Natural Products and Altered Metabolism in Cancer: Therapeutic Targets and Mechanisms of Action
Abstract
1. Introduction
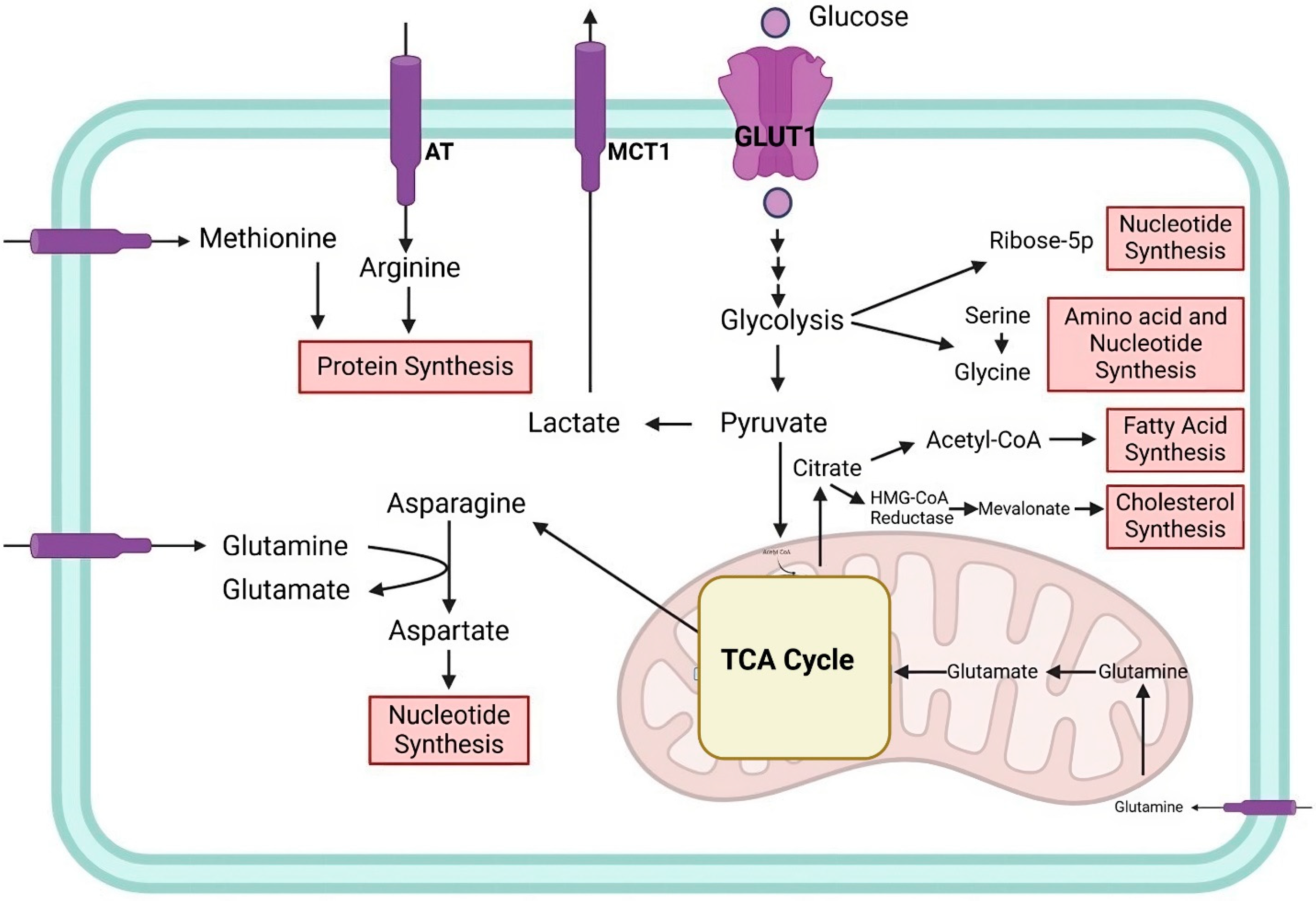
2. Cellular and Cancer Metabolism
3. Natural Products Targeting Cancer Metabolism
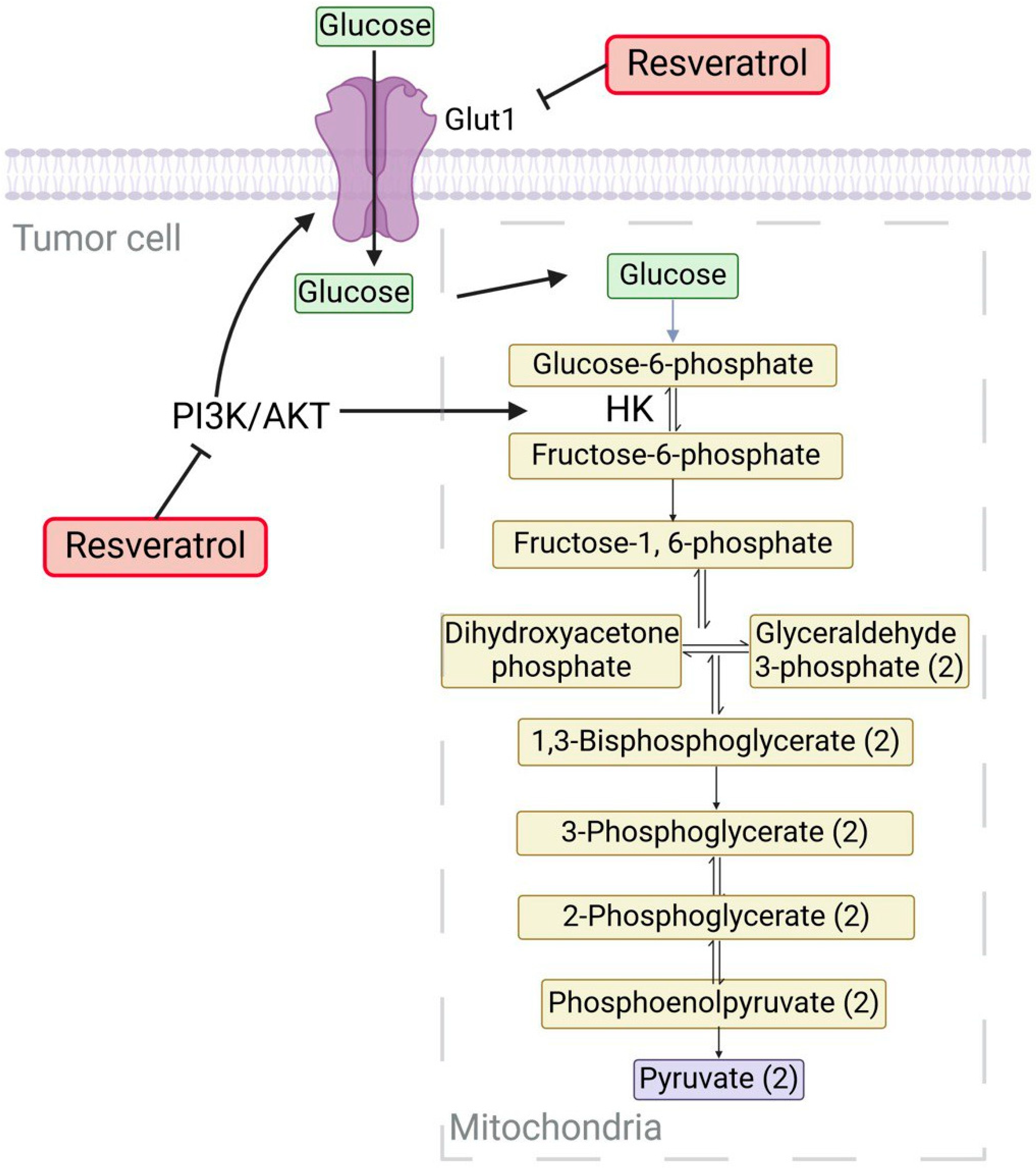
3.1. Resveratrol (RV)
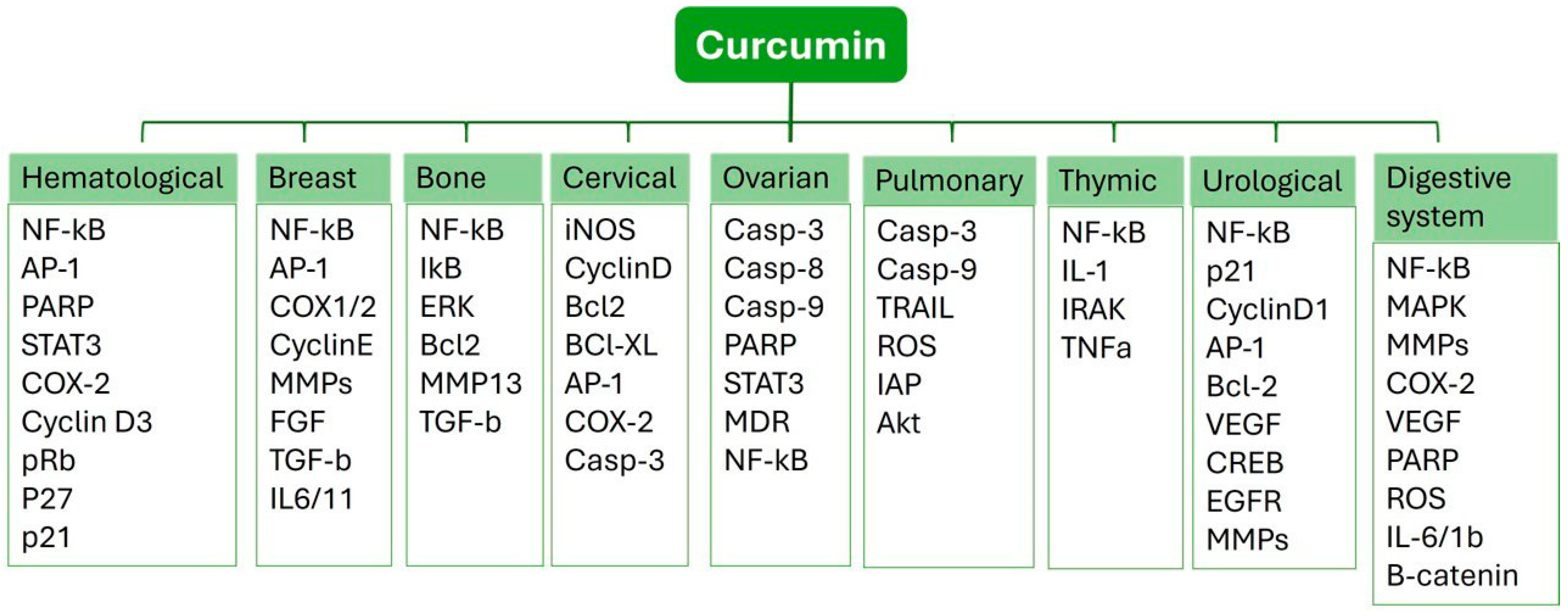
3.2. Curcumin (CUR)
3.3. Thymoquinone (TQ)
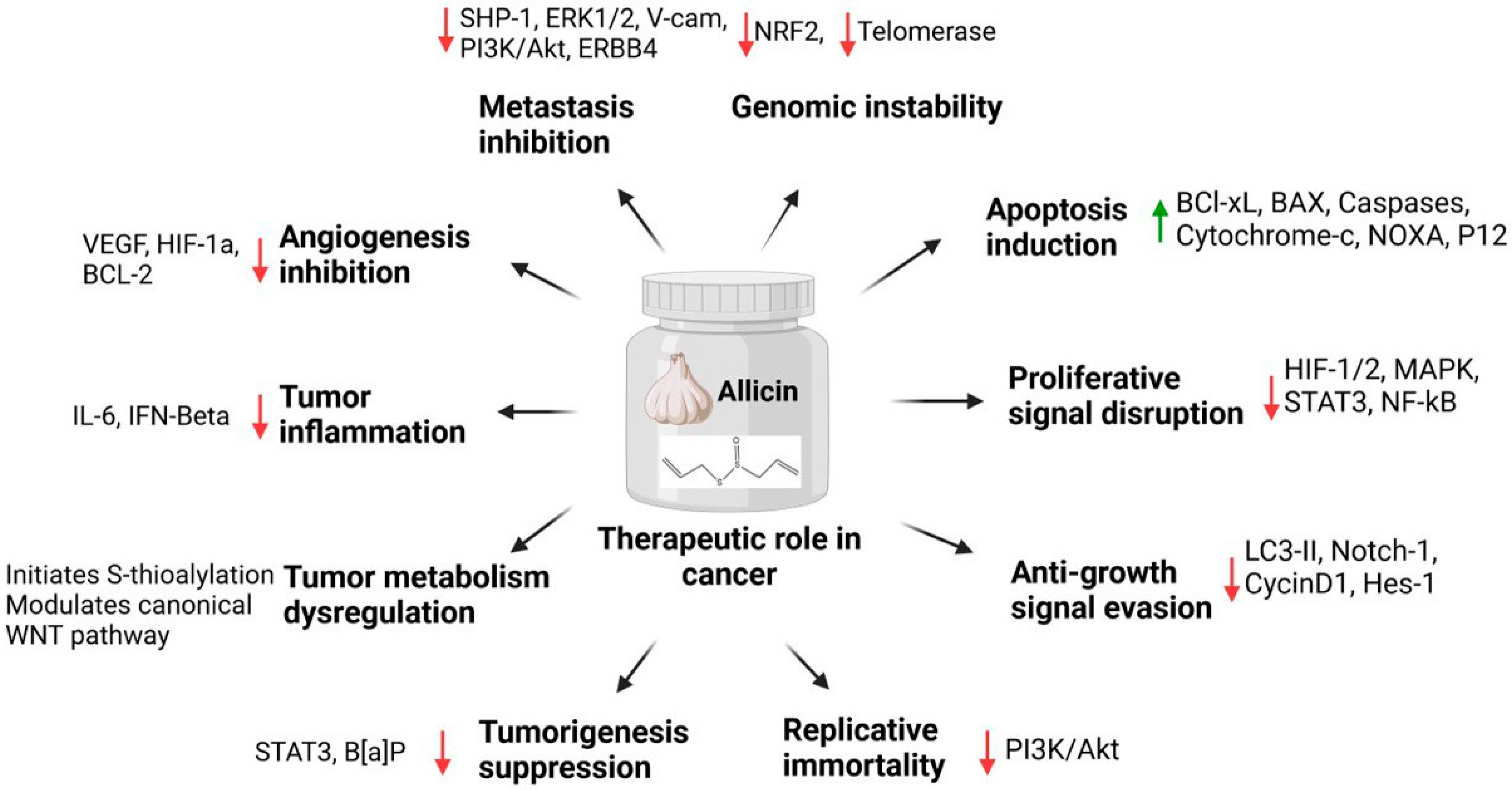
3.4. Allicin
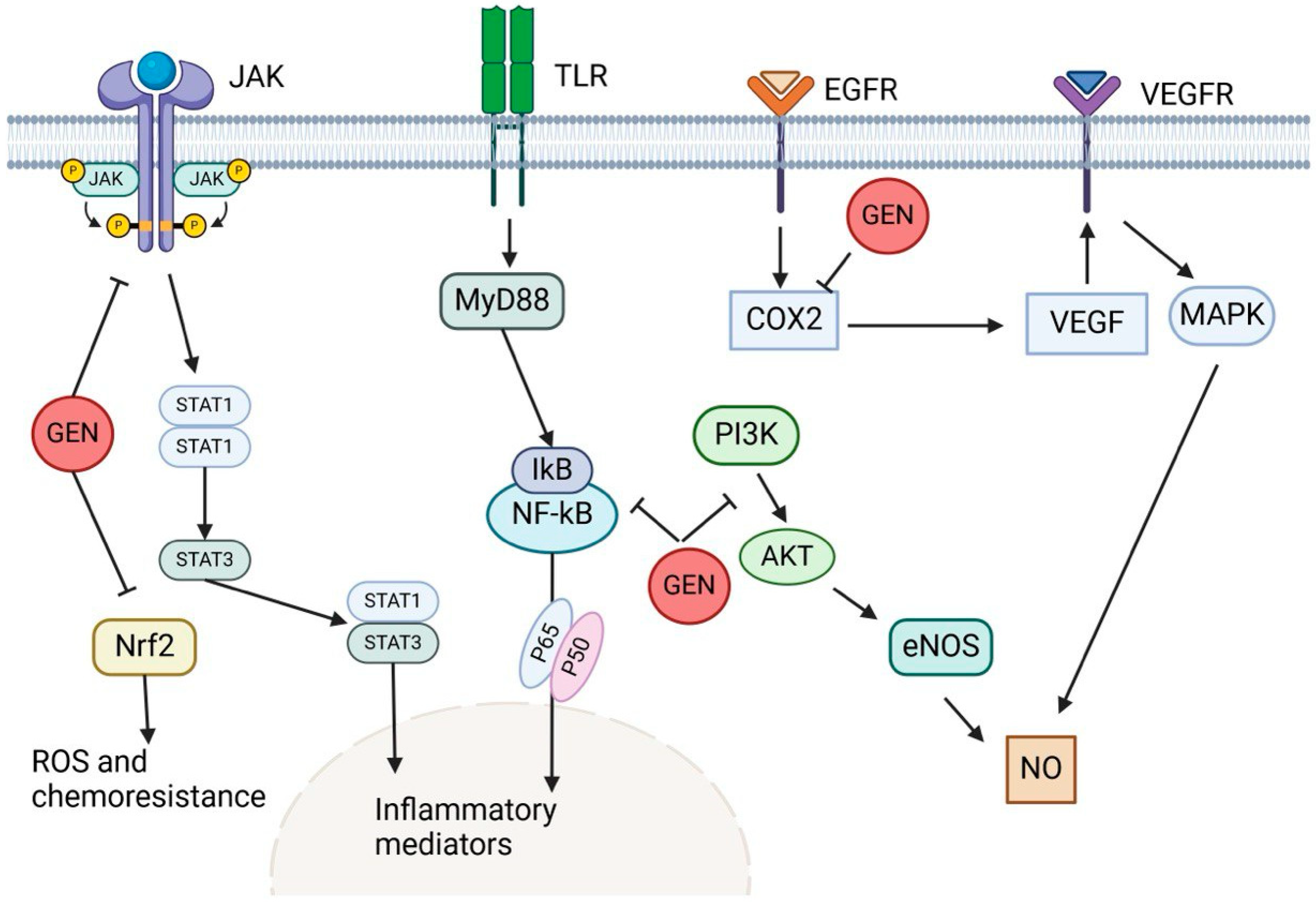
3.5. Genistein
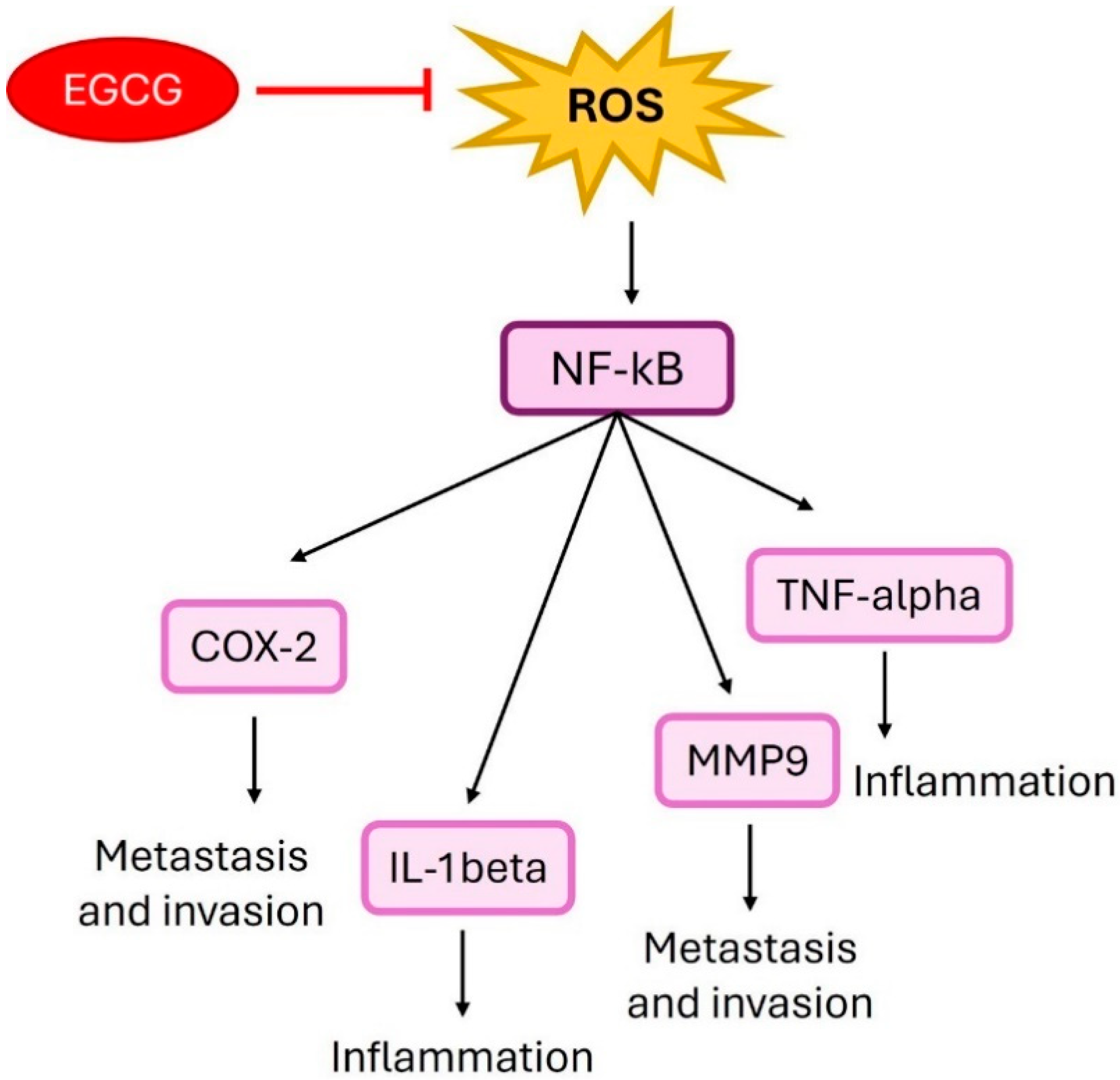
4. Epigallocatechin Gallate
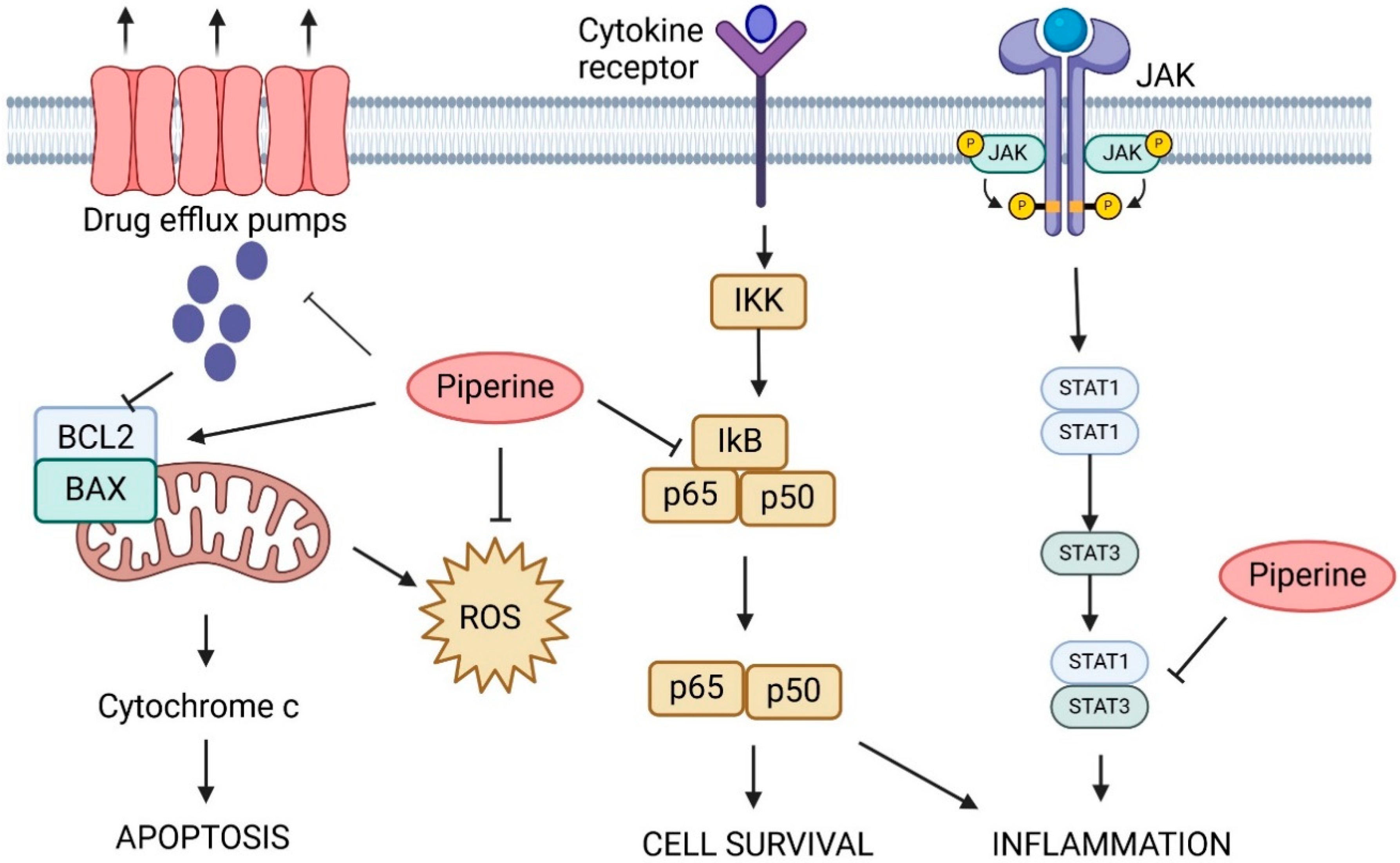
5. Piperine
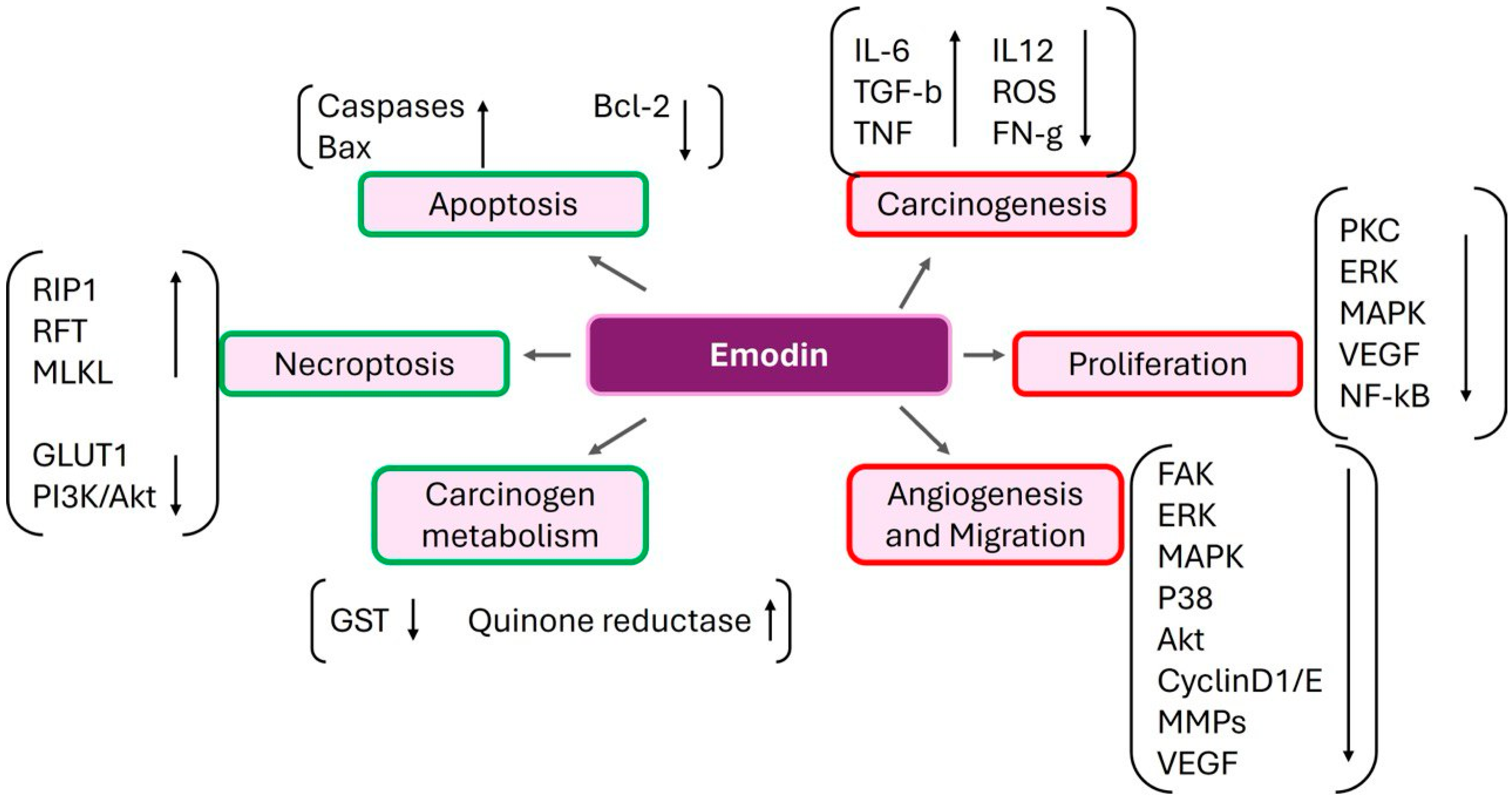
6. Emodin
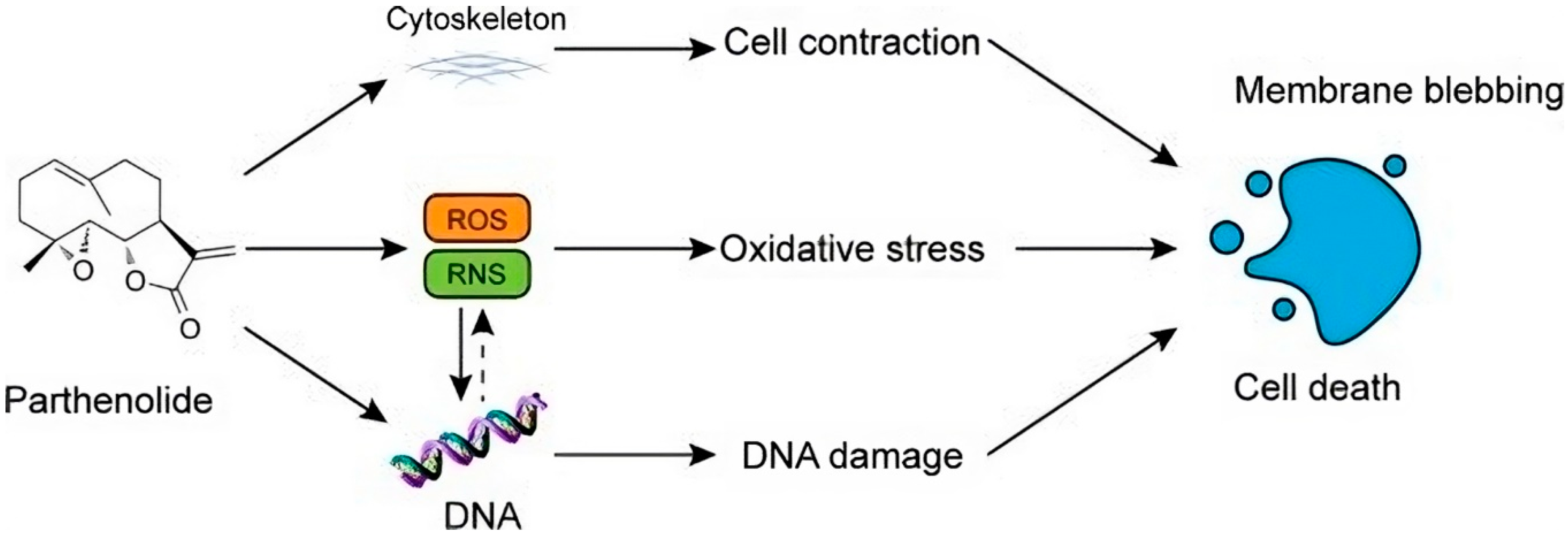
7. Parthenolide
8. Luteolin
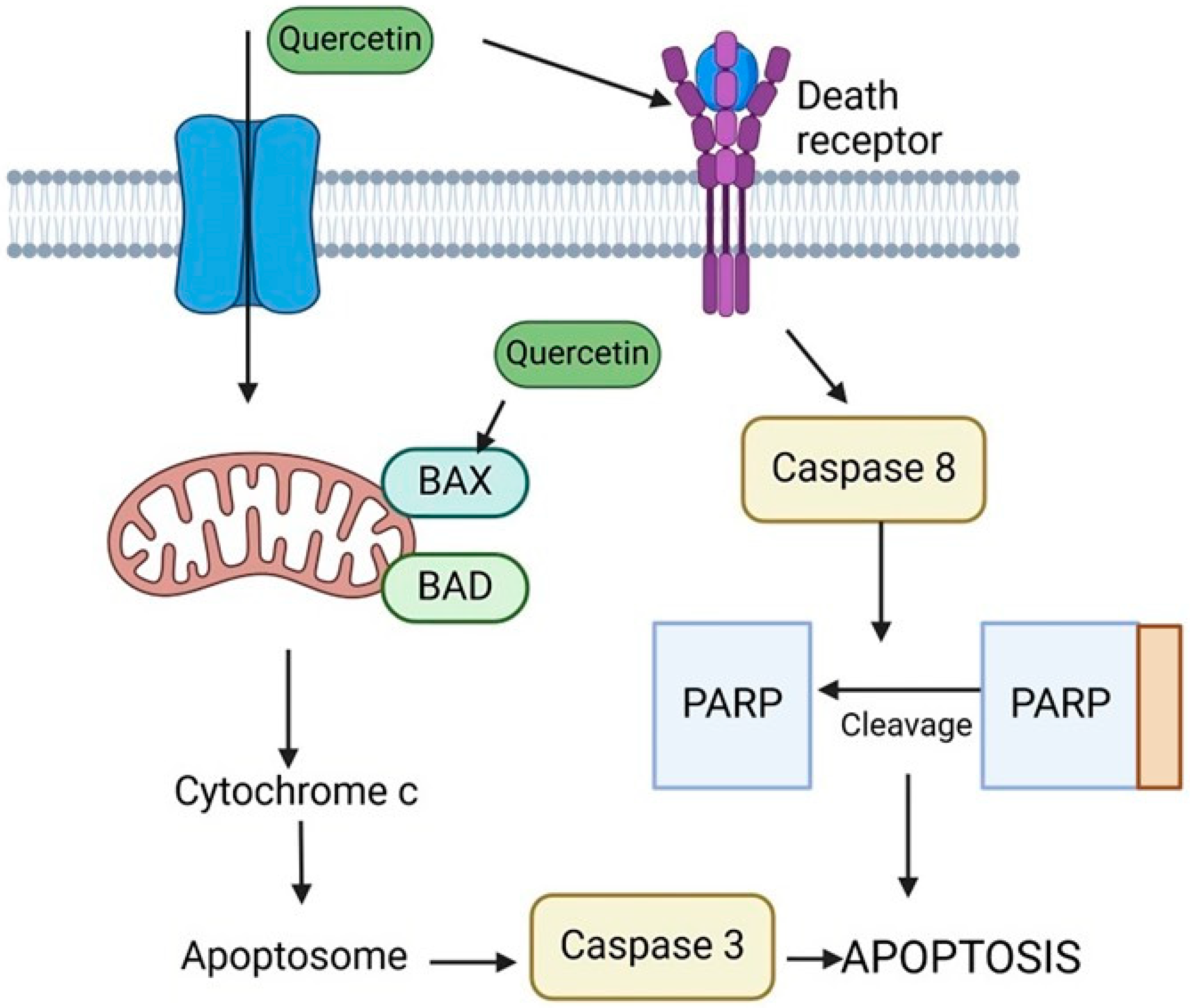
9. Quercetin
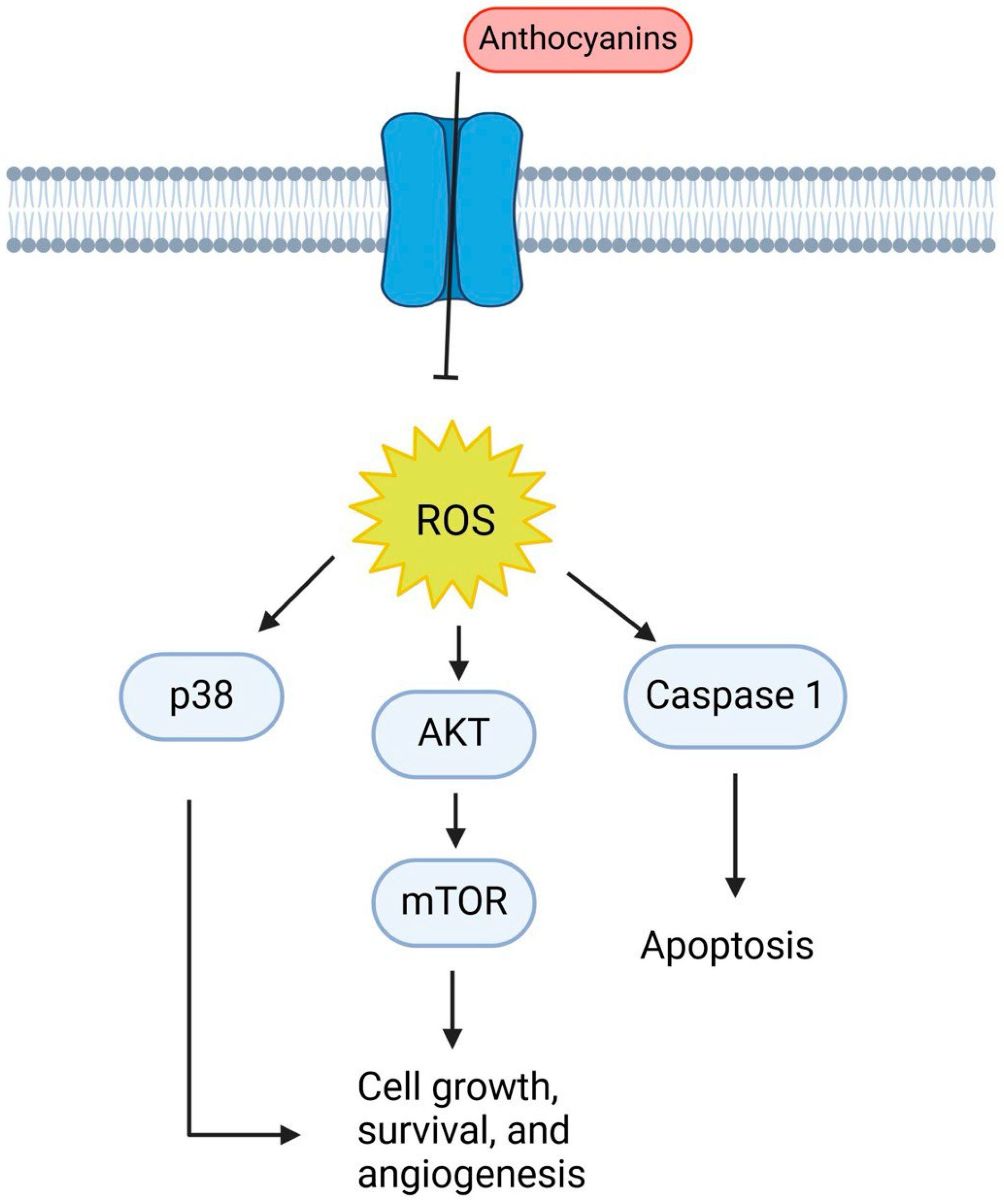
10. Anthocyanins
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vernieri, C.; Casola, S.; Foiani, M.; Pietrantonio, F.; de Braud, F.; Longo, V. Targeting cancer metabolism: Dietary and pharmacologic interventions. Cancer Discov. 2016, 6, 1315–1333. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Warburg, O. On respiratory impairment in cancer cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.R.; Sabatini, D.M. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef]
- Hossain, M.S.; Kader, M.A.; Goh, K.W.; Islam, M.; Khan, M.S.; Harun-Ar Rashid, M.; Ooi, D.J.; Melo Coutinho, H.D.; Al-Worafi, Y.M.; Moshawih, S. Herb and spices in colorectal cancer prevention and treatment: A narrative review. Front. Pharmacol. 2022, 13, 865801. [Google Scholar] [CrossRef]
- Reddy, L.; Odhav, B.; Bhoola, K. Natural products for cancer prevention: A global perspective. Pharmacol. Ther. 2003, 99, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chard Dunmall, L.S.; Cheng, Z.; Wang, Y.; Si, L. Natural products targeting glycolysis in cancer. Front. Pharmacol. 2022, 13, 1036502. [Google Scholar] [CrossRef]
- Saunier, E.; Antonio, S.; Regazzetti, A.; Auzeil, N.; Laprévote, O.; Shay, J.W.; Coumoul, X.; Barouki, R.; Benelli, C.; Huc, L. Resveratrol reverses the Warburg effect by targeting the pyruvate dehydrogenase complex in colon cancer cells. Sci. Rep. 2017, 7, 6945. [Google Scholar] [CrossRef] [PubMed]
- Baudot, A.; De La Torre, V.; Valencia, A. Mutated genes, pathways and processes in tumours. EMBO Rep. 2010, 11, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sancho, P.; Barneda, D.; Heeschen, C. Hallmarks of cancer stem cell metabolism. Br. J. Cancer 2016, 114, 1305–1312. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Dang, C.V. Rethinking the Warburg effect with Myc micromanaging glutamine metabolism. Cancer Res. 2010, 70, 859–862. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1: Upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51–56. [Google Scholar] [CrossRef]
- Tennant, D.A.; Durán, R.V.; Gottlieb, E. Targeting metabolic transformation for cancer therapy. Nat. Rev. Cancer 2010, 10, 267–277. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Lien, E.C.; Dibble, C.C.; Toker, A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 2017, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.C.; Lyssiotis, C.A.; Cantley, L.C. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In Metabolism in Cancer; Springer: Berlin/Heidelberg, Germany, 2016; pp. 39–72. [Google Scholar]
- Marbaniang, C.; Kma, L. Dysregulation of glucose metabolism by oncogenes and tumor suppressors in cancer cells. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 2377. [Google Scholar] [PubMed]
- Dejure, F.R.; Eilers, M. MYC and tumor metabolism: Chicken and egg. EMBO J. 2017, 36, 3409–3420. [Google Scholar] [CrossRef]
- Soni, S.; Padwad, Y.S. HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. 2017, 56, 503–515. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Cantley, L.C. Cancer’s fuel choice: New flavors for a picky eater. Mol. Cell 2015, 60, 514–523. [Google Scholar] [CrossRef]
- Lu, H.; Forbes, R.A.; Verma, A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem. 2002, 277, 23111–23115. [Google Scholar] [CrossRef] [PubMed]
- Desideri, E.; Vegliante, R.; Ciriolo, M.R. Mitochondrial dysfunctions in cancer: Genetic defects and oncogenic signaling impinging on TCA cycle activity. Cancer Lett. 2015, 356, 217–223. [Google Scholar] [CrossRef]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Chow, C.C.; Harada, H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factor 1: Oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 2001, 7, 345–350. [Google Scholar] [CrossRef]
- Shrestha, A.; Pandey, R.P.; Sohng, J.K. Biosynthesis of resveratrol and piceatannol in engineered microbial strains: Achievements and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 2959–2972. [Google Scholar] [CrossRef]
- Stagos, D.; Amoutzias, G.D.; Matakos, A.; Spyrou, A.; Tsatsakis, A.M.; Kouretas, D. Chemoprevention of liver cancer by plant polyphenols. Food Chem. Toxicol. 2012, 50, 2155–2170. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.S.; Xia, C.; Jiang, B.-H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D. Cellular signaling perturbation by natural products. Cell. Signal. 2009, 21, 1541–1547. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, T.; Oda, Y.; Seino, Y.; Yamamoto, T.; Inagaki, N.; Yano, H.; Imura, H.; Shigemoto, R.; Kikuchi, H. Distribution of the glucose transporters in human brain tumors. Cancer Res. 1992, 52, 3972–3979. [Google Scholar] [PubMed]
- Yamamoto, T.; Seino, Y.; Fukumoto, H.; Koh, G.; Yano, H.; Inagaki, N.; Yamada, Y.; Inoue, K.; Manabe, T.; Imura, H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem. Biophys. Res. Commun. 1990, 170, 223–230. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Zhang, W.; Bergmeier, S.; Qian, Y.; Akbar, H.; Colvin, R.; Ding, J.; Tong, L.; Wu, S. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol. Cancer Ther. 2012, 11, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Cao, Y.; Liu, Y.; Bergmeier, S.; Chen, X. Small compound inhibitors of basal glucose transport inhibit cell proliferation and induce apoptosis in cancer cells via glucose-deprivation-like mechanisms. Cancer Lett. 2010, 298, 176–185. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Bamezai, R.N. Resveratrol inhibits cancer cell metabolism by down regulating pyruvate kinase M2 via inhibition of mammalian target of rapamycin. PLoS ONE 2012, 7, e36764. [Google Scholar] [CrossRef]
- Lin, J.-N.; Lin, V.C.-H.; Rau, K.-M.; Shieh, P.-C.; Kuo, D.-H.; Shieh, J.-C.; Chen, W.-J.; Tsai, S.-C.; Way, T.-D. Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J. Agric. Food Chem. 2010, 58, 1584–1592. [Google Scholar] [CrossRef]
- Kueck, A.; Opipari Jr, A.W.; Griffith, K.A.; Tan, L.; Choi, M.; Huang, J.; Wahl, H.; Liu, J.R. Resveratrol inhibits glucose metabolism in human ovarian cancer cells. Gynecol. Oncol. 2007, 107, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Opipari Jr, A.W.; Tan, L.; Boitano, A.E.; Sorenson, D.R.; Aurora, A.; Liu, J.R. Resveratrol-induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004, 64, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Gwak, H.; Haegeman, G.; Tsang, B.K.; Song, Y.S. Cancer-specific interruption of glucose metabolism by resveratrol is mediated through inhibition of Akt/GLUT1 axis in ovarian cancer cells. Mol. Carcinog. 2015, 54, 1529–1540. [Google Scholar] [CrossRef]
- Thompson, C. Rethinking the regulation of cellular metabolism. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Berlin/Heidelberg, Germany, 2011; pp. 23–29. [Google Scholar]
- Rolfe, D.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519. [Google Scholar] [CrossRef]
- Brockmueller, A.; Sameri, S.; Liskova, A.; Zhai, K.; Varghese, E.; Samuel, S.M.; Büsselberg, D.; Kubatka, P.; Shakibaei, M. Resveratrol’s Anti-Cancer Effects through the Modulation of Tumor Glucose Metabolism. Cancers 2021, 13, 188. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, J.; Qin, S.; Zhou, Z.; Zeng, Q.; Long, R.; Mao, Z.; Dong, X.; Zhao, R.; Zhang, R. Resveratrol induces autophagy impeding BAFF-stimulated B-cell proliferation and survival by inhibiting the Akt/mTOR pathway. Biochem. Pharmacol. 2022, 202, 115139. [Google Scholar] [CrossRef] [PubMed]
- Shamim, U.; Hanif, S.; Albanyan, A.; Beck, F.W.; Bao, B.; Wang, Z.; Banerjee, S.; Sarkar, F.H.; Mohammad, R.M.; Hadi, S.M. Resveratrol-induced apoptosis is enhanced in low pH environments associated with cancer. J. Cell. Physiol. 2012, 227, 1493–1500. [Google Scholar] [CrossRef]
- Ahn, Y.S.; Chemeris, G.Y.; Turusov, V.S.; Bannasch, P. Enzymic pattern of preneoplastic and neoplastic lesions induced in the kidney of CBA mice by 1, 2-dimethylhydrazine. Toxicol. Pathol. 1994, 22, 415–422. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Amelio, I.; Berkers, C.R.; Antonov, A.; Vousden, K.H.; Melino, G.; Zolla, L. Metabolic effect of TAp63α: Enhanced glycolysis and pentose phosphate pathway, resulting in increased antioxidant defense. Oncotarget 2014, 5, 7722. [Google Scholar] [CrossRef][Green Version]
- Jonas, S.; Benedetto, C.; Flatman, A.; Hammond, R.; Micheletti, L.; Riley, C.; Riley, P.; Spargo, D.; Zonca, M.; Slater, T. Increased activity of 6-phosphogluconate dehydrogenase and glucose-6-phosphate dehydrogenase in purified cell suspensions and single cells from the uterine cervix in cervical intraepithelial neoplasia. Br. J. Cancer 1992, 66, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Galleggiante, V.; Rutigliano, M.; Sanguedolce, F.; Cagiano, S.; Bufo, P.; Lastilla, G.; Maiorano, E.; Ribatti, D.; Giglio, A. Metabolomic profile of glycolysis and the pentose phosphate pathway identifies the central role of glucose-6-phosphate dehydrogenase in clear cell-renal cell carcinoma. Oncotarget 2015, 6, 13371. [Google Scholar] [CrossRef]
- Sukhatme, V.P.; Chan, B. Glycolytic cancer cells lacking 6-phosphogluconate dehydrogenase metabolize glucose to induce senescence. FEBS Lett. 2012, 586, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Vander Jagt, D.L.; Semenza, G.L.; Dang, C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef]
- Mazurek, S.; Boschek, C.B.; Hugo, F.; Eigenbrodt, E. Pyruvate kinase type M2 and its role in tumor growth and spreading. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 300–308. [Google Scholar]
- DeBerardinis, R.J.; Sayed, N.; Ditsworth, D.; Thompson, C.B. Brick by brick: Metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 2008, 18, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Duncan, R.E.; Jaworski, K.; Sarkadi-Nagy, E.; Sook Sul, H. Triacylglycerol metabolism in adipose tissue. Future Lipidol. 2007, 2, 229–237. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Models Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef]
- Harris, D.M.; Li, L.; Chen, M.; Lagunero, F.T.; Go, V.L.W.; Boros, L.G. Diverse mechanisms of growth inhibition by luteolin, resveratrol, and quercetin in MIA PaCa-2 cells: A comparative glucose tracer study with the fatty acid synthase inhibitor C75. Metabolomics 2012, 8, 201–210. [Google Scholar] [CrossRef]
- Buckley, D.; Duke, G.; Heuer, T.S.; O’Farrell, M.; Wagman, A.S.; McCulloch, W.; Kemble, G. Fatty acid synthase–modern tumor cell biology insights into a classical oncology target. Pharmacol. Ther. 2017, 177, 23–31. [Google Scholar] [CrossRef]
- Flavin, R.; Peluso, S.; Nguyen, P.L.; Loda, M. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010, 6, 551–562. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, H.; Liu, Y.; Guo, A.; Xu, X.; Qu, X.; Wang, S.; Zhao, J.; Li, Y.; Cao, Y. Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/Akt/NF-κB signaling pathway. Nutrients 2015, 7, 4383–4402. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aljarbou, A.N.; Aldebasi, Y.H.; Faisal, S.M.; Khan, M.A. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014, 38, 765–772. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y. mTORC1 signaling in hepatic lipid metabolism. Protein Cell 2018, 9, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Chai, R.; Fu, H.; Zheng, Z.; Liu, T.; Ji, S.; Li, G. Resveratrol inhibits proliferation and migration through SIRT1 mediated post-translational modification of PI3K/AKT signaling in hepatocellular carcinoma cells. Mol. Med. Rep. 2017, 16, 8037–8044. [Google Scholar] [CrossRef]
- Jing, X.; Cheng, W.; Wang, S.; Li, P.; He, L. Resveratrol induces cell cycle arrest in human gastric cancer MGC803 cells via the PTEN-regulated PI3K/Akt signaling pathway. Oncol. Rep. 2016, 35, 472–478. [Google Scholar] [CrossRef]
- Long, J.; Zhang, C.-J.; Zhu, N.; Du, K.; Yin, Y.-F.; Tan, X.; Liao, D.-F.; Qin, L. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018, 8, 778. [Google Scholar]
- Wong, D.H.; Villanueva, J.A.; Cress, A.B.; Sokalska, A.; Ortega, I.; Duleba, A.J. Resveratrol inhibits the mevalonate pathway and potentiates the antiproliferative effects of simvastatin in rat theca-interstitial cells. Fertil. Steril. 2011, 96, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [CrossRef]
- Fan, L.; Cacicedo, J.M.; Ido, Y. Activation of AMPKK-AMPK cascade by silence information regulator 2 (sir2). Diabetes 2005, 54, A383. [Google Scholar]
- Lan, F.; Weikel, K.A.; Cacicedo, J.M.; Ido, Y. Resveratrol-induced AMP-activated protein kinase activation is cell-type dependent: Lessons from basic research for clinical application. Nutrients 2017, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Zhang, X.; Yi, J.; Huang, J.; He, J.; Tao, Y. Sirtuins in metabolism, DNA repair and cancer. J. Exp. Clin. Cancer Res. 2016, 35, 182. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Blander, G.; Tse, J.G.; Krieger, M.; Guarente, L. SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol. Cell 2007, 28, 91–106. [Google Scholar] [CrossRef]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöp, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef]
- Shehzad, A.; Ha, T.; Subhan, F.; Lee, Y.S. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur. J. Nutr. 2011, 50, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef]
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef]
- Shehzad, A.; Khan, S.; Shehzad, O.; Lee, Y.S. Curcumin therapeutic promises and bioavailability in colorectal cancer. Drugs Today 2010, 46, 523–532. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef]
- Qiao, Q.; Jiang, Y.; Li, G. Inhibition of the PI3K/AKT-NF-κB pathway with curcumin enhanced radiation-induced apoptosis in human Burkitt’s lymphoma. J. Pharmacol. Sci. 2013, 121, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Du, H.; Huang, Z.; Zhang, P.; Yue, Y.; Wang, W.; Liu, W.; Zeng, J.; Ma, J.; Chen, G.; et al. Thymoquinone induces apoptosis in bladder cancer cell via endoplasmic reticulum stress-dependent mitochondrial pathway. Chem.-Biol. Interact. 2018, 292, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Hsieh, D.J.; Lin, Y.M.; Chen, L.M.; Kuo, W.W.; Huang, C.Y. Thymoquinone induces caspase-independent, autophagic cell death in CPT-11-resistant lovo colon cancer via mitochondrial dysfunction and activation of JNK and p38. J. Agric. Food Chem. 2015, 63, 1540–1546. [Google Scholar] [CrossRef]
- Pandita, A.; Kumar, B.; Manvati, S.; Vaishnavi, S.; Singh, S.K.; Bamezai, R.N. Synergistic combination of gemcitabine and dietary molecule induces apoptosis in pancreatic cancer cells and down regulates PKM2 expression. PLoS ONE 2014, 9, e107154. [Google Scholar] [CrossRef] [PubMed]
- Salim, L.Z.A.; Mohan, S.; Othman, R.; Abdelwahab, S.I.; Kamalidehghan, B.; Sheikh, B.Y.; Ibrahim, M.Y. Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules 2013, 18, 11219–11240. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.; Goldberg, A.; Brinckmann, J. Herbal Medicine. Expanded Commission E Monographs; Integrative Medicine Communications: Newton, MA, USA, 2000. [Google Scholar]
- Gardner, C.D.; Lawson, L.D.; Block, E.; Chatterjee, L.M.; Kiazand, A.; Balise, R.R.; Kraemer, H.C. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: A randomized clinical trial. Arch. Intern. Med. 2007, 167, 346–353. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, B.; Zhao, L.; Liu, Y.; Zhao, F.; Feng, J.; Jin, Y.; Sun, J.; Geng, R.; Wei, Y. Allicin inhibits proliferation and invasion in vitro and in vivo via SHP-1-mediated STAT3 signaling in cholangiocarcinoma. Cell. Physiol. Biochem. 2018, 47, 641–653. [Google Scholar] [CrossRef]
- Li, X.; Ni, J.; Tang, Y.; Wang, X.; Tang, H.; Li, H.; Zhang, S.; Shen, X. Allicin inhibits mouse colorectal tumorigenesis through suppressing the activation of STAT3 signaling pathway. Nat. Prod. Product. Res. 2019, 33, 2722–2725. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Zaitseva, G.; Padilla, C.; Puebla, A.M. Antitumoral activity of allicin in murine lymphoma L5178Y. Asian Pac. J. Cancer Prev. 2010, 11, 1241–1244. [Google Scholar]
- Zhang, Z.-M.; Zhong, N.; Gao, H.-Q.; Zhang, S.-Z.; Wei, Y.; Xin, H.; Mei, X.; Hou, H.-S.; Lin, X.-Y.; Shi, Q. Inducing apoptosis and upregulation of Bax and Fas ligand expression by allicin in hepatocellular carcinoma in Balb/c nude mice. Chin. Med. J. 2006, 119, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Pavese, J.M.; Farmer, R.L.; Bergan, R.C. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010, 29, 465–482. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An integrative overview of its mode of action, pharmacological properties, and health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017; 119, 13–22. [Google Scholar]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Russo, G.L.; Daglia, M.; Kasi, P.D.; Ravi, S.; Nabavi, S.F.; Nabavi, S.M. Understanding genistein in cancer: The “good” and the “bad” effects: A review. Food Chem. 2016, 196, 589–600. [Google Scholar] [CrossRef]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Wood, C.G. Multimodal approaches in the management of locally advanced and metastatic renal cell carcinoma: Combining surgery and systemic therapies to improve patient outcome. Clin. Cancer Res. 2007, 13, 697s–702s. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Feng, K. EGCG adjuvant chemotherapy: Current status and future perspectives. Eur. J. Med. Chem. 2023, 250, 115197. [Google Scholar] [CrossRef]
- Rather, R.A.; Bhagat, M. Cancer chemoprevention and piperine: Molecular mechanisms and therapeutic opportunities. Front. Cell Dev. Biol. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Zadorozhna, M.; Tataranni, T.; Mangieri, D. Piperine: Role in prevention and progression of cancer. Mol. Biol. Rep. 2019, 46, 5617–5629. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, U.; Jha, N.K.; Shekhawat, M.S.; Saha, S.C.; Nongdam, P.; Rengasamy, K.R.R.; Proćków, J.; Dey, A. Anticancer applications and pharmacological properties of piperidine and piperine: A comprehensive review on molecular mechanisms and therapeutic perspectives. Front. Pharmacol. 2022, 12, 772418. [Google Scholar] [CrossRef] [PubMed]
- Benayad, S.; Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Oudghiri, M.; Saad, E.M.; Duval, R.E.; Limami, Y. The promise of Piperine in cancer chemoprevention. Cancers 2023, 15, 5488. [Google Scholar] [CrossRef] [PubMed]
- Turrini, E.; Sestili, P.; Fimognari, C. Overview of the anticancer potential of the “king of spices” piper nigrum and its main constituent piperine. Toxins 2020, 12, 747. [Google Scholar] [CrossRef]
- Wei, W.T.; Lin, S.Z.; Liu, D.L.; Wang, Z.H. The distinct mechanisms of the antitumor activity of emodin in different types of cancer (Review). Oncol. Rep. 2013, 30, 2555–2562. [Google Scholar] [CrossRef]
- Hsu, S.C.; Chung, J.G. Anticancer potential of emodin. Biomed. Pharmacother. 2012, 2, 108–116. [Google Scholar] [CrossRef]
- Hu, N.; Liu, J.; Xue, X.; Li, Y. The effect of emodin on liver disease—Comprehensive advances in molecular mechanisms. Eur. J. Pharmacol. 2020, 882, 173269. [Google Scholar] [CrossRef]
- Iwanowycz, S.; Wang, J.; Hodge, J.; Wang, Y.; Yu, F.; Fan, D. Emodin Inhibits Breast Cancer Growth by Blocking the Tumor-Promoting Feedforward Loop between Cancer Cells and Macrophages. Mol. Cancer Ther. 2016, 15, 1931–1942. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Wei, X.; Zhao, Z.; Xue, J.; Liu, P. Emodin Inhibits the Epithelial to Mesenchymal Transition of Epithelial Ovarian Cancer Cells via ILK/GSK-3<i>β</i>/Slug Signaling Pathway. BioMed Res. Int. 2016, 2016, 6253280. [Google Scholar]
- Galiardi-Campoy, A.E.B.; Machado, F.C.; Carvalho, T.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effects of photodynamic therapy mediated by emodin in cervical carcinoma cells. Photodiagn. Photodyn. Ther. 2021, 35, 102394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, J.; Sheng, A.; Huang, S.; Tang, Y.; Ma, S.; Hong, G. Emodin Inhibits the proliferation of MCF-7 human breast cancer cells through activation of aryl hydrocarbon receptor (AhR). Front. Pharmacol. 2021, 11, 622046. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-j.; Meng, X.-y.; Chen, J.-f.; Wang, K.-y.; Zhou, C.; Yu, R.; Ma, Q. Emodin induced necroptosis and inhibited glycolysis in the renal cancer cells by enhancing ROS. Oxidative Med. Cell. Longev. 2021, 2021, 8840590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hodge, J.; Wang, J.; Wang, Y.; Wang, L.; Singh, U.; Li, Y.; Yao, Y.; Wang, D.; Ai, W.; et al. Emodin reduces Breast Cancer Lung Metastasis by suppressing Macrophage-induced Breast Cancer Cell Epithelial-mesenchymal transition and Cancer Stem Cell formation. Theranostics 2020, 10, 8365–8381. [Google Scholar] [CrossRef]
- Dong, X.; Ni, B.; Fu, J.; Yin, X.; You, L.; Leng, X.; Liang, X.; Ni, J. Emodin induces apoptosis in human hepatocellular carcinoma HepaRG cells via the mitochondrial caspase-dependent pathway. Oncol. Rep. 2018, 40, 1985–1993. [Google Scholar] [CrossRef]
- Ma, Q.; Ding, Y.; Wu, Z.; Li, Y. Antitumor effects of emodin in CACO-2 human colon carcinoma cells are mediated via apoptosis, cell cycle arrest and downregulation of PI3K/AKT signalling pathway. J. Buon 2018, 23, 587–591. [Google Scholar]
- Dai, G.; Ding, K.; Cao, Q.; Xu, T.; He, F.; Liu, S.; Ju, W. Emodin suppresses growth and invasion of colorectal cancer cells by inhibiting VEGFR2. Eur. J. Pharmacol. 2019, 859, 172525. [Google Scholar] [CrossRef]
- Lin, W.; Zhong, M.; Yin, H.; Chen, Y.; Cao, Q.; Wang, C.; Ling, C. Emodin induces hepatocellular carcinoma cell apoptosis through MAPK and PI3K/AKT signaling pathways in vitro and in vivo. Oncol. Rep. 2016, 36, 961–967. [Google Scholar] [CrossRef]
- Su, J.; Yan, Y.; Qu, J.; Xue, X.; Liu, Z.; Cai, H. Emodin induces apoptosis of lung cancer cells through ER stress and the TRIB3/NF-κB pathway. Oncol. Rep. 2017, 37, 1565–1572. [Google Scholar] [CrossRef]
- Ma, L.; Chen, K.; Jiang, K.; Deng, G.; Jiang, P.; Shao, J.; Yu, Z. Emodin inhibits the proliferation and invasion of bladder cancer cells via down-regulating Notch1. Int. J. Clin. Exp. Pathol. 2017, 10, 9452–9459. [Google Scholar]
- Ghantous, A.; Sinjab, A.; Herceg, Z.; Darwiche, N. Parthenolide: From plant shoots to cancer roots. Drug Discov. Today 2013, 18, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Wiedhopf, R.M.; Young, M.; Bianchi, E.; Cole, J.R. Tumor Inhibitory Agent from Magnolia grandiflora (Magnoliaceae) I: Parthenolide. J. Pharm. Sci. 1973, 62, 345. [Google Scholar] [CrossRef] [PubMed]
- Nakshatri, H.; Sweeney, C.J. Use of Parthenolide to Inhibit Cancer. U.S. Patent No. 6,890,946, 10 May 2005. [Google Scholar]
- Araújo, T.G.; Vecchi, L.; Lima, P.; Ferreira, E.A.; Campos, I.M.; Brandão, D.C.; Guimarães, G.S.; Ribeiro, M.A.; Filho, A. Parthenolide and its Analogues: A New Potential Strategy for the Treatment of Triple-Negative Breast Tumors. Curr. Med. Chem. 2020, 27, 6628–6642. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Semin. Cancer Biol. 2016, 40–41, 192–208. [Google Scholar] [CrossRef]
- Freund, R.R.A.; Gobrecht, P.; Fischer, D.; Arndt, H.-D. Advances in chemistry and bioactivity of parthenolide. Nat. Prod. Product. Rep. 2020, 37, 541–565. [Google Scholar] [CrossRef]
- Koprowska, K.; Czyz, M. Molecular mechanisms of parthenolide’s action: Old drug with a new face. Postep. Hig. Med. Dosw. 2010, 64, 100–114. [Google Scholar]
- Kreuger, M.R.; Grootjans, S.; Biavatti, M.W.; Vandenabeele, P.; D’Herde, K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: Focus on parthenolide. Anticancer Drugs 2012, 23, 883–896. [Google Scholar] [CrossRef]
- Mathema, V.B.; Koh, Y.S.; Thakuri, B.C.; Sillanpää, M. Parthenolide, a sesquiterpene lactone, expresses multiple anti-cancer and anti-inflammatory activities. Inflammation 2012, 35, 560–565. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, J.; Kinghorn, A.D. Development of Anticancer Agents from Plant-Derived Sesquiterpene Lactones. Curr. Med. Chem. 2016, 23, 2397–2420. [Google Scholar] [CrossRef]
- Siveen, K.S.; Uddin, S.; Mohammad, R.M. Targeting acute myeloid leukemia stem cell signaling by natural products. Mol. Cancer 2017, 16, 13. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as Cooperating Agent for Anti-Cancer Treatment of Various Malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef]
- Wyrębska, A.; Gach, K.; Janecka, A. Combined effect of parthenolide and various anti-cancer drugs or anticancer candidate substances on malignant cells in vitro and in vivo. Mini Rev. Med. Chem. 2014, 14, 222–228. [Google Scholar] [CrossRef]
- Marino, S.; Bishop, R.T.; Carrasco, G.; Logan, J.G.; Li, B.; Idris, A.I. Pharmacological Inhibition of NFκB Reduces Prostate Cancer Related Osteoclastogenesis In Vitro and Osteolysis Ex Vivo. Calcif. Tissue Int. 2019, 105, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Wang, L.; Wang, X.Y.; Sun, J.; Yin, X.Y.; Hou, J.X.; Chen, J.; Xie, B.; Wei, H.L. Suppression of Aberrant Activation of NF-κB Pathway in Drug-resistant Leukemia Stem Cells Contributes to Parthenolide-potentiated Reversal of Drug Resistance in Leukemia. J. Cancer 2021, 12, 5519–5529. [Google Scholar] [CrossRef]
- Berdan, C.A.; Ho, R.; Lehtola, H.S.; To, M.; Hu, X.; Huffman, T.R.; Petri, Y.; Altobelli, C.R.; Demeulenaere, S.G.; Olzmann, J.A.; et al. Parthenolide Covalently Targets and Inhibits Focal Adhesion Kinase in Breast Cancer Cells. Cell Chem. Biol. 2019, 26, 1027–1035.e22. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, L.; Yang, Q.; Duan, A.; Ju, X.; Cai, B.; Chen, L.; An, T.; Li, Y. Parthenolide inhibits ubiquitin-specific peptidase 7 (USP7), Wnt signaling, and colorectal cancer cell growth. J. Biol. Chem. 2020, 295, 3576–3589. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Xiao, Y.; Ma, J.; Ou, W.; Wang, H.; Wu, J.; Tang, J.; Zhang, B.; Liao, X.; Yang, D.; et al. Parthenolide Inhibits Angiogenesis in Esophageal Squamous Cell Carcinoma Through Suppression of VEGF. Onco Targets Ther. 2020, 13, 7447–7458. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Z.; Zhang, D.; Wang, J. Metabonomic study of the intervention effects of Parthenolide on anti-thyroid cancer activity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1150, 122179. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, W.; Wen, G.; Yu, B.; Xu, F.; Gan, X.; Tang, J.; Zeng, Q.; Zhu, L.; Chen, C.; et al. Parthenolide inhibits human lung cancer cell growth by modulating the IGF-1R/PI3K/Akt signaling pathway. Oncol. Rep. 2020, 44, 1184–1193. [Google Scholar] [CrossRef]
- Liu, D.; Han, Y.; Liu, L.; Ren, X.; Zhang, H.; Fan, S.; Qin, T.; Li, L. Parthenolide inhibits the tumor characteristics of renal cell carcinoma. Int. J. Oncol. 2021, 58, 100–110. [Google Scholar] [CrossRef]
- Çetinkaya, M.; Baran, Y. Therapeutic Potential of Luteolin on Cancer. Vaccines 2023, 11, 554. [Google Scholar] [CrossRef]
- Formica, J.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, P.; Martini, C.; Bulzomi, P.; Leone, S.; Bolli, A.; Pallottini, V.; Marino, M. Quercetin-induced apoptotic cascade in cancer cells: Antioxidant versus estrogen receptor α-dependent mechanisms. Mol. Nutr. Food Res. 2009, 53, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; Chaffaut, L.; Mennen, L. An online comprehensive database on polyphenol contents in foods. Database Phenol.-Explor. 2010, 2010, bap024. [Google Scholar]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef]
- Araújo, K.C.F.; de MB Costa, E.M.; Pazini, F.; Valadares, M.C.; de Oliveira, V. Bioconversion of quercetin and rutin and the cytotoxicity activities of the transformed products. Food Chem. Toxicol. 2013, 51, 93–96. [Google Scholar] [CrossRef]
- Men, K.; Duan, X.; Wei Wei, X.; Ling Gou, M.; Juan Huang, M.; Juan Chen, L.; Yong Qian, Z.; Quan Wei, Y. Nanoparticle-delivered quercetin for cancer therapy. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2014, 14, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Jana, N.; Břetislav, G.; Pavel, S.; Pavla, U. Potential of the flavonoid quercetin to prevent and treat cancer-current status of research. Klin. Onkol. Cas. Casopis. Ceske A Slov. Onkol. Spol. 2018, 31, 184–190. [Google Scholar]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Gaza, H.V.; Kashuba, E. Role of the RB-interacting proteins in stem cell biology. Adv. Cancer Res. 2016, 131, 133–157. [Google Scholar] [PubMed]
- Ren, M.X.; Deng, X.H.; Ai, F.; Yuan, G.Y.; Song, H.Y. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp. Ther. Med. 2015, 10, 579–583. [Google Scholar] [CrossRef]
- Boly, R.; Gras, T.; Lamkami, T.; Guissou, P.; Serteyn, D.; Kiss, R.; Dubois, J. Quercetin inhibits a large panel of kinases implicated in cancer cell biology. Int. J. Oncol. 2011, 38, 833–842. [Google Scholar]
- Kim, H.; Moon, J.Y.; Ahn, K.S.; Cho, S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxidative Med. Cell. Longev. 2013, 2013, 596496. [Google Scholar] [CrossRef]
- Wang, K.; Liu, R.; Li, J.; Mao, J.; Lei, Y.; Wu, J.; Zeng, J.; Zhang, T.; Wu, H.; Chen, L. Quercetin induces protective autophagy in gastric cancer cells: Involvement of Akt-mTOR-and hypoxia-induced factor 1α-mediated signaling. Autophagy 2011, 7, 966–978. [Google Scholar] [CrossRef]
- Granato, M.; Rizzello, C.; Montani, M.S.G.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef]
- Voskas, D.; Ling, L.S.; Woodgett, J.R. Does GSK-3 provide a shortcut for PI3K activation of Wnt signalling? F1000 Biol. Rep. 2010, 2, 82. [Google Scholar] [CrossRef]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef]
- Kang, S.-Y.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Tart cherry anthocyanins inhibit tumor development in ApcMin mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003, 194, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.D.; Sreelakshmi, Y.; Sharma, R. Antioxidant ability of anthocyanins against ascorbic acid oxidation. Phytochemistry 1997, 45, 671–674. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-B.; Pan, X.-F.; Chen, J.; Cao, A.; Zhang, Y.-G.; Xia, L.; Wang, J.; Li, H.; Liu, G.; Pan, A. Combined lifestyle factors, incident cancer, and cancer mortality: A systematic review and meta-analysis of prospective cohort studies. Br. J. Cancer 2020, 122, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.-X.; Kai, K.; Li, J.-J.; Lin, S.; Terahara, N.; Wakamatsu, M.; Fujii, M.; Young, M.R.; Colburn, N. Anthocyanidins inhibit activator protein 1 activity and cell transformation: Structure–activity relationship and molecular mechanisms. Carcinogenesis 2004, 25, 29–36. [Google Scholar] [CrossRef]
- Xu, M.; Bower, K.A.; Wang, S.; Frank, J.A.; Chen, G.; Ding, M.; Wang, S.; Shi, X.; Ke, Z.; Luo, J. Cyanidin-3-glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Mol. Cancer 2010, 9, 285. [Google Scholar] [CrossRef]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Kalemba-Drożdż, M.; Cierniak, A.; Cichoń, I. Berry fruit juices protect lymphocytes against DNA damage and ROS formation induced with heterocyclic aromatic amine PhIP. J. Berry Res. 2020, 10, 95–113. [Google Scholar] [CrossRef]
- Esposito, D.; Chen, A.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J. Agric. Food Chem. 2014, 62, 7022–7028. [Google Scholar] [CrossRef]
- Peiffer, D.S.; Zimmerman, N.P.; Wang, L.-S.; Ransom, B.W.; Carmella, S.G.; Kuo, C.-T.; Siddiqui, J.; Chen, J.-H.; Oshima, K.; Huang, Y.-W. Chemoprevention of esophageal cancer with black raspberries, their component anthocyanins, and a major anthocyanin metabolite, protocatechuic acid. Cancer Prev. Res. 2014, 7, 574–584. [Google Scholar] [CrossRef]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin-and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer 2005, 113, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Suppression of inflammatory responses by black rice extract in RAW 264.7 macrophage cells via downregulation of NF-kB and AP-1 signaling pathways. Asian Pac. J. Cancer Prev. 2015, 16, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.S.; Zancan, P.; Marcondes, M.C.; Ramos-Santos, L.; Meyer-Fernandes, J.R.; Sola-Penna, M.; Da Silva, D. Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie 2013, 95, 1336–1343. [Google Scholar] [CrossRef]
- Mollerup, S.; Øvrebø, S.; Haugen, A. Lung carcinogenesis: Resveratrol modulates the expression of genes involved in the metabolism of PAH in human bronchial epithelial cells. Int. J. Cancer 2001, 92, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, X.; Li, N.; Liu, H.; Dong, Q.; Zhang, J.; Yang, C.; Liu, Y.; Liang, Q.; Zhang, S. Resveratrol inhibits Hexokinases II mediated glycolysis in non-small cell lung cancer via targeting Akt signaling pathway. Exp. Cell Res. 2016, 349, 320–327. [Google Scholar] [CrossRef]
- Dasari, S.K.; Bialik, S.; Levin-Zaidman, S.; Levin-Salomon, V.; Merrill, A.H.; Futerman, A.H.; Kimchi, A. Signalome-wide RNAi screen identifies GBA1 as a positive mediator of autophagic cell death. Cell Death Differ. 2017, 24, 1288–1302. [Google Scholar] [CrossRef]
- Vanamala, J.; Radhakrishnan, S.; Reddivari, L.; Bhat, V.B.; Ptitsyn, A. Resveratrol suppresses human colon cancer cell proliferation and induces apoptosis via targeting the pentose phosphate and the talin-FAK signaling pathways-A proteomic approach. Proteome Sci. 2011, 9, 49. [Google Scholar] [CrossRef]
- Jung, K.-H.; Lee, J.H.; Quach, C.H.T.; Paik, J.-Y.; Oh, H.; Park, J.W.; Lee, E.J.; Moon, S.-H.; Lee, K.-H. Resveratrol suppresses cancer cell glucose uptake by targeting reactive oxygen species–mediated hypoxia-inducible factor-1α activation. J. Nucl. Med. 2013, 54, 2161–2167. [Google Scholar] [CrossRef]
- Buhrmann, C.; Shayan, P.; Popper, B.; Goel, A.; Shakibaei, M. Sirt1 is required for resveratrol-mediated chemopreventive effects in colorectal cancer cells. Nutrients 2016, 8, 145. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Z.; Zhang, X. Resveratrol induces apoptosis in murine prostate cancer cells via hypoxia-inducible factor 1-alpha (HIF-1α)/reactive oxygen species (ROS)/P53 signaling. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 8970. [Google Scholar] [CrossRef]
- Mitani, T.; Harada, N.; Tanimori, S.; NAkANO, Y.; Inui, H.; Yamaji, R. Resveratrol inhibits hypoxia-inducible factor-1α-mediated androgen receptor signaling and represses tumor progression in castration-resistant prostate cancer. J. Nutr. Sci. Vitaminol. 2014, 60, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Roman, V.; Billard, C.; Kern, C.; Ferry-Dumazet, H.; Izard, J.C.; Mohammad, R.; Mossalayi, D.M.; Kolb, J.P. Analysis of resveratrol-induced apoptosis in human B-cell chronic leukaemia. Br. J. Haematol. 2002, 117, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, X.; Xiao, X.; Ma, Q.; Li, W. Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways. Int. J. Oncol. 2016, 49, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Srivani, G.; Behera, S.K.; Dariya, B.; Aliya, S.; Alam, A.; Nagaraju, G.P. Resveratrol binds and inhibits transcription factor HIF-1α in pancreatic cancer. Exp. Cell Res. 2020, 394, 112126. [Google Scholar] [CrossRef]
- Zhou, C.; Qian, W.; Ma, J.; Cheng, L.; Jiang, Z.; Yan, B.; Li, J.; Duan, W.; Sun, L.; Cao, J. Resveratrol enhances the chemotherapeutic response and reverses the stemness induced by gemcitabine in pancreatic cancer cells via targeting SREBP 1. Cell Prolif. 2019, 52, e12514. [Google Scholar] [CrossRef]
- Sayd, S.; Thirant, C.; El-Habr, E.A.; Lipecka, J.; Dubois, L.G.; Bogeas, A.; Tahiri-Jouti, N.; Chneiweiss, H.; Junier, M.-P. Sirtuin-2 activity is required for glioma stem cell proliferation arrest but not necrosis induced by resveratrol. Stem Cell Rev. Rep. 2014, 10, 103–113. [Google Scholar] [CrossRef]
- Akkoç, Y.; Berrak, Ö.; Arısan, E.D.; Obakan, P.; Çoker-Gürkan, A.; Palavan-Ünsal, N. Inhibition of PI3K signaling triggered apoptotic potential of curcumin which is hindered by Bcl-2 through activation of autophagy in MCF-7 cells. Biomed. Pharmacother. 2015, 71, 161–171. [Google Scholar] [CrossRef]
- Jin, H.; Qiao, F.; Wang, Y.; Xu, Y.; Shang, Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol. Rep. 2015, 34, 2782–2789. [Google Scholar] [CrossRef]
- Camacho-Barquero, L.; Villegas, I.; Sánchez-Calvo, J.M.; Talero, E.; Sánchez-Fidalgo, S.; Motilva, V.; Alarcón de la Lastra, C. Curcumin, a Curcuma longa constituent, acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis. Int. Immunopharmacol. 2007, 7, 333–342. [Google Scholar] [CrossRef]
- Ning, L.; Wentworth, L.; Chen, H.; Weber, S.M. Down-regulation of Notch1 signaling inhibits tumor growth in human hepatocellular carcinoma. Am. J. Transl. Res. 2009, 1, 358–366. [Google Scholar]
- Koo, J.Y.; Kim, H.J.; Jung, K.O.; Park, K.Y. Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J. Med. Food 2004, 7, 117–121. [Google Scholar] [CrossRef]
- Cort, A.; Ozben, T.; Melchiorre, M.; Chatgilialoglu, C.; Ferreri, C.; Sansone, A. Effects of bleomycin and antioxidants on the fatty acid profile of testicular cancer cell membranes. Biochim. Biophys. Acta 2016, 1858, 434–441. [Google Scholar] [CrossRef]
- Rawat, N.; Alhamdani, A.; McAdam, E.; Cronin, J.; Eltahir, Z.; Lewis, P.; Griffiths, P.; Baxter, J.N.; Jenkins, G.J. Curcumin abrogates bile-induced NF-κB activity and DNA damage in vitro and suppresses NF-κB activity whilst promoting apoptosis in vivo, suggesting chemopreventative potential in Barrett’s oesophagus. Clin. Transl. Oncol. 2012, 14, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Zanotto-Filho, A.; Braganhol, E.; Edelweiss, M.I.; Behr, G.A.; Zanin, R.; Schröder, R.; Simões-Pires, A.; Battastini, A.M.; Moreira, J.C. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J. Nutr. Biochem. 2012, 23, 591–601. [Google Scholar] [CrossRef]
- Paramasivam, A.; Sambantham, S.; Shabnam, J.; Raghunandhakumar, S.; Anandan, B.; Rajiv, R.; Vijayashree Priyadharsini, J.; Jayaraman, G. Anti-cancer effects of thymoquinone in mouse neuroblastoma (Neuro-2a) cells through caspase-3 activation with down-regulation of XIAP. Toxicol. Lett. 2012, 213, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Tyagi, G.; Kaur, P.; Pradhan, S.; Rajam, M.V.; Srivastava, T. Allicin overcomes hypoxia mediated cisplatin resistance in lung cancer cells through ROS mediated cell death pathway and by suppressing hypoxia inducible factors. Cell Physiol. Biochem. 2020, 54, 748–766. [Google Scholar] [PubMed]
- Zou, X.; Liang, J.; Sun, J.; Hu, X.; Lei, L.; Wu, D.; Liu, L. Allicin sensitizes hepatocellular cancer cells to anti-tumor activity of 5-fluorouracil through ROS-mediated mitochondrial pathway. J. Pharmacol. Sci. 2016, 131, 233–240. [Google Scholar] [CrossRef]
- He, W.; Fu, Y.; Zheng, Y.; Wang, X.; Liu, B.; Zeng, J. Diallyl thiosulfinate enhanced the anti-cancer activity of dexamethasone in the side population cells of multiple myeloma by promoting miR-127-3p and deactivating the PI3K/AKT signaling pathway. BMC Cancer 2021, 21, 125. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Liu, X.; Shen, D.; Qiu, Y.; Zhang, X.; Song, J. Influence of pH, concentration and light on stability of allicin in garlic (Allium sativum L.) aqueous extract as measured by UPLC. J. Sci. Food Agric. 2015, 95, 1838–1844. [Google Scholar] [CrossRef]
- Lin, X.-L.; Liu, Y.; Liu, M.; Hu, H.; Pan, Y.; Fan, X.-J.; Hu, X.-M.; Zou, W.-W. Inhibition of hydrogen peroxide-induced human umbilical vein endothelial cells aging by allicin depends on Sirtuin1 activation. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 563. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, Z.; Wang, J.; Feng, X.; Hua, W.; Luo, R.; Wang, B.; Liao, Z.; Ma, L.; Li, G. Allicin attenuated advanced oxidation protein product-induced oxidative stress and mitochondrial apoptosis in human nucleus pulposus cells. Oxidative Med. Cell. Longev. 2020, 2020, 6685043. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Zhao, J.; Jiang, D. Allicin inhibits oxidative stress-induced mitochondrial dysfunction and apoptosis by promoting PI3K/AKT and CREB/ERK signaling in osteoblast cells. Exp. Ther. Med. 2016, 11, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, D. Allicin protects traumatic spinal cord injury through regulating the HSP70/Akt/iNOS pathway in mice. Mol. Med. Rep. 2016, 14, 3086–3092. [Google Scholar] [CrossRef]
- Cheng, B.; Li, T.; Li, F. Use of network pharmacology to investigate the mechanism by which allicin ameliorates lipid metabolism disorder in HepG2 cells. Evid.-Based Complement. Altern. Med. 2021, 2021, 3956504. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.; Sheng, Y.; Xu, J.; Yang, C.; Zheng, S.; Liu, J.; Li, H.; Ge, J.; Yang, M. Allicin regulates energy homeostasis through brown adipose tissue. IScience 2020, 23, 101113. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Sharma, M.; Tiwari, M. Inhibitory effect of novel diallyldisulfide analogs on HMG-CoA reductase expression in hypercholesterolemic rats: CREB as a potential upstream target. Life Sci. 2009, 85, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, Q.; Wang, X.; Yang, X.; Wang, X.; Huang, Z.; Jiao, Y.; Wang, J. Quantitative phosphoproteomics reveals genistein as a modulator of cell cycle and DNA damage response pathways in triple-negative breast cancer cells. Int. J. Oncol. 2016, 48, 1016–1028. [Google Scholar] [CrossRef]
- Tian, T.; Li, J.; Li, B.; Wang, Y.; Li, M.; Ma, D.; Wang, X. Genistein exhibits anti-cancer effects via down-regulating FoxM1 in H446 small-cell lung cancer cells. Tumor Biol. 2014, 35, 4137–4145. [Google Scholar] [CrossRef]
- Wang, S.; Wei, H.; Cai, M.; Lu, Y.; Hou, W.; Yang, Q.; Dong, H.; Xiong, L. Genistein attenuates brain damage induced by transient cerebral ischemia through up-regulation of ERK activity in ovariectomized mice. Int. J. Biol. Sci. 2014, 10, 457. [Google Scholar] [CrossRef]
- Dong, X.; Xu, W.; Sikes, R.A.; Wu, C. Combination of low dose of genistein and daidzein has synergistic preventive effects on isogenic human prostate cancer cells when compared with individual soy isoflavone. Food Chem. 2013, 141, 1923–1933. [Google Scholar] [CrossRef]
- Gao, F.; Wei, D.; Bian, T.; Xie, P.; Zou, J.; Mu, H.; Zhang, B.; Zhou, X. Genistein attenuated allergic airway inflammation by modulating the transcription factors T-bet, GATA-3 and STAT-6 in a murine model of asthma. Pharmacology 2012, 89, 229–236. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhang, G.-Q.; Yang, Y.; Zhang, C.-Y.; Fu, R.-X.; Yang, Y.-M. Genistein induces G2/M arrest in gastric cancer cells by increasing the tumor suppressor PTEN expression. Nutr. Cancer 2013, 65, 1034–1041. [Google Scholar] [CrossRef]
- Morris, C.; Thorpe, J.; Ambrosio, L.; Santin, M. The soybean isoflavone genistein induces differentiation of MG63 human osteosarcoma osteoblasts. J. Nutr. 2006, 136, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Borcan, F.; Bojin, F.; Zupko, I.; Dehelean, C. Effect of the isoflavone genistein on tumor size, metastasis potential and melanization in a B16 mouse model of murine melanoma. Nat. Prod. Product. Commun. 2013, 8, 1934578X1300800318. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Yang, J.; Yi, P.; Xu, P.; Yi, M.; Peng, W. Integrated transcriptomic and metabolomic analyses to characterize the anti-cancer effects of (−)-epigallocatechin-3-gallate in human colon cancer cells. Toxicol. Appl. Pharmacol. 2020, 401, 115100. [Google Scholar] [CrossRef] [PubMed]
- Moradzadeh, M.; Hosseini, A.; Erfanian, S.; Rezaei, H. Epigallocatechin-3-gallate promotes apoptosis in human breast cancer T47D cells through down-regulation of PI3K/AKT and Telomerase. Pharmacol. Rep. 2017, 69, 924–928. [Google Scholar] [CrossRef]
- Yokoyama, M.; Noguchi, M.; Nakao, Y.; Pater, A.; Iwasaka, T. The tea polyphenol, (−)-epigallocatechin gallate effects on growth, apoptosis, and telomerase activity in cervical cell lines. Gynecol. Oncol. 2004, 92, 197–204. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, P.; Gao, F.; Yang, J.; Yao, K. Effects of epigallocatechin gallate on the proliferation and apoptosis of the nasopharyngeal carcinoma cell line CNE2. Exp. Ther. Med. 2014, 8, 1783–1788. [Google Scholar] [CrossRef]
- Yokoyama, M.; Noguchi, M.; Nakao, Y.; Ysunaga, M.; Yamasaki, F.; Iwasaka, T. Antiproliferative effects of the major tea polyphenol, (−)-epigallocatechin gallate and retinoic acid in cervical adenocarcinoma. Gynecol. Oncol. 2008, 108, 326–331. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef]
- Samykutty, A.; Shetty, A.V.; Dakshinamoorthy, G.; Bartik, M.M.; Johnson, G.L.; Webb, B.; Zheng, G.; Chen, A.; Kalyanasundaram, R.; Munirathinam, G. Piperine, a bioactive component of pepper spice exerts therapeutic effects on androgen dependent and androgen independent prostate cancer cells. PLoS ONE 2013, 8, e65889. [Google Scholar] [CrossRef]
- Si, L.; Yang, R.; Lin, R.; Yang, S. Piperine functions as a tumor suppressor for human ovarian tumor growth via activation of JNK/p38 MAPK-mediated intrinsic apoptotic pathway. Biosci. Rep. 2018, 38, BSR20180503. [Google Scholar] [CrossRef]
- Yaffe, P.B.; Power Coombs, M.R.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinog. 2015, 54, 1070–1085. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xu, J.; Liao, H.; Li, L.; Pan, L. Piperine induces apoptosis of lung cancer A549 cells via p53-dependent mitochondrial signaling pathway. Tumor Biol. 2014, 35, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wen, S.; Chen, G.; Wang, S. Antiproliferative potential of piperine and curcumin in drug-resistant human leukemia cancer cells are mediated via autophagy and apoptosis induction, S-phase cell cycle arrest and inhibition of cell invasion and migration. J. BUON 2020, 25, 401–406. [Google Scholar]
- Zhang, Y.; Pu, W.; Bousquenaud, M.; Cattin, S.; Zaric, J.; Sun, L.-K.; Rüegg, C. Emodin inhibits inflammation, carcinogenesis, and cancer progression in the AOM/DSS model of colitis-associated intestinal tumorigenesis. Front. Oncol. 2021, 10, 564674. [Google Scholar] [CrossRef]
- Liu, M.; Yang, Y.; Liu, D.; Cao, Y.; Li, Y. Parthenolide increases the sensitivity of gastric cancer cells to chemotherapy. J. Tradit. Chin. Med. 2020, 40, 908–916. [Google Scholar]
- Che, S.T.; Bie, L.; Li, X.; Qi, H.; Yu, P.; Zuo, L. Parthenolide inhibits the proliferation and induces the apoptosis of human uveal melanoma cells. Int. J. Ophthalmol. 2019, 12, 1531–1538. [Google Scholar] [CrossRef]
- Singh Tuli, H.; Rath, P.; Chauhan, A.; Sak, K.; Aggarwal, D.; Choudhary, R.; Sharma, U.; Vashishth, K.; Sharma, S.; Kumar, M. Luteolin, a potent anticancer compound: From chemistry to cellular interactions and synergetic perspectives. Cancers 2022, 14, 5373. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Matić, I.Z.; Mraković, A.; Rakočević, Z.; Stoiljković, M.; Pavlović, V.B.; Momić, T. Anticancer effect of novel luteolin capped gold nanoparticles selectively cytotoxic towards human cervical adenocarcinoma HeLa cells: An in vitro approach. J. Trace Elem. Med. Biol. 2023, 80, 127286. [Google Scholar] [CrossRef]
- Lei, C.-S.; Hou, Y.-C.; Pai, M.-H.; Lin, M.-T.; Yeh, S.-L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: In vitro and in vivo studies. J. Nutr. Biochem. 2018, 51, 105–113. [Google Scholar] [CrossRef]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Turkekul, K.; Dibirdik, I.; Doganlar, O.; Doganlar, Z.B.; Bilir, A.; Oktem, G. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed. Pharmacother. 2018, 107, 793–805. [Google Scholar] [CrossRef]
- Sturza, A.; Pavel, I.; Ancușa, S.; Danciu, C.; Dehelean, C.; Duicu, O.; Muntean, D. Quercetin exerts an inhibitory effect on cellular bioenergetics of the B164A5 murine melanoma cell line. Mol. Cell. Biochem. 2018, 447, 103–109. [Google Scholar] [CrossRef]
- Xintaropoulou, C.; Ward, C.; Wise, A.; Marston, H.; Turnbull, A.; Langdon, S.P. A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget 2015, 6, 25677. [Google Scholar] [CrossRef]
- Amorim, R.; Pinheiro, C.; Miranda-Gonçalves, V.; Pereira, H.; Moyer, M.P.; Preto, A.; Baltazar, F. Monocarboxylate transport inhibition potentiates the cytotoxic effect of 5-fluorouracil in colorectal cancer cells. Cancer Lett. 2015, 365, 68–78. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. Quercetin regresses Dalton’s lymphoma growth via suppression of PI3K/AKT signaling leading to upregulation of p53 and decrease in energy metabolism. Nutr. Cancer 2015, 67, 354–363. [Google Scholar] [CrossRef]
- Suolinna, E.; Buchsbaum, R.; Racker, E. The effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Res. 1975, 35, 1865–1872. [Google Scholar]
- Lang, D.R.; Racker, E. Effects of quercetin and F1 inhibitor on mitochondrial ATPase and energy-linked reactions in submitochondrial particles. Biochim. Biophys. Acta (BBA)-Bioenerg. 1974, 333, 180–186. [Google Scholar] [CrossRef]
- Suolinna, E.-M.; Lang, D.R.; Racker, E. Quercetin, an artificial regulator of the high aerobic glycolysis of tumor cells. J. Natl. Cancer Inst. 1974, 53, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- McCoy, G.D.; Racker, E. Protein synthesis in dextran sulfate-treated ascites tumor cells. Cancer Res. 1976, 36 Pt 1, 3346–3349. [Google Scholar]
- Hume, D.; Dean, J.; Weidemann, M.; Ferber, E. Preferential inhibition by quercetin of mitogen-stimulated thymocyte glucose transport. J. Natl. Cancer Inst. 1979, 62, 1243–1246. [Google Scholar] [PubMed]
- Belt, J.A.; Thomas, J.A.; Buchsbaum, R.N.; Racker, E. Inhibition of lactate transport and glycolysis in Ehrlich ascites tumor cells by bioflavonoids. Biochemistry 1979, 18, 3506–3511. [Google Scholar] [CrossRef] [PubMed]
- de Blas, E.; Estañ, M.C.; del Carmen Gómez de Frutos, M.; Ramos, J.; del Carmen Boyano-Adanez, M.; Aller, P. Selected polyphenols potentiate the apoptotic efficacy of glycolytic inhibitors in human acute myeloid leukemia cell lines. Regulation by protein kinase activities. Cancer Cell Int. 2016, 16, 70. [Google Scholar] [CrossRef]
- Aslan, E.; Guler, C.; Adem, S. In vitro effects of some flavonoids and phenolic acids on human pyruvate kinase isoenzyme M2. J. Enzym. Inhib. Med. Chem. 2016, 31, 314–317. [Google Scholar] [CrossRef]
- Lu, J.N.; Lee, W.S.; Nagappan, A.; Chang, S.-H.; Choi, Y.H.; Kim, H.J.; Kim, G.S.; Ryu, C.H.; Shin, S.C.; Jung, J.-M.; et al. Anthocyanins from the fruit of Vitis coignetiae Pulliat potentiate the cisplatin activity by inhibiting PI3K/Akt signaling pathways in human gastric cancer cells. J. Cancer Prev. 2015, 20, 50–56. [Google Scholar] [CrossRef]
- Kausar, H.; Jeyabalan, J.; Aqil, F.; Chabba, D.; Sidana, J.; Singh, I.P.; Gupta, R.C. Berry anthocyanidins synergistically suppress growth and invasive potential of human non-small-cell lung cancer cells. Cancer Lett. 2012, 325, 54–62. [Google Scholar] [CrossRef]
- Lage, N.N.; Layosa, M.A.A.; Arbizu, S.; Chew, B.P.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark sweet cherry (Prunus avium) phenolics enriched in anthocyanins exhibit enhanced activity against the most aggressive breast cancer subtypes without toxicity to normal breast cells. J. Funct. Foods 2020, 64, 103710. [Google Scholar] [CrossRef]
- Mazewski, C.; Kim, M.S.; Gonzalez de Mejia, E. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci. Rep. 2019, 9, 11560. [Google Scholar] [CrossRef]
- Charepalli, V.; Reddivari, L.; Radhakrishnan, S.; Vadde, R.; Agarwal, R.; Vanamala, J.K. Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J. Nutr. Biochem. 2015, 26, 1641–1649. [Google Scholar] [CrossRef]
- Longo, L.; Platini, F.; Scardino, A.; Alabiso, O.; Vasapollo, G.; Tessitore, L. Autophagy inhibition enhances anthocyanin-induced apoptosis in hepatocellular carcinoma. Mol. Cancer Ther. 2008, 7, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Ha, U.-S.; Bae, W.J.; Kim, S.J.; Yoon, B.I.; Hong, S.H.; Lee, J.Y.; Hwang, T.-K.; Hwang, S.Y.; Wang, Z.; Kim, S.W. Anthocyanin induces apoptosis of DU-145 cells in vitro and inhibits xenograft growth of prostate cancer. Yonsei Med. J. 2015, 56, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Do Thi, N.; Hwang, E.-S. Effects of black chokeberry extracts on metastasis and cell-cycle arrest in SK-Hep1 human liver cancer cell line. Asian Pac. J. Trop. Biomed. 2018, 8, 285–291. [Google Scholar]


| Natural Product | Compound Structure | Concentration Used | Model | Mechanism of Action | Reference |
|---|---|---|---|---|---|
| Resveratrol | 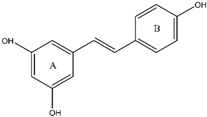 | 1, 5, 15, 50 or 100 μM | MCF-7 (breast cancer) | Resveratrol suppresses HIF-1α buildup, impairing glucose metabolism and lowering breast cancer cell survival. It also directly inhibits PFK activity, GLUT1-mediated glucose absorption, and intracellular ROS. | [179] |
| 5–150 µM (IC50 ~ 80 µM) | SKBR-3 (breast cancer) | In SKBR-3 cells, Resveratrol down-regulates FASN and HER2 genes, triggering apoptosis. Resveratrol inhibits the Akt/PI3K/mTOR pathway by suppressing Akt phosphorylation. Resveratrol inhibits FASN function, which down-regulates HER2 production. | [65] | ||
| 0, 20 or 50 μM | BEP2D cells (breast cancer) | Resveratrol inhibits glycolytic flow and GLUT1 expression by targeting ROS-mediated HIF-1α activation. | [180] | ||
| (0, 25, 50, 100) μM | NSCLC cells. (lung cancer) | Resveratrol causes A549 cancer cells to undergo autophagy through the upregulation of glucosylceramidase beta1 (GBA1), the gene linked to Gaucher disease that produces glucocerebrosidase, an enzyme that breaks down glucosylceramide into ceramide and glucose. | [181] | ||
| 200 μM | A549 cells (lung cancer) | Resveratrol modulates polycyclic aromatic hydrocarbon metabolism genes, potentially preventing lung cancer by inhibiting CYP1A1 and 1B1 enzymes and overexpressing microsomal epoxide hydrolase, altering carcinogenic metabolite synthesis. | [182] | ||
| 10 µM | PCA model (colorectal cancer) | Resveratrol targets the pyruvate dehydrogenase complex in colorectal cancer cells, modulating lipidomic activity, increasing oxidative activity, and reversing the Warburg effect by decreasing glycolysis and pentose phosphate activity. | [11] | ||
| 50–150 μM | HT-29 colon cancer cells | Resveratrol induces apoptosis by targeting the talin-FAK and pentose phosphate signaling pathways. | [183] | ||
| 50 mM | LLC, HT-29, and T47D cells (Colorectal Cancer) | Resveratrol reduces glucose absorption by targeting ROS-induced HIF-1α activation. | [184] | ||
| 1, 5, 10, 20, and 50 µM | HCT116 SW480 (Colorectal Cancer) | Resveratrol increases Sirt1 protein expression in CRC cells, but Sirt1 knockdown eliminates its inhibitory effects on cell viability and proliferation. In CRC cells, Sirt1 knockdown prevents resveratrol-induced suppression of NF-κB-dependent gene products associated with proliferation and metastasis. | [185] | ||
| 50 µM | TRAMP cells (prostate cancer) | Resveratrol promotes cell death by increasing ROS formation, expressing apoptotic biomarkers (Bax, p53, HIF-1α), and inhibiting the anti-apoptotic protein Bcl2. It induces apoptosis in prostate cancer cells through the HIF-1α/ROS/p53 signaling pathway. | [186] | ||
| (0–25) µM | LNCAP cells (prostate cancer) | Resveratrol inhibits HIF-1α, reducing nuclear β-catenin protein and suppressing the transcriptional activity of androgen receptor (AR) signaling, thereby inhibiting tumor development and inducing apoptosis in CRPC. | [187] | ||
| 50 and 100 μM | A2780 cells (ovarian cancer) | Resveratrol significantly reduces glucose absorption and lactate generation while lowering phosphorylated Akt and mTOR, which promote glucose uptake and glycolysis. | [42] | ||
| 0–50 μM | WSU-CLL and ESKOL cells (B-CLL) | Resveratrol induces apoptosis in B-cell lines and B-CLL patients by affecting annexin V binding, caspase activation, DNA fragmentation, and mitochondrial transmembrane potential. It also inhibits iNOS protein expression and NO release, showing potential for B-CLL therapy. | [188] | ||
| 80–200 µM | HepG2, Bel7402 and SMMC7721 (Hepatocellular Carcinoma) | Resveratrol induces apoptosis by inhibiting the PI3K/Akt pathway and lowering FoxO3a phosphorylation. Resveratrol activates SIRT1 and prevents post-translational changes in PI3K/Akt signaling. Resveratrol reduces cell migration and increases DLC1 phosphorylation by Akt. | [68] | ||
| 50 μM | Panc-1 cells (Pancreatic Cancer) | Resveratrol prevents Panc-1 cell proliferation induced by hyperglycemia. Resveratrol reduces hyperglycemia-induced ROS and H2O2 generation. Resveratrol inhibits the ERK and p38 MAPK pathways activated by hyperglycemia. Resveratrol prevents pancreatic cancer cell migration and invasion when exposed to H2O2. | [189] | ||
| Not mentioned | MIA Paca-2&PANC 1 (Pancreatic Cancer) | Resveratrol suppresses HIF-1α and prevents HIF-1α-mediated cellular abnormalities in Pancreatic Cancer cells, as demonstrated by MDS and in vitro studies. | [190] | ||
| 50 and 100 µM | MIA PaCa-2 (Pancreatic Cancer) | Resveratrol increases sensitivity to Gemcitabine by blocking lipid synthesis through SREBP1, reducing sphere formation, stemness, and CSC marker expression. | [191] | ||
| 6.25, 12.5, 25, 50, 100, 200, and 400 μM | MGC803 (gastric Cancer) | In MGC803 cells, resveratrol suppresses the cyclin D1 signaling pathway that is dependent on GSK3β. In MGC803 cells, resveratrol suppresses PI3K/Akt signaling. Resveratrol inhibits the PI3K/Akt pathway by controlling PTEN activity. | [68] | ||
| 0–300 µM | GSCs derived from human biopsies. (Glioblastoma Cancer) | change in cell shape following RES activation of GSC necrosis at dosages greater than 150 µM of resveratrol. Resveratrol had no effect on the cell shape or proliferation of human NSCs nor on their viability. SIRT2 activity blockage or downregulation of SIRT2 expression using siRNAs mitigated the proliferative effect of RES. | [192] | ||
| Curcumin | 0–100 μM | Wild-type and Bcl-2 + MCF-7 (breast cancer) | It has been proposed that curcumin inhibits the PI3K/Akt signaling pathway in breast cancer cells to cause apoptosis and autophagy. | [193] | |
| 5, 10, 20 and 40 µM | A549 cells (lung cancer) | Through the overexpression of microRNA-192-5p and the inhibition of the PI3K/Akt signaling pathway, curcumin decreased the growth of human non-small cell lung cancer cells and induced apoptosis. | [194] | ||
 | (CUR, 50 or 100 mg/kg p.o.) | positive model rats with TNBS-induced colitis (Colorectal cancer) | It was demonstrated that curcumin’s apoptotic effects were mediated by the production of ROS and the reduction of the transcriptional activities of JNK, P38, MAPK, and AP-1. Moreover, curcumin was found to reduce PGE2 expression, which eventually caused apoptosis and stopped the cell cycle. | [195] | |
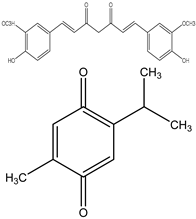 | 0–50 μM | Akt (Burkitt’s lymphoma) | Inhibition of the PI3K/Akt-dependent NF-κB pathway. | [85] | |
| 10–40 μM | HEP3B, SK-Hep-1 and SNU449 cells. (Hepatocellular Carcinoma) | Curcumin interferes with Notch-1 signaling in the HEP3B, SK-Hep-1, and SNU449 cell lines within the Notch intracellular domain. Studies on the pharmacological effects of curcumin in carcinogenic-induced HCC have been carried out both in vitro and in vivo. Through decreased expression of p21-Ras, P53, and NF-κB, curcumin protected animals against diethylnitrosamine (DENA)-induced hyperplasia and HCC. | [196] | ||
| 5 μM | AGS cells (gastric cancer) | It has been shown that curcumin acts by inhibiting the Akt/mTOR/p70S6 signal pathway and activating caspase-3, a mediator of apoptosis. | [197] | ||
| 20 μM | NTera-2 cells/human (Testicular germ cancer) | Following curcumin incubation, there was a decrease in Cis 18:2, MUFA, and PUFA and an increase in trans 16:1 and 18:2, SFA, SFA/MUFA. Trans 16:1, 20:4, and trans 18:2 were higher and lower, respectively, after bleomycin and curcumin were incubated together as opposed to bleomycin alone. | [198] | ||
| 50 μM | oesophageal cell lines (OE33) | curcumin enhanced apoptosis and inhibited bile-induced NF-κB activity and DNA damage in vivo, indicating that it may be used as a chemopreventative in the rat esophagus. | [199] | ||
| 10, 15, 30 μM | C6 glioma (Brain cancer) | Curcumin increased mitochondrial dysfunction accompanied by caspase-3 activation, downregulated Bcl-xL, and reduced PI3K/Akt and NF-κB activation. | [200] | ||
| 2, 4, 6, and 8 μM | CPT-11-R LoVo colon cancer cells | Stress-activated protein kinases JNK and p38 were found to be activated by TQ; it was thought that these kinases caused TQ-induced ACD. The 8 μM TQ cleaved caspase-3 and decreased cytochrome c. | [87] | ||
| Thymoquinone | 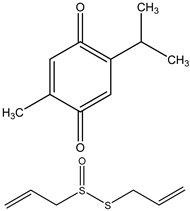 | 23, 36, 77 µM respectively | MIA PaCa-2, PANC-1, and FR2 (Pancreatic Cancer) | There was a decrease in expression of PKM2 when treated in combination doses. There was a decrease in Pro-Caspase-3 followed by a decrease in PARP. A decrease in the pyruvate kinase activity in treated cells followed by a greater decrease in combination. | [88] |
| (0, 40, 60, and 80) μM | bladder cancer cell lines (T24 and 253J) (Bladder cancer) | TQ activates caspase to cause human 1 cancer cells to undergo apoptosis. In bladder cancer, TQ causes mitochondrial malfunction and triggers the mitochondrion-mediated apoptotic pathway. TQ, in a dose-dependent way, enhances downstream molecules, including p-eIF2a and ATF4, as well as the expression of PERK, IRE1, and ATF6. As a feedback effect, GRP78 (BIP), which is linked to PERK, IRE1, and ATF6 on ER, is also deregulated. | [86] | ||
| (0–70) μM | Neuro-2a &normal cells (neuroblastoma) | TQ causes the release of cytochrome c from the mitochondria and depolarization of the mitochondrial membrane. | [201] | ||
| 0, 5, 10 and 20 μg/mL TQ | CEMss cells (ALL) | TQ raises the level of ROS on CEMss cell lines by acting as a concentration-dependent stimulator of ROS generation. Overexposure to ROS can result in oxidative damage to proteins, lipids, and DNA, which can promote tumor development or cell death. | [89] | ||
| Allicin 10 µg/mL + cisplatin 2 µg/mL for 24 h | A549 | Allicin increases apoptosis in a ROS-dependent way by enhancing the growth-inhibitory effect of cisplatin on A549 cells. Allicin induces p21, which in turn triggers ROS-dependent and p53-mediated cell cycle arrest. | [202] | ||
| Allicin | 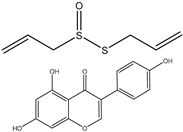 | Allicin 3–10 µg/mL + 5-FU 100–300 µg/mL for 48 h | SK-Hep-1 | In vitro, 5-fluorouracil synergistically sensitizes hepatocellular carcinoma cells when combined with allicin. Allicin increases the ROS-mediated mitochondrial pathway that triggers 5-FU-induced apoptosis. | [203] |
| Allicin 3–10 µg/mL + 5-FU 100–300 µg/mL for 48 h | BEL-7402 | Same result as above | [203] | ||
| Allicin 10 µg/mL + dexamethasone 50 µM 24–72 h | Side population sorted from RPMI-8226 and NCI-H929 cells | In SP cells, cotreatment with DATS+Dex suppresses PI3K/Akt/mTOR activation. The effects of cotreatment with diallyl thiosulfinate and dexamethasone (DATS + Dex) on the phosphoinositide 3-kinase (PI3K) pathway of multiple myeloma (MM) side population (SP) cells are reversed by silencing miR-127-3p expression. | [204] | ||
| Endothelial cells of mouse aorta | 2.5, 5, 10, 20, and 50 µg/mL | Cell viability is increased, superoxide dismutase (ROS) levels are decreased, protein levels of 8-OHDG are decreased, NF-κB is decreased, Nox4 is decreased, HIF-1α is decreased, mRNA expression of NF-κB is decreased, Nox4 is decreased, and HIF-1α is decreased by PKC pathway and the regulation of HIF-1α. | [205] | ||
| Human umbilical vein endothelial cells (HUVECs) | 2.5, 5, 10, and 20 ng/mL; 5 ng/mL | Cell viability is increased, phosphorylation and activity of Sirt1 are increased, protein expression of plasminogen activator inhibitor-1 (PAI-1) is decreased, and the aging process of HUVECs is decreased by its activity on Sirt-1. | [206] | ||
| Nucleus pulposus (NP) cells | 0, 5, 10, 20, and 40 μM; 0, 5, 10, and 20 μM | Oxidative stress and mitochondrial dysfunction are decreased by its effect on the P38-MAPK signaling pathway. | [207] | ||
| Mouse osteoblast MC3T3-E1 cells | 0, 10, 30, and 100 µg/mL | ROS production and apoptosis are decreased, and mitochondrial dysfunction is decreased by its effect on PI3K/Akt and CREB/ERK signaling pathways. | [208] | ||
| Adult BALB/c mice | 1, 5, and 10 mg/kg | BBB (Basso, Beattie, and Bresnahan) scores are increased, spinal cord water content is decreased, heat shock protein 70 (HSP70) protein levels are increased, Akt phosphorylation is increased, expression of iNOS protein is decreased, ROS levels are decreased, NADH levels are increased (HSP70, Akt, and iNOS signaling pathway). | [209] | ||
| HepG2 cells | 50, 100, and 200 μM | Palmitic acid (PA)-induced hepatocyte injury is decreased, and lipid accumulation is decreased by the PPAR signaling pathway. | [210] | ||
| High-fat-diet (HFD)-induced obesity and genetically leptin-receptor-deficient obese (Db/Db) mouse models | 4.2, 2.52, and 1.26 mg/day/mouse; 0.1, 1, 10, 50, and 100 μM; 50 μM | Body weight gain is decreased, adiposity is decreased, glucose homeostasis is maintained, insulin resistance is increased, and hepatic steatosis is decreased. Brown adipose tissue (BAT) activation, Sirt1-PGC1α-Tfam pathway, succinylation levels of UCP1 in BAT are increased, sirt5. | [211] | ||
| Adult male Wistar rats | 20 mg/kg wt | Total lipid levels are decreased, levels of oxidized low-density lipoprotein (ox-LDL) are decreased, lipid peroxidation is decreased, and NF-κB activity is decreased. | [212] | ||
| 40 μM of genistein for 0, 3, or 24 h, respectively. | Breast BCSCs (In vitro) | CD44+/CD24−/ESA+ are decreased, PI3K/Akt is increased, MEK/ERK is increased, G2/M cell cycle arrest is increased, apoptosis is increased, BRCA1 is increased, ATR complex is increased, DNA damage is increased, TNBC is decreased, cell proliferation is decreased. | [213] | ||
| Genistein | 0, 25, 50, and 75 μM exposure for 48 h. | Lung H446 (In vitro) | Apoptosis is increased, cell proliferation is decreased, FOXM1 protein is decreased, and proliferation is decreased. | [214] | |
| 10-mM. | Liver BNL CL2, Huh7, HepG2, HA22T (In vitro) | MMP-9 is decreased, NF-κB is decreased, P-1 is decreased, AP-1 is increased, JNK is decreased, ERK is decreased, NF-κB is decreased, and phosphatidylinositol/ERK3-kinase/Akt is increased. Metastasis is decreased. | [215] | ||
| 100 μM and 200 μM. | Prostate C4-2B, LNCaP (In vitro) | Cell proliferation is decreased, and apoptosis is increased. | [216] | ||
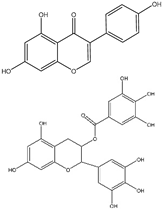 | (10−14) to (10−6) M | Brain Neuroblastoma SK-N-SH (In vitro) | Cell cycle arrest at phase G2/M is increased, proliferation is decreased, Akt is increased, CHD5 is increased, and p53 is increased. | [217] | |
| 140 mg/kg | Colon LoVo SW480, HCT116 (In vitro) | Apoptosis is increased, and NF-κB is decreased. G2/M cell cycle arrest is increased, p21waf1/cip1 is increased, and GADD45α is increased. | [218] | ||
| Range of concentrations assessed was 2.5–30 μmol/L. | Osteosarcoma MG63 osteosarcoma osteoblast cells (In vitro) | Akt is increased, and NF-κB is decreased. | [219] | ||
| 15 mg/kg/day for 15 days | Melanoma LiBr (In vivo) | Caspase-3 is decreased, and apoptosis is increased. | [220] | ||
| 10–300 μM for 48 h | Cell lines HCT-116, and SW-480 | Cancer cell growth inhibition. | [103] | ||
| EGCG | 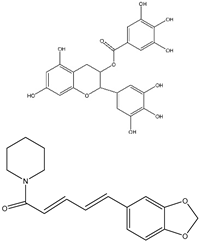 | 10, 20, 30, 40, 50, and 60 μg/mL | HT-29 cells for 24, 48, or 72 h | Reduces cell proliferation significantly by 61% after 48 h of treatment with 50 μg/mL of EGCG, relative to untreated. | [221] |
| 5 Μm EGCG with PDE3A inhibitor trequinsin | CSCs (Human) | Reduces the protein levels of FOXO3 and CD44 and upregulated cGMP expression, resulting in a significant reduction in the ability of CSCs to form colonies and spheroids. | [105] | ||
| EGCG + drug IC50 (Cisplatin) (μM) 3.48 ± 0.24 | OVCAR3 cell line | EGCG enhances cisplatin sensitivity by regulating the expression of the copper and cisplatin influx transporter CTR1 in ovary cancer. | [105] | ||
| EGCG + drug IC50 (Cisplatin) (μM) 6.14 ± 0.38 | SKOV3 cell line | EGCG enhances cisplatin sensitivity by regulating the expression of the copper and cisplatin influx transporter CTR1 in ovary cancer. | [105] | ||
| EGCG + drug IC50 (5-FU) (μM) 5 ± 0.36 | HCT-116 cell line | (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-kappaB/miR-155-5p/MDR1 pathway. | [105] | ||
| EGCG + drug IC50 (5-FU) 11 ± 0.96 | DLD1 cell line | (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-kappaB/miR-155-5p/MDR1 pathway. | [105] | ||
| EGCG + drug IC50 (Doxorubicin) 0.66 ± 0.14 (μM) | BEL-7404 Cell line | Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. | [105] | ||
| EGCG + drug IC50 (Doxorubicin) 3.28 ± 0.34 (μM) | BEL-7404/DOX cell line | Green tea catechins augment the antitumor activity of doxorubicin in an in vivo mouse model for chemoresistant liver cancer. | [105] | ||
| EGCG + drug IC50 (Doxorubicin) 29.89 ± 6.09 (μM) | CEM/ADR 5000 cell line | Modulation of multidrug-resistant cancer cells by EGCG, tannic acid, and curcumin. | [105] | ||
| EGCG + drug IC50 (Tamoxifen) 5.8 ± 0.4 (μM) | MCF-7/TAM cell line | Combination of siRNA-directed gene silencing with epigallocatechin-3-gallate (EGCG) reverses drug resistance in human breast cancer cells. | [105] | ||
| 80 µM | T47D breast cancer cells | A significant decrease in hTERT gene expression causing apoptosis was observed. | [222] | ||
| 100 µM | HEC-18, HEC-18T, HEN-18, HEN-18S | Growth inhibition greater than 90% and induction of apoptosis was observed in HEC-18 and HEN-18. Telomerase was inhibited in all 4 cells. | [223] | ||
| 100, 200 µg/mL | Nasopharyngeal carcinoma cell line CNE2 | Prevents CNE2 cells from proliferating, causes cell cycle block, apoptosis of the cells was promoted, and downregulation of the mRNA and protein expression of hTERT as well as c-Myc protein. | [224] | ||
| EGCG: 100 µM RA: 1 µM | HeLa and TMCC-1 | Combination treatment caused inhibition of telomerase, induction of apoptosis, and prevented cell proliferation. | [225] | ||
| (50, 100 or 150 µM) of piperine for 72 h. | Breast cancer (Triple-negative breast cancer cells) (MDA-MB-231 and MDA-MB-468) in vitro | Inhibits the growth of p53-deficient cell lines by inhibiting the G1-S transition of the cell cycle and inhibiting CDK activity, leading to apoptosis. | [226] | ||
| Piperine | 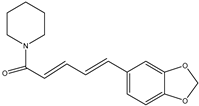 | (50–200 mM) of piperine for 24, 48, and 72 h respectively. | Prostate cancer (LNCaP, PC-3, DU-145 and 22RV1 PCa cells) | Activate the caspase-3-dependent apoptotic pathway by suppressing the activation of phosphorylated STAT-3. | [227] |
| (8, 16, and 20 μM) of piperine. | Ovarian cancer (A2780 cells) | Increase in mitochondrial cytochrome c release, which increased caspase-9 and caspase-3 activity (intrinsic pathway). | [228] | ||
| (75–150 µM) of piperine. | Rectal cancer [Human rectal adenocarcinoma cells (HRT-18)] | Inhibition of G0/G1 cell cycle progression and formation of ROS. | [229] | ||
| serial concentrations of piperine (25, 50, 100, 200, and 400 μg/mL) | Lung cancer (A549 cells) | Cell arrest of the phase G2-M in the cell cycle because of elevated levels of P53. | [230] | ||
| 0, 9, 18 of piperine and 36 µM curcumin for 24 h | Leukemia (HL60) | Release of mitochondrial cytochrome-c, which further initiates caspase-9/3 mediated cell apoptosis. | [231] | ||
| Piperine and curcumin | 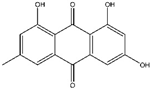 | 0–80 μM | Ovarian cancer A2780 and SK-OV-3 | Inhibits cancer cells’ epithelial-mesenchymal transition by blocking the ILK/GSK-3β/slug signaling pathway. | [123] |
| Emodin | 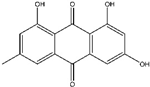  | 30 µmol/L | Cervical cancer SiHa, CaSki, and HaCaT | Increases caspase-3 activity and ROS production. | [116] |
| 25, 50, 100 μmol/L | Breast cancer
| Aryl hydrocarbon receptor (AhR) activators (agonists of AhR) increase the expression of CYP1A1. Suppressed TGF-β1 production. | |||
| 0, 25, 50, and 100 μM | Kidney cancer 786-O and OS-RC-2 | Increases ROS and the consequent activation of the JNK pathway. Increased phosphorylation levels of necroptosis-related proteins MLKL and RIP1. | [118] | ||
| Hepatocellular carcinoma
| Increase in BAX expression and a decrease in BCL-2 expression. Increased of cleaved caspase-3, -9, and PARP level. Induce the phosphorylation of ERK and p38. Inhibit the expression of p-c-Jun-N-terminal kinase (p-JNK). Suppress the activation of p-Akt. | |||
| Colon cancer
| Increased PI3K, p-Akt, and VEGFR2 expressions. Inhibition of PI3K/Akt signalling pathway. | |||
| 60 and 80 µmol/L | lung cancer A549 and H1299 | Inhibition of ER stress with 4-PBA. | [124] | ||
| 20, 40 and 80 µmol/L | Bladder cancer T24 and T5637 | Inhibits the expression of notch1. | [125] | ||
| 2.5–25 μM | Prostate cancer LNCaP | Caspase-3 activation. | [139] | ||
| Parthenolide |  | 0–20 μM | Leukemia K562/ADM | Downregulation of NF-κB activity and mediated P-gp expression, increasing ROS, Bax/Bcl-2 ratio, and cytochrome C expression. | [140] |
| 50 μM | Breast cancer 231MFP and HCC38 | Inhibits FAK1 activity and FAK1-dependent signaling pathway; activation of caspase-3/7. | [141] | ||
| 3–10 μM | Colorectal cancer HCT116, SW480, SW620, HT29, and Caco-2 | Reduces the activity of USP7 and Wnt signaling, resulting in caspase-8 and-9 activation. | [142] | ||
| UNK | Gastric cancer SGC7901 | Downregulation of NF-κB activity and Bcl-2 expression, and upregulation of Caspase-8 activity. | [233] | ||
| 5, 10 and 20 μM | Squamous Cell Carcinoma Eca109 and KYSE-510 | Reduces the levels of NF-κB, AP-1, and VEGF. | [143] | ||
| 4, 8 and 10 μM | Thyroid cancer TPC-1 | Increase ROS levels and Bax expression; downregulation of Bcl-2. | [144] | ||
| 5–50 μM | Lung cancer A549 and H1299 | Inhibits the IGF-1R Mediated PI3K/Akt/ FoxO3α signaling. | [145] | ||
| 4-8 μM | Renal cell carcinoma 786-O and ACHN | Inhibits the EMT, cancer stem cell markers, and the PI3K/Akt pathway. | [146] | ||
| 50, 100, and 200 µmol/L | Uveal melanoma C918 and SP6.5 | Increases the expression of p21, Bax, caspase-3, and caspase-9; downregulation of Cyclin D1, Bcl-XL and Bcl-2. | [234] | ||
| 25 μM | p53-deficient cell lines | Reduces cell viability by inducing apoptosis in p53-deficient cell lines by significantly increasing the cell proportion at the sub-G0/G1-phase of the cell cycle and decreasing the cell proportion at S-phase. | [235] | ||
| Luteolin |  | IC50 of 66.70 µmol/L (24 h) and 30.47 µmol/L (in 72 h) | colon cancerous LoVo cells | Cell cycle arrest at the G2/M phase that ultimately resulted in cellular apoptosis. | [235] |
| in vitro with IC50 from about 3 to 50 μM | tumor cells | Suppress the proliferation of various kinds of tumor cells and inhibit tumor growth effectively in vivo when administered, e.g., in concentrations of 50 to 200 ppm in food. | [236] | ||
| (10 mg/kg) | Sprague-Dawley rats | Inhibits the development of large tumors and can significantly lower the levels of vascular endothelial growth factor (VEGF). | [236] | ||
| 15 μM, 24 h | BxPC-3 human pancreatic cancer cells | Reduces nuclear GSK-3β and NF-κB p65 expression. | [237] | ||
| 30 μM | prostate carcinoma LNCaP cells | Induces cell apoptosis, up-regulating prostate-derived Ets factor (PDEF), and down-regulates androgen receptor (AR) gene expression. | [237] | ||
| 1–50 μM | Neuro-2a mouse neuroblastoma cells | Reduces cell viability by ~ 50%. Because the cytotoxic effect of luteolin was only modestly increased at the higher concentration (50 μM) of luteolin. | [237] | ||
| 6.25 to 100 µM | AGS cell line | Decreases the cell viability in a concentration-dependent way; 50% growth has been inhibited at a dose of 50 µM. When combined with SN-38, it enhances the anti-proliferation effect of the compound and enhances apoptosis, working synergistically with SN-38 to modulate GSK-3β/β-catenin signaling. | [238] | ||
| Quercetin |  | 20 mg/kg BW | BALB/c nude mice injected with AGS cells | The administration of QUE alone (thrice weekly) or in conjunction with irinotecan (10 mg/kg once weekly) has resulted in a noteworthy decrease in tumor size by day 28. Additionally, tumor VEGF-R and VEGF-A levels, protein levels, and COX-2 gene expression have all decreased. Furthermore, this combination has reduced the TEM population. | [238] |
| 0 to 100 µM | MCF-7 and MDA-MB-231 cell lines | IC50 = 30 µM has decreased viability, elevated autophagy, suppressed migration rate, and decreased levels of MMP-2, MMP-9, and VEGF proteins. Moreover, decreased GLUT1, PKM2, LDHA, and glucose uptake have been all observed, as well as lactate production. In a similar vein, QUE has inhibited mTOR, p70-S6K, and Akt activation. | [239] | ||
| 50 mg/kg BW | Mice injected with MCF-7 cells | Prevents the progression of breast cancer and the metastasis of tumors. In tumor tissue, it also reduces the levels of p-Akt, PKM2, and VEGF. | [239] | ||
| 1.78 to 100 µM | PC3 cells | suppressed survival in a way that is time- and dose-dependent. QUE has shown an increased level of Cyt c, casp 3, casp 8, Bax, Bcl-2, p21Cip1, p27Kip1, and p53 at a concentration of 40 µM. QUE has also enhanced MKsi’s apoptotic effect, raised casp 3, and lowered the expression of the Survivin gene. Moreover, QUE has reduced the number of cells in the S-phase and encouraged the arrest of G1 phase cells. QUE has also reduced PI3K, Akt, and ERK1/2 phosphorylation and elevated PTEN expression while lowering p38, NFκB, and Survivin protein levels. When paired with MKsi, QUE has shown an outperformance in ERK1/2, p38, NFκB, and Survivin. | [240] | ||
| 150 µM at 72 h | B164A5 murine melanoma cells | Decreasing ECAR and OCR. | [241] | ||
| 12 to 100 µM | BC3, BCBL1, and BC1 PEL cell lines. | In a dose-dependent manner, QUE for 24 h has shown a decrease in cell growth and survival while having no effect on healthy B lymphocytes. The apoptotic rate has been elevated by QUE 50 µM, leading to an increase in the G1 cell phase, PARP cleavage, and nuclear fragmentation/condensation. QUE has also promoted the degradation of β-catenin and inhibited Aktser473 and mTOR at this concentration. | [164] | ||
| 0.6 to 300 µM | MCF-7, MDA-MB-231, HBL100 and BT549 breast cancer cells, and OVCAR5, TOV112D, OVCAR3, CAOV3 ovarian cancer cells. | Decreasing in concentration-dependent cell proliferation. | [242] | ||
| 50 µM for 24 h | HBL100 cells | Increasing the lactate depletion in the culture media and increasing the intracellular glucose accumulation. | [242] | ||
| 300 µM for 48 h | MCF-7 cells | Apoptosis has increased by 25%. | [242] | ||
| 0 to 200µM | HCT-15 and RKO cells | Promoting apoptosis and inhibiting cell viability and proliferation in cancer cells in a concentration-dependent manner, but not in normal cells. | [243] | ||
| 142.7 µM (IC50) | HCT-15 cells | Decreasing the production of lactate and consumption of glucose following a 4 h incubation. By increasing sensitization to 5-FU, QUE has enhanced the inhibitory effects of the compound on glucose metabolism. | [243] | ||
| 121.9 µM (IC50) | RKO cells | QUE has also improved the inhibition of glucose metabolism by increasing sensitization to 5-FU. | [243] | ||
| 25 to 75 mg/kg BW | DL mice | Reducing LDH-A activity, mRNA expression, and cell viability in a dose-dependent way without causing hepatic toxicity. Additionally, QUE has increased p53 mRNA expression while downregulating Akt gene/protein expression and p85a phosphorylation. | [244] | ||
| 26.5 µM | Ehrlich ascites tumor cells | Reducing the synthesis of lactate by 78% and Na+-K+-ATPase by 85%. | [245] | ||
| 13.25 to 66.17 µM | Ehrlich ascites tumor cells | After 10 min of treatment and at a concentration of 26.5 µM, QUE has inhibited Na+-K+-ATPase activity by 50%. In a concentration-dependent manner, QUE has inhibited both oxidative phosphorylation and aerobic glycolysis. | [246,247] | ||
| 33.09 µM | Ascites tumor cells | Inhibiting glycolysis and protein synthesis. | [248] | ||
| 25 µM | Rat thymocytes | Preventing the uptake of glucose brought on by a mitogenic stimulus. | [249] | ||
| 0.1 µg/mg protein | Ascites tumor cells | Lactate efflux has been 50% inhibited, resulting in an increase in the internal lactate concentration while decreasing intracellular pH. | [250] | ||
| 5 to 40 µM | HL60 cells | QUE and 2-DG together have been the main cause for the reduction in mitochondrial membrane potential, an increase in mIMP, caspase-dependent late apoptosis, and attenuated phosphorylation of Akt and rpS6. The rate of apoptosis is increased when PI3K/Akt phosphorylation inhibitors are used in conjunction with QUE (10 µM) and 2-DG (2 mM) at low concentrations. | [251] | ||
| 9.24 µM | Recombinant human PKM2 enzyme | 50% less PKM2 activity has been mainly observed. | [252] | ||
| Anthocyanins extracts (400 µg/mL) | A549 (lung cancer cells) | Inhibiting the invasion and migration of cancer (MMP-2 and MMP-9) and preventing it from proliferating (COX-2, C-myc, and cyclin D1). | [253] | ||
| Anthocyanins | Anthocyanidins, cyanidin, malvidin, peonidin, petunidin, and delphinidin (12.5–100 µM) | A549, H1299 (lung cancer cells) | Inducing apoptosis (Bcl2, PARP), inhibited the WNT and oncogenic Notch pathways (VEGF, pERK, cyclin D1, cyclin B1, b-catenin, c-myc, and MMP9). | [254] | |
 | Anthocyanins extracts (40–320 µg GAE/mL, equivalent to 10.8–86.2 µg C3G/mL) | BT4747, MDA-MB-453, MDA-MB-231(Breast cancer cells) | The inhibition of proliferation and apoptosis by downregulating the Akt/mTOR and pro-survival Sirt1/survivin pathways, as well as a decrease in invasive/metastatic markers Sp1, Sp4, and VCAM-1. | [255] | |
 | Anthocyanins extracts, delphinidin, cyanidin (100–600 mg/mL) | HCT-116, HT-29 (colon cancer cells), in silico analysis | Decreasing the levels of PD-1, PD-L1, and PVGF expression. | [256] | |
| Anthocyanin extracts (5 mg/mL; 5 mg/kg) | colon cancer stem cells; AOM induced carcinogen in A/J mice | Apoptosis induction and down-regulation of Wnt/b-catenin signaling. | [257] | ||
| Anthocyanins extracts (400 µg/mL) | SNU-1, SNU-16 (gastric cancer cell) | Apoptosis induction (Caspase-3, Akt, Bax, XIAP). | [253] | ||
| Anthocyanins extracts (0.2 mg/mL | PLC/PRF/5, HepG2, McArdle (liver cancer cell) | Induced autophagy (eIF2a, mTOR, Bcl-2) and apoptosis (Bax, cytochrome C, caspase-3). | [258] | ||
| Anthocyanins (60–120 mM) | DU-145 (prostate cancer cell), tumor xenograft in nude mice | Inhibiting the androgen signaling (AR) and triggering apoptosis (p53, Bax, and Bcl 2). | [259] | ||
| Anthocyanins extracts (100–200 µg/mL) | SK-Hep1 (liver cancer cell) | Reducing migration, decreasing adhesion, and suppressing proliferation to prevent metastasis (MMP-2, MMP-9). | [260] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talib, W.H.; Baban, M.M.; Bulbul, M.F.; Al-Zaidaneen, E.; Allan, A.; Al-Rousan, E.W.; Ahmad, R.H.Y.; Alshaeri, H.K.; Alasmari, M.M.; Law, D. Natural Products and Altered Metabolism in Cancer: Therapeutic Targets and Mechanisms of Action. Int. J. Mol. Sci. 2024, 25, 9593. https://doi.org/10.3390/ijms25179593
Talib WH, Baban MM, Bulbul MF, Al-Zaidaneen E, Allan A, Al-Rousan EW, Ahmad RHY, Alshaeri HK, Alasmari MM, Law D. Natural Products and Altered Metabolism in Cancer: Therapeutic Targets and Mechanisms of Action. International Journal of Molecular Sciences. 2024; 25(17):9593. https://doi.org/10.3390/ijms25179593
Chicago/Turabian StyleTalib, Wamidh H., Media Mohammad Baban, Mais Fuad Bulbul, Esraa Al-Zaidaneen, Aya Allan, Eiman Wasef Al-Rousan, Rahaf Hamed Yousef Ahmad, Heba K. Alshaeri, Moudi M. Alasmari, and Douglas Law. 2024. "Natural Products and Altered Metabolism in Cancer: Therapeutic Targets and Mechanisms of Action" International Journal of Molecular Sciences 25, no. 17: 9593. https://doi.org/10.3390/ijms25179593
APA StyleTalib, W. H., Baban, M. M., Bulbul, M. F., Al-Zaidaneen, E., Allan, A., Al-Rousan, E. W., Ahmad, R. H. Y., Alshaeri, H. K., Alasmari, M. M., & Law, D. (2024). Natural Products and Altered Metabolism in Cancer: Therapeutic Targets and Mechanisms of Action. International Journal of Molecular Sciences, 25(17), 9593. https://doi.org/10.3390/ijms25179593








