SARS-CoV-2 Spike Protein Enhances Carboxypeptidase Activity of Angiotensin-Converting Enzyme 2
Abstract
1. Introduction
2. Results
2.1. Blood Glucose and Systolic Blood Pressure (SBP)
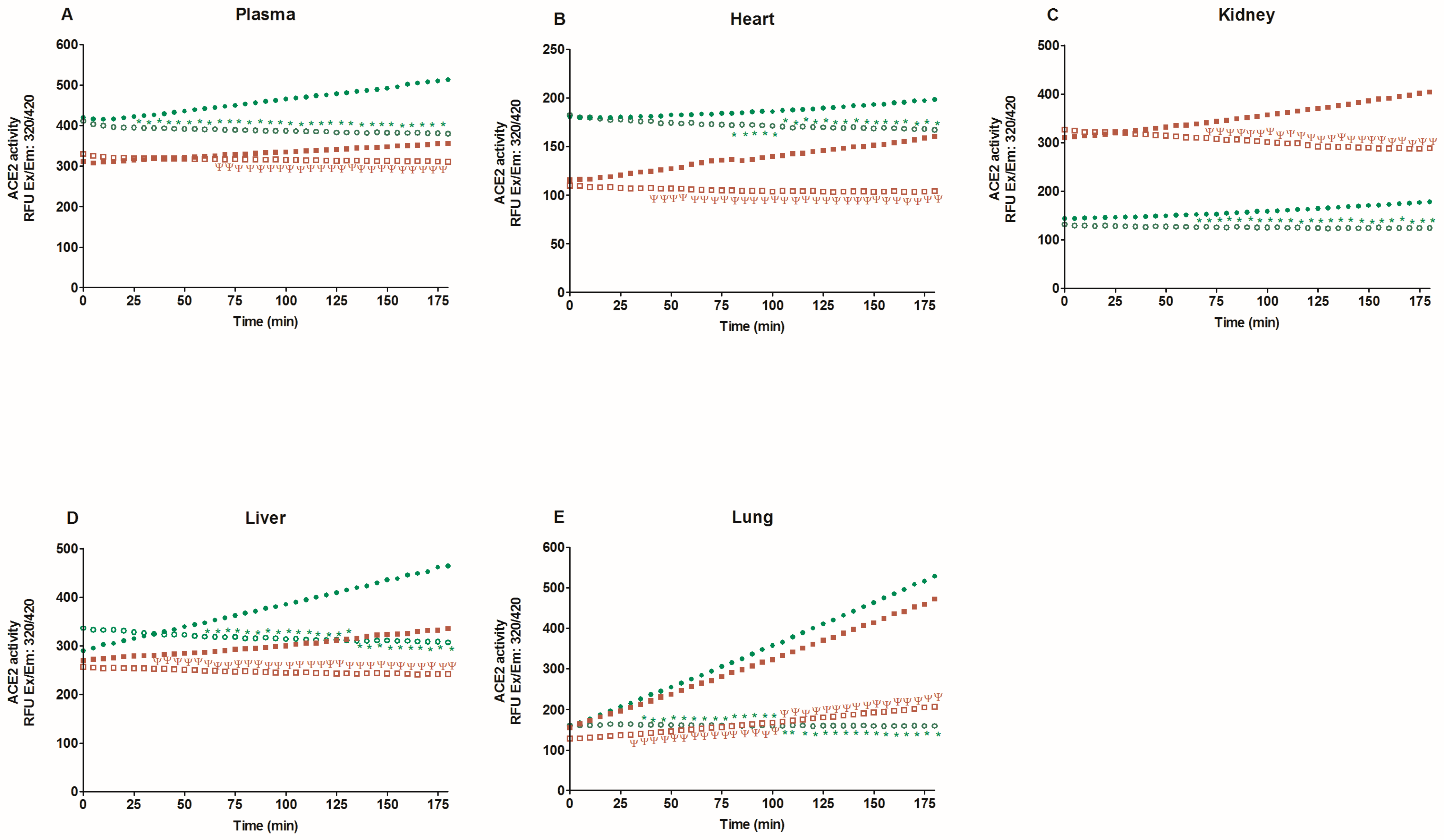
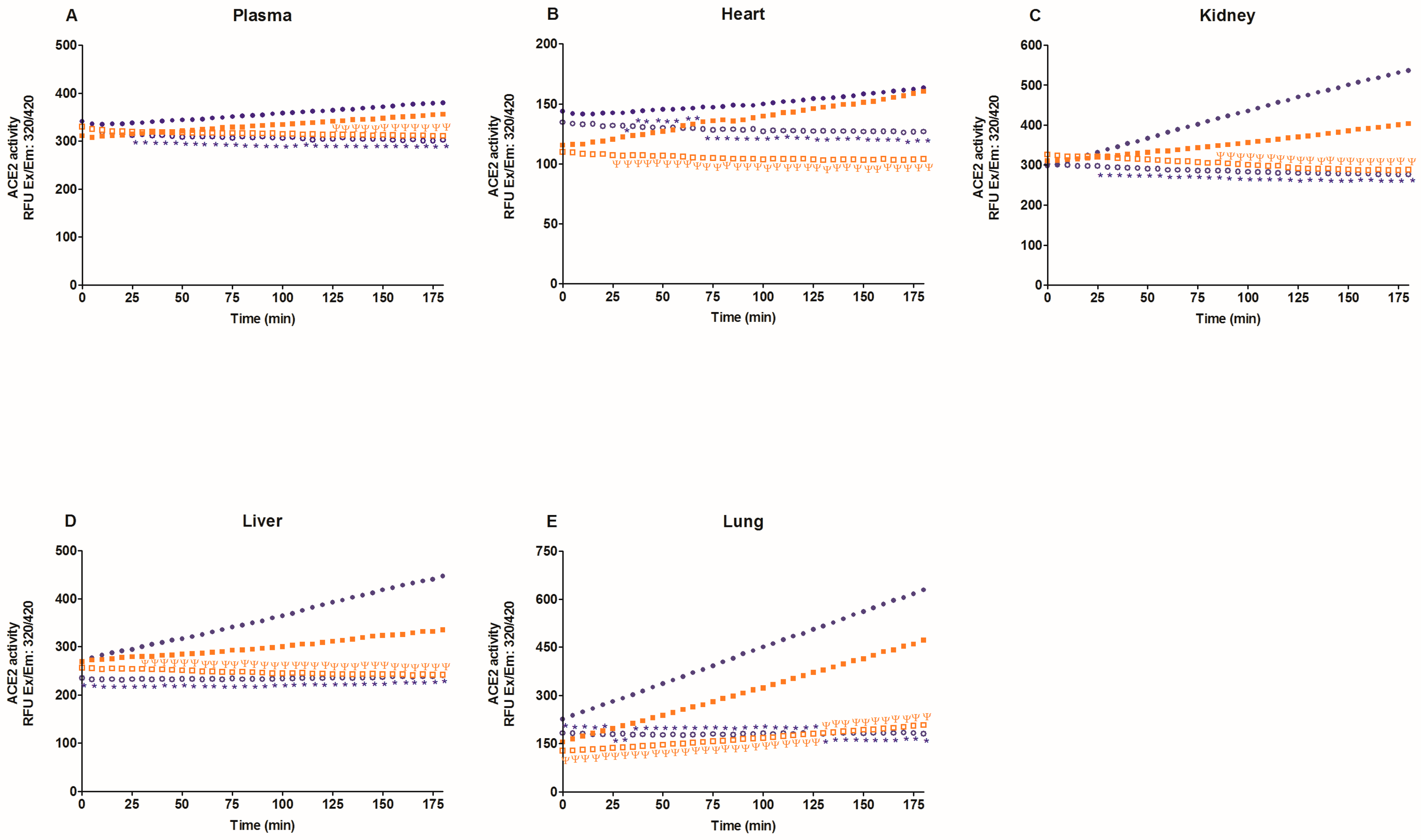
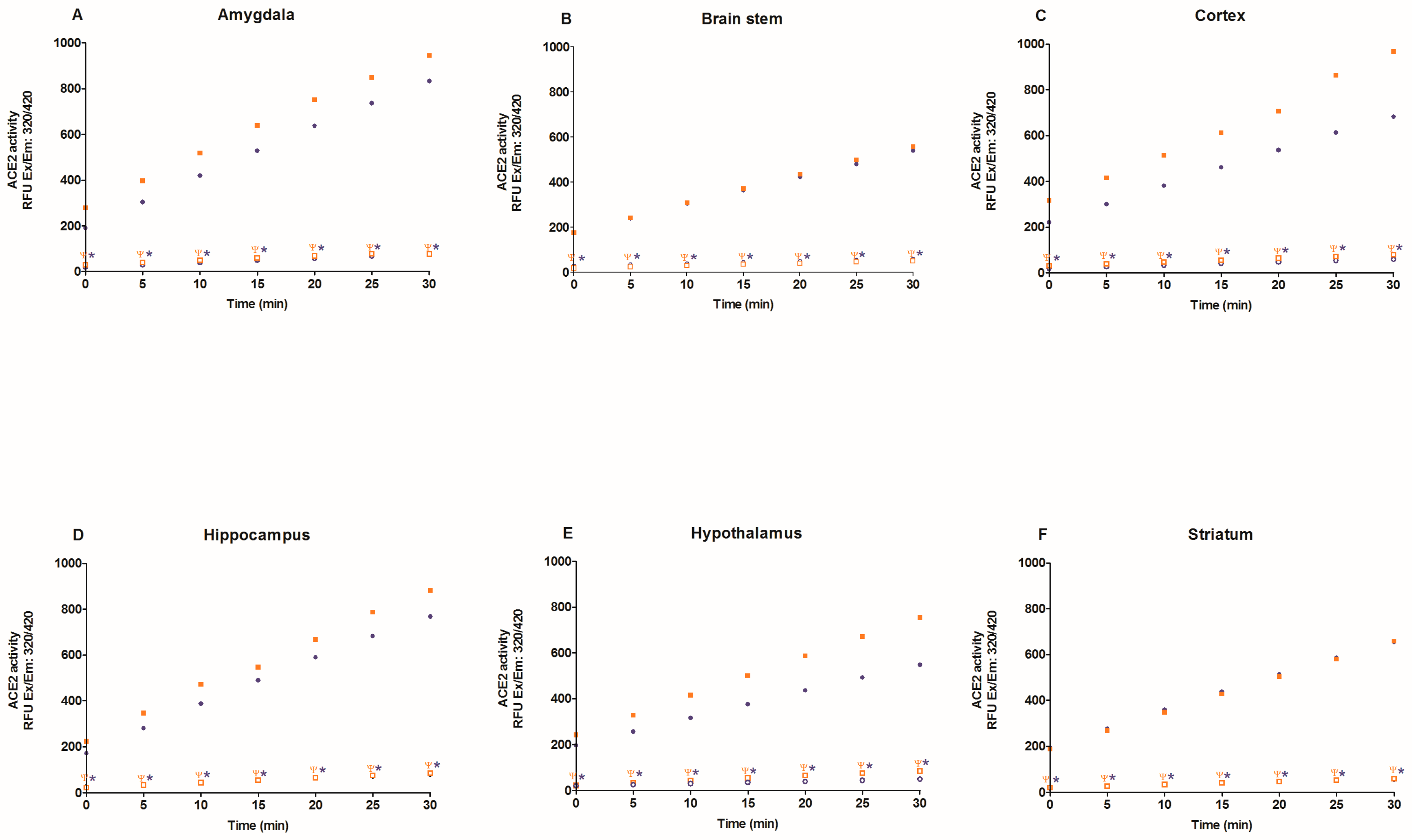
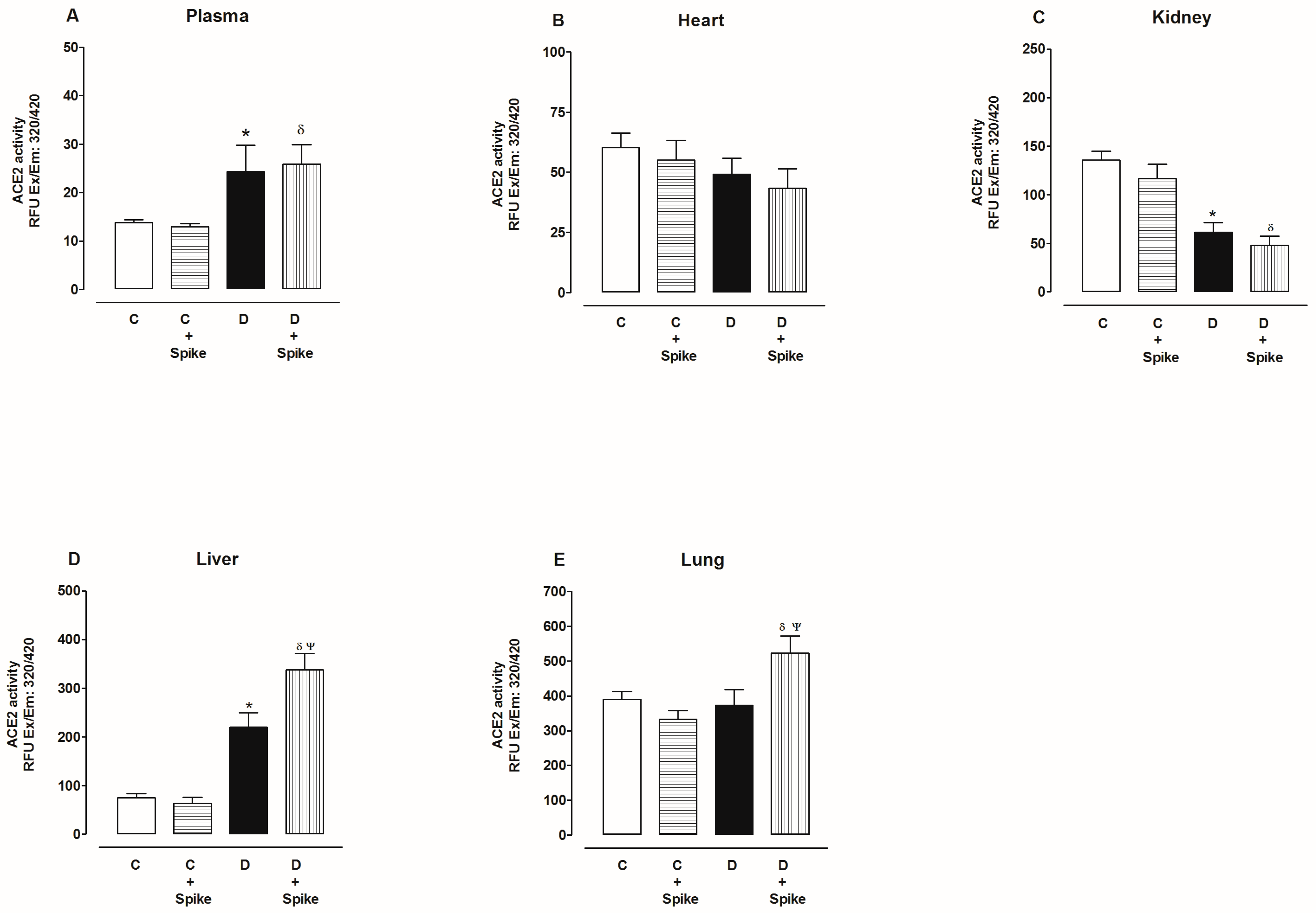
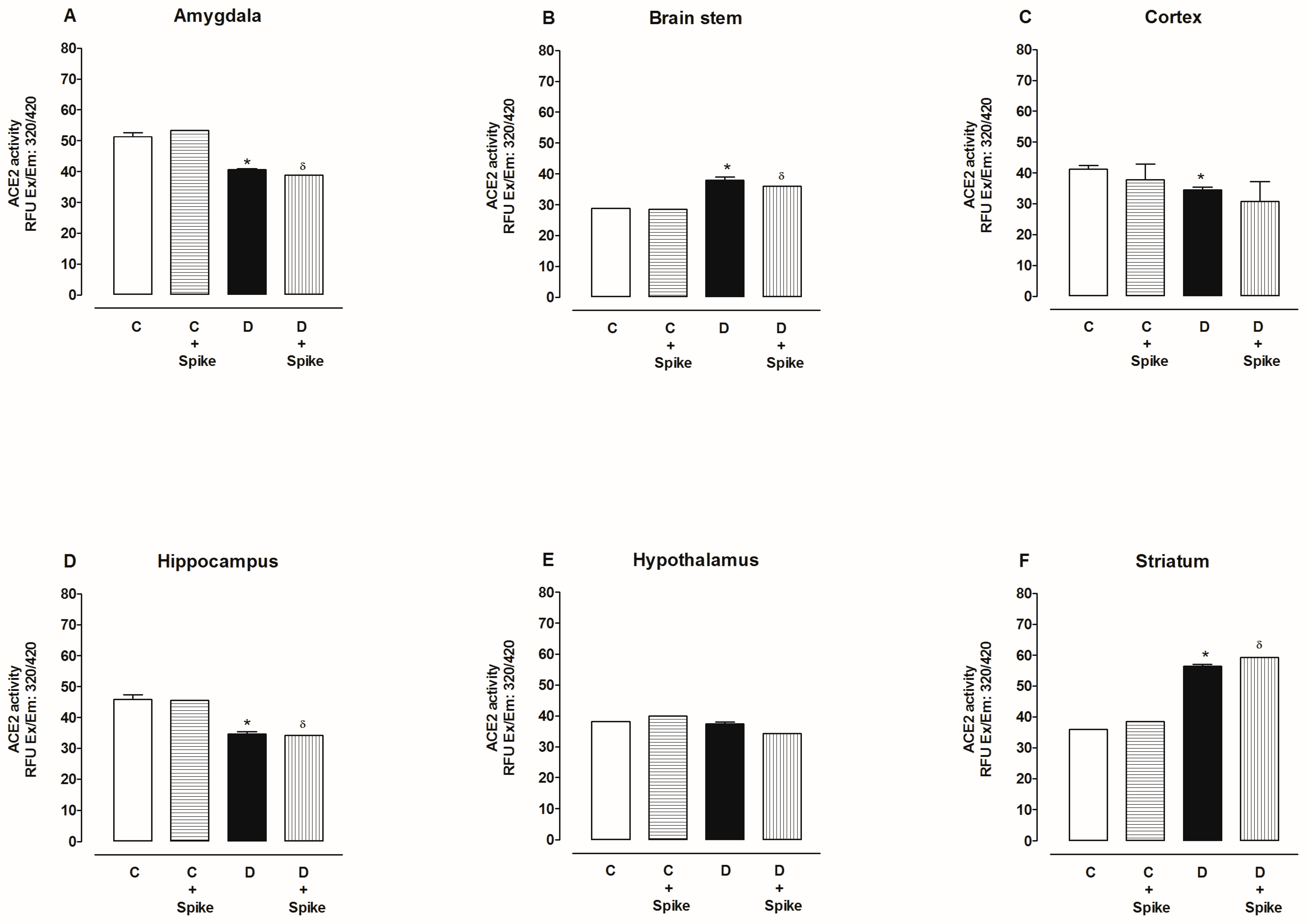
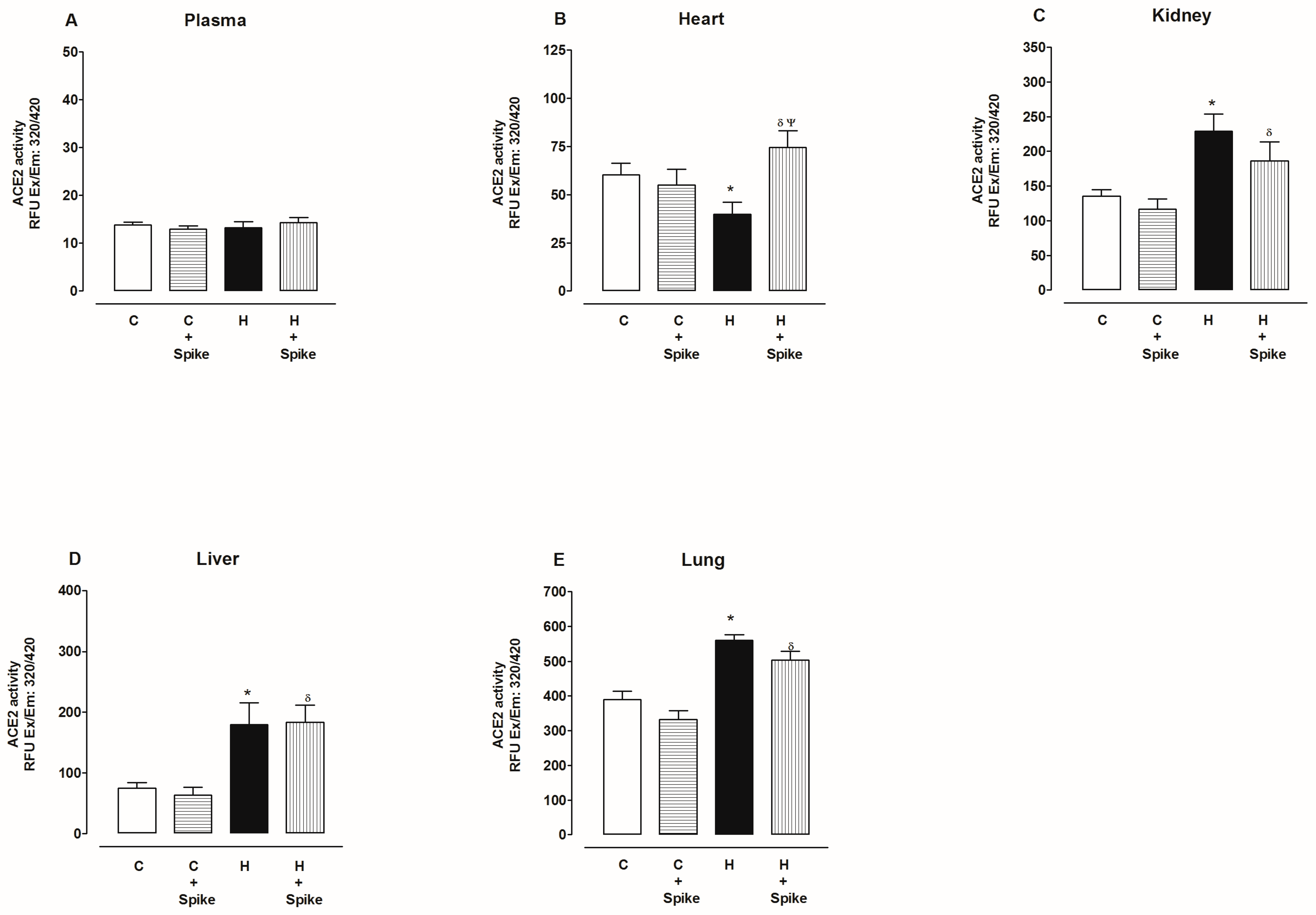
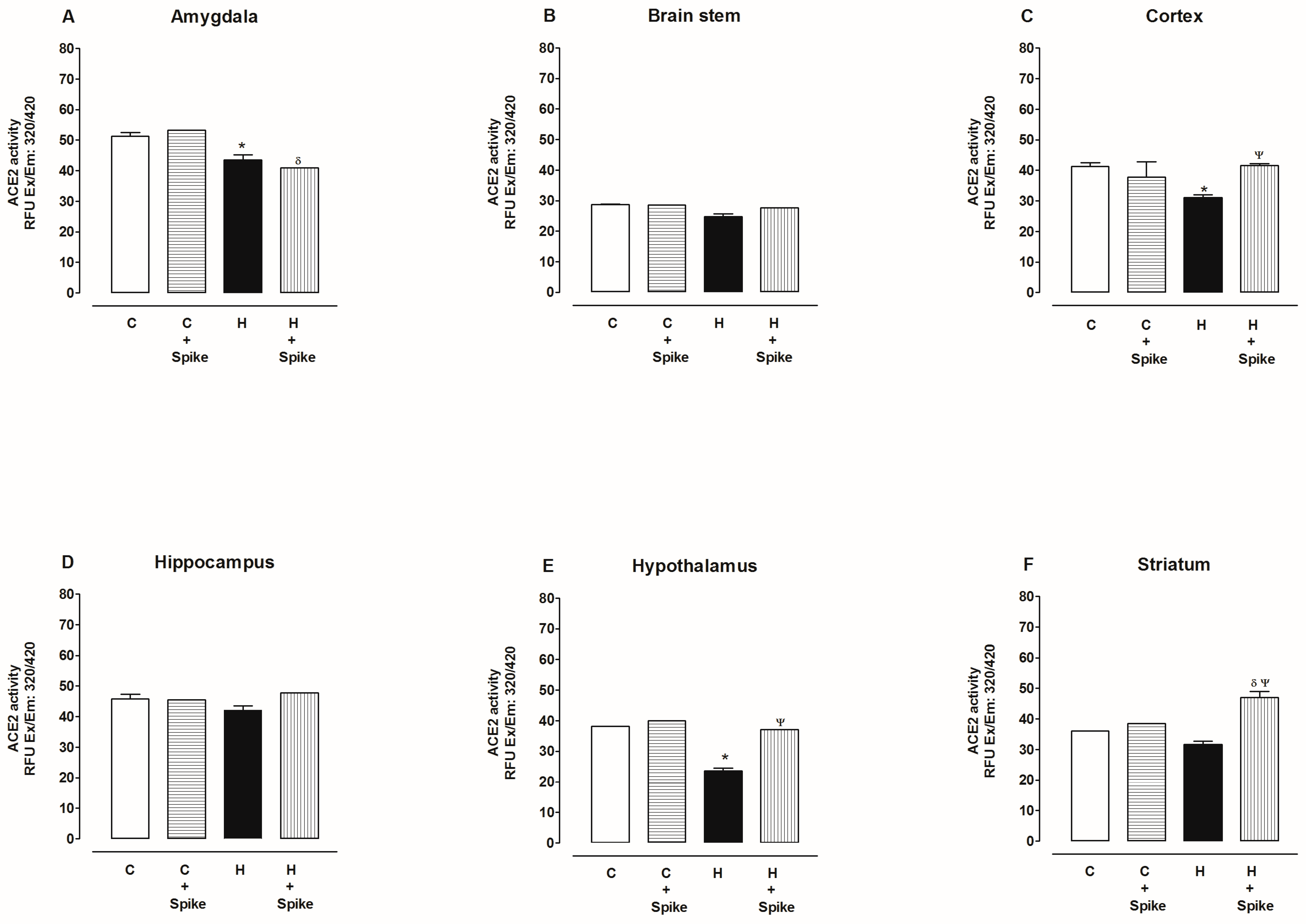
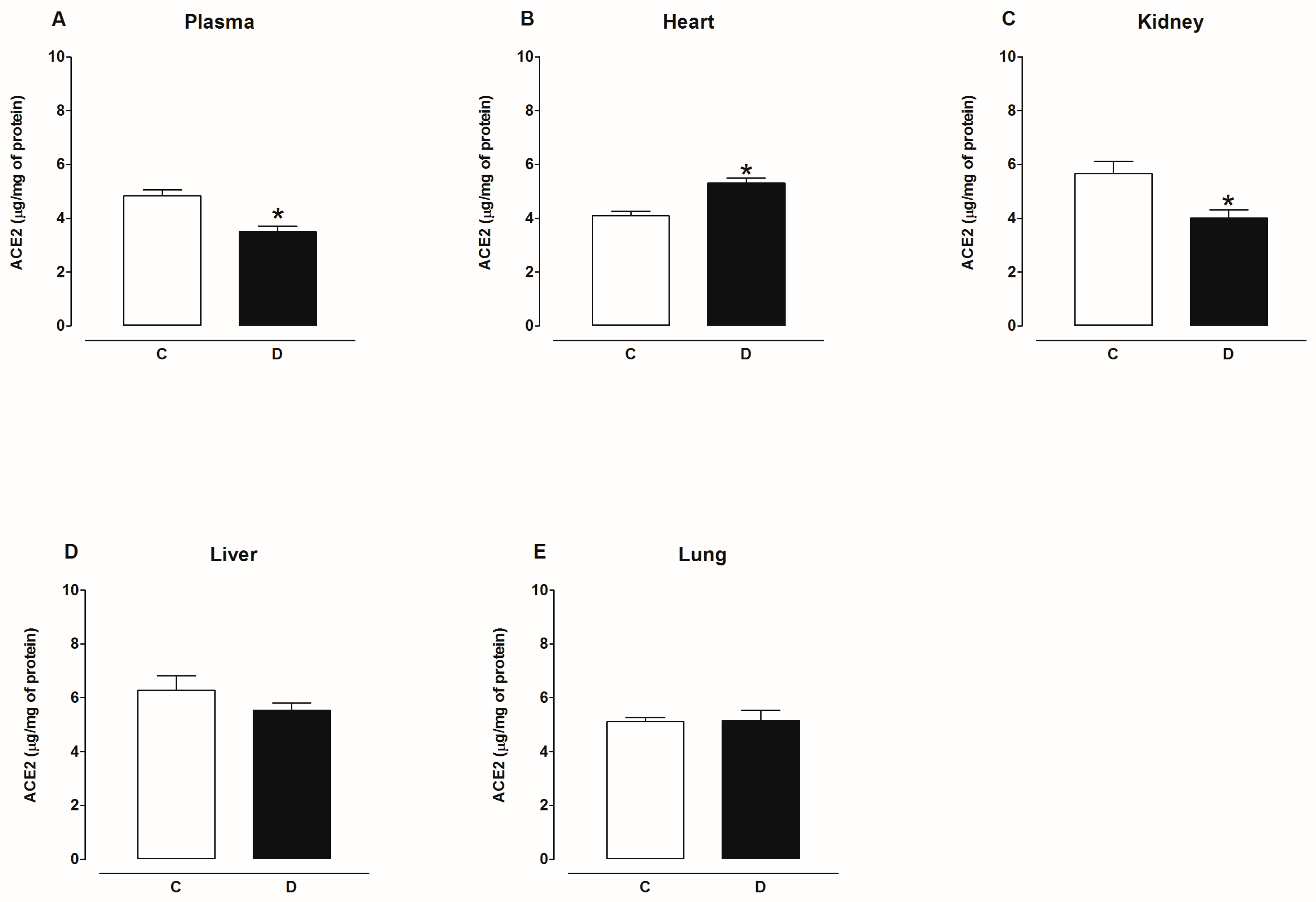
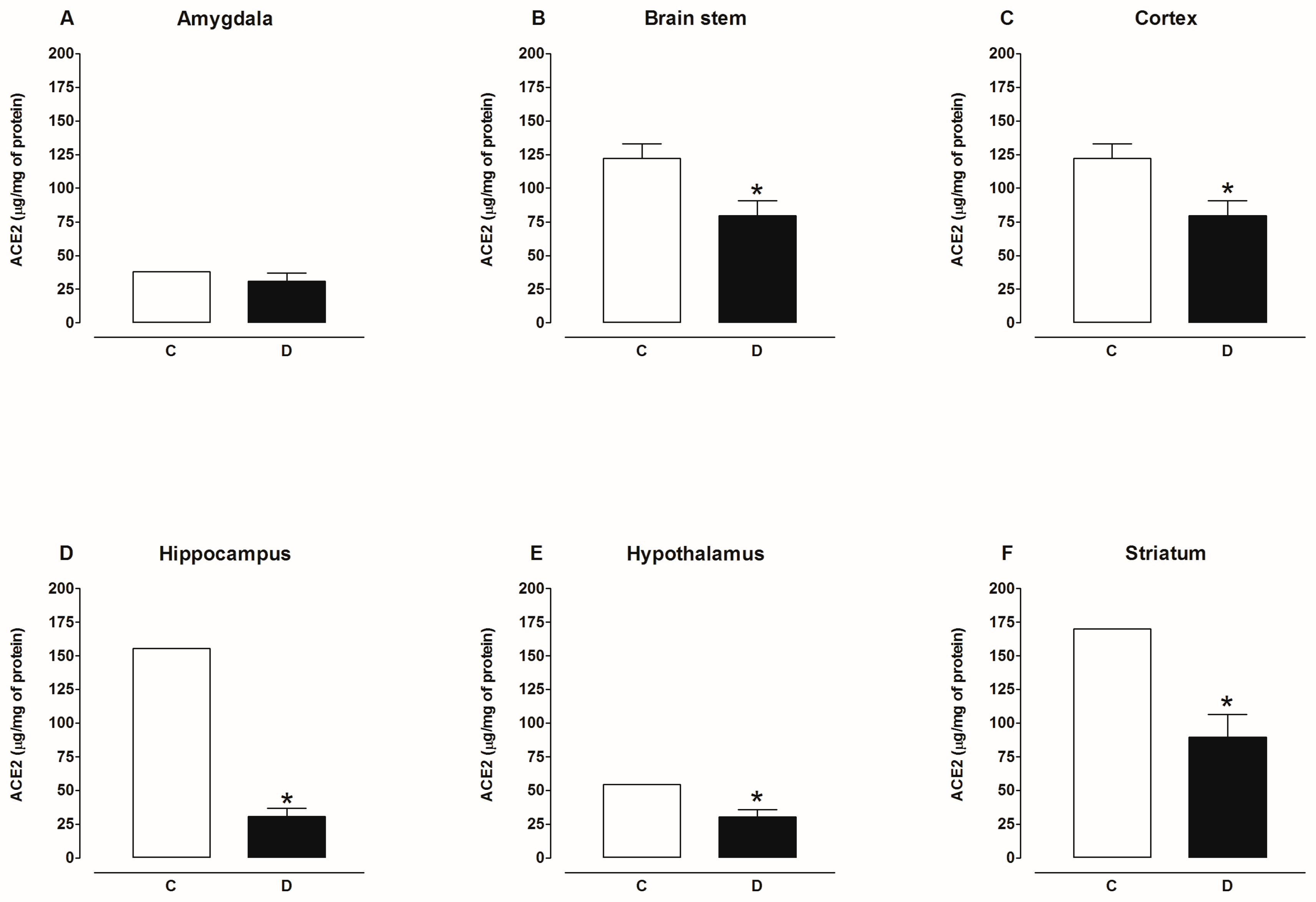
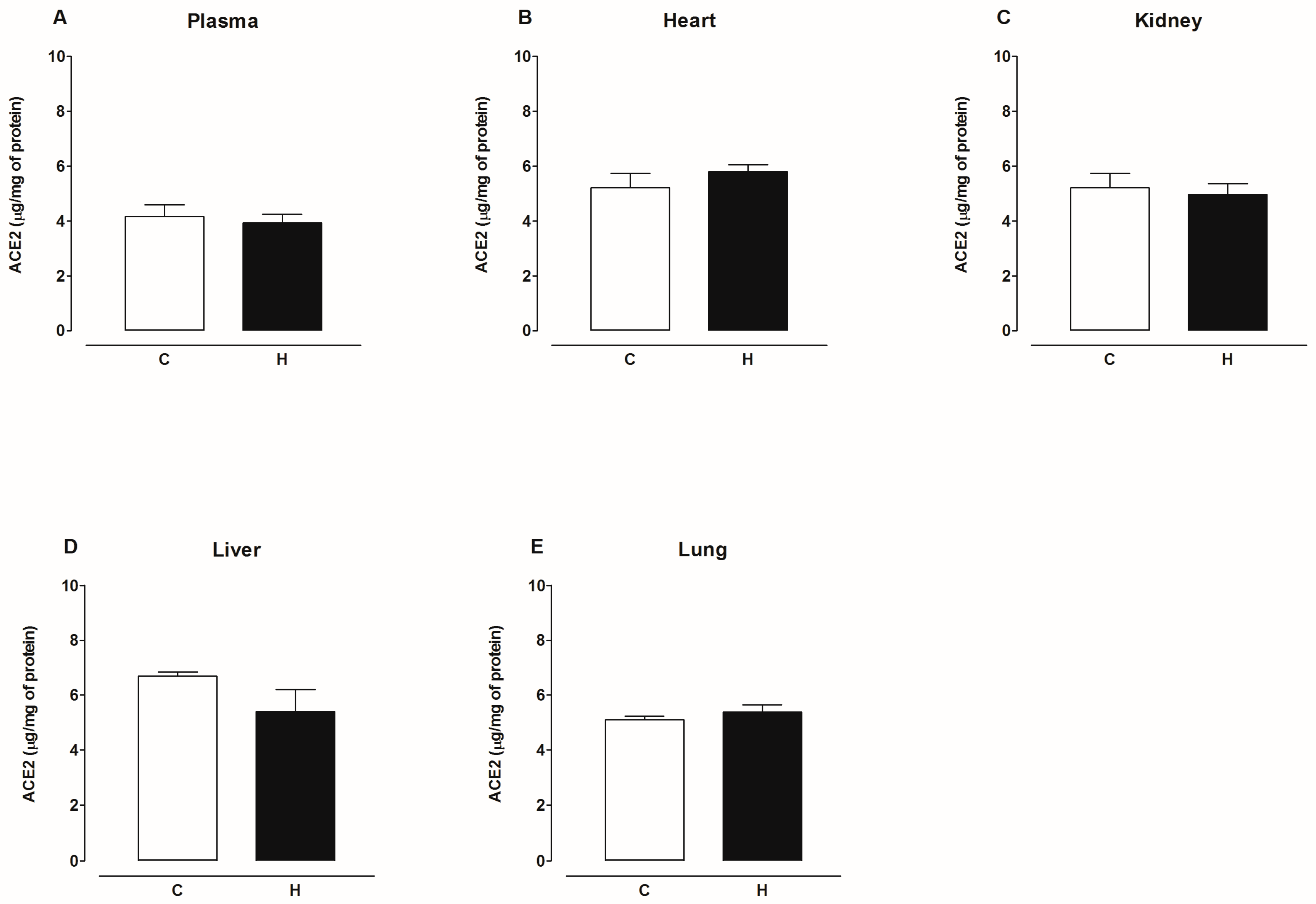
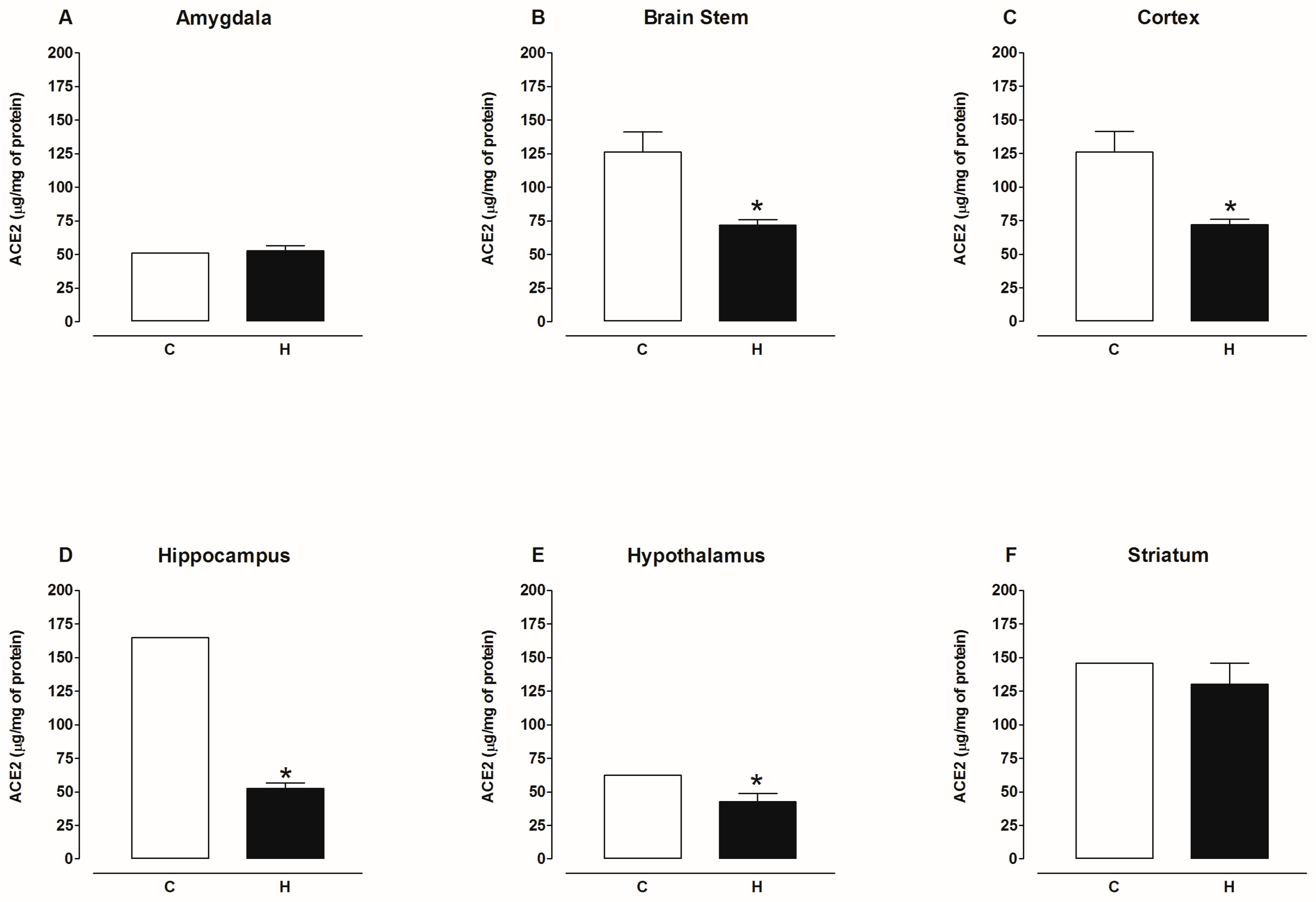
2.2. Activity of Angiotensin-Converting Enzyme 2 (ACE2)
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.1.1. Streptozotocin-Induced Diabetic Rats
4.1.2. Angiotensin II-Induced Hypertension Rats
4.1.3. Sample Collection and Tissue Preparation
4.2. Determination of ACE2 Activity
4.3. Enzyme-Linked Immunosorbent Assay (ELISA) for ACE2 Determination
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Islam, N.; Gutierrez, J.P.; Gutiérrez-Barreto, S.E.; Castañeda Prado, A.; Moolenaar, R.L.; Lacey, B.; Richter, P. Associations of diabetes, hypertension and obesity with COVID-19 mortality: A systematic review and meta-analysis. BMJ Glob. Health 2023, 8, e012581. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Segev, R.; Francis, B.; Abu-Saleh, N.; Awad, H.; Lazarovich, A.; Kabala, A.; Aronson, D.; Abassi, Z. Cardiac and renal distribution of ACE and ACE-2 in rats with heart failure. Acta Histochem. 2014, 116, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Khoury, E.E.; Knaney, Y.; Fokra, A.; Kinaneh, S.; Azzam, Z.; Heyman, S.N.; Abassi, Z. Pulmonary, cardiac and renal distribution of ACE2, furin, TMPRSS2 and ADAM17 in rats with heart failure: Potential implication for COVID-19 disease. J. Cell Mol. Med. 2021, 25, 3840–3855. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, K.; Reinhard, S.; Galván, J.A.; Wartenberg, M.; Hewer, E.; Schürch, C.M. Systematic Investigation of SARS-CoV-2 Receptor Protein Distribution along Viral Entry Routes in Humans. Respiration 2022, 101, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Herman-Edelstein, M.; Guetta, T.; Barnea, A.; Waldman, M.; Ben-Dor, N.; Barac, Y.D.; Kornowski, R.; Arad, M.; Hochhauser, E.; Aravot, D. Expression of the SARS-CoV-2 receptorACE2 in human heart is associated with uncontrolled diabetes, obesity, and activation of the renin angiotensin system. Cardiovasc. Diabetol. 2021, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- Sidarta-Oliveira, D.; Jara, C.P.; Ferruzzi, A.J.; Skaf, M.S.; Velander, W.H.; Araujo, E.P.; Velloso, L.A. SARS-CoV-2 receptor is co-expressed with elements of the kinin-kallikrein, renin-angiotensin and coagulation systems in alveolar cells. Sci. Rep. 2020, 10, 19522. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, V.; Ranganadin, P.; Shanmugam, L.; Rao, S.R.; Balakrishna Pillai, A. Early shedding of membrane-bounded ACE2 could be an indicator for disease severity in SARS-CoV-2. Biochimie 2022, 201, 139–147. [Google Scholar] [CrossRef]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000, 87, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [CrossRef] [PubMed]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Shrestha, K.; Troughton, R.W.; Francis, G.S.; Sen, S.; Klein, A.L.; Tang, W.H. Soluble angiotensin-converting enzyme 2 in human heart failure: Relation with myocardial function and clinical outcomes. J. Card. Fail. 2009, 15, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Pérez, J.T.; Riera, M.; Bosch, X.; De Caralt, T.M.; Perea, R.J.; Pascual, J.; Soler, M.J. Role of circulating angiotensin converting enzyme 2 in left ventricular remodeling following myocardial infarction: A prospective controlled study. PLoS ONE 2013, 8, e61695. [Google Scholar] [CrossRef] [PubMed]
- Úri, K.; Fagyas, M.; Mányiné Siket, I.; Kertész, A.; Csanádi, Z.; Sándorfi, G.; Clemens, M.; Fedor, R.; Papp, Z.; Édes, I.; et al. New perspectives in the renin-angiotensin-aldosterone system (RAAS) IV: Circulating ACE2 as a biomarker of systolic dysfunction in human hypertension and heart failure. PLoS ONE 2014, 9, e87845. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Z.; Yang, X.; Hu, B.; Huang, Y.; Fan, S. Association between circulating angiotensin-converting enzyme 2 and cardiac remodeling in hypertensive patients. Peptides 2017, 90, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Yamamoto, K.; Ohishi, M.; Tatara, Y.; Takeya, Y.; Shiota, A.; Oguro, R.; Iwamoto, Y.; Takeda, M.; Rakugi, H. The counterregulating role of ACE2 and ACE2-mediated angiotensin 1–7 signaling against angiotensin II stimulation in vascular cells. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2010, 33, 1182–1185. [Google Scholar] [CrossRef]
- Fernandes, F.B.; Fernandes, A.B.; Febba, A.C.S.; Leite, A.P.O.; Leite, C.A.; Vitalle, M.S.S.; Jung, F.F.; Casarini, D.E. Association of Ang-(1–7) and des-Arg9BK as new biomarkers of obesity and cardiometabolic risk factors in adolescents. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2021, 44, 969–977. [Google Scholar] [CrossRef]
- Rostamzadeh, F.; Najafipour, H.; Nakhaei, S.; Yazdani, R.; Langari, A.A. Low Ang-(1–7) and high des-Arg9 bradykinin serum levels are correlated with cardiovascular risk factors in patients with COVID-19. Open Med. 2023, 18, 20230741. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.; Javadi, A.; Aalikhani, M. Des-Arg9 bradykinin and bradykinin potentially trigger cytokine storm in patients with COVID-19. Iran. J. Immunol. IJI 2021, 18, 93–94. [Google Scholar] [CrossRef]
- Wang, W.; McKinnie, S.M.; Farhan, M.; Paul, M.; McDonald, T.; McLean, B.; Llorens-Cortes, C.; Hazra, S.; Murray, A.G.; Vederas, J.C.; et al. Angiotensin-Converting Enzyme 2 Metabolizes and Partially Inactivates Pyr-Apelin-13 and Apelin-17, Physiological Effects in the Cardiovascular System. Hypertension 2016, 68, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chakraborti, S.; Dimitrov, A.S.; Gramatikoff, K.; Dimitrov, D.S. The SARS-CoV S glycoprotein: Expression and functional characterization. Biochem. Biophys. Res. Commun. 2003, 312, 1159–1164. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sun, P.D. High affinity binding of SARS-CoV-2 spike protein enhances ACE2 carboxypeptidase activity. J. Biol. Chem. 2020, 295, 18579–18588. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, A.A.; Troisi, E.M.; Hensley, S.E.; Kohli, R.M.; Epstein, J.A. SARS-CoV-2 spike protein binding selectively accelerates substrate-specific catalytic activity of ACE2. J. Biochem. 2021, 170, 299–306. [Google Scholar] [CrossRef]
- Mensah, G.A.; Croft, J.B.; Giles, W.H. The heart, kidney, and brain as target organs in hypertension. Cardiol. Clin. 2002, 20, 225–247. [Google Scholar] [CrossRef]
- Kozakova, M.; Morizzo, C.; Goncalves, I.; Natali, A.; Nilsson, J.; Palombo, C. Cardiovascular organ damage in type 2 diabetes mellitus: The role of lipids and inflammation. Cardiovasc. Diabetol. 2019, 18, 61. [Google Scholar] [CrossRef]
- Klein, O.L.; Meltzer, D.; Carnethon, M.; Krishnan, J.A. Type II diabetes mellitus is associated with decreased measures of lung function in a clinical setting. Respir. Med. 2011, 105, 1095–1098. [Google Scholar] [CrossRef]
- Crackower, M.A.; Sarao, R.; Oudit, G.Y.; Yagil, C.; Kozieradzki, I.; Scanga, S.E.; Oliveira-dos-Santos, A.J.; da Costa, J.; Zhang, L.; Pei, Y.; et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 2002, 417, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.W.; Chen, Y.J.; Zhang, R.P.; Chen, Y.M.; Huang, B.W. Angiotensin-converting enzyme 2 alleviates liver fibrosis through the renin-angiotensin system. World J. Gastroenterol. 2024, 30, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, J.; Yan, Y.; Ruan, Z.; Su, Y.; Wang, J.; Huang, H.; Zhang, Y.; Wang, W.; Gao, J.; et al. Angiotensin-converting enzyme 2 regulates autophagy in acute lung injury through AMPK/mTOR signaling. Arch. Biochem. Biophys. 2019, 672, 108061. [Google Scholar] [CrossRef]
- Tikellis, C.; Brown, R.; Head, G.A.; Cooper, M.E.; Thomas, M.C. Angiotensin-converting enzyme 2 mediates hyperfiltration associated with diabetes. Am. J. Physiol. Ren. Physiol. 2014, 306, F773–F780. [Google Scholar] [CrossRef] [PubMed]
- Sriramula, S.; Xia, H.; Xu, P.; Lazartigues, E. Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension 2015, 65, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Bree, A.J.; Puente, E.C.; Daphna-Iken, D.; Fisher, S.J. Diabetes increases brain damage caused by severe hypoglycemia. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E194–E201. [Google Scholar] [CrossRef] [PubMed]
- Reno, C.M.; Tanoli, T.; Bree, A.; Daphna-Iken, D.; Cui, C.; Maloney, S.E.; Wozniak, D.F.; Fisher, S.J. Antecedent glycemic control reduces severe hypoglycemia-induced neuronal damage in diabetic rats. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1331–E1337. [Google Scholar] [CrossRef]
- Muela, H.C.; Costa-Hong, V.A.; Yassuda, M.S.; Moraes, N.C.; Memória, C.M.; Machado, M.F.; Macedo, T.A.; Shu, E.B.; Massaro, A.R.; Nitrini, R.; et al. Hypertension Severity Is Associated With Impaired Cognitive Performance. J. Am. Heart Assoc. 2017, 6, e004579. [Google Scholar] [CrossRef] [PubMed]
- Towler, P.; Staker, B.; Prasad, S.G.; Menon, S.; Tang, J.; Parsons, T.; Ryan, D.; Fisher, M.; Williams, D.; Dales, N.A.; et al. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004, 279, 17996–18007. [Google Scholar] [CrossRef] [PubMed]
- Soro-Paavonen, A.; Gordin, D.; Forsblom, C.; Rosengard-Barlund, M.; Waden, J.; Thorn, L.; Sandholm, N.; Thomas, M.C.; Groop, P.H. FinnDiane Study Group Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J. Hypertens. 2012, 30, 375–383. [Google Scholar] [CrossRef]
- Úri, K.; Fagyas, M.; Kertész, A.; Borbély, A.; Jenei, C.; Bene, O.; Csanádi, Z.; Paulus, W.J.; Édes, I.; Papp, Z.; et al. Circulating ACE2 activity correlates with cardiovascular disease development. J. Renin-Angiotensin-Aldosterone Syst. JRAAS 2016, 17, 1470320316668435. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Wysocki, J.; Naaz, P.; Salabat, M.R.; LaPointe, M.S.; Batlle, D. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: A renoprotective combination? Hypertension 2004, 43, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Burrell, L.M.; Risvanis, J.; Kubota, E.; Dean, R.G.; MacDonald, P.S.; Lu, S.; Tikellis, C.; Grant, S.L.; Lew, R.A.; Smith, A.I.; et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005, 26, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, J.; Ye, M.; Soler, M.J.; Gurley, S.B.; Xiao, H.D.; Bernstein, K.E.; Coffman, T.M.; Chen, S.; Batlle, D. ACE and ACE2 activity in diabetic mice. Diabetes 2006, 55, 2132–2139. [Google Scholar] [CrossRef]
- Kamilic, J.; Hamming, I.; Kreutz, R.; Bolbrinker, J.; Siems, W.E.; Nassar, I.; Sluimer, J.C.; Walther, T.; Navis, G.J.; van Goor, H. Renal ACE2 expression and activity is unaltered during established hypertension in adult SHRSP and TGR(mREN2)27. Hypertens. Res. 2010, 33, 123–128. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, X.; Cheng, L.; Miao, L.Y.; Li, H.X.; Han, L.H.; Jiang, W.P. Effects of enalapril on the expression of cardiac angiotensin-converting enzyme and angiotensin-converting enzyme 2 in spontaneously hypertensive rats. Arch. Cardiovasc. Dis. 2013, 106, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Roca-Ho, H.; Riera, M.; Palau, V.; Pascual, J.; Soler, M.J. Characterization of ACE and ACE2 Expression within Different Organs of the NOD Mouse. Int. J. Mol. Sci. 2017, 18, 563. [Google Scholar] [CrossRef] [PubMed]
- Vergara, A.; Jacobs-Cachá, C.; Molina-Van den Bosch, M.; Domínguez-Báez, P.; Benito, B.; García-Carro, C.; Serón, D.; Soler, M.J. Effect of ramipril on kidney, lung and heart ACE2 in a diabetic mice model. Mol. Cell Endocrinol. 2021, 529, 111263. [Google Scholar] [CrossRef] [PubMed]
- Elemam, N.M.; Hasswan, H.; Aljaibeji, H.; Sulaiman, N. Circulating Soluble ACE2 and Upstream microRNA Expressions in Serum of Type 2 Diabetes Mellitus Patients. Int. J. Mol. Sci. 2021, 22, 5263. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, V.C.; Son, S.J.; Ahmadi, S.; Filosa, J.A.; Stern, J.E. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 2014, 63, 572–579. [Google Scholar] [CrossRef]
- Janelidze, S.; Hertze, J.; Nägga, K.; Nilsson, K.; Nilsson, C.; Swedish BioFINDERStudy Group Wennström, M.; van Westen, D.; Blennow, K.; Zetterberg, H.; Hansson, O. Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. Neurobiol. Aging 2017, 51, 104–112. [Google Scholar] [CrossRef]
- Xu, J.; Sriramula, S.; Xia, H.; Moreno-Walton, L.; Culicchia, F.; Domenig, O.; Poglitsch, M.; Lazartigues, E. Clinical Relevance and Role of Neuronal AT1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ. Res. 2017, 121, 43–55. [Google Scholar] [CrossRef]
- Parekh, R.U.; Sriramula, S. Activation of Kinin B1R Upregulates ADAM17 and Results in ACE2 Shedding in Neurons. Int. J. Mol. Sci. 2020, 22, 145. [Google Scholar] [CrossRef]
- Xia, H.; Sriramula, S.; Chhabra, K.H.; Lazartigues, E. Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ. Res. 2013, 113, 1087–1096. [Google Scholar] [CrossRef]
- Koka, V.; Huang, X.R.; Chung, A.C.; Wang, W.; Truong, L.D.; Lan, H.Y. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am. J. Pathol. 2008, 172, 1174–1183. [Google Scholar] [CrossRef]
- Xiao, L.; Haack, K.K.; Zucker, I.H. Angiotensin II regulates ACE and ACE2 in neurons through p38 mitogen-activated protein kinase and extracellular signal-regulated kinase 1/2 signaling. Am. J. Physiol. Cell Physiol. 2013, 304, C1073–C1079. [Google Scholar] [CrossRef]
- Gowrisankar, Y.V.; Clark, M.A. Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J. Neurochem. 2016, 138, 74–85. [Google Scholar] [CrossRef]
- Deshotels, M.R.; Xia, H.; Sriramula, S.; Lazartigues, E.; Filipeanu, C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 2014, 64, 1368–1375. [Google Scholar] [CrossRef]
- Mohammed, M.; Ogunlade, B.; Elgazzaz, M.; Berdasco, C.; Lakkappa, N.; Ghita, I.; Guidry, J.J.; Sriramula, S.; Xu, J.; Restivo, L.; et al. Nedd4-2 up-regulation is associated with ACE2 ubiquitination in hypertension. Cardiovasc. Res. 2023, 119, 2130–2141. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, J.; Martin, M.; He, M.; Gongol, B.; Marin, T.L.; Chen, L.; Shi, X.; Yin, Y.; Shang, F.; et al. AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 509–520. [Google Scholar] [CrossRef]
- Mehdipour, A.R.; Hummer, G. Dual nature of human ACE2 glycosylation in binding to SARS-CoV-2 spike. Proc. Natl. Acad. Sci. USA 2021, 118, e2100425118. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Dong, C.; Kim, S.; Hou, D.; Tai, W.; Du, L.; Im, W.; Zhang, X.F. Biomechanical characterization of SARS-CoV-2 spike RBD and human ACE2 protein-protein interaction. Biophys. J. 2021, 120, 1011–1019. [Google Scholar] [CrossRef]
- Isobe, A.; Arai, Y.; Kuroda, D.; Okumura, N.; Ono, T.; Ushiba, S.; Nakakita, S.I.; Daidoji, T.; Suzuki, Y.; Nakaya, T.; et al. ACE2 N-glycosylation modulates interactions with SARS-CoV-2 spike protein in a site-specific manner. Commun. Biol. 2022, 5, 1188. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, D.; Szabla, R.; Zheng, M.; Li, G.; Du, P.; Zheng, S.; Li, X.; Song, C.; Li, R.; et al. Broad and Differential Animal Angiotensin-Converting Enzyme 2 Receptor Usage by SARS-CoV-2. J. Virol. 2020, 94, e00940-20. [Google Scholar] [CrossRef]
- Lupala, C.S.; Kumar, V.; Su, X.D.; Wu, C.; Liu, H. Computational insights into differential interaction of mammalian angiotensin-converting enzyme 2 with the SARS-CoV-2 spike receptor binding domain. Comput. Biol. Med. 2022, 141, 105017. [Google Scholar] [CrossRef]
- Guo, Y.; He, W.; Mou, H.; Zhang, L.; Chang, J.; Peng, S.; Ojha, A.; Tavora, R.; Parcells, M.S.; Luo, G.; et al. An Engineered Receptor-Binding Domain Improves the Immunogenicity of Multivalent SARS-CoV-2 Vaccines. mBio 2021, 12, e00930-21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, X.; Zhai, A.; Xu, H.; Ma, S.; Liu, Y. Expression of apelin-13 and its negative correlation with TGF-β1 in patients with diabetic kidney disease. Exp. Ther. Med. 2024, 27, 110. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; McKinnie, S.M.; Patel, V.B.; Haddad, G.; Wang, Z.; Zhabyeyev, P.; Das, S.K.; Basu, R.; McLean, B.; Kandalam, V.; et al. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: Therapeutic potential of synthetic Apelin analogues. J. Am. Heart Assoc. 2013, 2, e000249. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; Zhou, Y.; Zhang, S.; Yang, F.; Guo, H.; Fan, R.; Feng, N.; Jia, M.; Wang, Y.; et al. Role of dynorphin in hypoxic pulmonary hypertension. Eur. J. Pharmacol. 2016, 791, 78–84. [Google Scholar] [CrossRef]
- Lei, S.; Hu, B. Ionic and signaling mechanisms involved in neurotensin-mediated excitation of central amygdala neurons. Neuropharmacology 2021, 196, 108714. [Google Scholar] [CrossRef]
- Pérez-Villavicencio, R.; Flores-Estrada, J.; Franco, M.; Escalante, B.; Pérez-Méndez, O.; Mercado, A.; Bautista-Pérez, R. Effect of Empagliflozin on Sphingolipid Catabolism in Diabetic and Hypertensive Rats. Int. J. Mol. Sci. 2022, 23, 2883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cassis, L.A.; Thatcher, S.E. Use of a Fluorescent Substrate to Measure ACE2 Activity in the Mouse Abdominal Aorta. Methods Mol. Biol. 2017, 1614, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Burns, K.D. Measurement of Angiotensin Converting Enzyme 2 Activity in Biological Fluid (ACE2). Methods Mol. Biol. 2017, 1527, 101–115. [Google Scholar] [CrossRef]
- Jezova, D.; Karailiev, P.; Karailievova, L.; Puhova, A.; Murck, H. Food Enrichment with Glycyrrhiza glabra Extract Suppresses ACE2 mRNA and Protein Expression in Rats-Possible Implications for COVID-19. Nutrients 2021, 13, 2321. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendiola-Salazar, X.A.; Munguía-Laguna, M.A.; Franco, M.; Cano-Martínez, A.; Santamaría Sosa, J.; Bautista-Pérez, R. SARS-CoV-2 Spike Protein Enhances Carboxypeptidase Activity of Angiotensin-Converting Enzyme 2. Int. J. Mol. Sci. 2024, 25, 6276. https://doi.org/10.3390/ijms25116276
Mendiola-Salazar XA, Munguía-Laguna MA, Franco M, Cano-Martínez A, Santamaría Sosa J, Bautista-Pérez R. SARS-CoV-2 Spike Protein Enhances Carboxypeptidase Activity of Angiotensin-Converting Enzyme 2. International Journal of Molecular Sciences. 2024; 25(11):6276. https://doi.org/10.3390/ijms25116276
Chicago/Turabian StyleMendiola-Salazar, Xóchitl Andrea, Melanie A. Munguía-Laguna, Martha Franco, Agustina Cano-Martínez, José Santamaría Sosa, and Rocío Bautista-Pérez. 2024. "SARS-CoV-2 Spike Protein Enhances Carboxypeptidase Activity of Angiotensin-Converting Enzyme 2" International Journal of Molecular Sciences 25, no. 11: 6276. https://doi.org/10.3390/ijms25116276
APA StyleMendiola-Salazar, X. A., Munguía-Laguna, M. A., Franco, M., Cano-Martínez, A., Santamaría Sosa, J., & Bautista-Pérez, R. (2024). SARS-CoV-2 Spike Protein Enhances Carboxypeptidase Activity of Angiotensin-Converting Enzyme 2. International Journal of Molecular Sciences, 25(11), 6276. https://doi.org/10.3390/ijms25116276




