Evolving Strategies for Use of Phytochemicals in Prevention and Long-Term Management of Cardiovascular Diseases (CVD)
Abstract
1. Introduction and Main Topic Review
2. Cardiovascular Diseases (CVDs) and Ischemia-Reperfusion: A Paradigm of Oxidative Stress-Mediated Pathogenesis
3. Representative Pharmaceutical Agents Used for CVD
3.1. Statins
3.2. Beta Blockers (β-Blockers)
3.3. Blood Thinners (Anticoagulants)
3.4. Aspirin
4. Emerging Plant Phytochemical Supplements with Cardiovascular Applications and Functional Foods
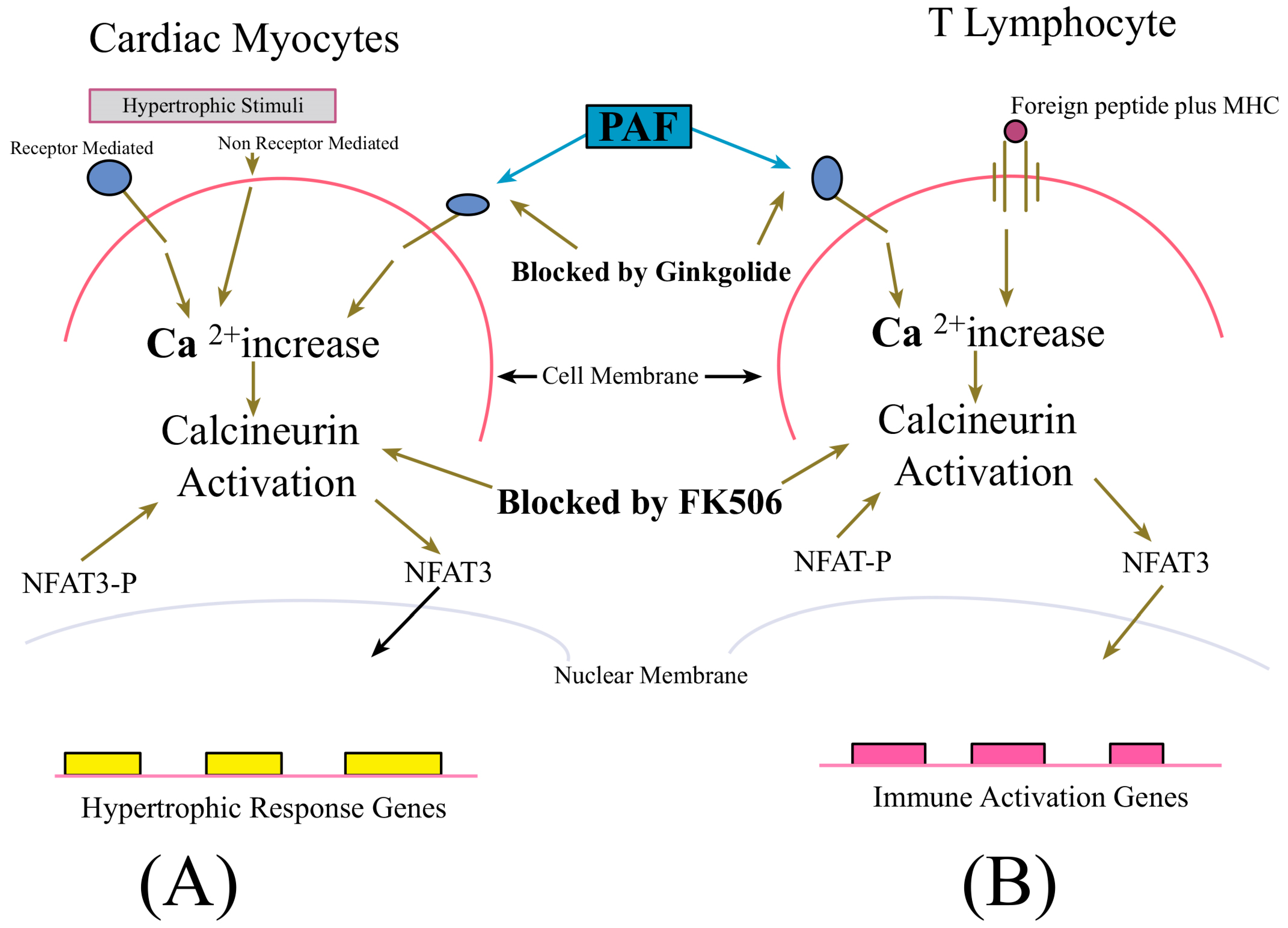
4.1. Ginkgolides
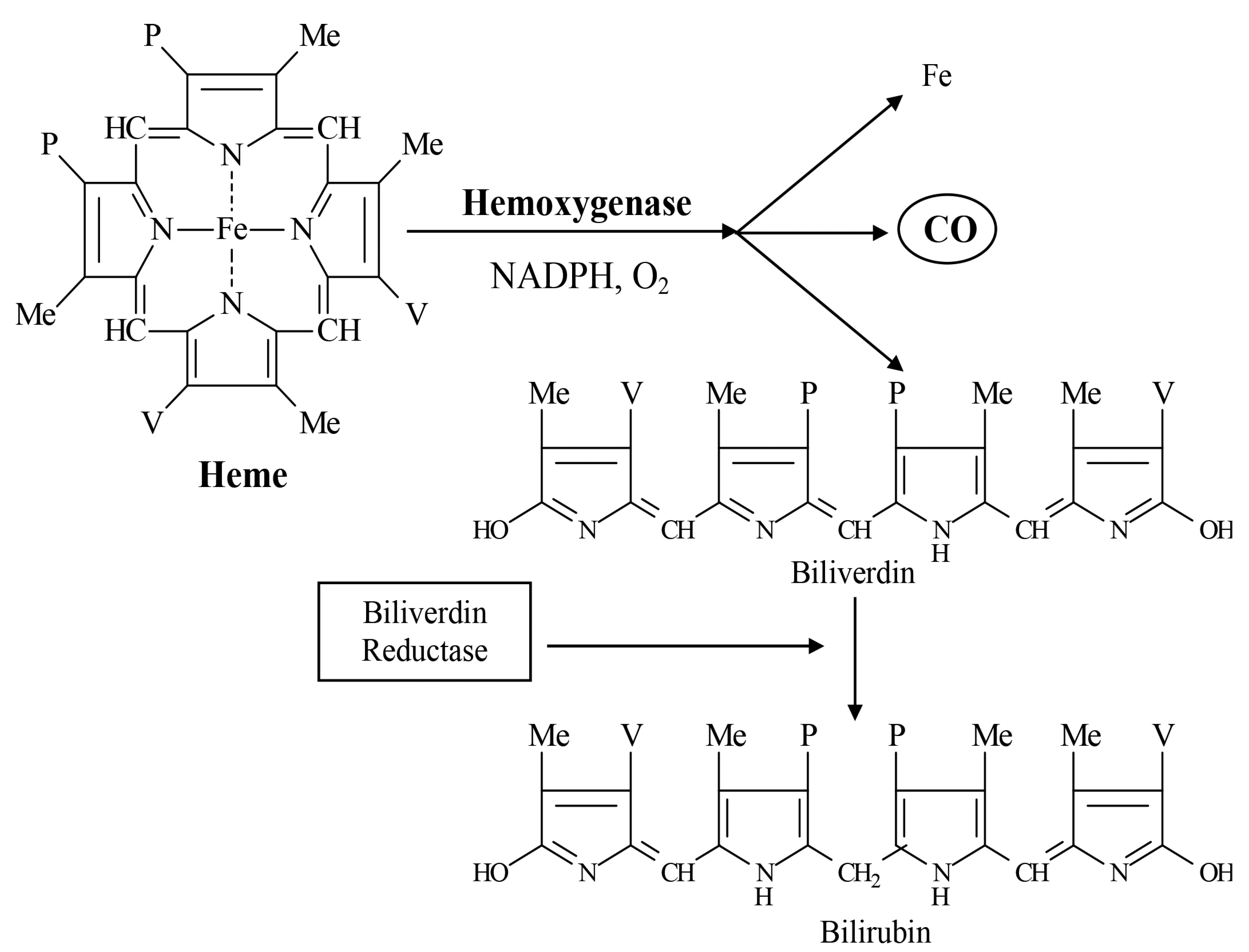
4.2. Heme Oxygenase Inducers
5. Major Physiologic Effects of HMOX Product Metabolites
6. Heme Oxygenase in Cardiovascular Disease: Limits of Clinical Use
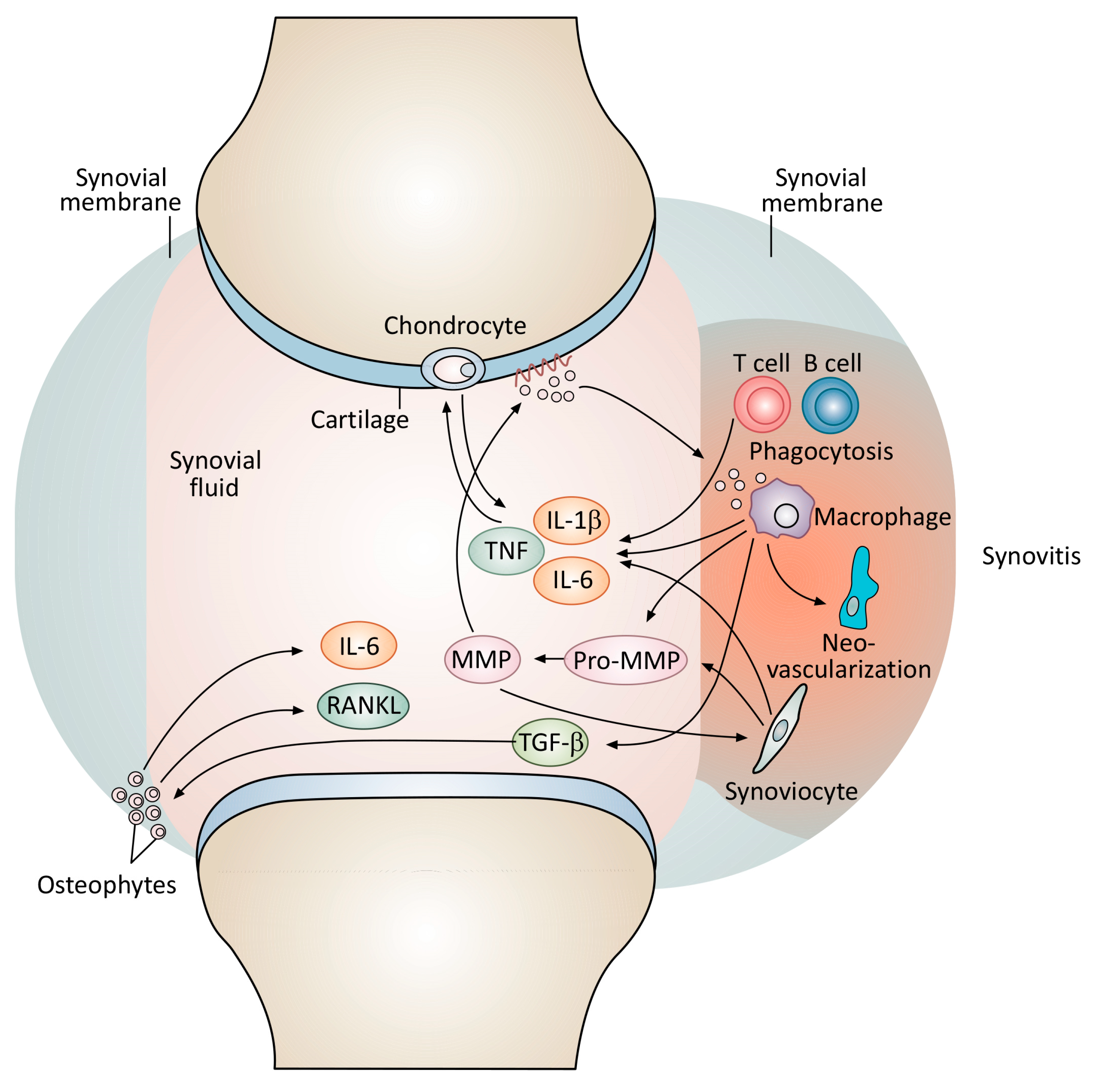
7. ‘Biotherapeutic’ Use of Heme Oxygenase in Osteoarthritis Treatment: Phase 1 Human Trials
8. Dietary Phytochemicals/Functional Foods
9. Future Directions
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef]
- Haines, D.; Tosaki, A. Emerging Clinical Applications of Heme Oxygenase. Curr. Pharm. Des. 2018, 24, 2227–2228. [Google Scholar] [CrossRef]
- Mahmoud, F.F.; Haines, D.D.; Abul, H.T.; Abal, A.T.; Onadeko, B.O.; Wise, J.A. In Vitro Effects of Astaxanthin Combined with Ginkgolide B on T Lymphocyte Activation in Peripheral Blood Mononuclear Cells from Asthmatic Subjects. J. Pharmacol. Sci. 2004, 94, 129–136. [Google Scholar] [CrossRef]
- Haines, D.D.; Tosaki, A. Heme Degradation in Pathophysiology of and Countermeasures to Inflammation-Associated Disease. Int. J. Mol. Sci. 2020, 21, 9698. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Suppression of the Nuclear Factor-kappaB Activation Pathway by Spice-Derived Phytochemicals: Reasoning for Seasoning. Ann. N. Y. Acad. Sci. 2004, 1030, 434–441. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Papageorgiou, S.G.; Piperi, C. Elucidating the Beneficial Effects of Ginger (Zingiber Officinale Roscoe) in Parkinson’s Disease. ACS Pharmacol. Transl. Sci. 2022, 5, 838–848. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-Dependent Transcription and Cell Survival by the SIRT1 Deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef]
- Yan, P.; Li, Z.; Xiong, J.; Geng, Z.; Wei, W.; Zhang, Y.; Wu, G.; Zhuang, T.; Tian, X.; Liu, Z.; et al. LARP7 Ameliorates Cellular Senescence and Aging by Allosterically Enhancing SIRT1 Deacetylase Activity. Cell Rep. 2021, 37, 110038. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, Y.; Lv, B.; Tian, Z.; Zhang, B. Intermittent Fasting Improves High-Fat Diet-Induced Obesity Cardiomyopathy via Alleviating Lipid Deposition and Apoptosis and Decreasing m6A Methylation in the Heart. Nutrients 2022, 14, 251. [Google Scholar] [CrossRef]
- Martin, K.R. Targeting Apoptosis with Dietary Bioactive Agents. Exp. Biol. Med. 2006, 231, 117–129. [Google Scholar] [CrossRef]
- Karaboga Arslan, A.K.; Uzunhisarcıklı, E.; Yerer, M.B.; Bishayee, A. The Golden Spice Curcumin in Cancer: A Perspective on Finalized Clinical Trials during the Last 10 Years. J. Cancer Res. Ther. 2022, 18, 19–26. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Bal, S.; Sharangi, A.B.; Upadhyay, T.K.; Khan, F.; Pandey, P.; Siddiqui, S.; Saeed, M.; Lee, H.-J.; Yadav, D.K. Biomedical and Antioxidant Potentialities in Chilli: Perspectives and Way Forward. Molecules 2022, 27, 6380. [Google Scholar] [CrossRef]
- Morré, D.J.; Morré, D.M. Synergistic Capsicum-Tea Mixtures with Anticancer Activity. J. Pharm. Pharmacol. 2003, 55, 987–994. [Google Scholar] [CrossRef]
- Abadi, A.J.; Mirzaei, S.; Mahabady, M.K.; Hashemi, F.; Zabolian, A.; Hashemi, F.; Raee, P.; Aghamiri, S.; Ashrafizadeh, M.; Aref, A.R.; et al. Curcumin and Its Derivatives in Cancer Therapy: Potentiating Antitumor Activity of Cisplatin and Reducing Side Effects. Phytother. Res. 2022, 36, 189–213. [Google Scholar] [CrossRef]
- Aftab, N.; Vieira, A. Antioxidant Activities of Curcumin and Combinations of This Curcuminoid with Other Phytochemicals. Phytother. Res. 2010, 24, 500–502. [Google Scholar] [CrossRef]
- Mishra, N.; Agarwal, R. Research Models of Sulfur Mustard- and Nitrogen Mustard-Induced Ocular Injuries and Potential Therapeutics. Exp. Eye Res. 2022, 223, 109209. [Google Scholar] [CrossRef]
- Haines, D.D.; Bak, I.; Ferdinandy, P.; Mahmoud, F.F.; Al-Harbi, S.A.; Blasig, I.E.; Tosaki, A. Cardioprotective Effects of the Calcineurin Inhibitor FK506 and the PAF Receptor Antagonist and Free Radical Scavenger, EGb 761, in Isolated Ischemic/Reperfused Rat Hearts. J. Cardiovasc. Pharmacol. 2000, 35, 37–44. [Google Scholar] [CrossRef]
- Cowan, F.M.; Broomfield, C.A.; Nealley, E.W.; Petrali, J.P. Anti-Inflammatory and Morphocytotoxic Actions of 4-Methyl-2-Mercaptopyridine-1-Oxide in Sulfur Mustard-Exposed Skin and Lung Epithelial Cell Cultures: Implications for Broad Spectrum Countermeasures; U.S. Army Medical Research and Materiel Command Medical Defense Bioscience Review: Baltimore, MD, USA, 2006. [Google Scholar]
- Zhang, L.; Li, G.; Tao, S.; Xia, P.; Chaudhry, N.; Kaura, S.; Stone, S.S.; Liu, M. Ginkgo Biloba Extract Reduces Cardiac and Brain Inflammation in Rats Fed a HFD and Exposed to Chronic Mental Stress through NF-κB Inhibition. Mediators Inflamm. 2022, 2022, 2408598. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Xu, X.; Wang, H.; Wang, D.; Yan, W.; Zhu, J.; Hao, H.; Wang, G.; Cao, L.; et al. Ginkgo Biloba Extract Ameliorates Atherosclerosis via Rebalancing Gut Flora and Microbial Metabolism. Phytother. Res. 2022, 36, 2463–2480. [Google Scholar] [CrossRef]
- Haines, D.D.; Varga, B.; Bak, I.; Juhasz, B.; Mahmoud, F.F.; Kalantari, H.; Gesztelyi, R.; Lekli, I.; Czompa, A.; Tosaki, A. Summative Interaction between Astaxanthin, Ginkgo Biloba Extract (EGb761) and Vitamin C in Suppression of Respiratory Inflammation: A Comparison with Ibuprofen. Phytother. Res. 2011, 25, 128–136. [Google Scholar] [CrossRef]
- Coutinho-Wolino, K.S.; Almeida, P.P.; Mafra, D.; Stockler-Pinto, M.B. Bioactive Compounds Modulating Toll-like 4 Receptor (TLR4)-Mediated Inflammation: Pathways Involved and Future Perspectives. Nutr. Res. 2022, 107, 96–116. [Google Scholar] [CrossRef]
- McCarty, M.F. Nutraceutical, Dietary, and Lifestyle Options for Prevention and Treatment of Ventricular Hypertrophy and Heart Failure. Int. J. Mol. Sci. 2021, 22, 3321. [Google Scholar] [CrossRef]
- Rafati-Rahimzadeh, M.; Rafati-Rahimzadeh, M.; Kazemi, S.; Jafarian Amiri, S.R.; Soleymani, A.; Moghadamnia, A.A. Ophthalmological Aspects of Mustard Gas Poisoning (Focus on Management). Caspian J. Intern. Med. 2022, 13, 458–468. [Google Scholar] [CrossRef]
- Cowan, F.M.; Broomfield, C.A.; Stojiljkovic, M.P.; Smith, W.J. A Review of Multi-Threat Medical Countermeasures against Chemical Warfare and Terrorism. Mil. Med. 2004, 169, 850–855. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Wang, Q.; Liu, F.; Yang, W.; Sui, X.; Yang, J.; Zhang, M.; Wang, S.; Xiao, Z.; et al. Potential Common Mechanisms of Cytotoxicity Induced by Amide Herbicides via TRPA1 Channel Activation. Int. J. Environ. Res. Public. Health 2022, 19, 7985. [Google Scholar] [CrossRef]
- Cowan, F. Revisiting Medical Implications of the Multi-Threat Medical Countermeasure Hypothesis; ResearchGate GmbH: Berlin, Germany, 2023. [Google Scholar]
- Cowan, F.M. Phytochemical Combinations That Regulate Pathological Immunity. U.S. Patent US20110305779A1, 1 December 2011. [Google Scholar]
- Dasuni Wasana, P.W.; Hasriadi; Muangnoi, C.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P.; Towiwat, P. Curcumin and Metformin Synergistically Modulate Peripheral and Central Immune Mechanisms of Pain. Sci. Rep. 2022, 12, 9713. [Google Scholar] [CrossRef]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short, G.F.; Staunton, J.E.; Jin, X.; et al. Synergistic Drug Combinations Tend to Improve Therapeutically Relevant Selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Effects of Once-Weekly Semaglutide 2.4 Mg on C-Reactive Protein in Adults with Overweight or Obesity (STEP 1, 2, and 3): Exploratory Analyses of Three Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trials—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/36467859/ (accessed on 10 May 2024).
- Surbatovic, M.; Filipovic, N.; Radakovic, S.; Stankovic, N.; Slavkovic, Z. Immune Cytokine Response in Combat Casualties: Blast or Explosive Trauma with or without Secondary Sepsis. Mil. Med. 2007, 172, 190–195. [Google Scholar] [CrossRef]
- Alharbi, S.H. Anti-Inflammatory Role of Glucagon-like Peptide 1 Receptor Agonists and Its Clinical Implications. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188231222367. [Google Scholar] [CrossRef]
- Shahriary, A.; Panahi, Y.; Shirali, S.; Rahmani, H. Relationship of Serum Levels of Interleukin 6, Interleukin 8, and C-Reactive Protein with Forced Expiratory Volume in First Second in Patients with Mustard Lung and Chronic Obstructive Pulmonary Diseases: Systematic Review and Meta-Analysis. Postepy Dermatol. Alergol. 2017, 34, 192–198. [Google Scholar] [CrossRef]
- Attaran, D.; Lari, S.M.; Khajehdaluee, M.; Ayatollahi, H.; Towhidi, M.; Asnaashari, A.; Marallu, H.G.; Mazloomi, M.; Mood, M.B. Highly Sensitive C-Reactive Protein Levels in Iranian Patients with Pulmonary Complication of Sulfur Mustard Poisoning and Its Correlation with Severity of Airway Diseases. Hum. Exp. Toxicol. 2009, 28, 739–745. [Google Scholar] [CrossRef]
- Attaran, D.; Lari, S.M.; Towhidi, M.; Marallu, H.G.; Ayatollahi, H.; Khajehdaluee, M.; Ghanei, M.; Basiri, R. Interleukin-6 and Airflow Limitation in Chemical Warfare Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct Pulmon Dis. 2010, 5, 335–340. [Google Scholar] [CrossRef]
- Pourfarzam, S.; Ghazanfari, T.; Yaraee, R.; Ghasemi, H.; Hassan, Z.M.; Faghihzadeh, S.; Ardestani, S.K.; Kariminia, A.; Fallahi, F.; Soroush, M.R.; et al. Serum Levels of IL-8 and IL-6 in the Long Term Pulmonary Complications Induced by Sulfur Mustard: Sardasht-Iran Cohort Study. Int. Immunopharmacol. 2009, 9, 1482–1488. [Google Scholar] [CrossRef]
- Etemad, L.; Moshiri, M.; Balali-Mood, M. Delayed Complications and Long-Term Management of Sulfur Mustard Poisoning: A Narrative Review of Recent Advances by Iranian Researchers Part ІІ: Clinical Management and Therapy. Iran. J. Med. Sci. 2018, 43, 235–247. [Google Scholar]
- Ramos, E.; Gil-Martín, E.; De Los Ríos, C.; Egea, J.; López-Muñoz, F.; Pita, R.; Juberías, A.; Torrado, J.J.; Serrano, D.R.; Reiter, R.J.; et al. Melatonin as Modulator for Sulfur and Nitrogen Mustard-Induced Inflammation, Oxidative Stress and DNA Damage: Molecular Therapeutics. Antioxidants 2023, 12, 397. [Google Scholar] [CrossRef]
- Czegledi, A.; Tosaki, A.; Gyongyosi, A.; Zilinyi, R.; Tosaki, A.; Lekli, I. Electrically-Induced Ventricular Fibrillation Alters Cardiovascular Function and Expression of Apoptotic and Autophagic Proteins in Rat Hearts. Int. J. Mol. Sci. 2019, 20, 1628. [Google Scholar] [CrossRef]
- GBD 2019 Stroke Collaborators. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- GBD 2013 Mortality and Causes of Death Collaborators. Global, Regional, and National Age-Sex Specific All-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Mendis, S.; Puska, P.; Norrving, B.; World Health Organization; World Heart Federation; World Stroke Organization. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Koltai, M.; Tosaki, A.; Hosford, D.; Braquet, P. Ginkgolide B Protects Isolated Hearts against Arrhythmias Induced by Ischemia but Not Reperfusion. Eur. J. Pharmacol. 1989, 164, 293–302. [Google Scholar] [CrossRef]
- Chen, C.; Yu, L.-T.; Cheng, B.-R.; Xu, J.-L.; Cai, Y.; Jin, J.-L.; Feng, R.-L.; Xie, L.; Qu, X.-Y.; Li, D.; et al. Promising Therapeutic Candidate for Myocardial Ischemia/Reperfusion Injury: What Are the Possible Mechanisms and Roles of Phytochemicals? Front. Cardiovasc. Med. 2021, 8, 792592. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, M.; Zheng, B.; Zhang, Y.; Chu, X.; Liu, Y.; Li, Z.; Han, X.; Chu, L. [8]-Gingerol Exerts Anti-myocardial Ischemic Effects in Rats via Modulation of the MAPK Signaling Pathway and L-type Ca2+ Channels. Pharmacol. Res. Perspect. 2021, 9, e00852. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, J.; Liu, X.; Tibenda, J.J.; Wang, X.; Zhao, Q. Therapeutic Targets by Traditional Chinese Medicine for Ischemia-Reperfusion Injury Induced Apoptosis on Cardiovascular and Cerebrovascular Diseases. Front. Pharmacol. 2022, 13, 934256. [Google Scholar] [CrossRef]
- Mandal, D.; Sarkar, T.; Chakraborty, R. Critical Review on Nutritional, Bioactive, and Medicinal Potential of Spices and Herbs and Their Application in Food Fortification and Nanotechnology. Appl. Biochem. Biotechnol. 2023, 195, 1319–1513. [Google Scholar] [CrossRef]
- Villa-Rivera, M.G.; Ochoa-Alejo, N. Chili Pepper Carotenoids: Nutraceutical Properties and Mechanisms of Action. Molecules 2020, 25, 5573. [Google Scholar] [CrossRef]
- Munjuluri, S.; Wilkerson, D.A.; Sooch, G.; Chen, X.; White, F.A.; Obukhov, A.G. Capsaicin and TRPV1 Channels in the Cardiovascular System: The Role of Inflammation. Cells 2021, 11, 18. [Google Scholar] [CrossRef]
- Castrejón-Téllez, V.; del Valle-Mondragón, L.; Pérez-Torres, I.; Guarner-Lans, V.; Pastelín-Hernández, G.; Ruiz-Ramírez, A.; Díaz-Juárez, J.A.; Varela-López, E.; Oidor-Chan, V.H.; Vargas-González, A.; et al. TRPV1 Contributes to Modulate the Nitric Oxide Pathway and Oxidative Stress in the Isolated and Perfused Rat Heart during Ischemia and Reperfusion. Molecules 2022, 27, 1031. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, X.; Zhang, X.; Jia, X.; Liu, J.; Wang, X.; Xiao, Y.; Xiao, Z.; Liu, T.; Dong, Y. The Application of Proteomics and Metabolomics to Reveal the Molecular Mechanism of Nutmeg-5 in Ameliorating Cardiac Fibrosis Following Myocardial Infarction. Phytomedicine 2022, 105, 154382. [Google Scholar] [CrossRef]
- Ayer, A.; Zarjou, A.; Agarwal, A.; Stocker, R. Heme Oxygenases in Cardiovascular Health and Disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Abdel Moneim, A.E.; Hafez, T.A.; Mubaraki, M.A.; Mohamed, W.F.; Thagfan, F.A.; Al-Quraishy, S. Myristica Fragrans Kernels Prevent Paracetamol-Induced Hepatotoxicity by Inducing Anti-Apoptotic Genes and Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2019, 20, 993. [Google Scholar] [CrossRef]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; et al. Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Mental Disorders Collaborators. Global, Regional, and National Burden of 12 Mental Disorders in 204 Countries and Territories, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Zhang, M.; Yang, H.; Wang, Y. Consumption of Coffee and Tea with All-Cause and Cause-Specific Mortality: A Prospective Cohort Study. BMC Med. 2022, 20, 449. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenges in Developing Countries; Fuster, V.; Kelly, B.B. Epidemiology of Cardiovascular Disease. In Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health; National Academies Press (US): Washington, DC, USA, 2010. [Google Scholar]
- Moran, A.E.; Forouzanfar, M.H.; Roth, G.A.; Mensah, G.A.; Ezzati, M.; Murray, C.J.L.; Naghavi, M. Temporal Trends in Ischemic Heart Disease Mortality in 21 World Regions, 1980 to 2010: The Global Burden of Disease 2010 Study. Circulation 2014, 129, 1483–1492. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.L.; Redline, S.; Emmons, K.M. Sleep as a Potential Fundamental Contributor to Disparities in Cardiovascular Health. Annu. Rev. Public. Health 2015, 36, 417–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dong, Y.; Weng, J.; Kontos, E.Z.; Chervin, R.D.; Rosen, C.L.; Marcus, C.L.; Redline, S. Associations among Neighborhood, Race, and Sleep Apnea Severity in Children. A Six-City Analysis. Ann. Am. Thorac. Soc. 2017, 14, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Kris-Etherton, P.M. Diet Quality Assessment and the Relationship between Diet Quality and Cardiovascular Disease Risk. Nutrients 2021, 13, 4305. [Google Scholar] [CrossRef] [PubMed]
- Ssekubugu, R.; Makumbi, F.; Enriquez, R.; Lagerström, S.R.; Yeh, P.T.; Kennedy, C.E.; Gray, R.H.; Negesa, L.; Serwadda, D.M.; Kigozi, G.; et al. Cardiovascular (Framingham) and Type II Diabetes (Finnish Diabetes) Risk Scores: A Qualitative Study of Local Knowledge of Diet, Physical Activity and Body Measurements in Rural Rakai, Uganda. BMC Public. Health 2022, 22, 2214. [Google Scholar] [CrossRef]
- Roldan, C.A.; Sibbitt, W.L.; Greene, E.R.; Qualls, C.R.; Jung, R.E. Libman-Sacks Endocarditis and Associated Cerebrovascular Disease: The Role of Medical Therapy. PLoS ONE 2021, 16, e0247052. [Google Scholar] [CrossRef]
- Heymans, S.; Lakdawala, N.K.; Tschöpe, C.; Klingel, K. Dilated Cardiomyopathy: Causes, Mechanisms, and Current and Future Treatment Approaches. Lancet 2023, 402, 998–1011. [Google Scholar] [CrossRef]
- Japp, A.G.; Gulati, A.; Cook, S.A.; Cowie, M.R.; Prasad, S.K. The Diagnosis and Evaluation of Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2996–3010. [Google Scholar] [CrossRef] [PubMed]
- Lampejo, T.; Durkin, S.M.; Bhatt, N.; Guttmann, O. Acute Myocarditis: Aetiology, Diagnosis and Management. Clin. Med. 2021, 21, e505–e510. [Google Scholar] [CrossRef]
- Cihakova, D.; Rose, N.R. Pathogenesis of Myocarditis and Dilated Cardiomyopathy. Adv. Immunol. 2008, 99, 95–114. [Google Scholar] [CrossRef]
- Pagura, L.; Imazio, M.; Merlo, M.; Sinagra, G. Ten questions about eosinophilic myocarditis. G Ital. Cardiol. 2022, 23, 259–267. [Google Scholar] [CrossRef]
- Brambatti, M.; Matassini, M.V.; Adler, E.D.; Klingel, K.; Camici, P.G.; Ammirati, E. Eosinophilic Myocarditis: Characteristics, Treatment, and Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2363–2375. [Google Scholar] [CrossRef]
- Khurshid, S.; Al-Alusi, M.A.; Churchill, T.W.; Guseh, J.S.; Ellinor, P.T. Accelerometer-Derived “Weekend Warrior” Physical Activity and Incident Cardiovascular Disease. JAMA 2023, 330, 247–252. [Google Scholar] [CrossRef]
- McGill, H.C.; McMahan, C.A.; Gidding, S.S. Preventing Heart Disease in the 21st Century: Implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Study. Circulation 2008, 117, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Chin, S.L.; Rangarajan, S.; Xavier, D.; Liu, L.; Zhang, H.; Rao-Melacini, P.; Zhang, X.; Pais, P.; Agapay, S.; et al. Global and Regional Effects of Potentially Modifiable Risk Factors Associated with Acute Stroke in 32 Countries (INTERSTROKE): A Case-Control Study. Lancet 2016, 388, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Schulz, R. Pleiotropic Effects of Dronedarone on Ischemia/Reperfusion Injury in Heart and Brain. Cardiovasc. Drugs Ther. 2012, 26, 257–263. [Google Scholar] [CrossRef]
- Costa, C.H.S.; Oliveira, A.R.S.; Dos Santos, A.M.; da Costa, K.S.; Lima, A.H.L.E.; Alves, C.N.; Lameira, J. Computational Study of Conformational Changes in Human 3-Hydroxy-3-Methylglutaryl Coenzyme Reductase Induced by Substrate Binding. J. Biomol. Struct. Dyn. 2019, 37, 4374–4383. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.A. Aggressive Lipid Therapy in the Statin Era. Prog. Cardiovasc. Dis. 1998, 41, 71–94. [Google Scholar] [CrossRef]
- Brandts, J.; Ray, K.K. Novel and Future Lipid-Modulating Therapies for the Prevention of Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 600–616. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory From the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; Brugts, J.; Ades, T. Comparative Tolerability and Harms of Individual Statins: A Study-Level Network Meta-Analysis of 246 955 Participants from 135 Randomized, Controlled Trials. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Sirtori, C.R.; Carugo, S.; Banach, M.; Corsini, A. Bempedoic Acid: For Whom and When. Curr. Atheroscler. Rep. 2022, 24, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Abd, T.T.; Jacobson, T.A. Statin-Induced Myopathy: A Review and Update. Expert. Opin. Drug Saf. 2011, 10, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Arcangelo, V.P.; Peterson, A.M. Pharmacotherapeutics for Advanced Practice: A Practical Approach; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; ISBN 978-0-7817-5784-3. [Google Scholar]
- Arnold, S.V. Beta-Blockers: The Constantly Swinging Pendulum. J. Am. Coll. Cardiol. 2023, 81, 2312–2314. [Google Scholar] [CrossRef] [PubMed]
- Freemantle, N.; Cleland, J.; Young, P.; Mason, J.; Harrison, J. Beta Blockade after Myocardial Infarction: Systematic Review and Meta Regression Analysis. BMJ 1999, 318, 1730–1737. [Google Scholar] [CrossRef]
- Frishman, W.H.; Cheng-Lai, A.; Nawarskas, J. Current Cardiovascular Drugs, 4th ed.; Current Medicine Group: London, UK, 2005; ISBN 978-1-57340-221-7. [Google Scholar]
- Stapleton, M.P. Sir James Black and Propranolol. The Role of the Basic Sciences in the History of Cardiovascular Pharmacology. Tex. Heart Inst. J. 1997, 24, 336–342. [Google Scholar]
- Schneier, F.R. Clinical Practice. Social Anxiety Disorder. N. Engl. J. Med. 2006, 355, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xu, S.; Xu, J.; Xin, Y.; Lu, Y.; Zhang, H.; Zhou, B.; Xu, H.; Sheu, S.-S.; Tian, R.; et al. Elevated MCU Expression by CaMKIIδB Limits Pathological Cardiac Remodeling. Circulation 2022, 145, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Tyrer, P. Anxiolytics Not Acting at the Benzodiazepine Receptor: Beta Blockers. Prog. Neuropsychopharmacol. Biol. Psychiatry 1992, 16, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Tindall, B. Better Playing through Chemistry; The New York Times: New York, NY, USA, 2004. [Google Scholar]
- Elliott, W.J.; Meyer, P.M. Incident Diabetes in Clinical Trials of Antihypertensive Drugs: A Network Meta-Analysis. Lancet 2007, 369, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Pope, E. Commentary: Beta-Blockers and Sleep Problems. Pediatr. Dermatol. 2021, 38, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Cojocariu, S.A.; Maștaleru, A.; Sascău, R.A.; Stătescu, C.; Mitu, F.; Leon-Constantin, M.M. Neuropsychiatric Consequences of Lipophilic Beta-Blockers. Medicina 2021, 57, 155. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, B.B.; Daly, M.C.; Freniere, B.; Higgins, J.P.; Safa, B.; Eberlin, K.R. Leech Therapy Following Digital Replantation and Revascularization. J. Hand Surg. Am. 2020, 45, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, E.A.; Whitaker, I.S.; Rozen, W.M.; Naderi, N.; Kon, M. Chemical and Mechanical Alternatives to Leech Therapy: A Systematic Review and Critical Appraisal. J. Reconstr. Microsurg. 2011, 27, 481–486. [Google Scholar] [CrossRef]
- Camaj, A.; Fuster, V.; Giustino, G.; Bienstock, S.W.; Sternheim, D.; Mehran, R.; Dangas, G.D.; Kini, A.; Sharma, S.K.; Halperin, J.; et al. Left Ventricular Thrombus Following Acute Myocardial Infarction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 1010–1022. [Google Scholar] [CrossRef]
- Drop, J.G.F.; Wildschut, E.D.; Gunput, S.T.G.; de Hoog, M.; van Ommen, C.H. Challenges in Maintaining the Hemostatic Balance in Children Undergoing Extracorporeal Membrane Oxygenation: A Systematic Literature Review. Front. Pediatr. 2020, 8, 612467. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Safe Anticoagulation When Heart and Lungs Are “on Vacation”. Ann. Transl. Med. 2015, 3, S11. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.; Kaide, C.G. Emergency Reversal of Anticoagulation. West. J. Emerg. Med. 2019, 20, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Zareh, M.; Davis, A.; Henderson, S. Reversal of Warfarin-Induced Hemorrhage in the Emergency Department. West. J. Emerg. Med. 2011, 12, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Bahit, M.C.; Vora, A.N.; Li, Z.; Wojdyla, D.M.; Thomas, L.; Goodman, S.G.; Aronson, R.; Jordan, J.D.; Kolls, B.J.; Dombrowski, K.E.; et al. Apixaban or Warfarin and Aspirin or Placebo After Acute Coronary Syndrome or Percutaneous Coronary Intervention in Patients With Atrial Fibrillation and Prior Stroke: A Post Hoc Analysis From the AUGUSTUS Trial. JAMA Cardiol. 2022, 7, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Ageno, W.; Donadini, M. Breadth of Complications of Long-Term Oral Anticoagulant Care. Hematology Am. Soc. Hematol. Educ. Program. 2018, 2018, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Schrag, D.; Uno, H.; Rosovsky, R.; Rutherford, C.; Sanfilippo, K.; Villano, J.L.; Drescher, M.; Jayaram, N.; Holmes, C.; Feldman, L.; et al. Direct Oral Anticoagulants vs Low-Molecular-Weight Heparin and Recurrent VTE in Patients With Cancer: A Randomized Clinical Trial. JAMA 2023, 329, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; Sobieraj-Teague, M.; Eikelboom, J.W. Direct Oral Anticoagulants: Evidence and Unresolved Issues. Lancet 2020, 396, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Marston, X.L.; Wang, R.; Yeh, Y.-C.; Zimmermann, L.; Ye, X.; Gao, X.; Brüggenjürgen, B.; Unverdorben, M. Comparison of Clinical Outcomes of Edoxaban versus Apixaban, Dabigatran, Rivaroxaban, and Vitamin K Antagonists in Patients with Atrial Fibrillation in Germany: A Real-World Cohort Study. Int. J. Cardiol. 2022, 346, 93–99. [Google Scholar] [CrossRef]
- Kahale, L.A.; Hakoum, M.B.; Tsolakian, I.G.; Matar, C.F.; Barba, M.; Yosuico, V.E.D.; Terrenato, I.; Sperati, F.; Schünemann, H.; Akl, E.A. Oral Anticoagulation in People with Cancer Who Have No Therapeutic or Prophylactic Indication for Anticoagulation. Cochrane Database Syst. Rev. 2017, 12, CD006466. [Google Scholar] [CrossRef]
- Jeffreys, D. Aspirin: The Remarkable Story of a Wonder Drug; Bloomsbury: London, UK, 2005; ISBN 978-0-7475-7083-7. [Google Scholar]
- Elwood, P.; Protty, M.; Morgan, G.; Pickering, J.; Delon, C.; Watkins, J. Aspirin and Cancer: Biological Mechanisms and Clinical Outcomes. Open Biol. 2016, 12, 220124. [Google Scholar] [CrossRef]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. JAMA 2021, 325, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Aspirin Monograph for Professionals. Available online: https://www.drugs.com/monograph/aspirin.html (accessed on 14 August 2023).
- Tasoudis, P.T.; Kyriakoulis, I.G.; Sagris, D.; Diener, H.C.; Ntaios, G. Clopidogrel Monotherapy versus Aspirin Monotherapy in Patients with Established Cardiovascular Disease: Systematic Review and Meta-Analysis. Thromb. Haemost. 2022, 122, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Soodi, D.; VanWormer, J.J.; Rezkalla, S.H. Aspirin in Primary Prevention of Cardiovascular Events. Clin. Med. Res. 2020, 18, 89–94. [Google Scholar] [CrossRef]
- Yeomans, N.D. Editorial: Risk of Gastric and Duodenal Ulcers among New Users of Low-Dose Aspirin. Aliment. Pharmacol. Ther. 2022, 56, 334–335. [Google Scholar] [CrossRef]
- Stevens, W.W.; Jerschow, E.; Baptist, A.P.; Borish, L.; Bosso, J.V.; Buchheit, K.M.; Cahill, K.N.; Campo, P.; Cho, S.H.; Keswani, A.; et al. The Role of Aspirin Desensitization Followed by Oral Aspirin Therapy in Managing Patients with Aspirin-Exacerbated Respiratory Disease: A Work Group Report from the Rhinitis, Rhinosinusitis and Ocular Allergy Committee of the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2021, 147, 827–844. [Google Scholar] [CrossRef]
- Singh, R.; Tiwari, M.K.; Gangopadhyay, D.; Mishra, P.C.; Mishra, H.; Srivastava, A.; Singh, R.K. Detection and Monitoring of in Vitro Formation of Salicylic Acid from Aspirin Using Fluorescence Spectroscopic Technique and DFT Calculations. J. Photochem. Photobiol. B 2018, 189, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Crane, P. Ginkgo: The Tree That Time Forgot; Yale University Press: London, UK, 2013; ISBN 978-0-300-18751-9. [Google Scholar]
- Chen, Y.; Fu, C.; Wu, Z.; Xu, H.; Liu, H.; Schneider, H.; Lin, J. Ginkgo Biloba . Trends Genet. 2021, 37, 488–489. [Google Scholar] [CrossRef]
- Varga, E.; Bodi, A.; Ferdinandy, P.; Droy-Lefaix, M.-T.; Blasig, I.E.; Tosaki, A. The Protective Effect of EGb 761 in Isolated Ischemic/Reperfused Rat Hearts: A Link Between Cardiac Function and Nitric Oxide Production J Cardiovasc Pharmacol. 1999, 34, 711–717. 34. [CrossRef]
- Montes, P.; Ruiz-Sanchez, E.; Rojas, C.; Rojas, P. Ginkgo Biloba Extract 761: A Review of Basic Studies and Potential Clinical Use in Psychiatric Disorders. CNS Neurol. Disord. Drug Targets 2015, 14, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Xiang, Y.-Q.; Ng, C.H.; Ungvari, G.S.; Chiu, H.F.K.; Xiang, Y.-T. Extract of Ginkgo Biloba for Tardive Dyskinesia: Meta-Analysis of Randomized Controlled Trials. Pharmacopsychiatry 2016, 49, 107–111. [Google Scholar] [CrossRef]
- Satoh, H.; Nishida, S. Electropharmacological Actions of Ginkgo Biloba Extract on Vascular Smooth and Heart Muscles. Clin. Chim. Acta 2004, 342, 13–22. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.H.; de Groot, A.; Evstatieva, L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- van Beek, T.A.; Montoro, P. Chemical Analysis and Quality Control of Ginkgo Biloba Leaves, Extracts, and Phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.; Tosaki, A.; Mahmoud, F.F. Immunosuppressive Compositions Comprising an Immunophilin-Binding Compound and a Gingkolide Compound. Application PCT/US2001/014718 (WO2001085206A2 WIPO (PCT), 8 May 2001. [Google Scholar]
- Molkentin, J.D.; Lu, J.R.; Antos, C.L.; Markham, B.; Richardson, J.; Robbins, J.; Grant, S.R.; Olson, E.N. A Calcineurin-Dependent Transcriptional Pathway for Cardiac Hypertrophy. Cell 1998, 93, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Lekli, I.; Teissier, P.; Bak, I.; Tosaki, A. Role of Haeme Oxygenase-1 in Resolution of Oxidative Stress-Related Pathologies: Focus on Cardiovascular, Lung, Neurological and Kidney Disorders. Acta Physiol. 2012, 204, 487–501. [Google Scholar] [CrossRef]
- Ulengin-Talkish, I.; Cyert, M.S. A Cellular Atlas of Calcineurin Signaling. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119366. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Chen, Y.; Zhang, L.; Wu, Y.; Li, H.; Wu, Y.; Wang, B.; Sun, Z.; Li, Y.; Lv, Q.; et al. Delivery of FK506-Loaded PLGA Nanoparticles Prolongs Cardiac Allograft Survival. Int. J. Pharm. 2020, 575, 118951. [Google Scholar] [CrossRef] [PubMed]
- Jahr, H.; van der Windt, A.E.; Timur, U.T.; Baart, E.B.; Lian, W.-S.; Rolauffs, B.; Wang, F.-S.; Pufe, T. Physosmotic Induction of Chondrogenic Maturation Is TGF-β Dependent and Enhanced by Calcineurin Inhibitor FK506. Int. J. Mol. Sci. 2022, 23, 5110. [Google Scholar] [CrossRef] [PubMed]
- Tosaki, A.; Droy-Lefaix, M.T.; Pali, T.; Das, D.K. Effects of SOD, Catalase, and a Novel Antiarrhythmic Drug, EGB 761, on Reperfusion-Induced Arrhythmias in Isolated Rat Hearts. Free Radic. Biol. Med. 1993, 14, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Tosaki, A. ArrhythmoGenoPharmacoTherapy. Front. Pharmacol. 2020, 11, 616. [Google Scholar] [CrossRef]
- Yao, L.; He, F.; Zhao, Q.; Li, D.; Fu, S.; Zhang, M.; Zhang, X.; Zhou, B.; Wang, L. Spatial Multiplexed Protein Profiling of Cardiac Ischemia-Reperfusion Injury. Circ. Res. 2023, 133, 86–103. [Google Scholar] [CrossRef]
- Tosaki, A.; Engelman, D.T.; Engelman, R.M.; Das, D.K. The Evolution of Diabetic Response to Ischemia/Reperfusion and Preconditioning in Isolated Working Rat Hearts. Cardiovasc. Res. 1996, 31, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Murohara, Y.; Yui, Y.; Hattori, R.; Kawai, C. Effects of Superoxide Dismutase on Reperfusion Arrhythmias and Left Ventricular Function in Patients Undergoing Thrombolysis for Anterior Wall Acute Myocardial Infarction. Am. J. Cardiol. 1991, 67, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, G.; Zweier, J.L.; Becker, L.C. Apoptosis Is Prevented by Administration of Superoxide Dismutase in Dogs with Reperfused Myocardial Infarction. Basic. Res. Cardiol. 1998, 93, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.D.; Gooijers, I.; Campostrini, G.; Verkerk, A.O.; Honkoop, H.; Bouwman, M.; de Bakker, D.E.M.; Koopmans, T.; Vink, A.; Lamers, G.E.M.; et al. Interplay between Calcium and Sarcomeres Directs Cardiomyocyte Maturation during Regeneration. Science 2023, 380, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Bagnis, C.; Deray, G.; Dubois, M.; Pirotzky, E.; Jacquiaud, C.; Baghos, W.; Aupetit, B.; Braquet, P.; Jacobs, C. Prevention of Cyclosporin Nephrotoxicity with a Platelet-Activating Factor (PAF) Antagonist. Nephrol. Dial. Transplant. 1996, 11, 507–513. [Google Scholar] [CrossRef] [PubMed]
- NaveenKumar, S.K.; SharathBabu, B.N.; Hemshekhar, M.; Kemparaju, K.; Girish, K.S.; Mugesh, G. The Role of Reactive Oxygen Species and Ferroptosis in Heme-Mediated Activation of Human Platelets. ACS Chem. Biol. 2018, 13, 1996–2002. [Google Scholar] [CrossRef]
- Hopper, C.P.; Meinel, L.; Steiger, C.; Otterbein, L.E. Where Is the Clinical Breakthrough of Heme Oxygenase-1 / Carbon Monoxide Therapeutics? Curr. Pharm. Des. 2018, 24, 2264–2282. [Google Scholar] [CrossRef] [PubMed]
- Costa Silva, R.C.M.; Correa, L.H.T. Heme Oxygenase 1 in Vertebrates: Friend and Foe. Cell Biochem. Biophys. 2022, 80, 97–113. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Almalki, W.H.; Albratty, M.; Meraya, A.M.; Najmi, A.; Vyas, G.; Singh, S.K.; Dua, K.; Gupta, G. The Therapeutic Role of Nutraceuticals Targeting the Nrf2/HO-1 Signaling Pathway in Liver Cancer. J. Food Biochem. 2022, 46, e14357. [Google Scholar] [CrossRef]
- Dai, Y.; Fleischhacker, A.S.; Liu, L.; Fayad, S.; Gunawan, A.L.; Stuehr, D.J.; Ragsdale, S.W. Heme Delivery to Heme Oxygenase-2 Involves Glyceraldehyde-3-Phosphate Dehydrogenase. Biol. Chem. 2022, 403, 1043–1053. [Google Scholar] [CrossRef]
- Hopper, C.P.; De La Cruz, L.K.; Lyles, K.V.; Wareham, L.K.; Gilbert, J.A.; Eichenbaum, Z.; Magierowski, M.; Poole, R.K.; Wollborn, J.; Wang, B. Role of Carbon Monoxide in Host-Gut Microbiome Communication. Chem. Rev. 2020, 120, 13273–13311. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Choi, A.M. Heme Oxygenase: Colors of Defense against Cellular Stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1029–L1037. [Google Scholar] [CrossRef] [PubMed]
- Hopper, C.P.; Zambrana, P.N.; Goebel, U.; Wollborn, J. A Brief History of Carbon Monoxide and Its Therapeutic Origins. Nitric Oxide 2021, 111–112, 45–63. [Google Scholar] [CrossRef] [PubMed]
- McClung, J.A.; Levy, L.; Garcia, V.; Stec, D.E.; Peterson, S.J.; Abraham, N.G. Heme-Oxygenase and Lipid Mediators in Obesity and Associated Cardiometabolic Diseases: Therapeutic Implications. Pharmacol. Ther. 2022, 231, 107975. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Ascorbic Acid as Antioxidant. Vitam. Horm. 2023, 121, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Zaaboul, F.; Liu, Y. Vitamin E in Foodstuff: Nutritional, Analytical, and Food Technology Aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 964–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J. Vitamin E and Its Function in Membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, Y.; Sato, Y.; Takayama, T. Implications of Glutathione-S Transferase P1 in MAPK Signaling as a CRAF Chaperone: In Memory of Dr. Irving Listowsky. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2022, 98, 72–86. [Google Scholar] [CrossRef]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The Changing Faces of Glutathione, a Cellular Protagonist. Biochem. Pharmacol. 2003, 66, 1499–1503. [Google Scholar] [CrossRef]
- Malik, A.; Alshehri, M.A.; Alamery, S.F.; Khan, J.M. Impact of Metal Nanoparticles on the Structure and Function of Metabolic Enzymes. Int. J. Biol. Macromol. 2021, 188, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of Structures and Properties among Catalases. Cell Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of Oxidative Stress and DNA Damage in Human Carcinogenesis. Mutat. Res. 2011, 711, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Chibaya, L.; Karim, B.; Zhang, H.; Jones, S.N. Mdm2 Phosphorylation by Akt Regulates the P53 Response to Oxidative Stress to Promote Cell Proliferation and Tumorigenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2003193118. [Google Scholar] [CrossRef]
- Barta, T.; Tosaki, A.; Haines, D.; Balla, G.; Lekli, I.; Tosaki, A. Endothelin-1-Induced Hypertrophic Alterations and Heme Oxygenase-1 Expression in Cardiomyoblasts Are Counteracted by Beta Estradiol: In Vitro and in Vivo Studies. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Vitek, L.; Hinds, T.D.; Stec, D.E.; Tiribelli, C. The Physiology of Bilirubin: Health and Disease Equilibrium. Trends Mol. Med. 2023, 29, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L.; Ho, Y.-C.; Yet, S.-F. A Central Role of Heme Oxygenase-1 in Cardiovascular Protection. Antioxid. Redox Signal 2011, 15, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Alhashim, A.; Abdelbary, M.; Sullivan, J.C.; Naeini, S.E.; Elmarakby, A.A. Sexual Dimorphism in Renal Heme Oxygenase-1 and Arachidonic Acid Metabolizing Enzymes in Spontaneously Hypertensive Rats versus Normotensive Wistar Kyoto Rats. Prostagland. Other Lipid Mediat. 2022, 161, 106650. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Choi, A.M.K. Heme Oxygenase-1: The “Emerging Molecule” Has Arrived. Am. J. Respir. Cell Mol. Biol. 2002, 27, 8–16. [Google Scholar] [CrossRef]
- Satoh, T.; Wang, L.; Espinosa-Diez, C.; Wang, B.; Hahn, S.A.; Noda, K.; Rochon, E.R.; Dent, M.R.; Levine, A.R.; Baust, J.J.; et al. Metabolic Syndrome Mediates ROS-miR-193b-NFYA-Dependent Downregulation of Soluble Guanylate Cyclase and Contributes to Exercise-Induced Pulmonary Hypertension in Heart Failure With Preserved Ejection Fraction. Circulation 2021, 144, 615–637. [Google Scholar] [CrossRef]
- Szabo, M.E.; Gallyas, E.; Bak, I.; Rakotovao, A.; Boucher, F.; de Leiris, J.; Nagy, N.; Varga, E.; Tosaki, A. Heme Oxygenase-1-Related Carbon Monoxide and Flavonoids in Ischemic/Reperfused Rat Retina. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Bertolatus, J.A.; Klinzman, D.; Bronsema, D.A.; Ridnour, L.; Oberley, L.W. Evaluation of the Role of Reactive Oxygen Species in Doxorubicin Hydrochloride Nephrosis. J. Lab. Clin. Med. 1991, 118, 435–445. [Google Scholar] [PubMed]
- Somarathna, M.; Isayeva-Waldrop, T.; Al-Balas, A.; Guo, L.; Lee, T. A Novel Model of Balloon Angioplasty Injury in Rat Arteriovenous Fistula. J. Vasc. Res. 2020, 57, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Varadi, J.; Lekli, I.; Juhasz, B.; Bacskay, I.; Szabo, G.; Gesztelyi, R.; Szendrei, L.; Varga, E.; Bak, I.; Foresti, R.; et al. Beneficial Effects of Carbon Monoxide-Releasing Molecules on Post-Ischemic Myocardial Recovery. Life Sci. 2007, 80, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.; Foresti, R.; Sarathchandra, P.; Kaur, H.; Green, C.J.; Motterlini, R. Heme Oxygenase-1-Derived Bilirubin Ameliorates Postischemic Myocardial Dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H643–H651. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.M.; Luke, P.P.W.; Bhattacharjee, R.N. Carbon Monoxide Mechanism of Protection against Renal Ischemia and Reperfusion Injury. Biochem. Pharmacol. 2022, 202, 115156. [Google Scholar] [CrossRef] [PubMed]
- ALTamimi, J.Z.; AlFaris, N.A.; Alshammari, G.M.; Alagal, R.I.; Aljabryn, D.H.; Yahya, M.A. Esculeoside A Decreases Diabetic Cardiomyopathy in Streptozotocin-Treated Rats by Attenuating Oxidative Stress, Inflammation, Fibrosis, and Apoptosis: Impressive Role of Nrf2. Medicina 2023, 59, 1830. [Google Scholar] [CrossRef] [PubMed]
- Brunt, K.R.; Tsuji, M.R.; Lai, J.H.; Kinobe, R.T.; Durante, W.; Claycomb, W.C.; Ward, C.A.; Melo, L.G. Heme Oxygenase-1 Inhibits Pro-Oxidant Induced Hypertrophy in HL-1 Cardiomyocytes. Exp. Biol. Med. 2009, 234, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.D.; Tosaki, A. Role of Heme Oxygenases in Cardiovascular Syndromes and Co-Morbidities. Curr. Pharm. Des. 2018, 24, 2322–2325. [Google Scholar] [CrossRef]
- Czompa, A.; Gyongyosi, A.; Czegledi, A.; Csepanyi, E.; Bak, I.; Haines, D.D.; Tosaki, A.; Lekli, I. Cardioprotection Afforded by Sour Cherry Seed Kernel: The Role of Heme Oxygenase-1. J. Cardiovasc. Pharmacol. 2014, 64, 412–419. [Google Scholar] [CrossRef]
- Vecsernyes, M.; Szokol, M.; Bombicz, M.; Priksz, D.; Gesztelyi, R.; Fulop, G.A.; Varga, B.; Juhasz, B.; Haines, D.; Tosaki, A. Alpha-Melanocyte-Stimulating Hormone Induces Vasodilation and Exerts Cardioprotection through the Heme-Oxygenase Pathway in Rat Hearts. J. Cardiovasc. Pharmacol. 2017, 69, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Csepanyi, E.; Czompa, A.; Haines, D.; Lekli, I.; Bakondi, E.; Balla, G.; Tosaki, A.; Bak, I. Cardiovascular Effects of Low versus High-Dose Beta-Carotene in a Rat Model. Pharmacol. Res. 2015, 100, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, F.F.; Al-Awadhi, A.M.; Haines, D.D. Amelioration of Human Osteoarthritis Symptoms with Topical ‘Biotherapeutics’: A Phase I Human Trial. Cell Stress. Chaperones 2015, 20, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.; Lekli, I.; Juhasz, B.; Varga, E.; Varga, B.; Gesztelyi, R.; Szendrei, L.; Tosaki, A. Isolation and Analysis of Bioactive Constituents of Sour Cherry (Prunus Cerasus) Seed Kernel: An Emerging Functional Food. J. Med. Food 2010, 13, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and Vegetable Consumption and Mortality from All Causes, Cardiovascular Disease, and Cancer: Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef]
- Benatar, J.R.; Stewart, R.A.H. Cardiometabolic Risk Factors in Vegans; A Meta-Analysis of Observational Studies. PLoS ONE 2018, 13, e0209086. [Google Scholar] [CrossRef] [PubMed]
- Dybvik, J.S.; Svendsen, M.; Aune, D. Vegetarian and Vegan Diets and the Risk of Cardiovascular Disease, Ischemic Heart Disease and Stroke: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Eur. J. Nutr. 2023, 62, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Glenn, A.J.; Li, J.; Lo, K.; Jenkins, D.J.A.; Boucher, B.A.; Hanley, A.J.; Kendall, C.W.C.; Shadyab, A.H.; Tinker, L.F.; Chessler, S.D.; et al. The Portfolio Diet and Incident Type 2 Diabetes: Findings From the Women’s Health Initiative Prospective Cohort Study. Diabetes Care 2023, 46, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, A.J.; Suter-Zimmermann, K.; Bucher, H.C.; Shai, I.; Tuttle, K.R.; Estruch, R.; Briel, M. Meta-Analysis Comparing Mediterranean to Low-Fat Diets for Modification of Cardiovascular Risk Factors. Am. J. Med. 2011, 124, 841–851.e2. [Google Scholar] [CrossRef]
- Guo, B.; Yu, Y.; Wang, M.; Li, R.; He, X.; Tang, S.; Liu, Q.; Mao, Y. Targeting the JAK2/STAT3 Signaling Pathway with Natural Plants and Phytochemical Ingredients: A Novel Therapeutic Method for Combatting Cardiovascular Diseases. Biomed. Pharmacother. 2024, 172, 116313. [Google Scholar] [CrossRef]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haines, D.D.; Cowan, F.M.; Tosaki, A. Evolving Strategies for Use of Phytochemicals in Prevention and Long-Term Management of Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2024, 25, 6176. https://doi.org/10.3390/ijms25116176
Haines DD, Cowan FM, Tosaki A. Evolving Strategies for Use of Phytochemicals in Prevention and Long-Term Management of Cardiovascular Diseases (CVD). International Journal of Molecular Sciences. 2024; 25(11):6176. https://doi.org/10.3390/ijms25116176
Chicago/Turabian StyleHaines, Donald David, Fred M. Cowan, and Arpad Tosaki. 2024. "Evolving Strategies for Use of Phytochemicals in Prevention and Long-Term Management of Cardiovascular Diseases (CVD)" International Journal of Molecular Sciences 25, no. 11: 6176. https://doi.org/10.3390/ijms25116176
APA StyleHaines, D. D., Cowan, F. M., & Tosaki, A. (2024). Evolving Strategies for Use of Phytochemicals in Prevention and Long-Term Management of Cardiovascular Diseases (CVD). International Journal of Molecular Sciences, 25(11), 6176. https://doi.org/10.3390/ijms25116176




