A Plasma Membrane Intrinsic Protein Gene OfPIP2 Involved in Promoting Petal Expansion and Drought Resistance in Osmanthus fragrans
Abstract
1. Introduction
2. Results
2.1. OfPIP2 Expression and Subcellular Localization Analysis
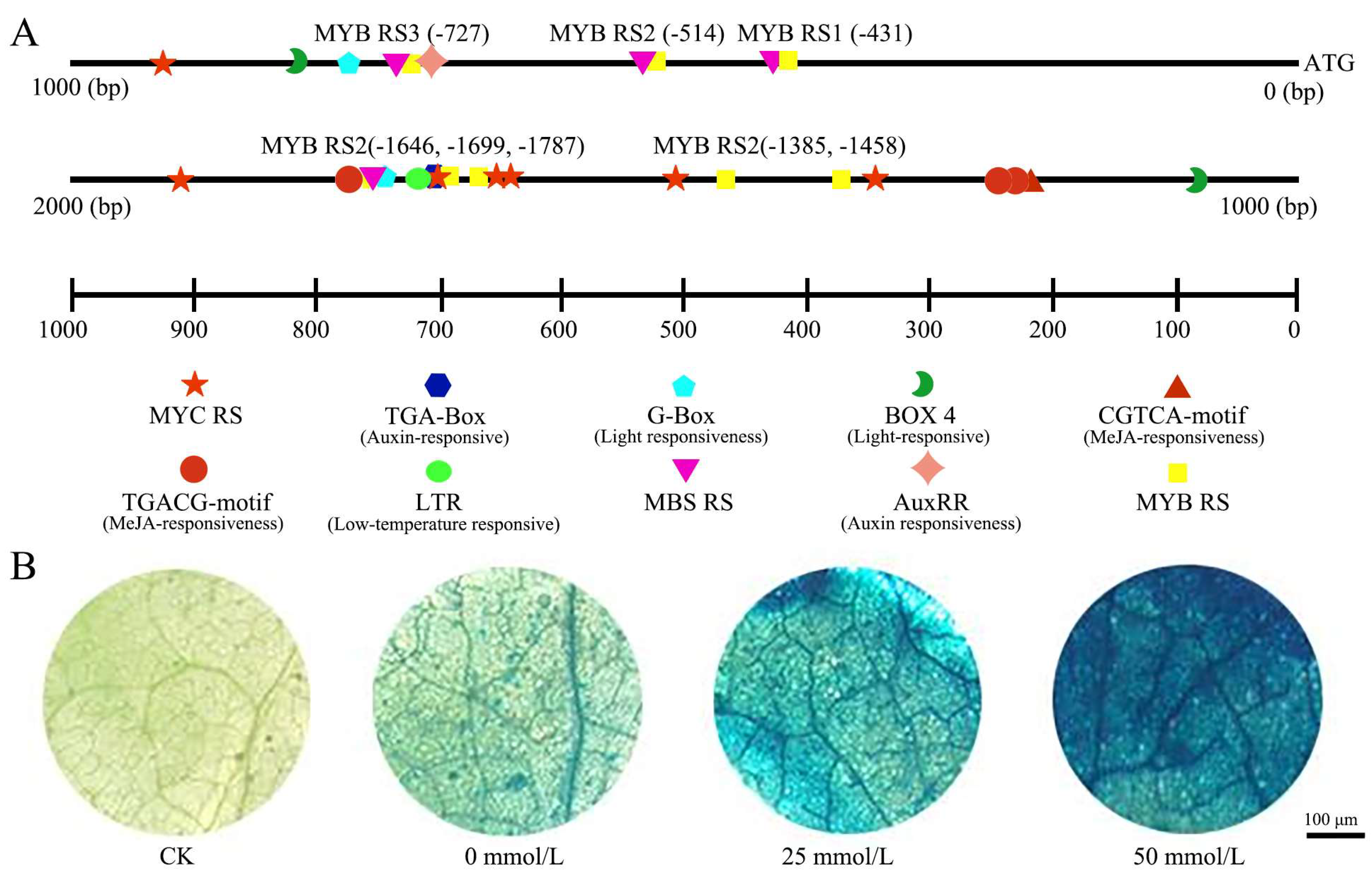
2.2. Promoter Analysis and Gus Staining
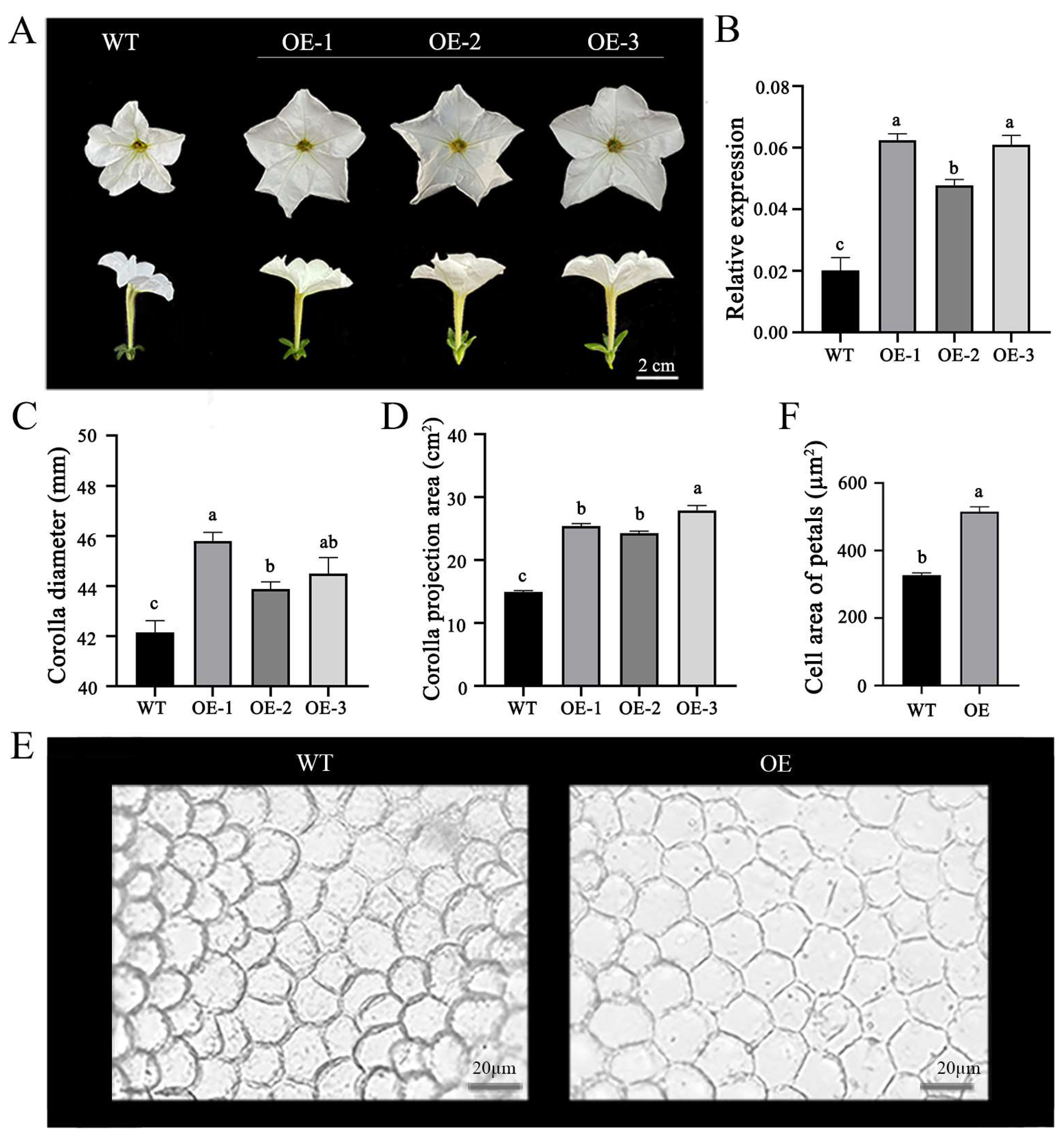
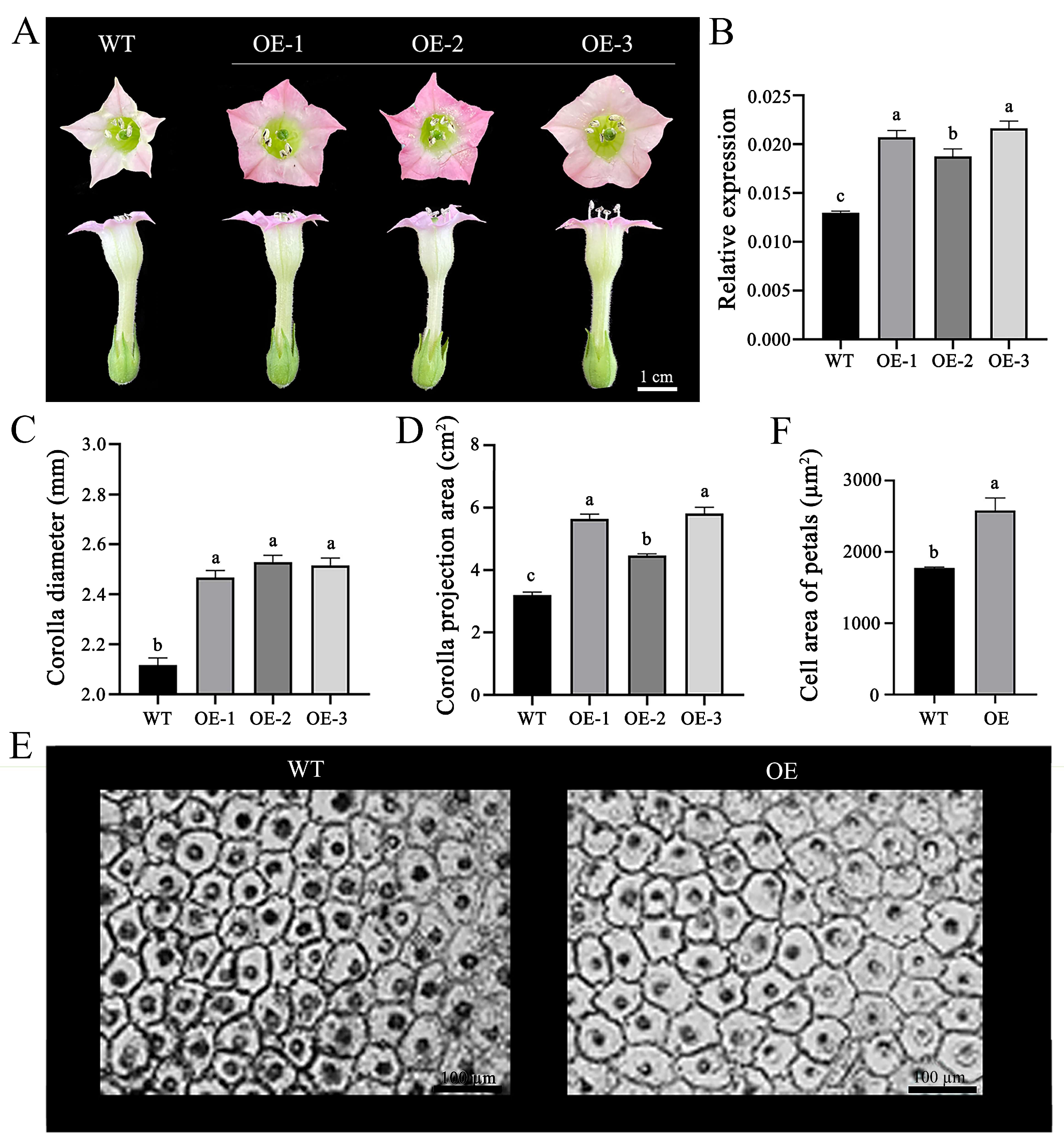
2.3. OfPIP2 Regulates Corolla Size in Petunia and Tobacco
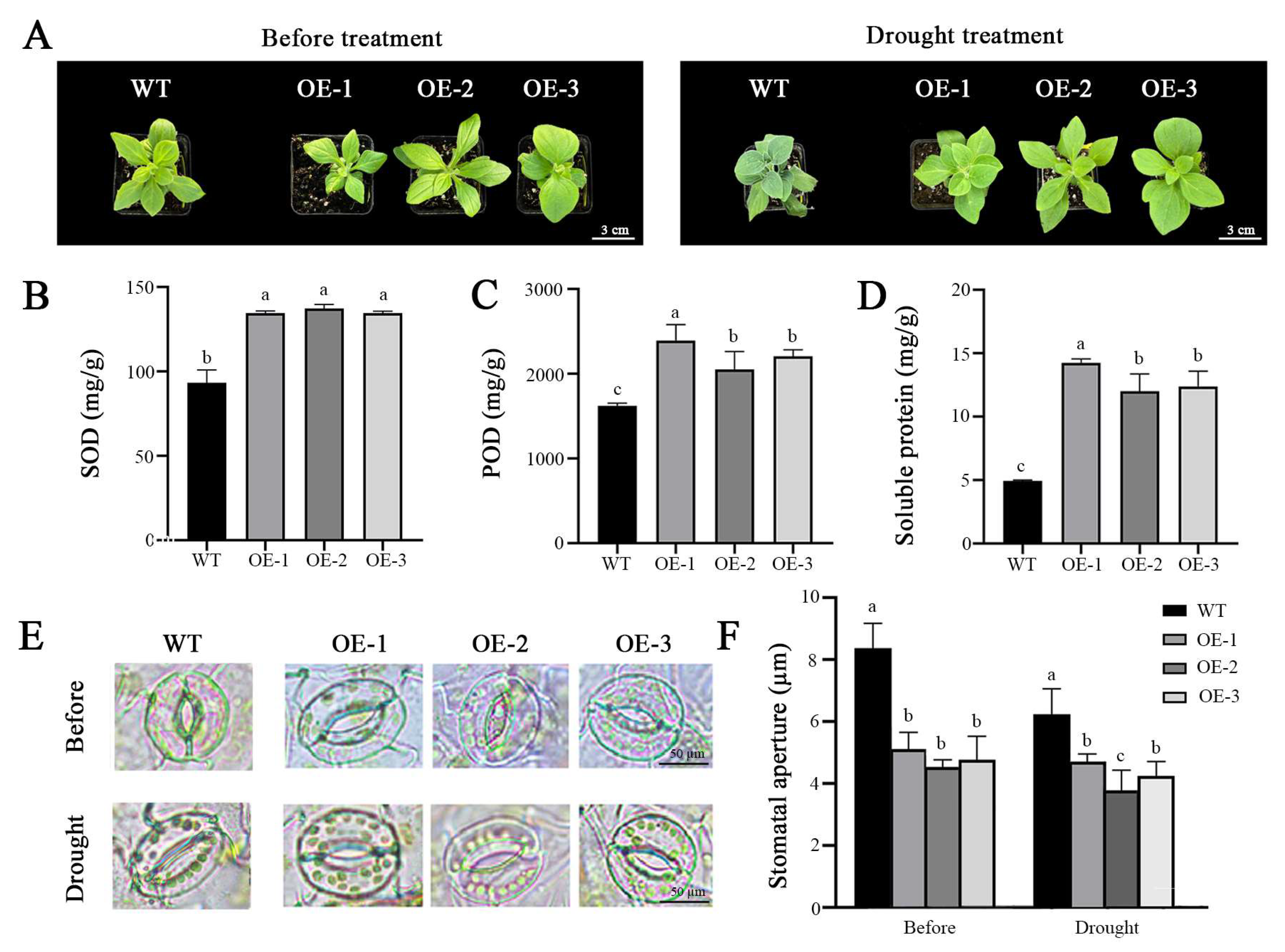
2.4. OfPIP2 Involved in the Regulation of Drought Tolerance in Petunia and Tobacco
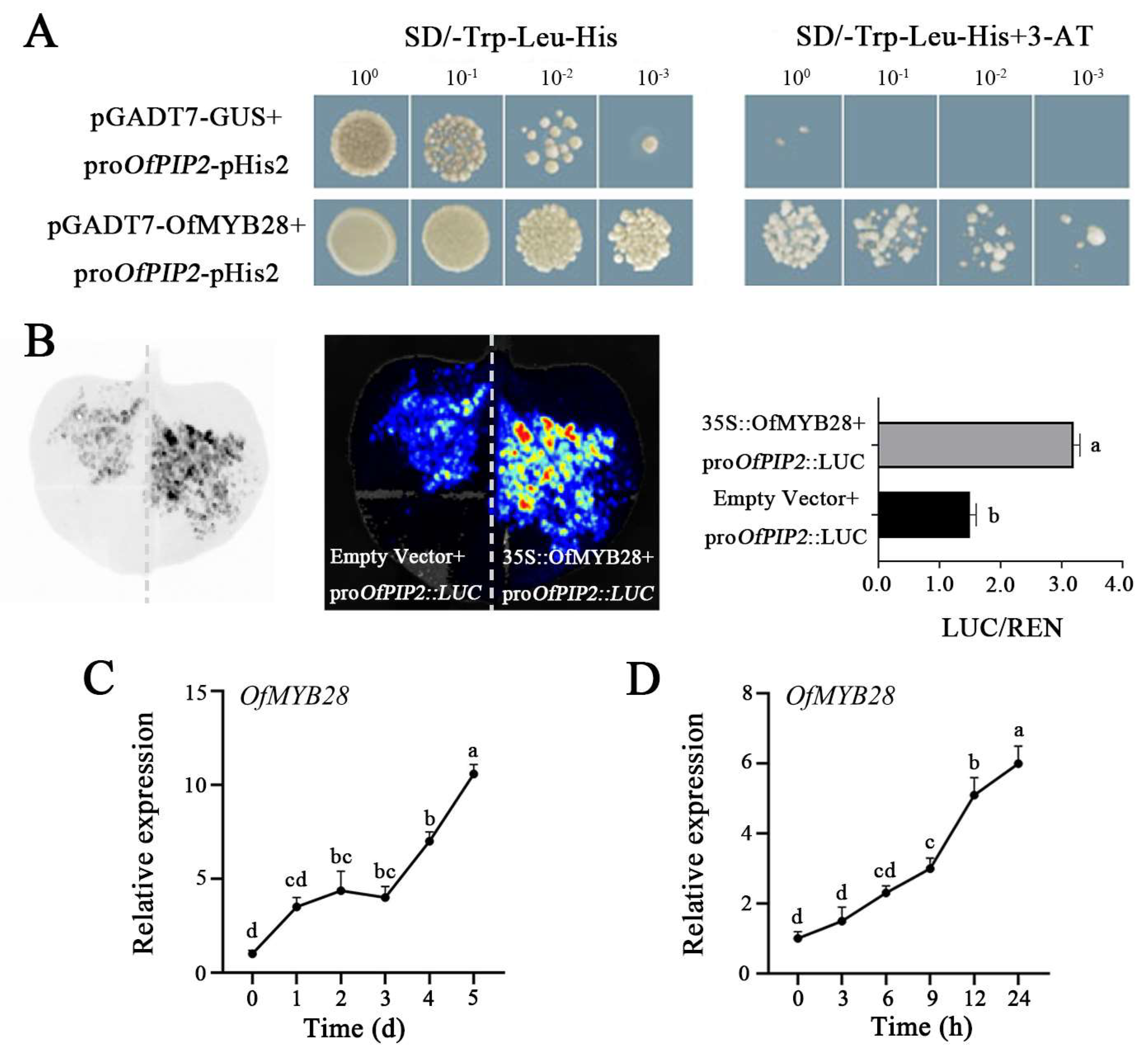
2.5. Transcription Factor OfMYB28 Positively Regulates OfPIP2 Expression
3. Discussion
3.1. OfPIP2 Involves in the Regulation of the Petal Size in O. fragrans
3.2. OfPIP2 Enhanced the Drought Tolerance in O. fragrans
3.3. MYB28 Positively Regulated the OfPIP2 Expression
4. Materials and Methods
4.1. Growth Conditions and Treatments of O. frangrans
4.2. RNA Extraction and First Strand cDNA Synthesis
4.3. Quantitative Real-Time PCR (qRT-PCR)
4.4. Subcellular Localization
4.5. Promoter Analysis and Histochemical GUS Staining
4.6. Transformation and Phenotype Investigation in Petunia and Tobacco
4.7. Physiological Index Measurement after Drought Treatment
4.8. Yeast One Hybrid (Y1H) Assay
4.9. Dual-Luciferase (Dual-LUC) Assays
4.10. Statistical Analysis
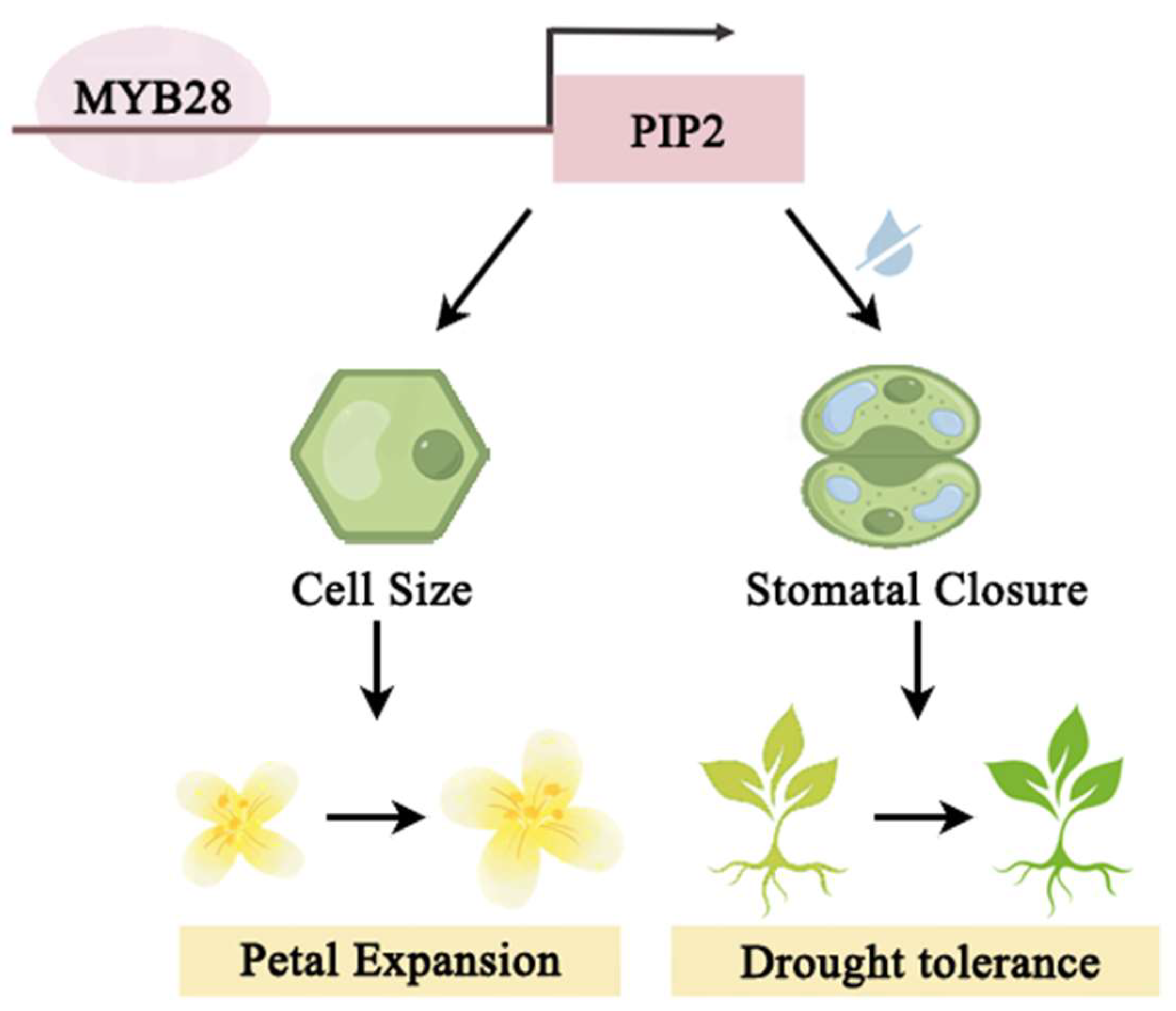
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bezerra-Neto, J.P.; De Araújo, F.C.; Ferreira-Neto, J.R.C.; Da Silva, M.D.; Pandolfi, V.; Aburjaile, F.F.; Sakamoto, T.; De Oliveira Silva, R.L.; Kido, E.A.; Barbosa Amorim, L.L.; et al. Plant Aquaporins: Diversity, Evolution and Biotechnological Applications. Curr. Protein Pept. Sci. 2019, 20, 368–395. [Google Scholar] [CrossRef] [PubMed]
- Chaumont, F.; Tyerman, S.D. Aquaporins: Highly Regulated Channels Controlling Plant Water Relations. Plant Physiol. 2014, 164, 1600–1618. [Google Scholar] [CrossRef] [PubMed]
- Kammerloher, W.; Fischer, U.; Piechottka, G.P.; Schäffner, A.R. Water Channels in the Plant Plasma Membrane Cloned by Immunoselection from a Mammalian Expression System. Plant J. 1994, 6, 187–199. [Google Scholar] [CrossRef]
- Ahmed, J.; Ismail, A.; Ding, L.; Yool, A.J.; Chaumont, F. A New Method to Measure Aquaporin-Facilitated Membrane Diffusion of Hydrogen Peroxide and Cations in Plant Suspension Cells. Plant Cell Environ. 2024, 47, 527–539. [Google Scholar] [CrossRef]
- Singh, R.K.; Deshmukh, R.; Muthamilarasan, M.; Rani, R.; Prasad, M. Versatile Roles of Aquaporin in Physiological Processes and Stress Tolerance in Plants. Plant Physiol. Biochem. 2020, 149, 178–189. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Kamdee, C. Flower Opening and Closure: An Update. J. Exp. Bot. 2014, 65, 5749–5757. [Google Scholar] [CrossRef]
- Li, Q.; Tong, T.; Jiang, W.; Cheng, J.; Deng, F.; Wu, X.; Chen, Z.-H.; Ouyang, Y.; Zeng, F. Highly Conserved Evolution of Aquaporin PIPs and TIPs Confers Their Crucial Contribution to Flowering Process in Plants. Front. Plant Sci. 2022, 12, 761713. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hussain, N.; Wang, Y.; Li, M.; Liu, L.; Qin, M.; Ma, N.; Gao, J.; Sun, X. An Ethylene-Inhibited NF-YC Transcription Factor RhNF-YC9 Regulates Petal Expansion in Rose. Hortic. Plant J. 2020, 6, 419–427. [Google Scholar] [CrossRef]
- Chen, W.; Yin, X.; Wang, L.; Tian, J.; Yang, R.; Liu, D.; Yu, Z.; Ma, N.; Gao, J. Involvement of Rose Aquaporin RhPIP1;1 in Ethylene-Regulated Petal Expansion through Interaction with RhPIP2;1. Plant Mol. Biol. 2013, 83, 219–233. [Google Scholar] [CrossRef]
- Azad, A.K.; Sawa, Y.; Ishikawa, T.; Shibata, H. Phosphorylation of Plasma Membrane Aquaporin Regulates Temperature-Dependent Opening of Tulip Petals. Plant Cell Physiol. 2004, 45, 608–617. [Google Scholar] [CrossRef]
- Leng, H.; Jiang, C.; Song, X.; Lu, M.; Wan, X. Poplar Aquaporin PIP1;1 Promotes Arabidopsis Growth and Development. BMC Plant Biol. 2021, 21, 253. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Uehlein, N.; Kaldenhoff, R.; Guo, S.; Zhu, Y.; Kai, L. Aquaporin PIP2;1 Affects Water Transport and Root Growth in Rice (Oryza sativa L.). Plant Physiol. Biochem. 2019, 139, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Milhiet, T.; Couvreur, V.; Nelissen, H.; Meziane, A.; Parent, B.; Aesaert, S.; Van Lijsebettens, M.; Inzé, D.; Tardieu, F.; et al. Modification of the Expression of the Aquaporin ZmPIP2;5 Affects Water Relations and Plant Growth. Plant Physiol. 2020, 182, 2154–2165. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Sugiyama, S.; Tateishi, A.; Satoh, S. Identification and Characterization of Plasma Membrane Intrinsic Protein (PIP) Aquaporin Genes in Petals of Opening Carnation Flowers. Hortic. J. 2017, 86, 78–86. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Li, J.; Li, Y.; Zeng, X.; Zhao, D. Overexpression of the Eucommia ulmoides Aquaporin, EuPIP1;1, Promotes Leaf Growth, Flowering and Bolting, and Stress Tolerance in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 11794. [Google Scholar] [CrossRef]
- Fürtauer, L.; Weiszmann, J.; Weckwerth, W.; Nägele, T. Dynamics of Plant Metabolism during Cold Acclimation. Int. J. Mol. Sci. 2019, 20, 5411. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.-M.; Li, Q.-S.; Liu, M.-Y.; Hashem, A.; Al-Arjani, A.-B.F.; Alenazi, M.M.; Abd_Allah, E.F.; Muthuramalingam, P.; Wu, Q.-S. Mycorrhizal Effects on Growth and Expressions of Stress-Responsive Genes (aquaporins and SOSs) of Tomato under Salt Stress. J. Fungi 2022, 8, 1305. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Jia, C.; Hu, W.; Song, S.; Xu, B.; Jin, Z. Overexpression of a Banana Aquaporin Gene MaPIP1;1 Enhances Tolerance to Multiple Abiotic Stresses in Transgenic Banana and Analysis of Its Interacting Transcription Factors. Front. Plant Sci. 2021, 12, 699230. [Google Scholar] [CrossRef]
- Sade, N.; Vinocur, B.J.; Diber, A.; Shatil, A.; Ronen, G.; Nissan, H.; Wallach, R.; Karchi, H.; Moshelion, M. Improving Plant Stress Tolerance and Yield Production: Is the Tonoplast Aquaporin SlTIP2;2 a Key to Isohydric to Anisohydric Conversion? New Phytol. 2009, 181, 651–661. [Google Scholar] [CrossRef]
- Mahdieh, M.; Mostajeran, A.; Horie, T.; Katsuhara, M. Drought Stress Alters Water Relations and Expression of PIP-Type Aquaporin Genes in Nicotiana tabacum Plants. Plant Cell Physiol. 2008, 49, 801–813. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Fu, J.; Zhao, H. Progresses on flower bud differentiation and flower opening in Osmanthus fragrans. J. Zhejiang AF Univ. 2016, 33, 340–347. [Google Scholar]
- Sun, Q.; Liu, X.; Kitagawa, Y.; Calamita, G.; Ding, X. Plant Aquaporins: Their Roles beyond Water Transport. Crop J. 2024, 12, 641–655. [Google Scholar] [CrossRef]
- Oh, B.-C. Phosphoinositides and Intracellular Calcium Signaling: Novel Insights into Phosphoinositides and Calcium Coupling as Negative Regulators of Cellular Signaling. Exp. Mol. Med. 2023, 55, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Claeys, H.; Inzé, D. The Agony of Choice: How Plants Balance Growth and Survival under Water-Limiting Conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef]
- Alexandersson, E.; Fraysse, L.; Sjövall-Larsen, S.; Gustavsson, S.; Fellert, M.; Karlsson, M.; Johanson, U.; Kjellbom, P. Whole Gene Family Expression and Drought Stress Regulation of Aquaporins. Plant Mol. Biol. 2005, 59, 469–484. [Google Scholar] [CrossRef]
- Horibe, T.; Yamada, K. Petal Growth Physiology of Cut Rose Flowers: Progress and Future Prospects. J. Hortic. Res. 2017, 25, 5–18. [Google Scholar] [CrossRef]
- Ma, N.; Xue, J.; Li, Y.; Liu, X.; Dai, F.; Jia, W.; Luo, Y.; Gao, J. Rh-PIP2;1, a Rose Aquaporin Gene, Is Involved in Ethylene-Regulated Petal Expansion. Plant Physiol. 2008, 148, 894–907. [Google Scholar] [CrossRef]
- Dong, B.; Wang, Q.; Zhou, D.; Wang, Y.; Miao, Y.; Zhong, S.; Fang, Q.; Yang, L.; Xiao, Z.; Zhao, H. Abiotic Stress Treatment Reveals Expansin like A Gene OfEXLA1 Improving Salt and Drought Tolerance of Osmanthus fragrans by Responding to Abscisic Acid. Hortic. Plant J. 2024, 10, 573–585. [Google Scholar] [CrossRef]
- McAdam, S.A.M.; Manandhar, A.; Kane, C.N.; Mercado-Reyes, J.A. Passive Stomatal Closure under Extreme Drought in an Angiosperm Species. J. Exp. Botany 2023, erad510. [Google Scholar] [CrossRef]
- Rosas-Santiago, P.; Zechinelli Pérez, K.; Gómez Méndez, M.F.; Vera López Portillo, F.; Ruiz Salas, J.L.; Cordoba Martínez, E.; Acosta Maspon, A.; Pantoja, O. A Differential Subcellular Localization of Two Copper Transporters from the COPT Family Suggests Distinct Roles in Copper Homeostasis in Physcomitrium patens. Plant Physiol. Biochem. 2021, 167, 459–469. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.; Carins Murphy, M.R. Xylem and Stomata, Coordinated through Time and Space. Plant Cell Environ. 2017, 40, 872–880. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.A.M.; Brodribb, T.J. Fern and Lycophyte Guard Cells Do Not Respond to Endogenous Abscisic Acid. Plant Cell 2012, 24, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, C.; Ji, Z.; Lu, J.; Zhang, L.; Li, C.; Huang, J.; Yang, G.; Yan, K.; Zhang, S.; et al. Regulation of Drought Tolerance in Arabidopsis Involves the PLATZ4-mediated Transcriptional Repression of Plasma Membrane Aquaporin PIP2;8. Plant J. 2023, 115, 434–451. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, M.; Chen, W.; Zhou, X.; Lu, J.; Wang, Y.; Li, Y.; Jiang, C.-Z.; Gan, S.-S.; Ma, N.; et al. In Rose, Transcription Factor PTM Balances Growth and Drought Survival via PIP2;1 Aquaporin. Nat. Plants 2019, 5, 290–299. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Feng, H.; Deng, J.; Zhang, R.; Wen, J.; Dong, J.; Wang, T. Dehydrin MtCAS31 Promotes Autophagic Degradation under Drought Stress. Autophagy 2020, 16, 862–877. [Google Scholar] [CrossRef]
- Zhong, S.; Zhu, H.; Li, W.; Wu, D.; Miao, Y.; Dong, B.; Wang, Y.; Xiao, Z.; Fang, Q.; Deng, J.; et al. DNA Methylome Analysis Reveals Novel Insights into Active Hypomethylated Regulatory Mechanisms of Temperature-Dependent Flower Opening in Osmanthus fragrans. Hortic. Res. 2024, 11, uhae010. [Google Scholar] [CrossRef] [PubMed]
- WANG, Q.; JIANG, Q.; FU, J.; DONG, B.; ZHAO, H. Screening reference genes of Osmanthus fragrans with differing photoperiod and temperature treatments. J. Zhejiang AF Univ. 2019, 36, 928–934. [Google Scholar]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, M.; Si, Y.; Li, W.; Wang, L.; Chen, S.; Chen, F.; Jiang, J. Transcriptome Analysis of Ethylene Response in Chrysanthemum moriflolium Ramat. with an Emphasis on Flowering Delay. Horticulturae 2023, 9, 428. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Reid, M.S.; Jiang, C.-Z. Controlling Plant Architecture by Manipulation of Gibberellic Acid Signalling in Petunia. Hortic. Res. 2014, 1, 14061. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Stier, G.; Lin, J.; Liu, G.; Zhang, Z.; Chang, Y.; Reid, M.S.; Jiang, C.-Z. Transcriptome Changes Associated with Delayed Flower Senescence on Transgenic Petunia by Inducing Expression of etr1-1, a Mutant Ethylene Receptor. PLoS ONE 2013, 8, e65800. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, Transient Expression of Fluorescent Fusion Proteins in Tobacco Plants and Generation of Stably Transformed Plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Kong, E.; Shen, L.; Ye, Y.; Wang, Y.; Dong, B.; Zhong, S. A Plasma Membrane Intrinsic Protein Gene OfPIP2 Involved in Promoting Petal Expansion and Drought Resistance in Osmanthus fragrans. Int. J. Mol. Sci. 2024, 25, 10716. https://doi.org/10.3390/ijms251910716
Lu X, Kong E, Shen L, Ye Y, Wang Y, Dong B, Zhong S. A Plasma Membrane Intrinsic Protein Gene OfPIP2 Involved in Promoting Petal Expansion and Drought Resistance in Osmanthus fragrans. International Journal of Molecular Sciences. 2024; 25(19):10716. https://doi.org/10.3390/ijms251910716
Chicago/Turabian StyleLu, Xinke, En Kong, Lixiao Shen, Yong Ye, Yiguang Wang, Bin Dong, and Shiwei Zhong. 2024. "A Plasma Membrane Intrinsic Protein Gene OfPIP2 Involved in Promoting Petal Expansion and Drought Resistance in Osmanthus fragrans" International Journal of Molecular Sciences 25, no. 19: 10716. https://doi.org/10.3390/ijms251910716
APA StyleLu, X., Kong, E., Shen, L., Ye, Y., Wang, Y., Dong, B., & Zhong, S. (2024). A Plasma Membrane Intrinsic Protein Gene OfPIP2 Involved in Promoting Petal Expansion and Drought Resistance in Osmanthus fragrans. International Journal of Molecular Sciences, 25(19), 10716. https://doi.org/10.3390/ijms251910716







