settepani, F.; Pagliaro, M.; Galletti, G. Fibrin sealant coated stents compared with non-coated stents in a porcine carotid artery model. Preliminary study report. J. Cardiovasc. Surg. 2001, 42, 543–549. [Google Scholar]
- Nzulumike, A.N.O.; Thormann, E. Fibrin Adsorption on Cardiovascular Biomaterials and Medical Devices. ACS Appl. Bio Mater. 2023, 6, 2667–2676. [Google Scholar] [CrossRef] [PubMed]
- McKenna, C.J.; Camrud, A.R.; Sangiorgi, G.; Kwon, H.M.; Edwards, W.D.; Holmes, D.R.; Schwartz, R.S. Fibrin-Film Stenting in a Porcine Coronary Injury Model: Efficacy and Safety Compared with Uncoated Stents 11This study was supported in part by Medtronic, Minneapolis, Minnesota and the J. Holden DeHaan Foundation, Indianapolis, Indiana. J. Am. Coll. Cardiol. 1998, 31, 1434–1438. [Google Scholar] [CrossRef][Green Version]
- Ząbczyk, M.; Ariëns, R.A.S.; Undas, A. Fibrin clot properties in cardiovascular disease: From basic mechanisms to clinical practice. Cardiovascular research 2023, 119, 94–111. [Google Scholar] [CrossRef]
- Rykowska, I.; Nowak, I.; Nowak, R. Drug-Eluting Stents and Balloons—Materials, Structure Designs, and Coating Techniques: A Review. Molecules 2020, 25, 4624. [Google Scholar] [CrossRef]
- Cherian, A.M.; Nair, S.V.; Maniyal, V.; Menon, D. Surface engineering at the nanoscale: A way forward to improve coronary stent efficacy. APL Bioeng. 2021, 5, 021508. [Google Scholar] [CrossRef]
- Driver, M. Coatings for cardiovascular devices: Coronary stents. In Coatings for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2012; pp. 223–250. [Google Scholar]
- Stiermaier, T.; Heinz, A.; Schloma, D.; Kleinertz, K.; Dänschel, W.; Erbs, S.; Linke, A.; Boudriot, E.; Lauer, B.; Schuler, G.; et al. Five-year clinical follow-up of a randomized comparison of a polymer-free sirolimus-eluting stent versus a polymer-based paclitaxel-eluting stent in patients with diabetes mellitus (LIPSIA Yukon trial). Catheter. Cardiovasc. Interv. 2014, 83, 418–424. [Google Scholar] [CrossRef]
- Ma, X.; Wu, T.; Robich, M.P. Drug-eluting stent coatings. Interv. Cardiol. 2012, 4, 73. [Google Scholar] [CrossRef]
- Adraider, Y.; Pang, Y.X.; Nabhani, F.; Hodgson, S.N.; Sharp, M.C.; Al-Waidh, A. Fabrication of zirconium oxide coatings on stainless steel by a combined laser/sol–gel technique. Ceram. Int. 2013, 39, 9665–9670. [Google Scholar] [CrossRef]
- Ma, Q.; Shi, X.; Tan, X.; Wang, R.; Xiong, K.; Maitz, M.F.; Cui, Y.; Hu, Z.; Tu, Q.; Huang, N.; et al. Durable endothelium-mimicking coating for surface bioengineering cardiovascular stents. Bioact. Mater. 2021, 6, 4786–4800. [Google Scholar] [CrossRef]
- Chen, W.; Habraken, T.C.; Hennink, W.E.; Kok, R.J. Polymer-Free Drug-Eluting Stents: An Overview of Coating Strategies and Comparison with Polymer-Coated Drug-Eluting Stents. Bioconjugate Chem. 2015, 26, 1277–1288. [Google Scholar] [CrossRef]
- Yao, S.; Cui, J.; Chen, S.; Zhou, X.; Li, J.; Zhang, K. Extracellular Matrix Coatings on Cardiovascular Materials—A Review. Coatings 2022, 12, 1039. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, S.; Rasser, C.; Mesnier, J.; Chevallier, P.; Gallet, R.; Choqueux, C.; Even, G.; Sayah, N.; Chaubet, F.; Nicoletti, A.; et al. Coronary stent CD31-mimetic coating favours endothelialization and reduces local inflammation and neointimal development in vivo. Eur. Heart J. 2021, 42, 1760–1769. [Google Scholar] [CrossRef]
- Tan, S.; White, H.D.; Layland, J. Heparin use in acute coronary syndromes and cardiovascular interventions: Habit or evidence based? Eur. Heart J. 2022, 43, 1008–1011. [Google Scholar] [CrossRef]
- Malucelli, G. Hybrid Organic/Inorganic Coatings Through Dual-Cure Processes: State of the Art and Perspectives. Coatings 2016, 6, 10. [Google Scholar] [CrossRef]
- Mani, G.; Feldman, M.D.; Patel, D.; Agrawal, C.M. Coronary stents: A materials perspective. Biomaterials 2007, 28, 1689–1710. [Google Scholar] [CrossRef]
- Yang, F.; Chang, R.; Webster, T.J. Atomic Layer Deposition Coating of TiO2 Nano-Thin Films on Magnesium-Zinc Alloys to Enhance Cytocompatibility for Bioresorbable Vascular Stents. Int. J. Nanomed. 2019, 14, 9955–9970. [Google Scholar] [CrossRef]
- Wibowo, D.; Muzakkar, M.Z.; Maulidiyah, M.; Nurdin, M.; Saad, S.K.M.; Umar, A.A. Morphological analysis of Ag doped on TiO2/Ti prepared via anodizing and thermal oxidation methods. Biointerface Res. Appl. Chem. 2022, 12, 1421–1437. [Google Scholar]
- Meyer-Kobbe, C.; Stekker, M.; Menze, R. Stent Made of a Bio-Degradable Magnesium Alloy with a Magnesium Fluoride Coating and an Organic Coating. U.S. Patent US20200139017A1, 7 May 2020. [Google Scholar]
- Maulidiyah, M.; Susilowati, P.E.; Mudhafar, N.K.d.; Salim, L.A.; Wibowo, D.; Muzakkar, M.Z.; Irwan, I.; Arham, Z.; Nurdin, M. Photo-inactivation Staphylococcus aureus by using formulation of Mn-N-TiO2 composite coated wall paint. Biointerface Res. Appl. Chem. 2022, 12, 1628–1637. [Google Scholar]
- Kröger, N.; Kopp, A.; Staudt, M.; Rusu, M.; Schuh, A.; Liehn, E.A. Hemocompatibility of plasma electrolytic oxidation (PEO) coated Mg-RE and Mg-Zn-Ca alloys for vascular scaffold applications. Mater. Sci. Eng. C 2018, 92, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, D.; Min, Z.; Pan, C. A Degradable Inorganic Coating on Vascular Stent to Used to Accelerate Endothelialization In Vivo. J. Biomater. Tissue Eng. 2014, 4, 725–729. [Google Scholar] [CrossRef]
- Justin Kunene, T.; Kwanda Tartibu, L.; Ukoba, K.; Jen, T.-C. Review of atomic layer deposition process, application and modeling tools. Mater. Today Proc. 2022, 62, S95–S109. [Google Scholar] [CrossRef]
- Xu, X.; Wang, L.; Wang, G.; Jin, Y. The effect of REDV/TiO(2) coating coronary stents on in-stent restenosis and re-endothelialization. J. Biomater. Appl. 2017, 31, 911–922. [Google Scholar] [CrossRef]
- Qi, Y.; Qi, H.; He, Y.; Lin, W.; Li, P.; Qin, L.; Hu, Y.; Chen, L.; Liu, Q.; Sun, H.; et al. Strategy of Metal–Polymer Composite Stent to Accelerate Biodegradation of Iron-Based Biomaterials. ACS Appl. Mater. Interfaces 2018, 10, 182–192. [Google Scholar] [CrossRef]
- Nazneen, F.; Herzog, G.; Arrigan, D.W.M.; Caplice, N.; Benvenuto, P.; Galvin, P.; Thompson, M. Surface chemical and physical modification in stent technology for the treatment of coronary artery disease. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1989–2014. [Google Scholar] [CrossRef]
- Mikhalovska, L.; Chorna, N.; Lazarenko, O.; Haworth, P.; Sudre, A.; Mikhalovsky, S. Inorganic coatings for cardiovascular stents: In vitro and in vivo studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96B, 333–341. [Google Scholar] [CrossRef]
- Tan, A.; Farhatnia, Y.; de Mel, A.; Rajadas, J.; Alavijeh, M.S.; Seifalian, A.M. Inception to actualization: Next generation coronary stent coatings incorporating nanotechnology. J. Biotechnol. 2013, 164, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.G.; Previtali, B.; Ge, Q.; Vedani, M.; Wu, W.; Migliavacca, F.; Petrini, L.; Biffi, C.A.; Bestetti, M. Biodegradable magnesium coronary stents: Material, design and fabrication. Int. J. Comput. Integr. Manuf. 2014, 27, 936–945. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Esteban-Lucia, M.; Martinez-Campos, E.; Mohedano, M.; Arrabal, R.; Blawert, C.; Zheludkevich, M.L.; Matykina, E. PEO coatings design for Mg-Ca alloy for cardiovascular stent and bone regeneration applications. Mater. Sci. Eng. C 2019, 105, 110026. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Cao, L.; Liu, Y.; Xu, X.; Wu, X. Evaluation of magnesium ions release, biocorrosion, and hemocompatibility of MAO/PLLA-modified magnesium alloy WE42. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 96B, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Echeverry-Rendon, M.; Echeverria, F.; Harmsen, M.C. Interaction of different cell types with magnesium modified by plasma electrolytic oxidation. Colloids Surf. B Biointerfaces 2020, 193, 111153. [Google Scholar] [CrossRef]
- Chen, C.; Tan, J.; Wu, W.; Petrini, L.; Zhang, L.; Shi, Y.; Cattarinuzzi, E.; Pei, J.; Huang, H.; Ding, W. Modeling and experimental studies of coating delamination of biodegradable magnesium alloy cardiovascular stents. ACS Biomater. Sci. Eng. 2018, 4, 3864–3873. [Google Scholar] [CrossRef]
- Dubrovskii, A.R.; Makarova, O.V.; Kuznetsov, S.A. Electrodeposition of Tantalum Coatings on Nitinol Stent and Composition of Intermetallic Compounds Forming During Electrolysis. J. Electrochem. Soc. 2021, 168, 046518. [Google Scholar] [CrossRef]
- Thierry, B.; Winnik, F.M.; Merhi, Y.; Silver, J.; Tabrizian, M. Bioactive coatings of endovascular stents based on polyelectrolyte multilayers. Biomacromolecules 2003, 4, 1564–1571. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Shen, S.; Li, Q.; Luo, X.; Xiong, P.; Gao, S.; Yan, J.; Cheng, Y.; Xi, T. In Vitro and in Vivo Studies on Two-Step Alkali-Fluoride-Treated Mg–Zn–Y–Nd Alloy for Vascular Stent Application: Enhancement in Corrosion Resistance and Biocompatibility. ACS Biomater. Sci. Eng. 2019, 5, 3279–3292. [Google Scholar] [CrossRef]
- Doro, F.G.; Ramos, A.P.; Schneider, J.F.; Rodrigues-Filho, U.P.; Veiga, M.A.M.S.; Yano, C.L.; Negreti, A.; Krieger, M.H.; Tfouni, E. Deposition of organic−inorganic hybrid coatings over 316L surgical stainless steel and evaluation on vascular cells. Can. J. Chem. 2014, 92, 987–995. [Google Scholar] [CrossRef]
- Zhai, C.; Dai, C.Y.; Lv, X.; Shi, B.; Li, Y.R.; Yang, Y.; Fan, D.; Lee, E.S.; Sun, Y.; Jiang, H.B. Fluoride Coatings on Magnesium Alloy Implants. Bioinorg. Chem. Appl. 2022, 2022, 7636482. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Zhang, S.; Li, J.; Wang, H.; Zhao, C.; Zhang, X. Effect of fluoride coating on in vitro dynamic degradation of Mg–Zn alloy. Mater. Lett. 2011, 65, 2568–2571. [Google Scholar] [CrossRef]
- Mao, L.; Shen, L.; Chen, J.; Wu, Y.; Kwak, M.; Lu, Y.; Xue, Q.; Pei, J.; Zhang, L.; Yuan, G.; et al. Enhanced Bioactivity of Mg–Nd–Zn–Zr Alloy Achieved with Nanoscale MgF2 Surface for Vascular Stent Application. ACS Appl. Mater. Interfaces 2015, 7, 5320–5330. [Google Scholar] [CrossRef] [PubMed]
- Drynda, A.; Seibt, J.; Hassel, T.; Bach, F.W.; Peuster, M. Biocompatibility of fluoride-coated magnesium-calcium alloys with optimized degradation kinetics in a subcutaneous mouse model. J. Biomed. Mater. Res. Part A 2013, 101A, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Nakamura, M.; Palmaz, J.C.; Schwartz, R.S. Role of stent design and coatings on restenosis and thrombosis. Adv. Drug Deliv. Rev. 2006, 58, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, P.; Huang, L.; Tan, X.; Zhou, N.; Yang, T.; Qiu, H.; Dai, X.; Michael, S.; Tu, Q.; et al. A tough nitric oxide-eluting hydrogel coating suppresses neointimal hyperplasia on vascular stent. Nat. Commun. 2021, 12, 7079. [Google Scholar] [CrossRef]
- Jafarzadeh, F.; McManus, D.; Barbolina, I.; Malik, N.; Casiraghi, C.; Holt, C. 145 Application of graphene based coating on coronary artery stents. Heart 2017, 103, A108. [Google Scholar] [CrossRef]
- ElSawy, A.M.; Attia, N.F.; Mohamed, H.I.; Mohsen, M.; Talaat, M.H. Innovative coating based on graphene and their decorated nanoparticles for medical stent applications. Mater. Sci. Eng. C 2019, 96, 708–715. [Google Scholar] [CrossRef]
- Fávaro, W.J.; de Souza, J.G.; Matsumoto, M.Y.; Durán, M.; Bockelmann, P.K.; Dias, Q.C.; Durán, N. Associating Graphene Oxide Derivatives to Treat Non-Muscle Invasive Bladder Cancer (NMIBC). Biointerface Res. Appl. Chem. 2021, 12, 196–210. [Google Scholar]
- Tóth, S.; Dobránszky, J.; Major, L.; Koós, M. Carbon-Based Coatings for Cardiovascular Stents. In Proceedings of the Fifth International Conference on Inorganic Materials, Ljubljana, Szlovénia, 23–26 September 2006. [Google Scholar]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Ge, S.; Xi, Y.; Du, R.; Ren, Y.; Xu, Z.; Tan, Y.; Wang, Y.; Yin, T.; Wang, G. Inhibition of in-stent restenosis after graphene oxide double-layer drug coating with good biocompatibility. Regen. Biomater. 2019, 6, 299–309. [Google Scholar] [CrossRef]
- Patelis, N.; Moris, D.; Matheiken, S.; Klonaris, C. The Potential Role of Graphene in Developing the Next Generation of Endomaterials. BioMed Res. Int. 2016, 2016, 3180954. [Google Scholar] [CrossRef]
- Nakano, K.; Egashira, K.; Masuda, S.; Funakoshi, K.; Zhao, G.; Kimura, S.; Matoba, T.; Sueishi, K.; Endo, Y.; Kawashima, Y.; et al. Formulation of Nanoparticle-Eluting Stents by a Cationic Electrodeposition Coating Technology. JACC Cardiovasc. Interv. 2009, 2, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Coronel-Meneses, D.; Sánchez-Trasviña, C.; Ratera, I.; Mayolo-Deloisa, K. Strategies for surface coatings of implantable cardiac medical devices. Front. Bioeng. Biotechnol. 2023, 11, 1173260. [Google Scholar] [CrossRef] [PubMed]
- Kobo, O.; Saada, M.; Meisel, S.R.; Hellou, E.; Frimerman, A.; Fanne, R.A.; Mohsen, J.; Danon, A.; Roguin, A. Modern Stents: Where Are We Going? Rambam Maimonides Med. J. 2020, 11, e0017. [Google Scholar] [CrossRef] [PubMed]
- Yerasi, C.; Case Brian, C.; Forrestal Brian, J.; Torguson, R.; Weintraub William, S.; Garcia-Garcia Hector, M.; Waksman, R. Drug-Coated Balloon for De Novo Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 75, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, D.; Alfonso, F.; Xu, B.; Claessen Bimmer, E.P.M.; Adriaenssens, T.; Jensen, C.; Pérez-Vizcayno María, J.; Kang, D.-Y.; Degenhardt, R.; Pleva, L.; et al. Drug-Coated Balloon Angioplasty Versus Drug-Eluting Stent Implantation in Patients with Coronary Stent Restenosis. J. Am. Coll. Cardiol. 2020, 75, 2664–2678. [Google Scholar] [CrossRef]
- Fahrni, G.; Scheller, B.; Coslovsky, M.; Gilgen, N.; Farah, A.; Ohlow, M.-A.; Mangner, N.; Weilenmann, D.; Wöhrle, J.; Cuculi, F.; et al. Drug-coated balloon versus drug-eluting stent in small coronary artery lesions: Angiographic analysis from the BASKET-SMALL 2 trial. Clin. Res. Cardiol. 2020, 109, 1114–1124. [Google Scholar] [CrossRef]
- Lindquist, E.M.; Gosnell, J.M.; Khan, S.K.; Byl, J.L.; Zhou, W.; Jiang, J.; Vettukattil, J.J. 3D printing in cardiology: A review of applications and roles for advanced cardiac imaging. Ann. 3d Print. Med. 2021, 4, 100034. [Google Scholar] [CrossRef]
- Alfonso, F.; Coughlan, J.J.; Giacoppo, D.; Kastrati, A.; Byrne, R.A. Management of in-stent restenosis. Eurointerv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2022, 18, e103–e123. [Google Scholar] [CrossRef]
- Giacoppo, D.; Alfonso, F.; Xu, B.; Claessen, B.; Adriaenssens, T.; Jensen, C.; Pérez-Vizcayno, M.J.; Kang, D.Y.; Degenhardt, R.; Pleva, L.; et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: A comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study). Eur. Heart J. 2020, 41, 3715–3728. [Google Scholar] [CrossRef]
- Verheye, S.; Vrolix, M.; Kumsars, I.; Erglis, A.; Sondore, D.; Agostoni, P.; Cornelis, K.; Janssens, L.; Maeng, M.; Slagboom, T.; et al. The SABRE Trial (Sirolimus Angioplasty Balloon for Coronary In-Stent Restenosis): Angiographic Results and 1-Year Clinical Outcomes. JACC Cardiovasc. Interv. 2017, 10, 2029–2037. [Google Scholar] [CrossRef]
- Lopez-Palop, R.; Pinar, E.; Lozano, I.; Saura, D.; Picó, F.; Valdés, M. Utility of the fractional flow reserve in the evaluation of angiographically moderate in-stent restenosis. Eur. Heart J. 2004, 25, 2040–2047. [Google Scholar] [CrossRef]
- Senst, B.; Goyal, A.; Basit, H.; Borger, J. Drug Eluting Stent Compounds. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kamberi, M.; Pinson, D.; Pacetti, S.; Perkins, L.E.L.; Hossainy, S.; Mori, H.; Rapoza, R.J.; Kolodgie, F.; Virmani, R. Evaluation of chemical stability of polymers of XIENCE everolimus-eluting coronary stents in vivo by pyrolysis-gas chromatography/mass spectrometry. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1721–1729. [Google Scholar] [CrossRef]
- Lewis, A.L.; Stratford, P.W. Phosphorylcholine-coated stents. J. Long-Term Eff. Med. Implant. 2002, 12, 231–250. [Google Scholar] [CrossRef]
- Gherasie, F.-A.; Valentin, C.; Busnatu, S.-S. Is There an Advantage of Ultrathin-Strut Drug-Eluting Stents over Second- and Third-Generation Drug-Eluting Stents? J. Pers. Med. 2023, 13, 753. [Google Scholar] [CrossRef]
- Kersani, D.; Mougin, J.; Lopez, M.; Degoutin, S.; Tabary, N.; Cazaux, F.; Janus, L.; Maton, M.; Chai, F.; Sobocinski, J.; et al. Stent coating by electrospinning with chitosan/poly-cyclodextrin based nanofibers loaded with simvastatin for restenosis prevention. Eur. J. Pharm. Biopharm. 2020, 150, 156–167. [Google Scholar] [CrossRef]
- Surovtseva, M.A.; Poveschenko, O.V.; Kuzmin, O.S.; Kim, I.I.; Kozhukhov, A.S.; Bondarenko, N.A.; Chepeleva, E.V.; Kolodin, A.N.; Lykov, A.P.; Shcheglov, D.V.; et al. Titanium oxide– and oxynitride–coated nitinol: Effects of surface structure and composition on interactions with endothelial cells. Appl. Surf. Sci. 2022, 578, 152059. [Google Scholar] [CrossRef]
- Zhao, Z.; Zong, L.; Liu, C.; Li, X.; Wang, C.; Liu, W.; Cheng, X.; Wang, J.; Jian, X. A novel Mg(OH)2/MgFx(OH)1−x composite coating on biodegradable magnesium alloy for coronary stent application. Corros. Sci. 2022, 208, 110627. [Google Scholar] [CrossRef]
- Jeong, D.-W.; Park, W.; Bedair, T.M.; Kang, E.Y.; Kim, I.H.; Park, D.S.; Sim, D.S.; Hong, Y.J.; Koh, W.-G.; Jeong, M.H.; et al. Augmented re-endothelialization and anti-inflammation of coronary drug-eluting stent by abluminal coating with magnesium hydroxide. Biomater. Sci. 2019, 7, 2499–2510. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, C.; Sun, B.; Zhang, Y.; Sun, X.; El-Newehy, M.; El-Hamshary, H.; Morsi, Y.; Gu, H.; Wang, W.; et al. 3D printed personalized, heparinized and biodegradable coronary artery stents for rabbit abdominal aorta implantation. Chem. Eng. J. 2022, 450, 138202. [Google Scholar] [CrossRef]


| Coating Type | Material | Observations | References |
|---|---|---|---|
| Synthetic polymer | Poly(ethylene-co-vinyl acetate) | Used in 1st generation of DESs Loaded with sirolimus Associated with late stent thrombosis Delayed wound healing due to poor reendothelialization and persistence of polymer coatings after drug release | [39,140] |
| Synthetic polymer | Poly(n-butyl methacrylate) | Used in 1st generation of DESs Loaded with sirolimus Maintains chemical integrity after multiple years of stent implantation Associated with late stent thrombosis Delayed wound healing due to poor reendothelialization and persistence of polymer coatings after drug release | [39,140,141] |
| Synthetic polymer | Tri-block copolymer poly(styrene-b-isobutylene-b-styrene) | Used in 1st generation of DESs Loaded with paclitaxel Allows for early burst release of the drug Delayed wound healing due to poor reendothelialization and persistence of polymer coatings after drug release | [39,140] |
| Synthetic polymer | Phosphorylcholine | Used in 2nd generation of DESs Ability to load and release a variety of therapeutic agents Reduces platelet adhesion and subsequential thrombosis | [140,142] |
| Synthetic polymer | Copolymer poly(vinylidene fluoride-co-hexafluoropropylene) | Used in 2nd generation of DESs Loaded with everolimus Maintains chemical integrity after multiple years of stent implantation | [140,141] |
| Synthetic polymer | Poly-lactic acid | Used in 3rd generation of DESs Degrades into harmless compounds that are further metabolized by the body Reduced risk of cardiac events compared to durable polymer coatings | [39,143] |
| Synthetic polymer | Poly(lactic-co-glycolic acid) | Used in 3rd generation of DESs Degrades into harmless compounds that are further metabolized by the body Capable of a sustained and directional release of high-molecular biological compounds | [48,143] |
| Natural polymer | Chitosan | Slows corrosion rate of stent platform Can be combined with other materials (e.g., poly-L-glutamic acid, graphene oxide, heparin) to provide synergistic outcomes | [62,144] |
| Natural polymer | Heparin | Anticoagulant properties Inhibitory effect on arterial smooth muscle cell proliferation Reduces stent thrombosis and restenosis | [68,92] |
| Natural polymer | Hyaluronic acid | Significantly reduces the formation of platelet thrombus Favorable antiproliferative effect and decreased anti-inflammatory response | [73,74] |
| Natural polymer | Fibrin | Provides complete endoluminal paving with anti-thrombogenic or antiproliferative therapy delivery Stimulates cell adhesion, spreading, migration, and alignment | [77,81] |
| Metallic | TiOxNy | Improved chemical/corrosion stability Improved mass loss, restenosis, and target vascularization Reduced platelet adhesion and fibrinogen binding | [14,145] |
| Metallic | Titanium dioxide | Strong corrosion resistance Slows down degradation of Mg–Zn alloy-based stents Encourages the adhesion and proliferation of endothelial cells Has anti-thrombotic properties | [102,105] |
| Metallic | Magnesium hydroxide | Improved corrosion resistance Augmented reendothelialization Anti-inflammatory and anti-thrombotic effects | [146,147] |
| Inorganic, non-metallic | Graphene oxide | Reduced adherence and activation of platelets Reduced migration and proliferation of VSMCs Promotes reendothelialization Suppresses thrombosis and intimal hyperplasia | [127,128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udriște, A.S.; Burdușel, A.C.; Niculescu, A.-G.; Rădulescu, M.; Grumezescu, A.M. Coatings for Cardiovascular Stents—An Up-to-Date Review. Int. J. Mol. Sci. 2024, 25, 1078. https://doi.org/10.3390/ijms25021078
Udriște AS, Burdușel AC, Niculescu A-G, Rădulescu M, Grumezescu AM. Coatings for Cardiovascular Stents—An Up-to-Date Review. International Journal of Molecular Sciences. 2024; 25(2):1078. https://doi.org/10.3390/ijms25021078
Chicago/Turabian StyleUdriște, Alexandru Scafa, Alexandra Cristina Burdușel, Adelina-Gabriela Niculescu, Marius Rădulescu, and Alexandru Mihai Grumezescu. 2024. "Coatings for Cardiovascular Stents—An Up-to-Date Review" International Journal of Molecular Sciences 25, no. 2: 1078. https://doi.org/10.3390/ijms25021078
APA StyleUdriște, A. S., Burdușel, A. C., Niculescu, A.-G., Rădulescu, M., & Grumezescu, A. M. (2024). Coatings for Cardiovascular Stents—An Up-to-Date Review. International Journal of Molecular Sciences, 25(2), 1078. https://doi.org/10.3390/ijms25021078







