Abstract
Aldehyde:ferredoxin oxidoreductases (AORs) have been isolated and biochemically-characterized from a handful of anaerobic or facultative aerobic archaea and bacteria. They catalyze the ferredoxin (Fd)-dependent oxidation of aldehydes to acids. Recently, the involvement of AOR in the reduction of organic acids to alcohols with electrons derived from sugar or synthesis gas was demonstrated, with alcohol dehydrogenases (ADHs) carrying out the reduction of the aldehyde to the alcohol (AOR-ADH pathway). Here, we describe the biochemical characterization of an AOR of the thermophilic fermentative bacterium Thermoanaerobacter sp. strain X514 (AORX514). The putative aor gene (Teth514_1380) including a 6x-His-tag was introduced into the genome of the genetically-accessible, related species Thermoanaerobacter kivui. The protein was purified to apparent homogeneity, and indeed revealed AOR activity, as measured by acetaldehyde-dependent ferredoxin reduction. AORX514 was active over a wide temperature (10 to 95 °C) and pH (5.5 to 11.5) range, utilized a wide variety of aldehydes (short and branched-chained, aliphatic, aromatic) and resembles archaeal sensu stricto AORs, as the protein is active in a homodimeric form. The successful, recombinant production of AORX514 in a related, well-characterized and likewise strict anaerobe paves the road towards structure-function analyses of this enzyme and possibly similar oxygen-sensitive or W/Mo-dependent proteins in the future.
1. Introduction
Aldehyde ferredoxin oxidoreductases (AORs) catalyze the reversible reduction of carboxylic acids to aldehydes [1,2]. These enzymes belong to the DSMO-family of oxidoreductases and depend on a bis-metallopterin cofactor, with most AORs containing the biologically rare element tungsten (W) [3,4]. However, there are a few known AORs with a molybdenum (Mo) instead [5,6].
AORs occur either as homodimer [3], or as complexes consisting of three different subunits [7,8], but there are also monomeric [9] and homotrimeric [5] AORs described. While the monomeric and homodimeric AORs consist of one ~67 kDa subunit in α2 conformation, the more complex AORs consist of three different subunits with multiple alpha and beta subunits being connected by one gamma subunit. In AOR from Moorella thermoacetica (AORMt), α3β3γ seems to be the most prominent conformation [7], whereas in AOR from Aromatoleum aromaticum (AORAa) (αβ)2γ and (αβ)3γ conformations are found more often [8]. The formation of multimers was proposed for the former, as up to 1600 kDa complexes were found [7], and just recently the structure of the latter was examined and is was found that AORAa forms long structures. These structures are similar to the spirosomes of AdhE [10] and even more similar to the recently described hydrogen-dependent carbon dioxide reductase (HDCR) from Thermoanaerobacter kivui [11]. The molecular function of these long filaments is not clear, but since the enzyme, as HDCR, accepts electrons from hydrogen or from an aldehyde, it may store and channel electrons along its backbone [8].
AORs have been isolated from mesophilic, thermophilic and hyperthermophilic archaea and bacteria, and are mainly found in anaerobic microorganisms [1,2,5,6,9,12,13,14,15,16]. They are described as strict oxygen sensitive proteins, containing iron-sulfur clusters and a metallopterin cofactor. Two biological functions of AORs have been proposed. In the hyperthermophilic archaeon Pyrococcus furiosus, it was initially suggested that AOR removes toxic aldehydes, produced as by-products of peptide oxidation in the energy metabolism of the organism, by oxidation to their corresponding acids [2,13] (Figure 1a). A similar function has been proposed for the AOR of the mesophilic bacterium Aromatoleum aromaticum in phenylacetaldehyde oxidation [17]. This may be explained by the broad substrate spectrum of most AORs, including oxidizing activities towards aliphatic, aromatic and branched chain aldehydes [5,9,13,14,16].
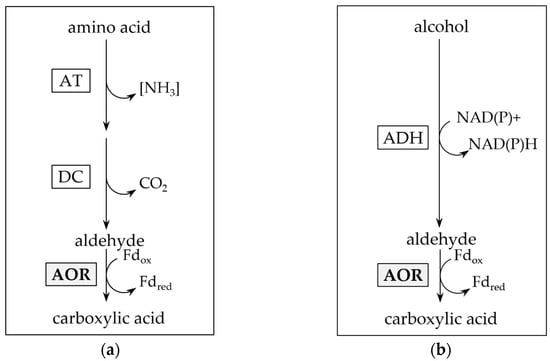
Figure 1.
Putative function of aldehyde:ferredoxin oxidoreductase (AOR) in aldehyde detoxification. Reductive detoxification of accumulated aldehydes as product of amino acid ((a); after [17,18]) or alcohol catabolism ((b); after [14,19]). AT: aminotransferase; DC: decarboxylase; ADH: alcohol dehydrogenase.
A second function of AOR is its involvement in the reductive branch of the catabolism in fermentative bacteria and lithotrophic acetogens. Organic acid reduction, as observed e.g., in thermophilic Moorella and Thermoanaerobacter sp. may be carried out [20,21], on the one hand, with an acyl-CoA thioester as intermediate, which may subsequently be reduced by aldehyde dehydrogenase (ALDH) and alcohol dehydrogenase (ADH) (Figure 2b) [22]. On the other hand, in the first reaction, AOR may alternatively catalyze a direct reduction of the acid, e.g., acetic acid, to the aldehyde, e.g., acetaldehyde (Figure 2a). Since the standard redox potential of the pair acid/aldehyde is in the range of −520 to −560 mV [2], this requires the low redox potential electron carrier ferredoxin (Fd) as electron donor. Subsequently, the aldehyde is reduced to the corresponding alcohol by an (NADH or NADPH)-dependent alcohol dehydrogenase (ADH) [21]. This AOR-ADH pathway for alcohol production from carboxylic acids was first proposed in the late 1980s by Simon and colleagues, that found biochemical evidence for the involvement of a carboxylic acid reductase (later reclassified as AOR) in the thermophilic acetogenic bacterium Moorella thermoacetica [23]. The essential role of AOR in acid reduction was ultimately proven in an engineered strain of the hyperthermophilic archaeon Pyrococcus furiosus that contained a foreign ADH, and produced ethanol from sugars and alcohols from their corresponding acids [24]. More recently, AOR has been shown to be essential for ethanol formation from synthesis gas in Clostridium autoethanogenum or/and Clostridium ljungdahlii [25,26,27,28], with increasing evidence that this pathway may be widespread among sugar fermenting and gas (H2 or CO) oxidizing microorganisms [21].
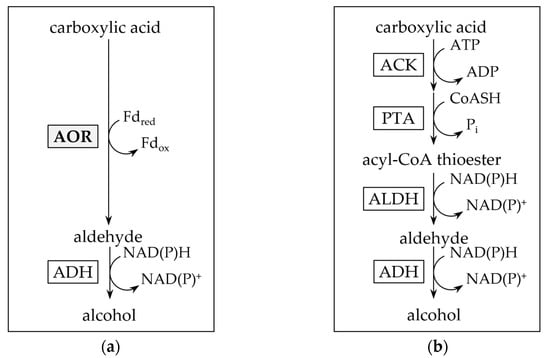
Figure 2.
Pathways for enzymatic carboxylic acid reduction. (a) Aldehyde:ferredoxin oxidoreductase and alcohol dehydrogenase involving (AOR-ADH) pathway for direct reduction of carboxylic acids to alcohols. Note that the pathway also reduces acetate produced from pyruvate oxidation in sugar fermentation. (b) Activation of carboxylic acids through acetate kinase (ACK) and phosphotransacetylase (PTA) to their acyl-CoA thioesters, and subsequent reduction by aldehyde dehydrogenase (ALDH) and ADH.
The organotrophic thermophilic bacterium Thermoanaerobacter sp. strain X514 converts sugars to mostly ethanol and organic acids [20]. When supplied with external organic acids such as propionate or isobutyrate, it produced up to 43 mM of the corresponding alcohol [20]. The ability to reduce organic acids to alcohols with electrons derived from sugars is widespread among Thermoanaerobacter species [20,29]. Toward elucidating organic acid reduction in Thermoanaerobacter sp. strain X514, and in the absence of a genetic system, we purified the most abundant NADH- and NADPH-dependent alcohol dehydrogenases, the bifunctional aldehyde dehydrogenase (ALDH)/alcohol dehydrogenase, AdhE, and the secondary alcohol dehydrogenase AdhB, from the organism and biochemically characterized both, as well as two primary AdhA-type alcohol dehydrogenases [30]. Ethanol production from sugars and alcohol production from organic acids may proceed via reduction of acyl-CoA to aldehydes catalyzed by the ALDH AdhE, followed by reduction to the alcohol catalyzed by one of the ADHs, likely AdhB or the ADH activity of the bifunctional AdhE (Figure 2b) [22]. The strain however, phylogenetically groups with a few Thermoanaerobacter species that contain genes putatively encoding AORs, and it is among the group of strains that produce the highest titers of alcohols from acids, at rates comparable to those of M. thermoacetica [20].
Aldehyde-dependent reduction of benzyl viologen (BV), previously associated with AOR activity [5,14,16,31] was measured in the cell-free extract of Thermoanaerobacter sp. strain X514 [20], and the gene, Teth514_1380 was transcribed at comparably high levels. This prompted us to propose that Teth514_1380 likely encodes an active AOR. One the one hand, the enzyme may be involved in carboxylic acid reduction in Thermoanaerobacter sp. strain X514 [30]. One the other hand, the metabolic function of AOR may be the oxidative detoxification of aldehydes accumulating during alcohol or peptide catabolism [32], as Thermoanaerobacter sp. have been reported to oxidize amino acids [18].
Here, we took advantage of the genetic accessibility of the related, acetogenic T. kivui [33], that natively neither contains an aor nor produces alcohols, but converts H2 + CO2 and CO [34,35] at high rates to acetate. We expressed a C-terminally His-tagged version of Teth514_1380 in T. kivui. The corresponding protein was characterized as a versatile AOR that may explain amino acid catabolism, and putatively, alcohol production in Thermoanaerobacter sp. strain X514 and that paves the road towards future metabolic engineering efforts in T. kivui towards ethanol production.
2. Results and Discussion
2.1. Recombinant AOR-His Obtained from a Related Strain Is Active
To obtain enough protein for a biochemical characterization, we initially considered two options, the ‘native’ purification from Thermoanaerobacter sp. strain X514 or a recombinant expression in a suitable host. However, the expression in a standard host like E. coli is difficult due to the formation of the Fe4S4 and bis-W/Mo-opterin cofactors. Only two AORs have been produced recombinantly before, the sensu stricto AOR of P. furiosus was functionally produced in the extremely thermophilic anaerobic bacterium Caldicellulosiruptor bescii [36] and, recently, the novel type AOR of A. aromaticum was produced in the related strain Aromatoleum evansii [37]. Here, we took advantage of the recently described genetic system for the acetogenic bacterium T. kivui. This bacterium produces large quantities of the W-opterin and FeS-containing HDCR [11,38,39]; therefore, we assumed that it may be able to assemble these cofactors into a foreign protein, too. Proteins of T. kivui have been homologously-produced in T. kivui, either plasmid-based [33], or by integration into its genome [40]. A plasmid-based method to overproduce tagged proteins was established, with genes under the control of the strong constitutive promoter of the S-layer protein (PSlp) [41]. Here, we cloned the putative aor (Teth514_1380) gene with a C-terminal 6xHis-tag and controlled by PSlp into plasmid pJM009 (Figure S1a). The plasmid also contained the pyrE gene, encoding a orotate phosphoribosyltranferase, which was transformed into the uracil auxotrophic strain T. kivui MB002 (old name TKV002; [33], and integrated into the genome (Figure S1b, strain MB014).
T. kivui MB014 cells were grown, harvested and lysed as described in the method section of this article. Subsequently, the putative AORX514 was purified by affinity chromatography to apparent homogeneity (Figure 3a) under strict anoxic conditions, since AORs have been reported to be extremely sensitive to oxygen [2,13]. Specific antibodies were used to verify the purification (Figure 3b). Using a protein standard, a subunit size of under 72 kDa was determined, which corresponds to the expected subunit size of AOR-His, 66.6 kDa. The purified protein is bound by the specific antibodies. Apart from AORMt and AORAa, as described above, most of the characterized tungsten-dependent AORs consist of only one type of subunit, and form homodimers, except for AOREa, which most likely stays monomeric [9]. This homodimeric conformation can be found for hyperthermophilic archaeal AORs, such as those from Pyrococcus furiosus, Thermococcus strain ES-1, Pyrococcus strain ES-1 and Pyrobaculum aerophilum [3,13,15,32], but also for mesophilic bacteria such as AOR from Clostridium formicoaceticum and Megalodesulfovibrio gigas (formerly Desulfovibrio gigas) [12,14].
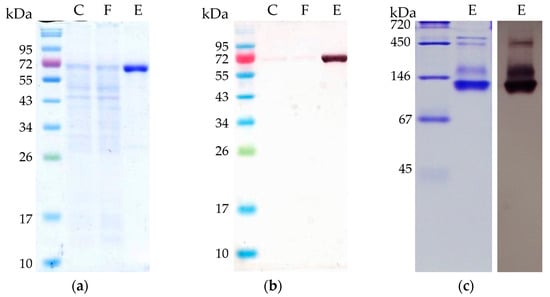
Figure 3.
Separation of purified AORX514 with C-terminal His-Tag by polyacrylamide gel electrophoresis (PAGE). C: 5 µg cell free MB014 extract before affinity chromatography; F: 5 µg flow-through of all proteins not binding to the His-tag column; E: 2 µg eluate containing AOR-His, eluted with 150 mM imidazole were analyzed (a) on a 10% SDS-polyacrylamide gel stained with Coomassie blue, (b) used for Western blot with specific anti-AOR antibodies and (c) on a 10% native polyacrylamide gel stained with Coomassie blue (c, left side) and used for Western blot with specific anti-AOR antibodies (c, right side). For SDS PAGE, NEB Color Prestained Protein Standard Broad Range (New England BioLabs) marker and for native gel Serva Native Marker (Serva) was loaded onto the gel.
To elucidate the native composition, we ran a native PAGE (Figure 3c) and observed the most prominent protein with a measured size between 105 and 124 kDa. This corresponds to the expected size of an AOR-His homodimer (134 kDa). In addition, there are bigger proteins observed (between 290 and 520 kDa), which may be AORX514 forming complexes. The formation of complexes has been described for other AORs, for example for AOR from Clostridium formicoaceticum (AORCf), where complexes of 240 kDa can be found in highly concentrated samples [12] and even bigger complexes of up to 1600 kDa were shown for AOR from Moorella thermoacetica (AORMt) [7]. And just recently, the formation of nanowires and thereby the formation of large complexes has also been demonstrated for AORAa [8] as well.
Initially, we determined that the protein is an aldehyde oxidoreductase as it catalyzed the acetaldehyde-dependent reduction of benzyl viologen (BV) as an artificial electron acceptor, matching the activity in the cell free extract reported before [20]. Unfortunately, there was no acetaldehyde oxidation measurable in cell free extracts of T. kivui MB014 cells, and the enzyme activity of the purified protein was low, compared to previously purified AORs (less than 0.5 U mg−1, one unit (U) corresponding to 1 µmol acetaldehyde oxidized per minute [16,36,37], therefore we tested whether it was possible to improve enzyme activity, by adding different supplements to the growing T. kivui cells and testing different assay conditions.
2.2. AOR-His Activity Can Be Improved by Media Optimization
Most of the previously characterized AORs are W-containing enzymes and the increase of AOR activity through the addition of tungsten to the growing cells has been described previously [1], while molybdenum (Mo) is the antagonist for tungsten (W) [32]. The standard T. kivui [33] media contains 12 nM W and 490 nM Mo, i.e., about 40-times more Mo than W was supplied. Moreover, the T. kivui cell contains vast amounts of HDCR, which comprises one FeS cluster and one W per formate dehydrogenase subunit [11].
To determine whether the aldehyde oxidoreductase is W dependent and whether the activity can be improved by adding more W or less Mo, we supplied the growing T. kivui MB014 cells with different concentrations of W and Mo. The addition of W did not affect growth of T. kivui cells (Figure S2, Table S1), however, we saw a significant increase in AOR activity of the purified protein (Table 1). In this experiment, the standard amount of W (12 nM) led to an acetaldehyde oxidation activity of 0.04 U mg−1, while the addition of 10-fold W (120 nM), increased the AOR activity by about 100-fold to 5.1 U mg−1. If 1.2 µM or 12 µM W was added, the activity was even higher (5.8 and 7.7 U mg−1, respectively), however, no further increase was observed with 120 µM or 1.2 mM. In contrast to W, the addition of Mo did influence growth of T. kivui and when adding 49 µM Mo (instead of 490 nM) the final optical density was much lower (Table S2) and AOR activity was reduced to 4% (Table 1), while omitting Mo did not improve the activity any further. Therefore, we routinely used the 1000-fold increased W concentration (12 µM) compared to the original T. kivui medium, with unchanged Mo concentration (490 nM), to optimize the amount of active protein, and achieved specific activities of up to 40 U mg−1.

Table 1.
Effect of different components in the growth media on activity of purified AORX514. The purification was routinely carried out with cells grown in media containing 12 µM W, 490 nM Mo, 11 µM Fe, 1 mM SO4, 0.2% (w/v) yeast (Roth) and 25 mM glucose. Changes in media composition and resulting relative specific activities are highlighted in bold in the table.
As the purified enzyme contained only 3.2 Fe per subunit, compared to the expected 4 Fe, there may not have been enough iron supplied for the formation and integration of iron/sulfur clusters. Since a lot more sulfur than iron is supplied in the media (2 mM sulfate, 7.2 µM iron), we tested to supply to the growing cells an about 10-times higher iron concentration. Through this increase of iron in the media, AOR activity of the purified enzyme increased from 26 U mg−1 to 42 U mg−1 and the amount of Fe per subunit increased to 3.8 Fe per subunit (Table 1). In addition to W, Mo and Fe, we also tested other supplements such as different sulfur components, glucose, yeast extract, but only the addition of sodium sulfate and yeast extract increased the AOR activity (Table 1).
The effects of the different sulfur sources are relatively minor, likely due to the fact that T. kivui utilized the cysteine in the medium. Apart from sulfide, no inorganic sulfur source has been described to support growth of T. kivui [34].
There are multiple possible reasons as to why higher AOR activity is measured with higher tungsten concentration in the growth medium. T. kivui has tungsten enzymes such as the HDCR [11], therefore, it must have a mechanism to transport W into the cell, and a TupABC transporter is also encoded in the genome (TKV_c18720–18740). As the activity of AOR became eventually saturated at higher tungstate concentrations, it is a possibility that the transportation rate is too low to channel enough tungsten into the cell for the production of functional metabolic tungsten enzymes native in T. kivui and the additional, highly expressed AORX514. A different explanation may be the cofactor synthesis. Unfortunately, there are not many studies focused on the maturation of the tungstopterin cofactor, but the tungstopterin in HDCR seems to be a tungstopterin guanine dinucleotide [11], while the tungstopterin of AORPf, for example, has no nucleotide appended [3]. These differences could potentially have an impact on the activity and further examination of the tungsten transporters and cofactor structure and its maturation in T. kivui may give more insights.
2.3. AORX514 Is a Versatile Ferredoxin-Dependent Aldehyde Oxidoreductase
Purified AORX514 had a very wide temperature range, and we were able to measure activity between 10 °C (0.16 U mg−1) and 95 °C (8.2 U mg−1) with a temperature optimum at about 75 to 80 °C (Figure 4a). This temperature is slightly higher than the optimal growth temperature (60 °C) of Thermoanaerobacter sp. strain X514, however this effect has often been observed for enzymes from thermophiles, as for example observed for alcohol dehydrogenases AdhB and AdhA of the same organism [30], and also for bacterial AORs, such as AOR from Megalodesulfovibrio gigas, which has an optimum between 48 and 65 °C, while the organism grows around 30 °C [14]. AORX514 is similar on amino acid level to AORPf (61% identity); P. furiosus has optimal growth temperature of 100 °C, but the optimum of AORPf is slightly lower (>90 °C) [2].
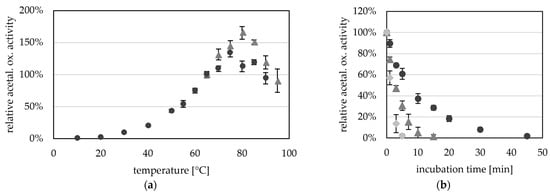
Figure 4.
Temperature-dependent aldehyde oxidation activity of AORX514. (a) AOR activity was measured between 10 °C and 90 °C (dark grey circles) and between 65 °C and 95 °C (grey triangles) as acetaldehyde-dependent reduction of BV (ε600 = 7.4 mM−1 cm−1). The reaction mixture contained in 50 mM TRIS, pH 7.5 with 1.2 mM BV, 12 µg (dark grey circles) or 15 µg (light grey squares) AORX514 and 1.2 mM acetaldehyde. AOR-His from two independent purifications was used, the average represents four technical replicates. The specific activities were normalized to the mean value of the activity at 65 °C. (b) Thermostability of AOR at 65 °C (dark grey circles), 75 °C (grey triangles) and 85 °C (light grey squares). AOR activity was measured in 50 mM TRIS with 2.4 mM benzyl viologen, 14 µg AOR-His and 1.2 mM acetaldehyde at 65 °C. The average represents three to five experiments, activities were normalized to the enzyme activity before heat treatment.
As bacterial AORs with the exception of AORMt have been purified from mesophilic organisms, the optimal temperature (TOPT) of these AORs is generally lower than the ideal temperature of AORX514 [4,12,14,16]. AORX514 has therefore the highest TOPT of all characterized, bacterial AORs, more comparable to the operating temperatures of archaeal AORs. An activation energy of 53 kJ mol−1 was calculated via Arrhenius plot for AORX514. This value is lower than the activation energies calculated for the archaeal AORs from Thermococcus strain ES-1 and Pyrobaculum aerophilum, which have a high temperature optimum as well.
Although AORX514 is more active at higher temperatures, AORX514 has a relatively low thermostability and the half life time is only 7.3 min at 65 °C, 2.9 min at 75 °C and 1.4 min at 85 °C (Figure 4b). This is comparable to the half-life time of AORES1 which is 5 min at 70 °C and 1 min at 85 °C [13]. Considering the high optimal growth temperature of the organisms that both of these AORs are found in, the thermostability is very low. Also compared to, for example, AdhE and AdhB from Thermoanaerobacter sp. strain X514, which have stable activities at 65 °C for at least two or three hours, respectively [30].
It seems like AORX514, and potentially even AORs in general, are extremely heat sensitive enzymes, which raises the question, whether they are more protected inside the cell, e.g., by a protein or by the osmolyte concentration, or whether this instability is the reason, AORX514 is always highly expressed. Even though the activity was higher at a higher temperature we continued to measure the activity at 65 °C since the AOR is more stable at this temperature.
Towards its pH optimum, acetaldehyde oxidation was observed at pH 5.5 (0.5 U mg−1) and increased activities up to pH 11.5 (36.3 U mg−1) (Figure 5a). A higher pH optimum for the aldehyde oxidation reaction has generally been reported for all AORs. However, the ideal pH for archaeal AORs from Pyrococcus furiosus and Thermococcus strain ES-1 is overall higher (pH 11 to 12) [2,13] than the ideal pH of bacterial AORs such as AOR from Aromatoleum aromaticum and Clostridium formicoaceticum (pH 8 to 10) [5,12,16].
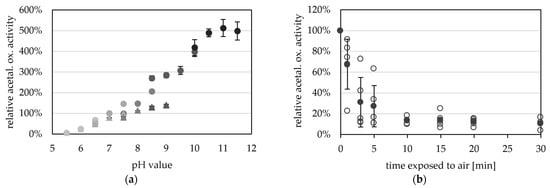
Figure 5.
pH- and oxygen-dependent aldehyde oxidation activity of AORX514. (a) AOR activity was measured between pH 5.5 and pH 11.5 with 0.2 mM benzyl viologen (BV, circles) and between pH 5.5 and pH 9.0 (triangles) with 2 mM BV at 65 °C, with 14 µg AOR-His and 1.2 mM acetaldehyde. The buffers used were at concentrations of 50 mM, MES (5.5, 6.0, 6.5), MOPS (6.5, 7.0, 7.5), TRIS (7.5, 8.0, 8.5, 9.0), CHES (8.5, 9.0, 9.5, 10.0) and CAPS (10.0, 10.5, 11.0, 11.5) were used (from lightest to darkest). The average represents four technical replicates. The specific activities were normalized to the mean value of 50 mM TRIS, pH7.5. (b) AORX514 was exposed to air and the activity was measured regularly. Specific AOR activity was measured in 50 mM TRIS at pH 7.5 with 2.4 mM BV, 17 µg AORX514 and 1.2 mM acetaldehyde at 65 °C. The average (filled circle) represents five separate experiments (empty circles), activities were normalized to the enzyme activity before air exposure.
The purified enzyme is extremely oxygen sensitive and has a half-life time of only 2.4 min when exposed to air at room temperature (Figure 5b). The oxygen sensitivity of purified AOR is reflected in the cell free extracts of Thermoanaerobacter sp. strain X514, or T. kivui MB014 (Figure S3), with half lives of 4 and 1.3 min in the CFE, respectively. This also reveals the necessity to purify the protein under anoxic conditions. Compared to other tungsten-dependent AORs, these values seem to be the norm, as AOR from Thermococcus strain ES-1, when exposed to air at room temperature, has a half-life time of 2 min in CFE and 1 min when purified [13]. AORPf has only less than 20% activity left after 5 min exposure to air [2]. However, normally the sensitivity to oxygen is less extreme in the bacterial AORs, and AOR from Megalodesulfovibrio gigas has 20% activity left after 20 to 30 min [14], AOR from Moorella thermoacetica has still 60% activity left after 2 min [4] and AORAa has a half life time of one hour, when exposed to air [16].
The protein, however, is very stable if kept at low temperatures in the absence of oxygen. AORX514 can be stored for up to three weeks at 4 °C without loss of activity and for up to one year at −20 °C. In comparison to that AORX514 only has 59% left after one week at room temperature, with only 2% activity left after one year at room temperature. As AORX514 showed activity even at temperatures as low as 10 °C, it makes sense that even colder temperatures were required for long-term storage.
To test whether the position of the tag is of importance, we introduced an N-terminally His-tagged version of Teth514_1380 (plasmid pMB009) (Figure S4), and subsequently treated the same way AOR-His was treated. The activity of His-AOR was a little higher (35.5 U mg−1) than that observed for AOR-His (25.1 U mg−1), but in a similar range, indicating that the position of the tag had a marginal influence.
Carboxylic acid reduction by microorganisms has gained attention in industry such as for bio-based polymers and production of alcohols as biofuels [42]. And, due to their broad substrate spectra, AORs have become more interesting for these applications. Along these lines, we tested a wide range of aldehydes as substrates. All of the supplied aldehydes, such as short-chained aliphatic (e.g., acetaldehyde, propionaldehyde) branched-chained aliphatic (e.g., isobutyraldehyde), aromatic (e.g., benzyaldehyde, phenylacetaldehyde, hydrocinnamaldehyde, trans-cinnamaldehyde) as well as unsaturated (crotonaldehyde), heterocyclic aldehydes (furfural) and naphthaldehyde were oxidized with comparably high specific activities (>80% of the activity with acetaldehyde, Figure 6 and Table S3). Although acetaldehyde was the best substrate tested, longer chained aldehydes such as nonanal and decanal were also oxidized (28% and 20% of activity towards acetaldehyde, respectively). Generally, AORX514 showed a lower specific activity with branched-chained aldehydes than with the linear aldehyde, e.g., 71% butyraldehyde and 62% valeraldehyde versus 26% for isovaleraldehyde. For AORX514 a wide variety of substrates were tested, also in comparison to other AORs, but overall, the high activity with short-chained linear aldehydes and short-chained aromatic aldehydes has also been described for other AORs, such as AOR from Aromatoleum aromaticum (AORAa) [16] and AOR from Peptoclostridium acidaminophilum (formerly Eubacterium acidaminophilum) [9]. Interestingly, furfural, which is toxic to most microorganisms and is a side product of pretreatment and hydrolysis or lignocellulosic feedstock and a problem in industrial wastewater [43,44], was oxidized.
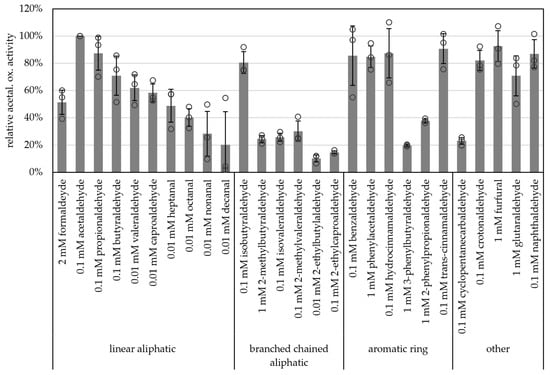
Figure 6.
Substrate specificity of AOR-His. AOR activity was measured as aldehyde oxidation at 65 °C with 2 mM BV, 8 to 14 µg AOR-His and 2 mM, 1 mM, 0.1 mM or 0.01 mM aldehyde, as depicted. For each aldehyde the activity with 1 mM, 0.1 mM and 0.01 mM were tested, when the highest activity was with 1 mM, 2 mM aldehyde was tested additionally. The highest activity per aldehyde is represented in the figure. The average represents three separate experiments; activities were normalized to the enzyme activity with acetaldehyde.
As substrate inhibition has been described for AORs [1,2,9,13], the affinity and substrate inhibition for acetaldehyde was determined. The Km for acetaldehyde was determined to be 8.4 ± 1.0 µM with a Vmax of 15.5 ± 2.1 U mg−1 through Michaelis-Menten kinetic and for substrate inhibition, the ki value was determined to be 8.7 ± 1.3 mM. Between about 0.03 and 3.0 mM the activity was constant (Figure S5a). In another experiment, a similar Km value of 9.7 µM was determined, but a higher Vmax value of 38 U mg−1 (Table 2, Figure S5b). Compared to other AORs, this high affinity was expected as a Km values for aldehydes between 10 and 200 µM had also been described for, for example AORAa and AOR from P. acidaminophilum [9,16]. With longer chain length of the aldehydes, the Vmax decreased, whereas the affinity stayed in the same range (Table 2). Based on a higher Km, formaldehyde might not be the preferred substrate for AOR514. Towards formaldehyde oxidation, other organisms such as P. furiosus have a separate formaldehyde oxidoreductase (FOR) [45,46].

Table 2.
Kinetic parameters of AOR-His. Values for Km and Vmax are given as mean values from two (*) or three different experiments.
When testing benzyl viologen and methyl viologen as artificial electron acceptors in acetaldehyde oxidation, a higher affinity for BV (Km: 2.1 mM; Vmax 17.7 µmol min−1 mg−1) than for MV (Km: 19.2 mM; Vmax 19.6 µmol min−1 mg−1) was measured (Table 2, Figures S6 and S7). Since MV has a lower redox potential (E = −450 mV; [47]) than BV (E = −374 mV; [47]), it might be less suitable to accept electrons during aldehyde oxidation. A similar difference between activity and affinity for BV and MV as electron acceptors has also been shown for AOR from Megalodesulfovibrio gigas [14] and for the AORs from Thermococcus strain ES-1 and P. acidaminophilum [9,13].
Fd is the putative native electron partner for all characterized AORs, since they are able to utilize BV, which is an indicator for Fd-dependence as archaeal AORs have been described to utilize both a viologen dye and Fd [32]. In these cases, ferredoxins native to the organism were purified and subsequently used for enzyme assays [2,13,15].
Here, we used four potential Fds from T. kivui (TKV_c16450; TKV_c09620; TKV_c10420; TKV_c19530), as they can be overproduced in T. kivui and subsequently purified using a His-tag [48]. Of these four putative Fds, two were used by the AORX514 for acetaldehyde oxidation (TKV_c16450; TKV_c09620) as electron acceptor (Figure 7), while the other two Fds (TKV_c10420; TKV_c19530) were not used (Figure S8). These findings correspond with the recently published characterization of these ferredoxins. Throughout a study carried out by Katsyv et al. (2023), it was found, that His- TKV_c16450 and His- TKV_c09620 are most likely ferredoxins involved in T. kivui metabolism [48]. The affinity for TKV_c09620 was higher than that for the artificial electron acceptors (50 to 200 µM) (Table 2), but since the absorption at 430 nm is saturated, when around 200 µM Fd are inserted into the assay, the absorption change cannot be measured reliably at higher concentrations and therefore no exact value was determined. Unlike the multimeric AORs from M. thermoaceticum [4] and A. aromaticum [16], AORX514 neither used NAD+ nor NADP+ (Figure S9).
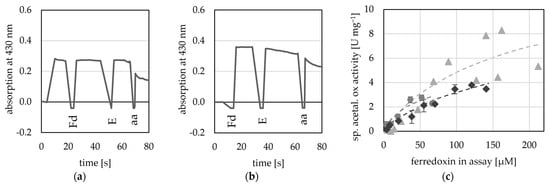
Figure 7.
Ferredoxin-dependent AOR activity. AOR activity was measured at 65 °C as acetaldehyde-dependent Fd reduction at 430 nm in 50 mM TRIS buffer at pH 7.5. (a) 20 µM His- TKV_c16450 was used as electron acceptor (b) 20 µM His-TKV_c09620 was used as electron acceptor, 50 µg AORX514 (E) were added and the reaction was started with the addition of 1 mM acetaldehyde (aa). (c) Between 4 to 212 µM His-TKV_c09620 were used as electron acceptor and 8 to 20 µg AOR-His, and 1 mM acetaldehyde. Three independent experiments with one to three technical replicates (grey circles, light grey triangles and dark grey diamonds).
The characterization of the enzyme was carried out with assays in the direction of oxidation of the (intermediate) aldehyde. Assaying AOR in the direction of carboxylic acid reduction is difficult due to the low redox potential of the reaction and has only been demonstrated for few AORs [7,12,13]. Here, we showed that AOR indeed carried out MV+-dependent reduction of acetate, albeit only at a low rate and at pH 5.0 (Table 3). This is very characteristic since AOR is believed to utilize the undissociated acid as a substrate [12]. As aldehydes are extremely reactive, the addition of chemicals such as semicarbazide can be used to bind the produced aldehyde and thereby eliminating it from the reaction and reducing re/oxidation of the aldehyde [1,4,9]. Interestingly, the addition of semicarbazide to the acetate reduction assay here, led to the re/oxidation of MV+, which could afterwards not be re-reduced with dithionite and therefore this approach did not work. To eliminate acetaldehyde from the assay AdhE-His from Thermoanaerobacter sp. strain X514, purified from E. coli [30] was utilized, which, with the addition of NADH, can reduce acetaldehyde further to ethanol [1]. The elimination of acetaldehyde, by AdhE, led to a two-fold increase of acetate reduction activity from 7 mU mg−1 to 14 mU mg−1 (Figure S10). A similar coupled assay was also used to determine benzoate reduction of AORAa, using a benzyl alcohol dehydrogenase (BaDH) for elimination of benzaldehyde [37]. Neither in the uncoupled nor in the coupled assay, activity was measured with either one of the two purified Fds (His-TKV_c16450; His-TKV_c09620). The low specific activity towards the acid points towards a function in oxidative aldehyde removal, as suggested for AOR of P. furiosus before [2]. These aldehydes may be derived e.g., from peptide catabolism (the medium contains yeast extract). The in vitro rates, however, may not fully reflect the in vivo function, since AOR in organic acid reducing organisms such as Moorella thermoacetica also have a much lower Km and Vmax (~1000-fold and 5%, respectively) [1]. Hence, AOR may therefore also be involved in alcohol production from organic acids.

Table 3.
Comparison of AORX514 acetaldehyde oxidation and acetate reduction activity with different redox Activities were normalized to the specific acetaldehyde oxidation activity with 2 mM oxidized BV. BV: benzyl viologen; MV: methyl viologen; Fd: ferredoxin.
3. Materials and Methods
3.1. Cultivation of Thermoanaerobacter kivui Strains
Thermoanaerobacter kivui strains were routinely grown anaerobically in modified DSMZ171 media at 66 °C, as previously described [33]. The medium contained 50 mM Na2HPO4, 50 mM NaH2PO4, 1.3 mM K2HPO4, 1.6 mM KH2PO4, 7.6 mM NaCl, 5.9 mM NH4Cl, 1.7 mM (NH4)2SO4, 0.4 mM MgSO4, 7.2 µM FeSO4, 55.6 µM CaCl2, 1% trace element solution DSMZ141, 1% mL vitamin solution DSMZ141, 54 mM KHCO3, 2.8 mM cysteine-HCl and 2 g L−1 yeast extract (Carl Roth GmbH + Co. KG, Karlsruhe, Germany). If not stated otherwise, 12 µM tungsten (as tungstate; Na2WO4) was routinely added to the media for AOR-His and His-AOR purification. The medium was purged with Protadur C20 (80%/20% [v/v] N2/CO2) and then autoclaved. If not described otherwise, cells were grown with 25 mM glucose as substrate, added from a sterile, anoxic stock solution. Growth was monitored by measuring the OD at 600 nm.
Thermoanaerobacter kivui strains containing pMU131_His-TKV_c09620, pMU131_His-TKV_c16450, pMU131_His-TKV_c10420 and pMU131_His-TKV_c19530 for overproduction of T. kivui ferredoxins (Fd) were kindly provided by Prof. Volker Müller (Goethe University Frankfurt/Main). The construction of the plasmids for Fd overexpression is described elsewhere [48].
Cultivation of Thermoanaerobacter sp. strain X514 was performed as previously described [30].
3.2. Transformation of Thermoanaerobacter kivui
To generate T. kivui strains for production of AOR from Thermoanaerobacter sp. strain X514, plasmid pJM008 and pJM009 were constructed (Figure S11). Both plasmids were derived from plasmid pJM006 for overexpression of genes under the control of the S-layer promoter after integration into the genome region between Tkv_c24500 and Tkv_c24520 via homologous recombination [40]. The plasmid allows for selection using uracil auxotrophy, since it contains the pyrE gene under the gyrase promoter from Thermoanaerobacter sp. strain X514 [33]. For the generation of plasmids pJM008 and pJM009, the gene in pJM006 (adhE) was replaced with the aor gene from Thermoanaerobacter sp. strain X514 (Teth514_1380) in which 18 nucleotides encoding His6-tag (5′-CACCATCACCATCACCAT-3′) are fused either at the 5′ end (N-terminal His-tag; pJM008) or at the 3′ end of aor (C-terminal His-tag; pJM009). For the construction of pJM008, the His6-aor insert and pJM006 backbone were amplified by PCR using primers JM039 (5′-GAGGATTGACTGTATGCACCATCACCATCACCATTTTGGGTATGCCGGC-3′) and JM011 (5′-AAAAGCATGCTTCCCTCAAATTCCCAATTTTTGTAAAGTT-3′), and JM012 (5′-CTTTACAAAAATTGGGAATTTGAGGGAAGCATGCTTTTTAAAACAT-3′) and JM040 (5′-ATGGTGATGGTGATGGTGCATACAGTCAATCCTCCTCCTTGTA-3′), respectively. For the construction of pJM009, the aor-His6 insert and pJM006 backbone were amplified by PCR using primers JM010 (5′-CAAGGAGGAGGATTGACTGTATGTTTGGGTATGCCGG-3′) and JM041 (5′-GCTTCCCTCAATGGTGATGGTGATGGTGAATTCCCAATTTTTGTAAAGTTTCTTT-3′), and JM042 (5′-GGAATTCACCATCACCATCACCATTGAGGGAAGCATGCTTTTTAAAACA-3′) and JM013 (5′-CCGGCATACCCAAACATACAGTCAATCCTCCTCCTTGTATTT-3′), respectively. Then, the aor inserts were fused to the pJM006 backbone using Hifi assembly (New England Biolabs., Frankfurt am Main, Germany) to yield the plasmids pJM008 and pJM009. Subsequently, pJM008 and pJM009 were transformed into the ∆pyrE strain MB TKV002 [33] and integration of His6-aor or aor-His6 was carried out on agar plates with minimal medium without uracil. The integrated genome region of single colonies was analyzed by PCR with primers LH028 (5′-CAGGCTGTGATAATTTGAGAA-3′) and LH029 (5′-GGTCACGATTTAAAGGACTTA-3′) which bind at the 5′ end of the upstream flanking region (UFR) and at the 3′ end of the downstream flanking region (DFR) used for genome integration. Furthermore, the integrated region was verified by Sanger sequencing. After verification, the strain carrying His6-aor was named as TKV_MB009 and the strain aor-His6 as TKV_MB014.
3.3. Purification of His-Tagged Proteins from Thermoanaerobacter kivui
AOR-His purification was conducted under strictly anoxic conditions. Towards that, all material was placed inside an anoxic chamber (95% N2/5% H2 atmosphere; Coy Laboratory Products, Inc., Grass Lake, MI, USA) at least one day before use; all buffers were flushed with N2 and supplemented with 2 mM 1,4-dithioerythritol (DTE). T. kivui MB014 cells were grown in one to ten 2 L bottles with 1 L media and 25 mM glucose as substrate as described above. Cells were harvested in stationary phase (after 18–24 h, OD 0.5 to 1) and centrifuged for 20 min at 2700× g and 4 °C. The cells were washed with wash buffer (50 mM TRIS, 20 mM MgSO4, 150 mM NaCl, 20% [v/v] glycerol, pH 7.5, 2 mM DTE) and then resuspended in 20 mL start buffer (50 mM TRIS, 20 mM MgSO4, 150 mM NaCl, 10 mM imidazole, 20% [v/v] glycerol, pH 7.5, 2 mM DTE). Transfer to an anoxic chamber minimized contact of the intact cells to air during the harvest and washing steps. For this, the cell cultures were transferred into anoxic centrifuge bottles inside the anoxic chamber, the lids were closed, and then the closed bottles were taken out for centrifugation. After centrifugation, the centrifuge bottles containing the cell pellets were brought back into the anoxic chamber, and the media or buffer was removed, and the cells were resuspended in anoxic buffer. After addition of a spatula tip of DNase I and 0.5 mM phenylmethylsulfonyl fluoride (PMSF) the cells were transferred to a pressure cell under anoxic conditions and subsequently disrupted by a French Press at 110 MPa. The extract was collected in a degassed bottle filled with N2 to avoid contact with air. Cell debris was removed by centrifugation for 30 min at 37,000× g and 4 °C. The cell free extract was diluted to 52 mL. A 2 mL subsample was taken for enzyme activity, SDS-PAGE and Western blot, while the remaining 50 mL were used for the purification via affinity chromatography. AOR-His was purified using an Äkta Pure system (Cytiva, Marlborough, MI, USA) equipped with HisTrap HP 1 mL or 5 mL columns (Cytiva, Marlborough, MI, USA), placed in the anoxic chamber. Equilibration was performed with start buffer, while unspecific bound proteins were eluted with 2% elution buffer (50 mM TRIS, 20 mM MgSO4, 150 mM NaCl, 300 mM imidazole, 20% [v/v] glycerol, pH 7.5, 2 mM DTE), AOR-His was eluted with 50% elution buffer in start buffer, at a concentration of 150 mM imidazol.
His-AOR was purified from cell pellets of T. kivui MB009; grown in 1 L media and 25 mM glucose as substrate as described above. The ferredoxins His-TKV_c16450 and His-TKV_c09620 were purified from up to 8 L culture. Cultivation and production of the cell free extract was carried out under the same conditions as described above. His-AOR was purified using HisTrap HP 1 mL column (Cytiva, Marlborough, MI, USA) whereas TKV_c16450 and His-TKV_c09620 were purified using HisTrap HP 1 mL or HisTrap HP 5 mL column (Cytiva, Marlborough, MI, USA).
Protein concentrations were determined in a colorimetric assay according to Bradford [49], using ROTI®Nanoquant reagent (Carl Roth GmbH + Co. KG, Karlsruhe, Germany). Proteins were separated by SDS-PAGE with acrylamide concentrations between 10% and 16% [50]. For Western blot, the acrylamide gels were then transferred onto a nitrocellulose membrane [51].
The specific AOR-His antibodies were generated by immunization of a rabbit (Davids Biotechnologie, Regensburg, Germany) and used for immunological detection of the native protein in Thermoanaerobacter sp. strain X514 and control of successful purification. The size of AOR-His was determined by native PAGE under anoxic conditions. Towards that, polyacrylamide gel was prepared under air, then transferred into the anoxic chamber, and was stored in anoxic running buffer (25 mM TRIS, 96 mM glycine, pH 8.3) over night before use.
3.4. Enzyme Assays
Enzyme Assays were usually carried out under anaerobic conditions at 65 °C. The 1.4 mL quarz glass high performance cuvettes (Hellma, Müllheim, Germany) were closed with a rubber plug, flushed with N2 and then filled with 1 mL anaerobic TRIS-buffer (50 mM TRIS, 0.005% [w/v] resazurin, 2 mM DTE, pH 7.5) by injection through the stopper. Routinely, AOR activity was measured as acetaldehyde-dependent reduction of benzyl viologen at 600 nm (ε600 = 7.4 µmol min−1 mg−1) at 65 °C. For the reaction 2 mM benzyl viologen were used and between 2 and 100 µg protein, finally, the assay was started by the addition of 1 mM acetaldehyde from an anoxic stock solution. Activity was measured between pH 5.5 and 11.0 and between 10 °C and 95 °C, and routinely calculated as micromoles aldehyde oxidized per minute per milligram protein.
To determine the ferredoxin-dependency, benzyl viologen was replaced by 4 to 200 µM His-TKV_c16450, His-TKV_c09620, His-TKV_c10420 and His-TKV_c19530 from T. kivui [48]. The reduction of ferredoxin was measured at 430 nm, and the activity was calculated as micromoles aldehyde oxidized per minute per milligram protein, under the assumption that His-TKV_c16450 transports one electron and His-TKV_c09620 transports two electrons.
To determine acid-reduction of AOR, methyl viologen (MV; ε600 = 13.1 µmol min−1 mg−1) was used as electron donor. First, 10 to 20 µM MV in 50 mM anoxic TRIS buffer was reduced using sodium dithionite (from an anoxic 1 M stock solution). Subsequently, 24 µg AOR-His was added, and the reaction was started by adding 230 to 460 mM acetate. To determine acid reduction of AOR coupled to further reduction of the aldehyde to the alcohol, methyl viologen (MV) and NADH were used as electron donors for AOR and AdhE, respectively. First, 10 to 20 µM MV in 50 mM anoxic TRIS buffer was reduced using sodium dithionite (from an anoxic 1 M stock solution), then 0.5 mM NADH was added. Subsequently, 15 µg AdhE-His and afterwards 60 µg AOR-His was added, and the reaction was started by adding 450 mM acetate.
E. coli with the inducible plasmid pet21a::AdhE-His was grown in LB media with 100 µg mL−1 ampicillin. Production and purification of AdhE-His as previously described [30].
3.5. Quantification of Elements
Iron content of the purified enzyme was determined using a colorimetric assay with ferrozine (Fish, 1988) using ferrozine (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). The color change was measured at 593 nm. A standard of 0 to 200 µM ammonium iron sulfate hexahydrate was used to calculate the amount of iron in the samples.
The sulfur content of the purified enzyme was determined using a colorimetric assay [52] using N,N-dimethyl-p-phenylenediamine (DMPD) HCl. The sodium sulfide standard was made freshly every time from an anaerobic 1 mM stock solution and the samples were taken freshly from an anoxically stored solution. The change in color of DMPD was measured after centrifugation at 670 nm. A standard of 0 to 200 µM sodium sulfide was used to calculate the amount of sulfur in the samples.
For the quantification of tungsten and molybdenum in the purified AOR-His, the samples were sent to Spurenanalytisches Laboratorium Dr. Baumann (Maxhütte-Haidhof, Germany) for ICP-MS.
4. Conclusions
The results prove that TethX514_1380 is a sensu stricto dimeric archaeal type AOR, and may be acquired by horizontal gene transfer from archaea. Since the observed in vitro activities in the forward direction (acid reduction) are low, a major function of the enzyme may be the detoxification of aldehydes derived from peptide metabolism, as originally suggested for AOR of P. furiosus. Nonetheless, the enzyme may in be responsible for organic acid reduction in vivo, and explain at least part of the alcohol production from corresponding acids in Thermoanaerobacter sp. strain X514. The recombinant production of AOR paves the road to engineering in T. kivui for alcohol production and for structural studies of the protein using enzyme variants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25021077/s1.
Author Contributions
Conceptualization, M.B.; methodology, all authors; validation, L.S.N. and M.B.; formal analysis, L.S.N.; investigation, L.S.N.; resources, L.H., J.M. and M.B.; data curation, L.S.N., L.H. and J.M.; writing—original draft preparation, L.S.N. and M.B.; writing—review and editing, L.H. and J.M.; visualization, L.S.N.; supervision, M.B.; project administration, M.B.; funding acquisition, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant of the Deutsche Forschungsgemeinschaft DFG to Mirko Basen (grant number DFG BA5757/1-1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets presented in this study can be found in the Supplementary Material.
Acknowledgments
We are grateful to Alexander Katsyv and Volker Müller for providing the tagged Fd-producing T. kivui strains. We want to thank Manja Henneberg for technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- White, H.; Strobl, G.; Feicht, R.; Simon, H. Carboxylic acid reductase: A new tungsten enzyme catalyses the reduction of non-activated carboxylic acids to aldehydes. Eur. J. Biochem. 1989, 184, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mukund, S.; Adams, M.W.W. The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase: Evidence for its participation in a unique glycolytic pathway. J. Biol. Chem. 1991, 266, 14208–14216. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.K.; Mukund, S.; Kletzin, A.; Adams, M.W.W.; Rees, D.C. Structure of a hyperthermophilic tungstopterin enzyme, aldehyde ferredoxin oxidoreductase. Science 1995, 267, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; Skopan, H.; Feicht, R.; White, H.; Simon, H. Pterin cofactor, substrate specificity, and observations on the kinetics of the reversible tungsten-containing aldehyde oxidoreductase from Clostridium thermoaceticum. Arch. Microbiol. 1995, 164, 110–118. [Google Scholar] [CrossRef]
- White, H.; Huber, C.; Feicht, R.; Simon, H. On a reversible molybdenum-containing aldehyde oxidoreductase from Clostridium formicoaceticum. Arch. Microbiol. 1993, 159, 244–249. [Google Scholar] [CrossRef]
- Trautwein, T.; Krauss, F.; Lottspeich, F.; Simon, H. The (2R)-hydroxycarboxylate-viologen-oxidoreductase from Proteus vulgaris is a molybdenum-containing iron-sulphur protein. Eur. J. Biochem. 1994, 222, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Strobl, G.; Feicht, R.; White, H.; Lottspeich, F.; Simon, H. The tungsten-containing aldehyde oxidoreductase from Clostridium thermoaceticum and its complex with a viologen-accepting NADPH oxidoreductase. Biol. Chem. Hoppe-Seyler 1992, 373, 123–132. [Google Scholar] [CrossRef]
- Winiarska, A.; Ramírez-Amador, F.; Hege, D.; Gemmecker, Y.; Prinz, S.; Hochberg, G.; Heider, J.; Szaleniec, M.; Schuller, J.M. A bacterial tungsten-containing aldehyde oxidoreductase forms an enzymatic decorated protein nanowire. bioRxiv 2023. [Google Scholar] [CrossRef]
- Rauh, D.; Graentzdoerffer, A.; Granderath, K.; Andreesen, J.R.; Pich, A. Tungsten-containing aldehyde oxidoreductase of Eubacterium acidaminophilum. Eur. J. Biochem. 2004, 271, 212–219. [Google Scholar] [CrossRef]
- Kim, G.; Azmi, L.; Jang, S.; Jung, T.; Hebert, H.; Roe, A.J.; Byron, O.; Song, J.-J. Aldehyde-alcohol dehydrogenase forms a high-order spirosome architecture critical for its activity. Nat. Commun. 2019, 10, 4527. [Google Scholar] [CrossRef]
- Dietrich, H.M.; Righetto, R.D.; Kumar, A.; Wietrzynski, W.; Trischler, R.; Schuller, S.K.; Wagner, J.; Schwarz, F.M.; Engel, B.D.; Müller, V.; et al. Membrane-anchored HDCR nanowires drive hydrogen-powered CO2 fixation. Nature 2022, 607, 823–830. [Google Scholar] [CrossRef] [PubMed]
- White, H.; Feicht, R.; Huber, C.; Lottspeich, F.; Simon, H. Purification and some properties of the tungsten-containing carboxylic acid reductase from Clostridium formicoaceticum. Biol. Chem. Hoppe-Seyler 1991, 372, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Heider, J.; Ma, K.; Adams, M.W.W. Purification, characterization, and metabolic function of tungsten-containing aldehyde ferredoxin oxidoreductase from the hyperthermophilic and proteolytic archaeon Thermococcus strain ES-1. J. Bacteriol. 1995, 177, 4757–4764. [Google Scholar] [CrossRef] [PubMed]
- Hensgens, C.M.; Hagen, W.R.; Hansen, T.A. Purification and characterization of a benzylviologen-linked, tungsten-containing aldehyde oxidoreductase from Desulfovibrio gigas. J. Bacteriol. 1995, 177, 6195–6200. [Google Scholar] [CrossRef] [PubMed]
- Hagedoorn, P.-L.; Chen, T.; Schröder, I.; Piersma, S.R.; de Vries, S.; Hagen, W.R. Purification and characterization of the tungsten enzyme aldehyde:ferredoxin oxidoreductase from the hyperthermophilic denitrifier Pyrobaculum aerophilum. J. Biol. Inorg. Chem. 2005, 10, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Arndt, F.; Schmitt, G.; Winiarska, A.; Saft, M.; Seubert, A.; Kahnt, J.; Heider, J. Characterization of an aldehyde oxidoreductase from the mesophilic bacterium Aromatoleum aromaticum EbN1, a member of a new subfamily of tungsten-containing enzymes. Front. Microbiol. 2019, 10, 71–83. [Google Scholar] [CrossRef]
- Debnar-Daumler, C.; Seubert, A.; Schmitt, G.; Heider, J. Simultaneous involvement of a tungsten-containing aldehyde:ferredoxin oxidoreductase and a phenylacetaldehyde dehydrogenase in anaerobic phenylalanine metabolism. J. Bacteriol. 2014, 196, 483–492. [Google Scholar] [CrossRef]
- Scully, S.M.; Iloranta, P.; Myllymaki, P.; Orlygsson, J. Branched-chain alcohol formation by thermophilic bacteria within the genera of Thermoanaerobacter and Caldanaerobacter. Extremophiles 2015, 19, 809–818. [Google Scholar] [CrossRef]
- Hensgens, C.M.H.; Nienhuis-Kuiper, M.E.; Hansen, T.A. Effects of tungstate on the growth of Desulfovibrio gigas NCIMB 9332 and other sulfate-reducing bacteria with ethanol as a substrate. Arch. Mikrobiol. 1994, 162, 143–147. [Google Scholar] [CrossRef]
- Hitschler, L.; Kuntz, M.; Langschied, F.; Basen, M. Thermoanaerobacter species differ in their potential to reduce organic acids to their corresponding alcohols. Appl. Microbiol. Biotechnol. 2018, 102, 8465–8476. [Google Scholar] [CrossRef]
- Nissen, L.S.; Basen, M. The emerging role of aldehyde:ferredoxin oxidoreductases in microbially-catalyzed alcohol production. J. Biotechnol. 2019, 306, 105–117. [Google Scholar] [CrossRef]
- Olson, D.G.; Sparling, R.; Lynd, L.R. Ethanol production by engineered thermophiles. Curr. Opin. Biotechnol. 2015, 33, 130–141. [Google Scholar] [CrossRef]
- Simon, H.; White, H.; Lebertz, H.; Thanos, I. Reduction of 2-enoates and alkanoates with carbon monoxide or formate, viologens, and Clostridium thermoaceticum to saturated acids and unsaturated and saturated alcohols. Angew. Chem. Int. Ed. Engl. 1987, 26, 785–787. [Google Scholar] [CrossRef]
- Basen, M.; Schut, G.J.; Nguyen, D.M.; Lipscomb, G.L.; Benn, R.A.; Prybol, C.J.; Vaccaro, B.J.; Poole, F.L.; Kelly, R.M.; Adams, M.W.W. Single gene insertion drives bioalcohol production by a thermophilic archaeon. Proc. Natl. Acad. Sci. USA 2014, 111, 17618–17623. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.; Zheng, Y.; Mueller, A.P.; Ly, S.; Tran, L.; Segovia, S.; Nagaraju, S.; Köpke, M.; Dürre, P.; Thauer, R.K. Energy conservation associated with ethanol formation from H2 and CO2 in Clostridium autoethanogenum involving electron bifurcation. J. Bacteriol. 2015, 197, 2965–2980. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.; Henstra, A.M.; Köpke, M.; Winzer, K.; Simpson, S.D.; Minton, N.P. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metab. Eng. 2017, 40, 104–114. [Google Scholar] [CrossRef]
- Perez, J.M.; Richter, H.; Loftus, S.E.; Angenent, L.T. Biocatalytic reduction of short-chain carboxylic acids into their corresponding alcohols with syngas fermentation. Biotechnol. Bioeng. 2013, 110, 1066–1077. [Google Scholar] [CrossRef]
- Richter, H.; Molitor, B.; Wei, H.; Chen, W.; Aristilde, L.; Angenent, L.T. Ethanol production in syngas-fermenting Clostridium ljungdahlii is controlled by thermodynamics rather than by enzyme expression. Energy Environ. Sci. 2016, 9, 2392–2399. [Google Scholar] [CrossRef]
- Scully, S.M.; Brown, A.E.; Ross, A.B.; Orlygsson, J. Biotransformation of organic acids to their corresponding alcohols by Thermoanaerobacter pseudoethanolicus. Anaerobe 2019, 57, 28–31. [Google Scholar] [CrossRef]
- Hitschler, L.; Nissen, L.S.; Kuntz, M.; Basen, M. Alcohol dehydrogenases AdhE and AdhB with broad substrate ranges are important enzymes for organic acid reduction in Thermoanaerobacter sp. strain X514. Biotechnol. Biofuels 2021, 14, 187. [Google Scholar] [CrossRef]
- Roy, R.; Menon, A.L.; Adams, M.W.W. Aldehyde oxidoreductases from Pyrococcus furiosus. Meth. Enzymol. 2001, 331, 132–144. [Google Scholar] [CrossRef]
- Kletzin, A.; Adams, M.W.W. Tungsten in biological systems. FEMS Microbiol. Rev. 1996, 18, 5–63. [Google Scholar] [CrossRef]
- Basen, M.; Geiger, I.; Henke, L.; Müller, V. A genetic system for the thermophilic acetogenic bacterium Thermoanaerobacter kivui. Appl. Environ. Microbiol. 2018, 84, e02210-17. [Google Scholar] [CrossRef]
- Leigh, J.A.; Mayer, F.; Wolfe, R.S. Acetogenium kivui, a new thermophilic hydrogen-oxidizing, acetogenic bacterium. Arch. Microbiol. 1981, 129, 275–280. [Google Scholar] [CrossRef]
- Weghoff, M.C.; Müller, V. CO metabolism in the thermophilic acetogen Thermoanaerobacter kivui. Appl. Environ. Microbiol. 2016, 82, 2312–2319. [Google Scholar] [CrossRef]
- Scott, I.M.; Rubinstein, G.M.; Lipscomb, G.L.; Basen, M.; Schut, G.J.; Rhaesa, A.M.; Lancaster, W.A.; Poole, F.L.; Kelly, R.M.; Adams, M.W.W. A new class of tungsten-containing oxidoreductase in Caldicellulosiruptor, a genus of plant biomass-degrading thermophilic bacteria. Appl. Environ. Microbiol. 2015, 81, 7339–7347. [Google Scholar] [CrossRef] [PubMed]
- Winiarska, A.; Hege, D.; Gemmecker, Y.; Kryściak-Czerwenka, J.; Seubert, A.; Heider, J.; Szaleniec, M. A tungsten enzyme using hydrogen as an electron donor to reduce carboxylic acids and NAD+. ACS Catal. 2022, 12, 8707–8717. [Google Scholar] [CrossRef] [PubMed]
- Schuchmann, K.; Müller, V. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Biochemistry 2013, 342, 1382–1385. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Energetics and application of heterotrophy in acetogenic bacteria. Appl. Environ. Microbiol. 2016, 82, 4056–4069. [Google Scholar] [CrossRef]
- Jain, S.; Dietrich, H.M.; Müller, V.; Basen, M. Formate is required for growth of the thermophilic acetogenic bacterium Thermoanaerobacter kivui lacking hydrogen-dependent carbon dioxide reductase (HDCR). Front. Microbiol. 2020, 11, 59. [Google Scholar] [CrossRef]
- Katsyv, A.; Schoelmerich, M.C.; Basen, M.; Müller, V. The pyruvate:ferredoxin oxidoreductase of the thermophilic acetogen, Thermoanaerobacter kivui. FEBS Open Bio 2021, 11, 1332–1342. [Google Scholar] [CrossRef]
- Napora-Wijata, K.; Strohmeier, G.A.; Winkler, M. Biocatalytic reduction of carboxylic acids. Biotechnol. J. 2014, 9, 822–843. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, J.; Yuan, J.; Jiang, X.; Jiang, L.; Zhao, G.; Huang, D.; Liu, B. Omics-based analyses revealed metabolic responses of Clostridium acetobutylicum to lignocellulose-derived inhibitors furfural, formic acid and phenol stress for butanol fermentation. Biotechnol. Biofuels 2019, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Feng, D.; Huang, Y.; Zhu, X.; Wang, Z.; Zhu, X.; Liao, Q. Activated carbon facilitates anaerobic digestion of furfural wastewater: Effect of direct interspecies electron transfer. ACS Sustain. Chem. Eng. 2022, 10, 8206–8215. [Google Scholar] [CrossRef]
- Roy, R.; Mukund, S.; Schut, G.J.; Dunn, D.M.; Weiss, R.; Adams, M.W.W. Purification and molecular characterization of the tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus: The third of a putative five-member tungstoenzyme family. J. Bacteriol. 1999, 181, 1171–1180. [Google Scholar] [CrossRef]
- Mukund, S.; Adams, M.W.W. Characteriztion of a novel tugsten containing formadehyde ferrodoxin oxidoreductase from the hyperthermophilic Archaeon. Thermococcus litoralis. J. Biol. Chem. 1993, 268, 13592–13600. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. The reduction potential of benzyl viologen: An important reference compound for oxidant/radical redox couples. Free Radic. Res. Commun. 1991, 14, 57–67. [Google Scholar] [CrossRef]
- Katsyv, A.; Essig, M.; Bedendi, G.; Sahin, S.; Milton, R.D.; Müller, V. Characterization of ferredoxins from the thermophilic, acetogenic bacterium Thermoanaerobacter kivui. FEBS J. 2023, 290, 4107–4125. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Beinert, H. Semi-micro methods for analysis of labile sulfide and of labile sulfide plus sulfane sulfur in unusually stable iron-sulfur proteins. Anal. Biochem. 1983, 131, 373–378. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).