Elevated Arterial Blood Pressure as a Delayed Complication Following COVID-19—A Narrative Review
Abstract
1. Introduction
1.1. Arterial Hypertension (HTN)
1.2. COVID-19 Cardiovascular Outcome
2. Materials and Methods
2.1. Results
2.2. New Arterial Hypertension Development
2.3. Retrospective Cohort Studies Based on Medical Record Databases
2.4. Elevated Blood Pressure
2.5. No Changes in Blood Pressure Values after COVID-19
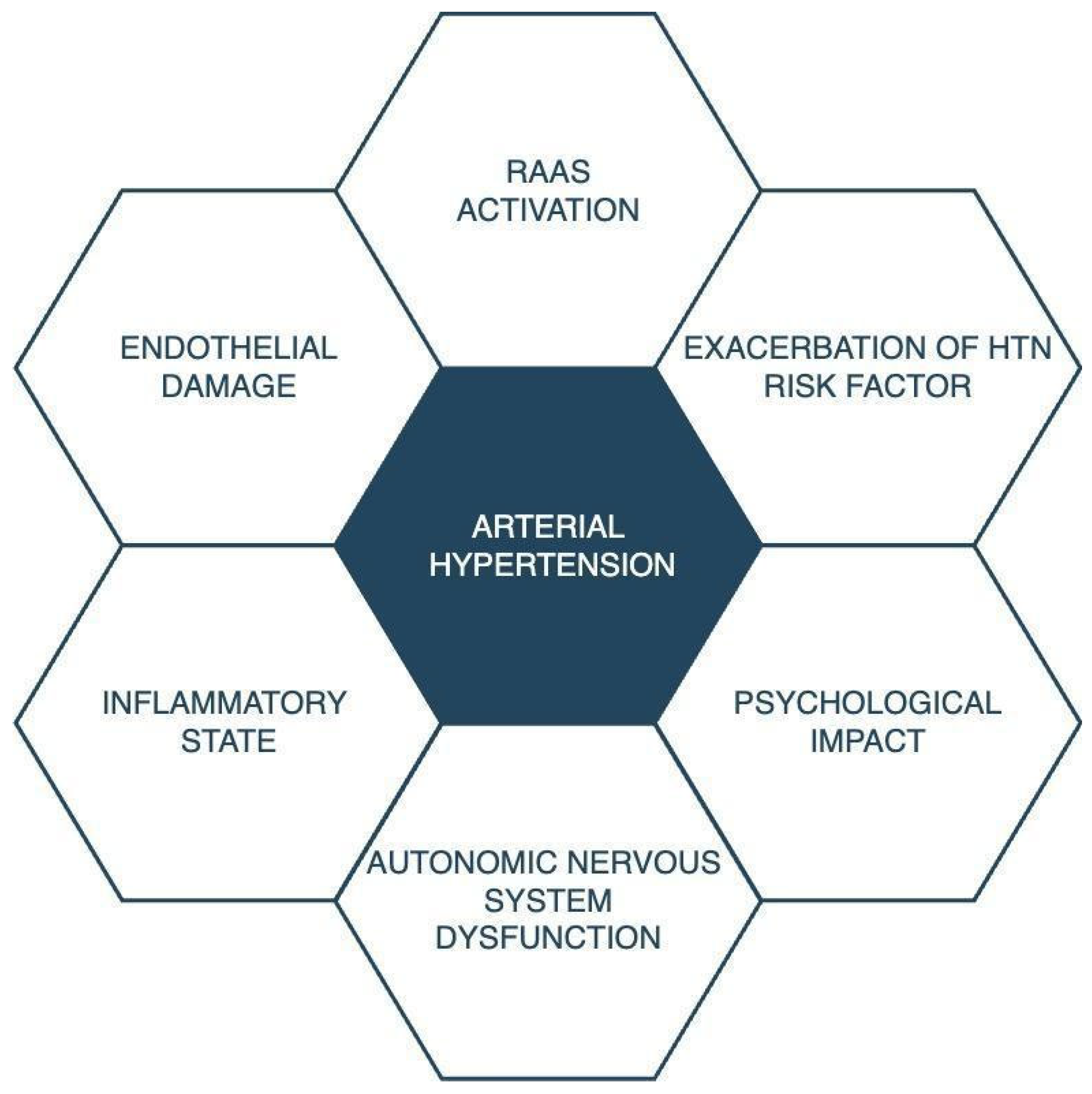
3. Discussion
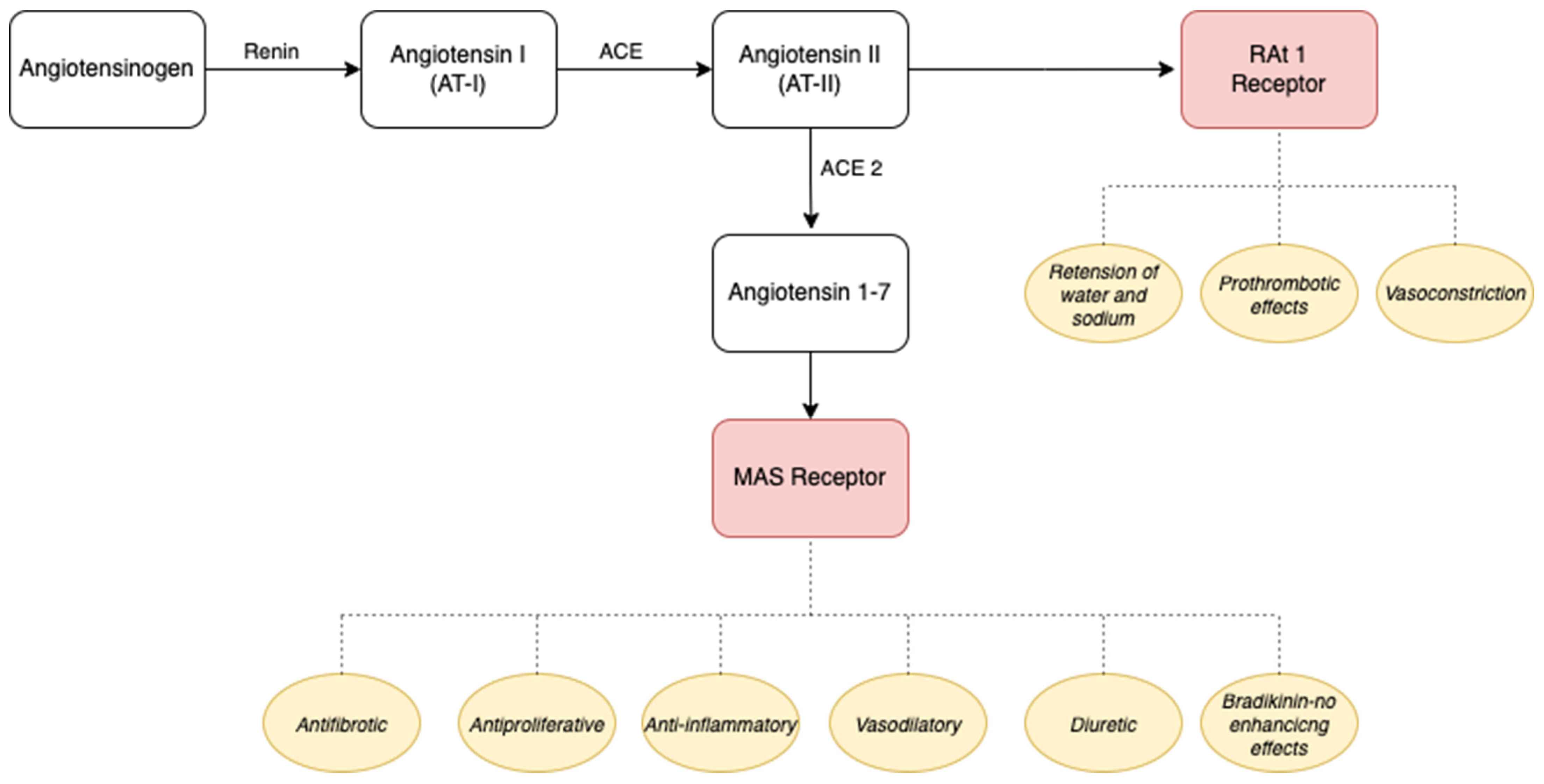
3.1. The Renin–Angiotensin–Aldosterone System, Inflammatory State, and Endothelial Damage
3.2. Exacerbation of Hypertension Risk
3.3. Psychological Impact
3.4. Autonomic Nervous System
4. Limitations
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Ghorani, H.; Götzinger, F.; Böhm, M.; Mahfoud, F. Arterial hypertension—Clinical trials update 2021. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef] [PubMed]
- Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; Azizi, M.; Benetos, A.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension global hypertension practice guidelines. J. Hypertens. 2020, 38, 982–1004. [Google Scholar] [CrossRef] [PubMed]
- Hypertension in Adults: Diagnosis and Management. National Institute for Health and Care Excellence (NICE). Available online: http://www.nice.org.uk/guidance/ng136 (accessed on 23 October 2020).
- Forman, J.P.; Stampfer, M.J.; Curhan, G.C. Diet and Lifestyle Risk Factors Associated with Incident Hypertension in Women. JAMA 2009, 302, 401–411. [Google Scholar] [CrossRef]
- Sonne-Holm, S.; Sorensen, T.I.; Jensen, G.; Schnohr, P. Independent effects of weight change and attained body weight on prevalence of arterial hypertension in obese and non-obese men. BMJ 1989, 299, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Rapsomaniki, E.; Timmis, A.; George, J.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; White, I.R.; Caulfield, M.J.; Deanfield, J.E.; Smeeth, L.; et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014, 383, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B. Blood pressure as a cardiovascular risk factor: Prevention and treatment. JAMA 1996, 275, 1571–1576. [Google Scholar] [CrossRef]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Miller, N.H.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Living with COVID-19. 2020. Available online: https://evidence.nihr.ac.uk/themedreview/living-with-covid19/ (accessed on 27 January 2024).
- Venkatesan, P. NICE guideline on long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 27 January 2024).
- Munblit, D.; O’Hara, M.E.; Akrami, A.; Perego, E.; Olliaro, P.; Needham, D.M. Long COVID: Aiming for a consensus. Lancet Respir. Med. 2022, 10, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Parhizgar, P.; Yazdankhah, N.; Rzepka, A.M.; Chung, K.Y.C.; Ali, I.; Fur, R.L.F.; Russell, V.; Cheung, A.M. Beyond Acute COVID-19: A Review of Long-term Cardiovascular Outcomes. Can. J. Cardiol. 2023, 39, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.; Sheerin, F.; Xie, C.; Mahmod, M.; et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021, 31, 100683. [Google Scholar] [CrossRef] [PubMed]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Leong, H.N.; Hsu, L.Y.; Tan, T.T.; Kurup, A.; Fook-Chong, S.; Tan, B.H. Autonomic dysfunction in recovered severe acute respiratory syndrome patients. Can. J. Neurol. Sci. 2005, 32, 264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Ahmad, S.J.; Feigen, C.M.; Vazquez, J.P.; Kobets, A.J.; Altschul, D.J. Neurological Sequelae of COVID-19. J. Integr. Neurosci. 2022, 21, 77. [Google Scholar] [CrossRef]
- Becker, R.C. Autonomic dysfunction in SARS-COV-2 infection acute and long-term implications COVID-19 editor’s page series. J. Thromb. Thrombolysis 2021, 52, 692–707. [Google Scholar] [CrossRef]
- Marques, K.C.; Silva, C.C.; Trindade, S.d.S.; Santos, M.C.d.S.; Rocha, R.S.B.; Vasconcelos, P.F.d.C.; Quaresma, J.A.S.; Falcão, L.F.M. Reduction of Cardiac Autonomic Modulation and Increased Sympathetic Activity by Heart Rate Variability in Patients with Long COVID. Front. Cardiovasc. Med. 2022, 9, 862001. [Google Scholar] [CrossRef]
- Balcom, E.F.; Nath, A.; Power, C. Acute and chronic neurological disorders in COVID-19: Potential mechanisms of disease. Brain 2021, 144, 3576–3588. [Google Scholar] [CrossRef]
- Goodman, B.P.; Khoury, J.A.; Blair, J.E.; Grill, M.F. COVID-19 Dysautonomia. Front. Neurol. 2021, 12, 624968. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jolkkoken, J.; Zhao, C. Neurotropism of SARS-CoV-2 and its neuropathological alterations: Similarities with other coronaviruses. Neurosci. Biobehav. Rev. 2020, 11, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Hultström, M.; von Seth, M.; Frithiof, R. Hyperreninemia and low total body water may contribute to acute kidney injury in COVID-19 patients in intensive care. J. Hypertens. 2020, 38, 1613–1614. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Tadic, M.; Larsen, T.H.; Grassi, G.; Mancia, G. Coronavirus disease 2019 and cardiovascular complications: Focused clinical review. J. Hypertens. 2021, 39, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, S.; Sun, G.; Yu, S.; Sun, Z.; Zheng, L.; Xu, C.; Li, J.; Sun, Y. Total and abdominal obesity among rural Chinese women and the association with hypertension. Nutrition 2012, 28, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Itoh, H. Hypertension as a Metabolic Disorder and the Novel Role of the Gut. Curr. Hypertens. Rep. 2019, 21, 63. [Google Scholar] [CrossRef]
- Muhamad, S.-A.; Ugusman, A.; Kumar, J.; Skiba, D.; Hamid, A.A.; Aminuddin, A. COVID-19 and Hypertension: The What, the Why, and the How. Front. Physiol. 2021, 12, 665064. [Google Scholar] [CrossRef]
- Ambrosino, P.; Parrella, P.; Formisano, R.; Perrotta, G.; D’anna, S.E.; Mosella, M.; Papa, A.; Maniscalco, M. Cardiopulmonary Exercise Performance and Endothelial Function in Convalescent COVID-19 Patients. J. Clin. Med. 2022, 11, 1452. [Google Scholar] [CrossRef]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study. Front. Cardiovasc. Med. 2021, 8, 745758. [Google Scholar] [CrossRef] [PubMed]
- Konukoglu, D.; Uzun, H. Endothelial dysfunction and hypertension. Adv. Exp. Med. Biol. 2017, 956, 511–540. [Google Scholar] [PubMed]
- Rogier van der Velde, A.; Meijers, W.C.; de Boer, R.A. Chapter 3.7.1—Cardiovascular biomarkers: Translational aspects of hypertension, atherosclerosis, and heart failure in drug development. In Principles of Translational Science in Medicine, 2nd ed.; Wehling, M., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 167–183. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin. Microbiol. Infect. 2021, 27, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Wang, F.; Yang, Y.; Hu, G.; Guo, S.; Zhang, Q.; Bryant, A.; Zhang, L.; Kurts, C.; Wei, L.; et al. Health Issues and Immunological Assessment Related to Wuhan’s COVID-19 Survivors: A Multicenter Follow-Up Study. Front. Med. 2021, 8, 617689. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.F.; Liu, T.; Yu, J.N.; Xu, X.R.; Zahid, K.R.; Wei, Y.C.; Wang, X.H.; Zhou, F.L. Half-year follow-up of patients recovering from severe COVID-19: Analysis of symptoms and their risk factors. J. Intern. Med. 2021, 290, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Boglione, L.; Meli, G.; Poletti, F.; Rostagno, R.; Moglia, R.; Cantone, M.; Esposito, M.; Scianguetta, C.; Domenicale, B.; Di Pasquale, F.; et al. Risk factors and incidence of long-COVID syndrome in hospitalized patients: Does remdesivir have a protective effect? QJM Int. J. Med. 2021, 114, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Özcan, S.; İnce, O.; Güner, A.; Katkat, F.; Dönmez, E.; Tuğrul, S.; Şahin, İ.; Okuyan, E.; Kayıkçıoğlu, M. Long-Term Clinical Consequences of Patients Hospitalized for COVID-19 Infection. Anatol. J. Cardiol. 2022, 26, 305–315. [Google Scholar] [CrossRef]
- Akpek, M. Does COVID-19 Cause Hypertension? Angiology 2022, 73, 682–687. [Google Scholar] [CrossRef]
- Delalić, Đ.; Jug, J.; Prkačin, I. Arterial hypertension following COVID-19: A retrospective study of patients in a Central European tertiary care center. Acta Clin. Croat. 2022, 61, 23–26. [Google Scholar] [CrossRef]
- Ogungbe, O.; Gilotra, N.A.; Davidson, P.M.; Farley, J.E.; Himmelfarb, C.R.D.; Post, W.S.; Commodore-Mensah, Y. Cardiac postacute sequelae symptoms of SARS-CoV-2 in community-dwelling adults: Cross-sectional study. Open Heart 2022, 9, e002084. [Google Scholar] [CrossRef]
- Fernández-Ortega, M.; Ponce-Rosas, E.R.; Muñiz-Salinas, D.A.; Rodríguez-Mendoza, O.; Chávez, P.N.; Sánchez-Pozos, V.; Dávila-Mendoza, R.; Barrell, A.E. Cognitive dysfunction, diabetes mellitus 2 and arterial hypertension: Sequelae up to one year of COVID-19. Travel. Med. Infect. Dis. 2023, 52, 102553. [Google Scholar] [CrossRef] [PubMed]
- Vyas, P.; Joshi, D.; Sharma, V.; Parmar, M.; Vadodariya, J.; Patel, K.; Modi, G. Incidence and predictors of development of new onset hypertension post COVID-19 disease. Indian. Heart J. 2023, 75, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Abumayyaleh, M.; Gil, I.J.N.; Viana-Llamas, M.C.; Roubin, S.R.; Romero, R.; Alfonso-Rodríguez, E.; Uribarri, A.; Feltes, G.; Becerra-Muñoz, V.M.; Santoro, F.; et al. Post-COVID-19 syndrome and diabetes mellitus: A propensity-matched analysis of the International HOPE-II COVID-19 Registry. Front. Endocrinol. 2023, 14, 1167087. [Google Scholar] [CrossRef] [PubMed]
- Maestre-Muñiz, M.M.; Arias, Á.; Mata-Vázquez, E.; Martín-Toledano, M.; López-Larramona, G.; Ruiz-Chicote, A.M.; Nieto-Sandoval, B.; Lucendo, A.J. Long-Term Outcomes of Patients with Coronavirus Disease 2019 at One Year after Hospital Discharge. J. Clin. Med. 2021, 10, 2945. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, S.E.; Guo, Y.; Heath, K.; Dasmariñas, M.C.; Jubilo, K.G.; Samranvedhya, J.; Lipsitch, M.; Cohen, K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021, 373, 1098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Dagliati, A.; Abad, Z.S.H.; Xiong, X.; Bonzel, C.-L.; Xia, Z.; Tan, B.W.Q.; Avillach, P.; Brat, G.A.; Hong, C.; et al. International electronic health record-derived post-acute sequelae profiles of COVID-19 patients. NPJ Digit. Med. 2022, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Ren, S.; Heath, K.; Dasmariñas, M.C.; Jubilo, K.G.; Guo, Y.; Lipsitch, M.; Daugherty, S.E. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2022, 376, e068414. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S.S.; Lerner, U.; Bivas-Benita, M.; et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: Nationwide cohort study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef]
- Jennifer, K.; Shirley, S.B.D.; Avi, P.; Daniella, R.-C.; Naama, S.S.; Anat, E.Z.; Miri, M.-R. Post-acute sequelae of COVID-19 infection. Prev. Med. Rep. 2023, 31, 102097. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Tisler, A.; Stirrup, O.; Pisarev, H.; Kalda, R.; Meister, T.; Suija, K.; Kolde, R.; Piirsoo, M.; Uusküla, A. Post-acute sequelae of COVID-19 among hospitalized patients in Estonia: Nationwide matched cohort study. PLoS ONE 2022, 17, e0278057. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Rafiullah, M.; Alkhowaiter, M.; Alotaibi, N.; Alzahrani, M.; Binkhamis, K.; Siddiqui, K.; Youssef, A.; Altalhi, H.; Almaghlouth, I.; et al. Clinical and biochemical characteristics of people experiencing post-coronavirus disease 2019-related symptoms: A prospective follow-up investigation. Front. Med. 2022, 9, 1067082. [Google Scholar] [CrossRef] [PubMed]
- Ternushchak, T.M.; Tovt-Korshynska, M.I.; Varvarynets, A.V. Ambulatory blood pressure variability in young adults with long-COVID syndrome. Wiadomości Lek. Mon. J. 2022, 75, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, R.; Conte, C.; Lanzani, C.; Benedetti, F.; Roveri, L.; Mazza, M.G.; Brioni, E.; Giacalone, G.; Canti, V.; Sofia, V.; et al. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PLoS ONE 2020, 15, e0239570. [Google Scholar] [CrossRef] [PubMed]
- Tanni, S.E.; Tonon, C.R.; Gatto, M.; Mota, G.A.; Okoshi, M.P. Post-COVID-19 syndrome: Cardiovascular manifestations. Int. J. Cardiol. 2022, 369, 80–81. [Google Scholar] [CrossRef]
- Gameil, M.A.; Marzouk, R.E.; Elsebaie, A.H.; Rozaik, S.E. Long-term clinical and biochemical residue after COVID-19 recovery. Egypt. Liver J. 2021, 11, 74. [Google Scholar] [CrossRef]
- Saloň, A.; Neshev, R.; Teraž, K.; Šimunič, B.; Peskar, M.; Marušič, U.; Pišot, S.; Šlosar, L.; Gasparini, M.; Pišot, R.; et al. A pilot study: Exploring the influence of COVID-19 on cardiovascular physiology and retinal microcirculation. Microvasc. Res. 2023, 150, 104588. [Google Scholar] [CrossRef]
- Nandadeva, D.; Skow, R.J.; Stephens, B.Y.; Grotle, A.K.; Georgoudiou, S.; Barshikar, S.; Seo, Y.; Fadel, P.J. Cardiovascular and cerebral vascular health in females with postacute sequelae of COVID-19. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, 713–720. [Google Scholar] [CrossRef]
- Mahmoud, Z.; East, L.; Gleva, M.; Woodard, P.K.; Lavine, K.; Verma, A.K. Cardiovascular symptom phenotypes of post-acute sequelae of SARS-CoV-2. Int. J. Cardiol. 2022, 366, 35–41. [Google Scholar]
- Nandadeva, D.; Skow, R.J.; Grotle, A.-K.; Stephens, B.Y.; Young, B.E.; Fadel, P.J. Impact of COVID-19 on ambulatory blood pressure in young adults: A cross-sectional analysis investigating time since diagnosis. J. Appl. Physiol. 2022, 133, 183–190. [Google Scholar] [CrossRef] [PubMed]
- van der Sluijs, K.M.; Bakker, E.A.; Schuijt, T.J.; Joseph, J.; Kavousi, M.; Geersing, G.-J.; Rutten, F.H.; Hartman, Y.A.W.; Thijssen, D.H.J.; Eijsvogels, T.M.H. Long-term cardiovascular health status and physical functioning of nonhospitalized patients with COVID-19 compared with non-COVID-19 controls. Am. J. Physiol. Circ. Physiol. 2023, 324, H47–H56. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P.; Clare, R.M.; Chiswell, K.; Navar, A.M.; Shah, B.R.; Peterson, E.D. Trends of blood pressure control in the U.S. during the COVID-19 pandemic. Am. Heart J. 2022, 247, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, W.; Rajzer, M.; Weber, T.; Prejbisz, A.; Dobrowolski, P.; Ostrowska, A.; Bilo, G.; Mancia, G.; Kreutz, R.; Januszewicz, A. Ambulatory blood pressure monitoring in treated patients with hypertension in the COVID-19 pandemic—The study of European society of hypertension (ESH ABPM COVID-19 study). Blood Press. 2023, 32, 2161998. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Díaz-Barreiro, L.; Cossio-Aranda, J.; Verdejo-Paris, J.; Odin-de-Los-Ríos, M.; Galván-Oseguera, H.; Álvarez-López, H.; Alcocer-Gamba, M.A. COVID-19 and the renin, angiotensin, aldosterone system. A complex relationship. Arch. Cardiol. Mex. 2020, 90, 19–25. [Google Scholar] [PubMed]
- Ferrario, C.M.; Chappell, M.C.; Tallant, E.A.; Brosnihan, K.B.; Diz, D.I. Counterregulatory Actions of Angiotensin-(1-7). Hypertension 1997, 30, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Roks, A.J.; van Geel, P.P.; Pinto, Y.M.; Buikema, H.; Henning, R.H.; de Zeeuw, D.; van Gilst, W.H. Angiotensin-(1-7) is a modulator of the human renin-angiotensin system. Hypertension 1999, 34, 296–301. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ohishi, M.; Katsuya, T.; Ito, N.; Ikushima, M.; Kaibe, M.; Tatara, Y.; Shiota, A.; Sugano, S.; Takeda, S.; et al. Deletion of Angiotensin-Converting Enzyme 2 Accelerates Pressure Overload-Induced Cardiac Dysfunction by Increasing Local Angiotensin II. Hypertension 2006, 47, 718–726. [Google Scholar] [CrossRef]
- Freel, E.M.; Connell, J.M. Mechanisms of hypertension: The expanding role of aldosterone. J. Am. Soc. Nephrol. 2004, 15, 1993–2001. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, T.M.; Lakkappa, N.; Lazartigues, E. ADAM17-Mediated Shedding of Inflammatory Cytokines in Hypertension. Front. Pharmacol. 2020, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Salomão, R.; Assis, V.; de Sousa Neto, I.V.; Petriz, B.; Babault, N.; Durigan, J.L.Q.; de Cássia Marqueti, R. Involvement of Matrix Metalloproteinases in COVID-19: Molecular Targets, Mechanisms, and Insights for Therapeutic Interventions. Biology 2023, 12, 843. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, V.; Gjymishka, A.; Jarajapu, Y.P.; Qi, Y.; Afzal, A.; Rigatto, K.; Ferreira, A.J.; Fraga-Silva, R.A.; Kearns, P.; Douglas, J.Y.; et al. Diminazene Attenuates Pulmonary Hypertension and Improves Angiogenic Progenitor Cell Functions in Experimental Models. Am. J. Respir. Crit. Care Med. 2013, 187, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.J.; Shenoy, V.; Yamazato, Y.; Sriramula, S.; Francis, J.; Yuan, L.; Castellano, R.K.; Ostrov, D.A.; Oh, S.P.; Katovich, M.J.; et al. Evidence for Angiotensin-converting Enzyme 2 as a Therapeutic Target for the Prevention of Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2009, 179, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of Biological Peptides by Human Angiotensin-converting Enzyme-related Carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xia, H.; Santos, R.A.; Speth, R.; Lazartigues, E. Angiotensin-converting enzyme 2: A new target for neurogenic hypertension. Exp. Physiol. 2010, 95, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Gurley, S.B.; Allred, A.; Le, T.H.; Griffiths, R.; Mao, L.; Philip, N.; Haystead, T.A.; Donoghue, M.; Breitbart, R.E.; Acton, S.L.; et al. Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J. Clin. Investig. 2006, 116, 2218–2225. [Google Scholar] [CrossRef]
- Glowacka, I.; Bertram, S.; Herzog, P.; Pfefferle, S.; Steffen, I.; Muench, M.O.; Simmons, G.; Hofmann, H.; Kuri, T.; Weber, F.; et al. Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. J. Virol. 2010, 84, 1198–1205. [Google Scholar] [CrossRef]
- Grimes, J.M.; Grimes, K.V. p38 MAPK inhibition: A promising therapeutic approach for COVID-19. J. Mol. Cell Cardiol. 2020, 144, 63–65. [Google Scholar] [CrossRef]
- Tomasoni, D.; Italia, L.; Adamo, M.; Inciardi, R.M.; Lombardi, C.M.; Solomon, S.D.; Metra, M. COVID-19 and heart failure: From infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur. J. Heart Fail. 2020, 22, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Oudit, G.Y.; Pfeffer, M.A. Plasma angiotensin-converting enzyme 2: Novel biomarker in heart failure with implications for COVID-19. Eur. Heart J. 2020, 41, 1818–1820. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Gui, M.; Wang, X.; Xiang, Y. Cryo-EM structure of the SARS coronavirus spike glycoprotein in complex with its host cell receptor ACE2. PLOS Pathog. 2018, 14, e1007236. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, W.; Farzan, M.; Harrison, S. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 2005, 309, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.P.; Look, D.C.; Tan, P.; Shi, L.; Hickey, M.; Gakhar, L.; Chappell, M.C.; Wohlford-Lenane, C.; McCray, P.B., Jr. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L84–L96. [Google Scholar] [CrossRef]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 Cleave ACE2 Differentially and Only Proteolysis by TMPRSS2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Coronavirus Spike Protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Yu, Y.; Chien, S.; Chen, I.; Lai, C.; Tsay, Y.; Chang, S.; Chang, M. Surface vimentin is critical for the cell entry of SARS-CoV. J. Biomed. Sci. 2016, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Tanaka, N.; Tanaka, Y.; Inoue, S.; Morita, K.; Zhuang, M.; Hattori, T.; Sugamura, K. Clathrin-Dependent Entry of Severe Acute Respiratory Syndrome Coronavirus into Target Cells Expressing ACE2 with the Cytoplasmic Tail Deleted. J. Virol. 2007, 81, 8722–8729. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.V.; Dveksler, G.; Gagneten, S.; Yeager, C.; Lin, S.H.; Beauchemin, N.; Look, A.T.; Ashmun, R.; Dieffenbach, C. Coronavirus receptor specificity. Adv. Exp. Med. Biol. 1993, 342, 261–266. [Google Scholar] [PubMed]
- Bernard, I.; Limonta, D.; Mahal, L.K.; Hobman, T.C. Endothelium Infection and Dysregulation by SARS-CoV-2: Evidence and Caveats in COVID-19. Viruses 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Avtaar Singh, S.S. Endothelial Dysfunction in SARS-CoV-2 Infection. Biomedicines 2022, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Meher, M.; Pradhan, S.; Pradhan, S.R. Risk Factors Associated with Hypertension in Young Adults: A Systematic Review. Cureus 2023, 15, e37467. [Google Scholar] [CrossRef] [PubMed]
- Cénat, J.M.; Blais-Rochette, C.; Kokou-Kpolou, C.K.; Noorishad, P.-G.; Mukunzi, J.N.; McIntee, S.-E.; Dalexis, R.D.; Goulet, M.-A.; Labelle, P.R. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 2021, 295, 113599. [Google Scholar] [CrossRef]
- Kaczyńska, A.; Gaciong, Z. Mental stress and hypertension. In Przewodnik Lekarza/Guide for GPs; Termedia: Poznań, Poland, 2008; pp. 62–67. [Google Scholar]
- Deng, H.-B.; Tam, T.; Zee, B.C.-Y.; Chung, R.Y.-N.; Su, X.; Jin, L.; Chan, T.-C.; Chang, L.-Y.; Yeoh, E.-K.; Lao, X.Q. Short Sleep Duration Increases Metabolic Impact in Healthy Adults: A Population-Based Cohort Study. Sleep 2017, 40, zsx130. [Google Scholar] [CrossRef]
- Albikawi, Z.F. Fear Related to COVID-19, Mental Health Issues, and Predictors of Insomnia among Female Nursing College Students during the Pandemic. Healthcare 2023, 11, 174. [Google Scholar] [CrossRef]
- Robles-Cabrera, A.; Michel-Chávez, A.; Callejas-Rojas, R.C.; Malamud-Kessler, C.; Delgado, G.; Estañol-Vidal, B. The cardiovagal, cardiosympathetic and vasosympathetic arterial baroreflexes and the neural control of short-term blood pressure. Rev. Neurol. 2014, 59, 508–516. [Google Scholar] [PubMed]
- Kirchheim, H.R. Systemic arterial baroreceptor reflexes. Physiol. Rev. 1976, 56, 100–177. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.C. COVID-19-associated vasculitis and vasculopathy. J. Thromb. Thrombolysis 2020, 50, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Pasquetto, G.; Mazza, A. Risk of Incident New-Onset Arterial Hypertension After COVID-19 Recovery: A Systematic Review and Meta-analysis. High. Blood Press. Cardiovasc. Prev. 2023, 30, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Szeghy, R.E.; Province, V.M.; Stute, N.L.; Augenreich, M.A.; Koontz, L.K.; Stickford, J.L.; Stickford, A.S.L.; Ratchford, S.M. Carotid stiffness, intima-media thickness and aortic augmentation index among adults with SARS-CoV-2. Exp. Physiol. 2022, 107, 694–707. [Google Scholar] [CrossRef]
- Gathiram, P.; Mackraj, I.; Moodley, J. The Renin-Angiotensin System, Hypertension, and SARS-CoV-2 Infection: A Review. Curr. Hypertens. Rep. 2021, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Vaughan, A.S.; Coronado, F.; Casper, M.; Loustalot, F.; Wright, J.S. County-Level Trends in Hypertension-Related Cardiovascular Disease Mortality-United States, 2000 to 2019. J. Am. Heart Assoc. 2022, 11, e024785. [Google Scholar] [CrossRef]
- Wytyczne Polskiego Towarzystwa Nadciśnie Polsce, Rules for the management of hypertension. Arter. Hypertens. 2008, 12, 317–342.

| Study/Quality Criteria | Study Design | Study Population | Control Group | Data Sources | Methods of Systematic Analysis | Reporting Statistical Significance | Ethics Committee Approval | Obtaining Informed Consent | Presenting Limitations |
|---|---|---|---|---|---|---|---|---|---|
| Xiong et al. [38] | + | + | + | + | + | + | + | - | + |
| Mei et al. [39] | + | + | - | + | + | + | + | + | + |
| Shang et al. [40] | + | + | - | + | + | + | + | - | + |
| Boglione et al. [41] | + | + | - | + | + | + | + | + | + |
| Ozcan et al. [42] | + | + | - | + | + | + | + | + | + |
| Akpek et al. [43] | + | + | - | + | + | + | + | - | + |
| Delalic et al. [44] | + | + | _ | + | - | - | - | - | - |
| Ogungbe et al. [45] | + | + | - | + | - | + | + | + | + |
| Fernandez-Ortega MA [46] | + | + | - | + | - | - | + | + | + |
| Vyas et al. [47] | + | + | - | + | + | + | + | + | + |
| Abumayyaleh et al. [48] | + | + | - | + | + | + | + | - | + |
| Maestre-Muniz et al. [49] | + | + | - | + | + | + | + | - | + |
| Daugherty et al. [50] | + | + | + | + | + | + | + * | - | + |
| Zhang HG et al. [51] | + | + | + | + | + | + | + | - | + |
| Cohen et al. [52] | + | + | + | + | + | + | + * | - | + |
| Al-Aly et al. [53] | + | + | + | + | + | + | + | - | + |
| Mizrahi B et al. [54] | + | + | + | + | + | + | + | - | + |
| Jennifer K et al. [55] | + | + | + | + | + | + | + | - ** | + |
| Ziyad Al-Aly et al. [56] | + | + | + | + | + | + | + | - | + |
| Tisler A et al. [57] | + | + | + | + | + | + | + | - | + |
| Alfadda et al. [58] | + | + | - | + | + | + | + | + | + |
| Tetiana et al. [59] | + | + | + | + | + | + | - | - | - |
| DeLorenzo et al. [60] | + | + | - | + | + | + | + | + | + |
| Tanni et al. [61] | + | + | - | + | - | - | - | - | + |
| Gameil et al. [62] | + | + | + | + | + | + | + | + | + |
| Saloň et al. [63] | + | + | + *** | + | + | + | + | + | + |
| Nandadeva et al. [64] | + | + | + | + | + | + | + | + | - |
| Mahmoud et al. [65] | + | + | - | + | + | + | + | - | + |
| Nandadeva et al. [66] | + | + | + | + | + | + | + | + | - |
| vas der Sluijs et al. [67] | + | + | + | + | + | + | + | + | + |
| Study, (Year) | City/ Country | Sample Size | Disease Severity | Median/Mean Follow-Up Periods | Median/ Mean Age, % of Male in Case Group | % of Patients with Newly Diagnosed HTN | Obtain Data |
|---|---|---|---|---|---|---|---|
| Xiong et al. [38] (2020) | Wuhan, China | 722 | Mostly Severe, Critical | Median 97 days (95–102) | Median 52 (41–62) 45.5% | 1.3 | Telephone surveys |
| Mei et al. [39] (2021) | Wuhan, China | 3677 | Mild, Severe, Critical | Median 144 days (135–157) | Median 59 (47–68) 45.9% | 0.16 | Case, medical and self-reports |
| Shang et al. [40] (2021) | Wuhan, China | 796 | Severe Critical | 6 months after infection | Median 62 (51–69) 50.8% | 0.4 | Telephone surveys |
| Boglione et al. [41] (2021) | Vercelli, Italy | 449 | Hospitalized | Median visit 1 32.5 days, visit 2 178.5 days | Median 65 (56–75.5) 78% | 25.8—First visit 14—Second visit | Visit with examination |
| Ozcan et al. [42] (2022) | Turkey | 406 | Hospitalized | 3 and 6 months | WHO-1: 46.8 ± 13.3 WHO-2 52.8 ± 13.1 WHO-3 54.8 ± 11.8 | 1 | Telephone surveys |
| Akpek et al. [43] (2021) | Turkey | 153 | Mild | Mean 31.6 ± 5.0 days | Mean 46.5 ± 12.7 34% | 11.76 | Visit, examination |
| Delalic et al. [44] (2022) | Croatia, Zagreb | 199 | No data | Median 1 month | Mean age 57.3 46% | 16.08 | Visit, examination |
| Ogungbe et al. [45] (2022) | No data | 442 | Mild | Median 12.4 months (10–15.2) | Mean 45.4 29% | 20 | Telephone surveys |
| Fernandez-Ortega MA [46] (2023) | Mexico | 70 | Hospitalized | Follow up 5 months and 12 months | No data 65.7% | 29.7 (5 months) 12.5 (12 months) | Telephone surveys |
| Vyas et al. [47] (2023) | India | 248 | Hospitalized | Follow-up length 1 year | Mean 51.16 ± 12.71 68.1% | 32.3 | Visit, examination |
| Abumayyaleh et al. [48] (2023) | International | 3096 | Severe | Follow-up time (months) diabetes 2.6 ± 4.6 non-diabetes 2.8 ± 4.9 | Mean 72.6 ± 12.7 63.5% | DM patients 0.5% non-DM patients 1.6% | Telephone surveys |
| Maestre-Muniz et al. [49] (2021) | Spain | 543 | Hospitalized | 12 months | Mean 65.1 (17.5; 18–98), 50.7% | 2% | Telephone surveys |
| Study, (Year) | City/ Country | Sample Size | Disease Severity | Median/Mean Follow-Up Periods | Median/Mean Age Małe % | HTN Risk |
|---|---|---|---|---|---|---|
| Daugherty et al. [50] (2021) | USA | 9,247,505 | Mild, Moderate, Severe | Median 87 days (45–124) | Mean 42.4 50.2% | risk ratio 1.81 (1.10 to 2.96) |
| Zhang HG et al. [51] (2022) | Germany, France, Italy, Singapore, USA | 2,745,130 | Hospitalized | Follow up 1 year | No data 74% | relative risk 1.14 (1.06–1.22) |
| Cohen et al. [52] (2022) | USA | 2,895,943 | Hospitalized | Median 78 days (30–175) | Mean 75.7 42% | risk difference 4.43 (2.27–6.37) |
| Al-Aly et al. [53] (2022) | USA | 501,743 | Mild, Moderate, Severe | Follow-up length 6 months | Mean 64.9 89.9% | hazard ratio 1.62 |
| Mizrahi B et al. [54] (2023) | Israel | 599,740 | Mild | Two time periods after infection Early (30–180 days) Late (180–360 days) | Median 25 years old 49.4% | hazard ratio 1.27 |
| Jennifer K et al. [55] (2022) | Israel | 185,924 | No data | Follow-up length: 14 months | No data | no difference |
| Ziyad Al-Aly et al. [56] (2021) | USA | 5,064,270 | Mild | Median 126 (81–203) | Mean 59.09 87.96% | hazard ratio 15.18 (11.53–18.62) |
| Tisler A et al. [57] (2022) | Estonia | 19,460 | Mild, Moderate, Severe | Mean 294.9 | Mean 65.4 45.7% | hazard ratio 2.85 |
| Study, (Year) | City/ Country | Sample Size | Disease Severity | Median/Mean Follow-Up Periods | Median/Mean Age Male % | BP |
|---|---|---|---|---|---|---|
| Alfadda et al. [58] (2022) | Saudi Arabia | 98 | Hospitalized | Mean 7.02 ± 1.6 months | Mean 48.87 ± 17.11 51% | SBP mmHg 124.68 ± 14.9 vs. in follow-up 131.26 ± 15.3 |
| Tetiana et al. [59] (2022) | Ukraine | 115 | Mild, Moderate | Mean 1.68 ± 1.2 months | Mean age 23.07 ± 1.54. | Patients with long COVID syndrome vs. control group SBP (127.1 ± 6.65 mmHg and 115.93 ± 6.24 mmHg and DBP 73.31 ± 5.30 mmHg vs. 68.79 ± 5.5 mmHg |
| DeLorenzo et al. [60] (2020) | Italy, Milan | 185 | Mild, Moderate, Severe | Median time from hospital discharge 23 days (20–29) | Mean age 57 male 66.5% | Uncontrolled BP requiring therapeutic change In 21.6% of patients |
| Tanni et al. [61] (2022) | Brazil | 100 | No data | Median 99 days | Mean age at 46.3. Mostly female | No data |
| Gameil et al. [62] (2021) | Egypt | 240 | Mild, Moderate | >3 months | Mean 38.29 55.9% | Control 120.63 ± 8.49 vs. research group 126.70 ± 10.31 |
| Saloň et al. [63] (2023) | Austria | 35 | Hospitalized | Measurements day: 0/10 occurred 2 months after hospitalization. | Mean 60 ± 10 85% | 142 mmHg to 150 mmHg |
| Nandadeva et al. [64] (2023) | USA, Texas | 23 | No Data | Median 15 months (3–30) | Mean 48 ± 9 0% | Systolic BP in COVID group 126 ± 19 vs. control: 109 ± 8 mmHg |
| Mahmoud et al. [65] (2022) | USA, Washington | 100 | Mild, Moderate, Severe | Median 99 days | Mean 46.3 19% | (Before COVID-19 disease vs. after) Median systolic BP 128 vs. 121.5 mmHg, median diastolic BP: 83.5 vs. 76 mmHg |
| Study, (Year) | City/ Country | Sample Size | Disease Severity | Median/Mean Follow Up Periods | Median/Mean Age Male % |
|---|---|---|---|---|---|
| Nandadeva et al. (2022) [66] | USA, Texas | 38 | Mild | Mean 11 ± 6 weeks | Control: 23 ± 3 yr COVID: 24.5 ± 4 yr 100% |
| vas der. Sluijs et al. (2022) [67] | The Netherlands | 202 | Mild | Median 175 days (126–235) | Mean 58 (54–65) 58% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielecka, E.; Sielatycki, P.; Pietraszko, P.; Zapora-Kurel, A.; Zbroch, E. Elevated Arterial Blood Pressure as a Delayed Complication Following COVID-19—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 1837. https://doi.org/10.3390/ijms25031837
Bielecka E, Sielatycki P, Pietraszko P, Zapora-Kurel A, Zbroch E. Elevated Arterial Blood Pressure as a Delayed Complication Following COVID-19—A Narrative Review. International Journal of Molecular Sciences. 2024; 25(3):1837. https://doi.org/10.3390/ijms25031837
Chicago/Turabian StyleBielecka, Emilia, Piotr Sielatycki, Paulina Pietraszko, Agnieszka Zapora-Kurel, and Edyta Zbroch. 2024. "Elevated Arterial Blood Pressure as a Delayed Complication Following COVID-19—A Narrative Review" International Journal of Molecular Sciences 25, no. 3: 1837. https://doi.org/10.3390/ijms25031837
APA StyleBielecka, E., Sielatycki, P., Pietraszko, P., Zapora-Kurel, A., & Zbroch, E. (2024). Elevated Arterial Blood Pressure as a Delayed Complication Following COVID-19—A Narrative Review. International Journal of Molecular Sciences, 25(3), 1837. https://doi.org/10.3390/ijms25031837







