CAFs-Associated Genes (CAFGs) in Pancreatic Ductal Adenocarcinoma (PDAC) and Novel Therapeutic Strategy
Abstract
1. Introduction
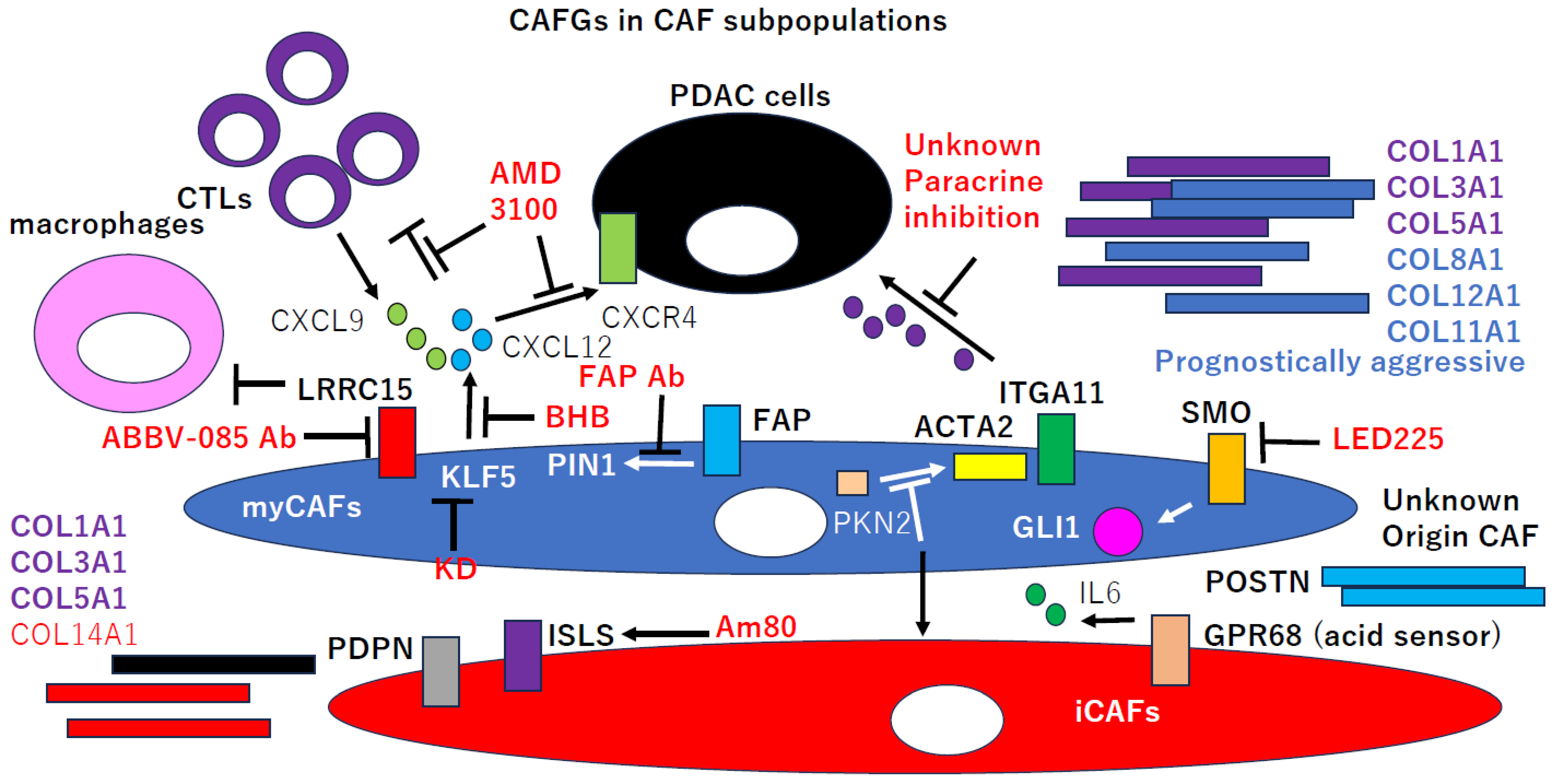
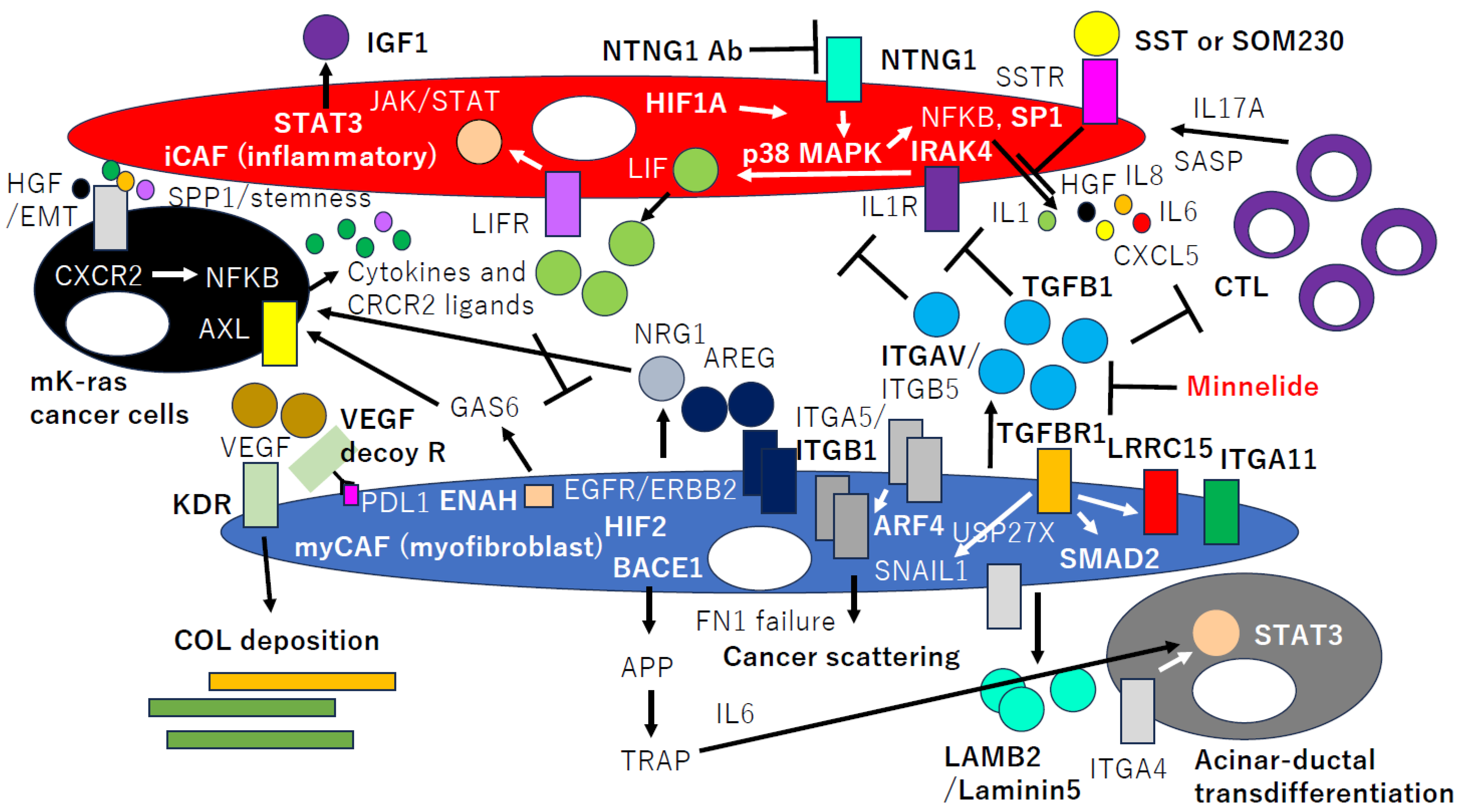
2. CAFGs in PDAC and Novel Therapeutic Potential
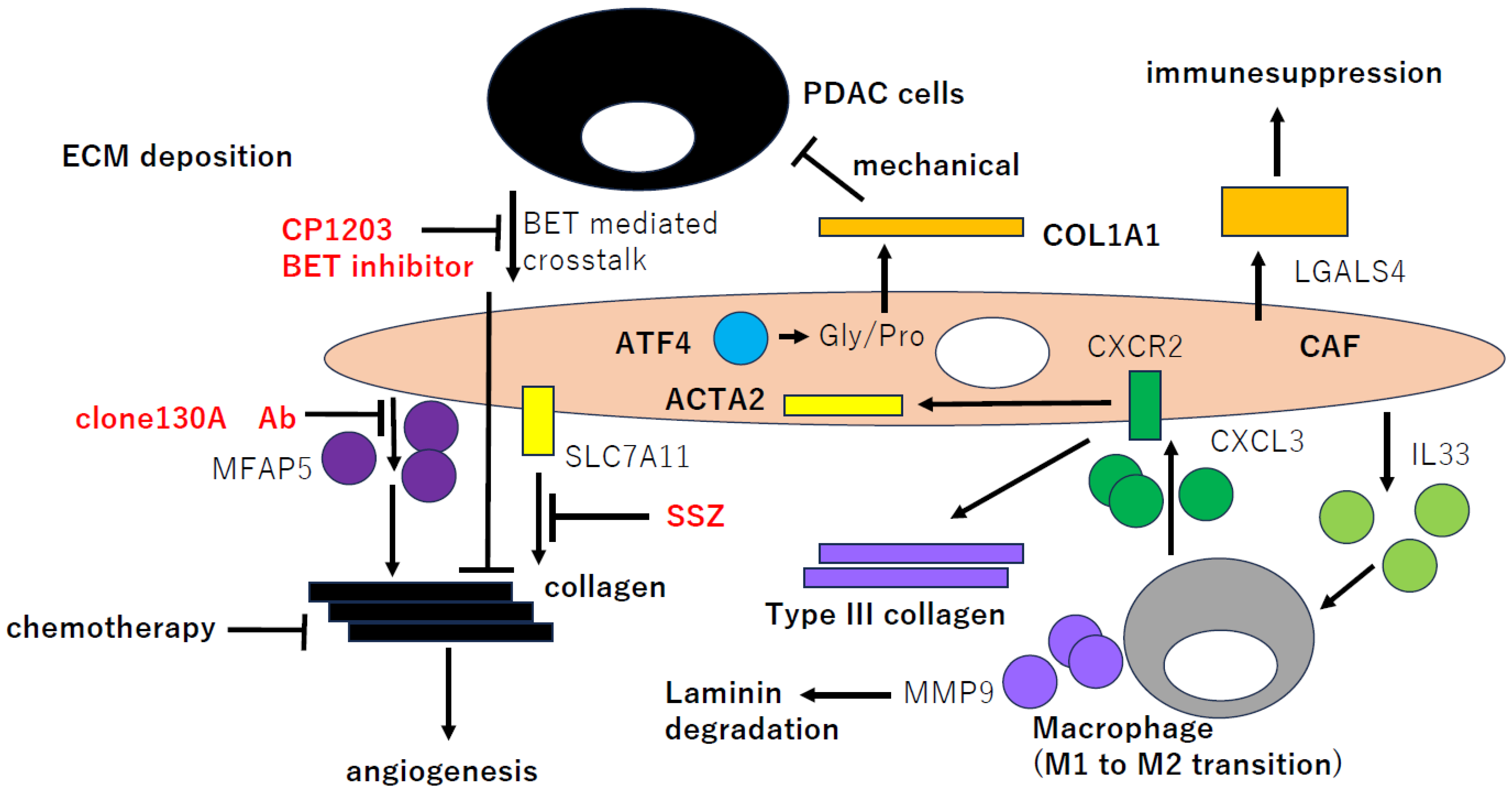
3. CAFGs Collagens in PDAC and Novel Therapeutic Potential
4. Semi-CAFGs in PDAC and Novel Therapeutic Potential
5. CAFGs with Low SE (L-CAFGs) and Novel Therapeutic Potential in PDAC
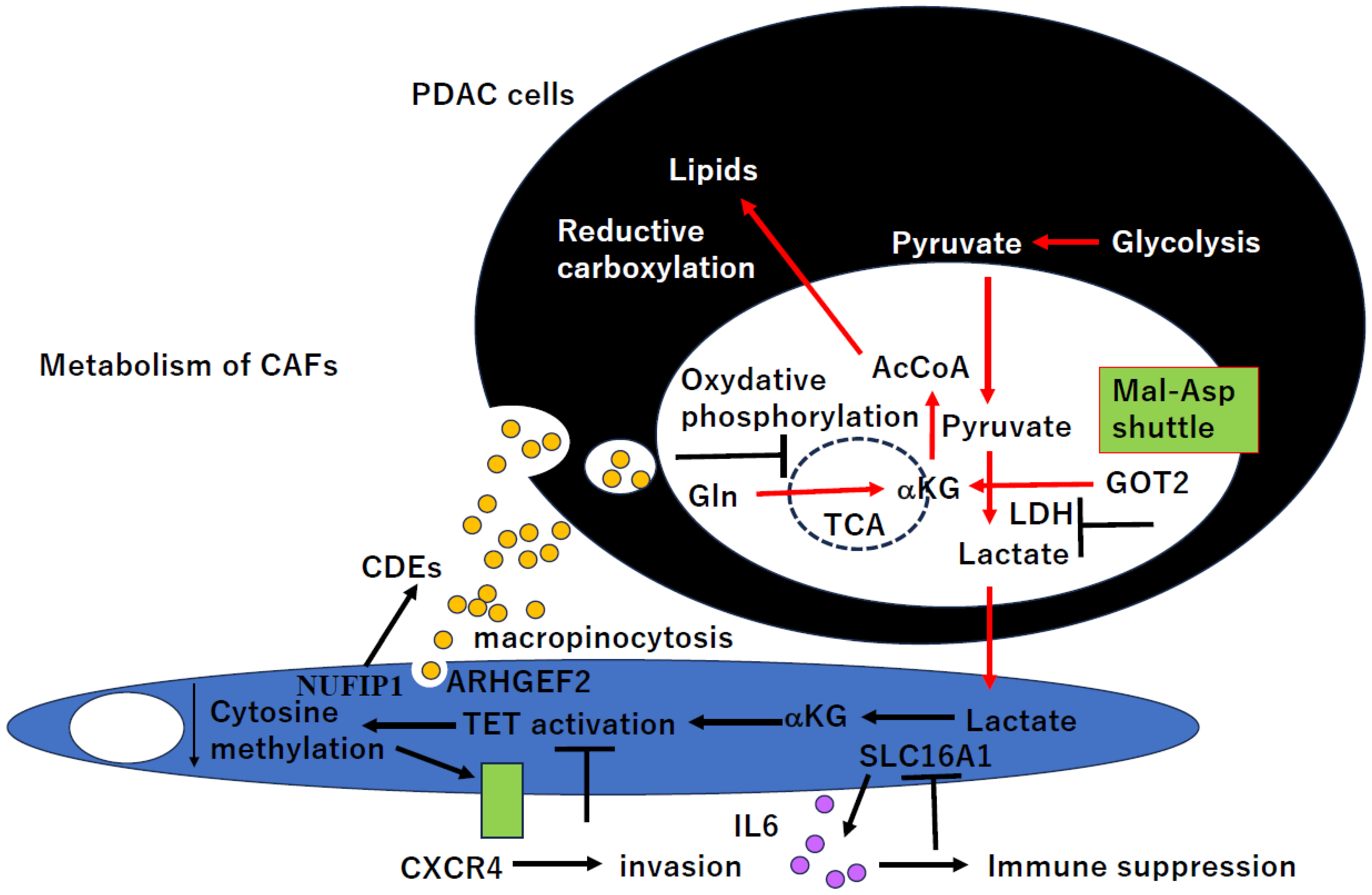
6. CAFs’ Metabolism in PDAC and Novel Therapeutic Potential
7. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Raphael, B.J.; Hruban, R.H.; Aguirre, A.J.; Moffitt, R.A.; Yeh, J.J.; Stewart, C.; Lolla, L. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e113. [Google Scholar] [CrossRef] [PubMed]
- Shaashua, L.; Ben-Shmuel, A.; Pevsner-Fischer, M.; Friedman, G.; Levi-Galibov, O.; Nandakumar, S.; Barki, D.; Nevo, R.; Brown, L.E.; Zhang, W.; et al. BRCA mutational status shapes the stromal microenvironment of pancreatic cancer linking clusterin expression in cancer associated fibroblasts with HSF1 signaling. Nat. Commun. 2022, 13, 6513. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Tijeras-Raballand, A.; Ragulan, C.; Cros, J.; Patil, Y.; Martinet, M.; Kocher, H.M. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J. Pathol. 2019, 248, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Porte, H.; Chastre, E.; Prevot, S.; Nordlinger, B.; Empereur, S.; Basset, P.; Gespach, C. Neoplastic progression of human colorectal cancer is associated with overexpression of the stromelysin-3 and BM-40/SPARC genes. Int. J. Cancer 1995, 64, 70–75. [Google Scholar] [CrossRef]
- Le Bail, B.; Faouzi, S.; Boussarie, L.; Guirouilh, J.; Blanc, J.F.; Carles, J.; Rosenbaum, J. Osteonectin/SPARC is overexpressed in human hepatocellular carcinoma. J. Pathol. 1999, 189, 46–52. [Google Scholar] [CrossRef]
- Yamashita, K.; Upadhay, S.; Mimori, K.; Inoue, H.; Mori, M. Clinical significance of secreted protein acidic and rich in cystein in esophageal carcinoma and its relation to carcinoma progression. Cancer 2003, 97, 2412–2419. [Google Scholar] [CrossRef]
- Massi, D.; Franchi, A.; Borgognoni, L.; Reali, U.M.; Santucci, M. Osteonectin expression correlates with clinical outcome in thin cuta-neous malignant melanomas. Hum. Pathol. 1999, 30, 339–344. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Brekken, R.A.; Sivridis, E.; Gatter, K.C.; Harris, A.L.; Sage, E.H. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003, 63, 5376–5380. [Google Scholar]
- Jones, C.; Mackay, A.; Grigoriadis, A.; Cossu, A.; Reis-Filho, J.S.; Fulford, L.; Lakhani, S.R. Expression profiling of purified normal human luminal and myoepithelial breast cells: Identification of novel prognostic markers for breast cancer. Cancer Res. 2004, 64, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Lin, K.H.; Chen, S.L.; Chan, Y.F.; Hsueh, S. Overexpression of SPARC gene in human gastric carcinoma and its clinic-pathologic significance. Br. J. Cancer 2004, 91, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Infante, J.R.; Matsubayashi, H.; Sato, N.; Tonascia, J.; Klein, A.P.; Riall, T.A.; Goggins, M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 2007, 25, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jeong, D.; Ahn, T.S.; Kim, H.J.; Park, D.S.; Park, S.Y.; Bae, S.B.; Lee, S.; Lee, S.S.; Lee, M.S.; et al. Expression of Secreted Protein Acidic and Rich in Cysteine in the Stroma of a Colorectal Carcinoma is Associated with Patient Prognosis. Ann. Coloproctol. 2013, 29, 93–99. [Google Scholar] [CrossRef]
- Alachkar, H.; Santhanam, R.; Maharry, K.; Metzeler, K.H.; Huang, X.; Kohlschmidt, J.; Mendler, J.H.; Benito, J.M.; Hickey, C.; Neviani, P.; et al. SPARC promotes leukemic cell growth and predicts acute myeloid leukemia outcome. J. Clin. Investig. 2014, 124, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Courtois, E.T.; Sengupta, D.; Tan, Y.; Chen, K.H.; Goh, J.J.L.; Kong, S.L.; Chua, C.; Hon, L.K.; Tan, W.S.; et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 2017, 49, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Okuno, K.; Ikemura, K.; Okamoto, R.; Oki, K.; Watanabe, A.; Kuroda, Y.; Kidachi, M.; Fujino, S.; Nie, Y.; Higuchi, T.; et al. CAF-associated genes putatively representing distinct prognosis by in silico landscape of stromal components of colon cancer. PLoS ONE 2024, 19, e0299827. [Google Scholar] [CrossRef] [PubMed]
- Walter, K.; Omura, N.; Hong, S.-M.; Griffith, M.; Vincent, A.; Borges, M.; Goggins, M. Overexpression of Smoothened Activates the Sonic Hedgehog Signaling Pathway in Pancreatic Cancer-Associated Fibroblasts. Clin. Cancer Res. 2010, 16, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Steele, N.G.; Biffi, G.; Kemp, S.B.; Zhang, Y.; Drouillard, D.; Syu, L.; Hao, Y.; Oni, T.E.; Brosnan, E.; Elyada, E.; et al. Inhibition of Hedgehog Signaling Alters Fibroblast Composition in Pancreatic Cancer. Clin. Cancer Res. 2021, 27, 2023–2037. [Google Scholar] [CrossRef]
- Purcell, J.W.; Tanlimco, S.G.; Hickson, J.A.; Fox, M.; Sho, M.; Durkin, L.; Uziel, T.; Powers, R.; Foster, K.; McGonigal, T.; et al. LRRC15 Is a Novel Mesenchymal Protein and Stromal Target for Antibody-Drug Conjugates. Cancer Res. 2018, 78, 4059–4072. [Google Scholar] [CrossRef]
- Dominguez, C.X.; Müller, S.; Keerthivasan, S.; Koeppen, H.; Hung, J.; Gierke, S.; Breart, B.; Foreman, O.; Bainbridge, T.W.; Castiglioni, A.; et al. Single-Cell RNA Sequencing Reveals Stromal Evolution into LRRC15+ Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov. 2020, 10, 232–253. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Tuveson, D.A. Distinct populations of inflammatory fibro-blasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurty, A.T.; Shyer, J.A.; Thai, M.; Gandham, V.; Buechler, M.B.; Yang, Y.A.; Pradhan, R.N.; Wang, A.W.; Sanchez, P.L.; Qu, Y.; et al. LRRC15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature 2022, 611, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Menezes, S.; Okail, M.H.; Jalil, S.M.A.; Kocher, H.M.; Cameron, A.J.M. Cancer-associated fibroblasts in pancreatic cancer: New subtypes, new markers, new targets. J. Pathol. 2022, 257, 526–544. [Google Scholar] [CrossRef]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef] [PubMed]
- Fearon, D.T. The Carcinoma-Associated Fibroblast Expressing Fibroblast Activation Protein and Escape from Immune Surveillance. Cancer Immunol. Res. 2014, 2, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Mu, C.; Li, M.; Li, K.; Li, S.; Wu, W.; Du, L.; Zhang, X.; Li, C.; et al. Pancreatic tumor eradication via selective Pin1 inhibition in cancer-associated fibroblasts and T lymphocytes engagement. Nat. Commun. 2022, 13, 4308. [Google Scholar] [CrossRef] [PubMed]
- Bartoschek, M.; Oskolkov, N.; Bocci, M.; Lövrot, J.; Larsson, C.; Sommarin, M.; Madsen, C.D.; Lindgren, D.; Pekar, G.; Karlsson, G.; et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 2018, 9, 5150. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Zhou, Y.; Li, C.; Rychahou, P.; Zhang, S.; Titlow, W.B.; Bauman, G.; Wu, Y.; Liu, J.; Wang, C.; et al. Ketogenesis Attenuates KLF5-Dependent Production of CXCL12 to Overcome the Immunosuppressive Tumor Microenvironment in Colorectal Cancer. Cancer Res. 2022, 82, 1575–1588. [Google Scholar] [CrossRef]
- Yan, R.; Moresco, P.; Gegenhuber, B.; Fearon, D.T. T cell-Mediated Development of Stromal Fibroblasts with an Immune-Enhancing Chemokine Profile. Cancer Immunol. Res. 2023, 11, 1044–1054. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, J.; Jensen, C.; Nissen, N.I.; Cox, T.R.; Kalluri, R.; Karsdal, M.; Willumsen, N. The collagen landscape in cancer: Profiling collagens in tumors and in circulation reveals novel markers of cancer-associated fibroblast subtypes. J. Pathol. 2024, 262, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Nicolle, R.; Raffenne, J.; Tijeras-Raballand, A.; Brunel, A.; Astorgues-Xerri, L.; Bousquet, C. Periostin- and podoplanin-positive cancer-associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma. J. Pathol. 2022, 258, 408–425. [Google Scholar] [CrossRef] [PubMed]
- Schnittert, J.; Bansal, R.; Mardhian, D.F.; Van Baarlen, J.; Östman, A.; Prakash, J. Integrin α11 in pancreatic stellate cells regulates tumor stroma interaction in pancreatic cancer. FASEB J. 2019, 33, 6609–6621. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.R.; Menezes, S.; Henry, J.C.; Williams, J.L.; Alba-Castellón, L.; Baskaran, P.; Cameron, A.J. Disruption of pancreatic stellate cell myofibroblast phenotype promotes pancreatic tumor invasion. Cell Rep. 2022, 38, 110227. [Google Scholar] [CrossRef] [PubMed]
- Helms, E.J.; Berry, M.W.; Chaw, R.C.; DuFort, C.C.; Sun, D.; Onate, M.K.; Sherman, M.H. Mesenchymal Lineage Heterogeneity Underlies Nonredundant Functions of Pancreatic Cancer-Associated Fibroblasts. Cancer Discov. 2022, 12, 484–501. [Google Scholar] [CrossRef]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, Z.; Zhang, Y.; Pradhan, R.N.; Ganguly, D.; Chandra, R.; Brekken, R.A. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell 2022, 40, 656–673.e7. [Google Scholar] [CrossRef] [PubMed]
- Wiley, S.Z.; Sriram, K.; Liang, W.; Chang, S.E.; French, R.; McCann, T.; Insel, P.A. GPR68, a proton-sensing GPCR, mediates interaction of cancer-associated fibroblasts and cancer cells. FASEB J. 2018, 32, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, A.C.; Tisdale, A.A.; Venkat, S.; Maraszek, K.E.; Alahmari, A.A.; George, A.; Attwood, K.; George, M.; Rempinski, D.; Franco-Barraza, J.; et al. Lorazepam Stimulates IL6 Production and Is Associated with Poor Survival Outcomes in Pancreatic Cancer. Clin. Cancer Res. 2023, 29, 3793–3812. [Google Scholar] [CrossRef]
- Mizutani, Y.; Kobayashi, H.; Iida, T.; Asai, N.; Masamune, A.; Hara, A.; Esaki, N.; Ushida, K.; Mii, S.; Shiraki, Y.; et al. Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis. Cancer Res. 2019, 79, 5367–5381. [Google Scholar] [CrossRef]
- Iida, T.; Mizutani, Y.; Esaki, N.; Ponik, S.M.; Burkel, B.M.; Weng, L.; Kuwata, K.; Masamune, A.; Ishihara, S.; Haga, H.; et al. Pharmacologic conversion of cancer-associated fibroblasts from a protumor phenotype to an antitumor phenotype improves the sensitivity of pancreatic cancer to chemotherapeutics. Oncogene 2022, 41, 2764–2777. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Ino, Y.; Naito, C.; Nara, S.; Shimasaki, M.; Ishimoto, U.; Iwasaki, T.; Doi, N.; Esaki, M.; Kishi, Y.; et al. Neoadjuvant therapy alters the collagen architecture of pancreatic cancer tissue via Ephrin-A5. Br. J. Cancer 2022, 126, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.-L.; Leung, C.S.; Yip, K.-P.; Sheng, J.; Vien, L.; Bover, L.C.; Birrer, M.J.; Wong, S.T.; Mok, S.C. Anticancer Immunotherapy by MFAP5 Blockade Inhibits Fibrosis and Enhances Chemosensitivity in Ovarian and Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 6417–6428. [Google Scholar] [CrossRef]
- Honselmann, K.C.; Finetti, P.; Birnbaum, D.J.; Monsalve, C.S.; Wellner, U.F.; Begg, S.K.; Nakagawa, A.; Hank, T.; Li, A.; Goldsworthy, M.A.; et al. Neoplastic-Stromal Cell Cross-talk Regulates Matrisome Expression in Pancreatic Cancer. Mol. Cancer Res. 2020, 18, 1889–1902. [Google Scholar] [CrossRef] [PubMed]
- Sharbeen, G.; McCarroll, J.A.; Akerman, A.; Kopecky, C.; Youkhana, J.; Kokkinos, J.; Holst, J.; Boyer, C.; Erkan, M.; Goldstein, D.; et al. Cancer-Associated Fibroblasts in Pancreatic Ductal Adenocarcinoma Determine Response to SLC7A11 Inhibition. Cancer Res. 2021, 81, 3461–3479. [Google Scholar] [CrossRef] [PubMed]
- Verginadis, I.I.; Avgousti, H.; Monslow, J.; Skoufos, G.; Chinga, F.; Kim, K.; Leli, N.M.; Karagounis, I.V.; Bell, B.I.; Velalopoulou, A.; et al. A stromal Integrated Stress Response activates perivascular cancer-associated fibroblasts to drive angiogenesis and tumour progression. Nat. Cell Biol. 2022, 24, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, X.; Zhang, Y.; Hosaka, K.; Andersson, P.; Wu, J.; Cao, Y. Inflammatory cell-derived CXCL3 promotes pancreatic cancer me-tastasis through a novel myofibroblast-hijacked cancer escape mechanism. Gut 2022, 71, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.; Yang, Y.; Hosaka, K.; Zhang, Y.; Fischer, C.; Braun, H.; Liu, S.; Yu, G.; Liu, S.; Beyaert, R.; et al. Molecular mechanisms of IL-33-mediated stromal interactions in cancer metastasis. J. Clin. Investig. 2018, 3, e122375. [Google Scholar] [CrossRef] [PubMed]
- Parte, S.; Kaur, A.B.; Nimmakayala, R.K.; Ogunleye, A.O.; Chirravuri, R.; Vengoji, R.; Ponnusamy, M.P. Cancer-Associated Fibroblast Induces Acinar-to-Ductal Cell Transdifferentiation and Pancreatic Cancer Initiation via LAMA5/ITGA4 Axis. Gastroenterology 2023, 166, 842–858.e5. [Google Scholar] [CrossRef]
- Franco-Barraza, J.; Francescone, R.; Luong, T.; Shah, N.; Madhani, R.; Cukierman, G.; Cukierman, E. Matrix-regulated integrin α(v)β(5) maintains α(5)β(1)-dependent desmoplastic traits prognostic of neoplastic recurrence. eLife 2017, 6, e20600. [Google Scholar] [CrossRef]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017, 216, 3799–3816. [Google Scholar] [CrossRef] [PubMed]
- Dauer, P.; Zhao, X.; Gupta, V.K.; Sharma, N.; Kesh, K.; Gnamlin, P.; Saluja, A. Inactivation of Cancer-Associated-Fibroblasts Disrupts Oncogenic Signaling in Pancreatic Cancer Cells and Promotes Its Regression. Cancer Res. 2018, 78, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Biffi, G.; Oni, T.E.; Spielman, B.; Hao, Y.; Elyada, E.; Park, Y.; Preall, J.; Tuveson, D.A. IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma. Cancer Discov. 2019, 9, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Lambies, G.; Miceli, M.; Martínez-Guillamon, C.; Olivera-Salguero, R.; Peña, R.; Frías, C.-P.; Calderón, I.; Atanassov, B.S.; Dent, S.Y.R.; Arribas, J.; et al. TGFβ-Activated USP27X Deubiquitinase Regulates Cell Migration and Chemoresistance via Stabilization of Snail1. Cancer Res. 2018, 79, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.E.; Zeleniak, A.E.; Fishel, M.L.; Wu, J.; Littlepage, L.E.; Hill, R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2017, 36, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Mucciolo, G.; Araos Henríquez, J.; Jihad, M.; Pinto Teles, S.; Manansala, J.S.; Li, W.; Biffi, G. EGFR-activated myofibroblasts promote me-tastasis of pancreatic cancer. Cancer Cell 2024, 42, 101–118.e11. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Xu, J.; Liu, Y.; Liang, S.; LaBella, K.A.; Chakravarti, D.; Spring, D.J.; Xia, Y.; DePinho, R.A. Stromal-derived NRG1 enables oncogenic KRAS bypass in pancreas cancer. Genes Dev. 2023, 37, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Leca, J.; Martinez, S.; Lac, S.; Nigri, J.; Secq, V.; Rubis, M.; Bressy, C.; Sergé, A.; Lavaut, M.-N.; Dusetti, N.; et al. Cancer-associated fibroblast-derived annexin A6+ extracellular vesicles support pancreatic cancer aggressiveness. J. Clin. Investig. 2016, 126, 4140–4156. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Jeong, J.; Lee, D.S.; Hyeon, D.Y.; Park, G.W.; Jeon, S.; Lee, K.B.; Jang, J.-Y.; Hwang, D.; Kim, H.M.; et al. PD-L1-directed PlGF/VEGF blockade synergizes with chemotherapy by targeting CD141+ cancer-associated fibroblasts in pancreatic cancer. Nat. Commun. 2022, 13, 6292. [Google Scholar] [CrossRef]
- Hutton, C.; Heider, F.; Blanco-Gomez, A.; Banyard, A.; Kononov, A.; Zhang, X.; Jørgensen, C. Single-cell analysis defines a pancreatic fibroblast lineage that supports anti-tumor immunity. Cancer Cell 2021, 39, 1227–1244.e20. [Google Scholar] [CrossRef]
- Rainero, E.; Howe, J.D.; Caswell, P.T.; Jamieson, N.B.; Anderson, K.; Critchley, D.R.; Machesky, L.; Norman, J.C. Ligand-Occupied Integrin Internalization Links Nutrient Signaling to Invasive Migration. Cell Rep. 2015, 10, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Munir, H.; Jones, J.O.; Janowitz, T.; Hoffmann, M.; Euler, M.; Martins, C.P.; Welsh, S.J.; Shields, J.D. Stromal-driven and Amyloid β-dependent induction of neutrophil extracellular traps modulates tumor growth. Nat. Commun. 2021, 12, 683. [Google Scholar] [CrossRef]
- de Castro Silva, I.; Bianchi, A.; Deshpande, N.U.; Sharma, P.; Mehra, S.; Garrido, V.T.; Datta, J. Neutrophil-mediated fibroblast-tumor cell il-6/stat-3 signaling underlies the association between neutrophil-to-lymphocyte ratio dynamics and chemotherapy response in localized pancreatic cancer: A hybrid clinical-preclinical study. eLife 2022, 11, e78921. [Google Scholar] [CrossRef] [PubMed]
- Datta, J.; Dai, X.; Bianchi, A.; De Castro Silva, I.; Mehra, S.; Garrido, V.T.; Merchant, N.B. Combined MEK and STAT3 Inhibition Uncovers Stromal Plasticity by Enriching for Cancer-Associated Fibroblasts with Mesenchymal Stem Cell-Like Features to Overcome Immuno-therapy Resistance in Pancreatic Cancer. Gastroenterology 2022, 163, 1593–1612. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.R.; Taniguchi, C.M. Turning Down Oxygen to Turn Up Inflammation in CAFs. Cancer Res. 2023, 83, 1560–1562. [Google Scholar] [CrossRef] [PubMed]
- Toste, P.A.; Nguyen, A.H.; Kadera, B.E.; Duong, M.; Wu, N.; Gawlas, I.; Tran, L.M.; Bikhchandani, M.; Li, L.; Patel, S.G.; et al. Chemotherapy-Induced Inflammatory Gene Signature and Protumorigenic Phenotype in Pancreatic CAFs via Stress-Associated MAPK. Mol. Cancer Res. 2016, 14, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Francescone, R.; Vendramini-Costa, D.B.; Franco-Barraza, J.; Wagner, J.; Muir, A.; Lau, A.N.; Gabitova, L.; Pazina, T.; Gupta, S.; Luong, T.; et al. Netrin G1 Promotes Pancreatic Tumorigenesis through Cancer-Associated Fibroblast-Driven Nutritional Support and Immunosuppression. Cancer Discov. 2021, 11, 446–479. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, D.; Rucki, A.A.; Williams, J.; Zhou, J.; Mo, G.; Zheng, L. Cancer-Associated Fibroblasts in Pancreatic Cancer Are Reprogrammed by Tumor-Induced Alterations in Genomic DNA Methylation. Cancer Res. 2016, 76, 5395–5404. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Jiang, H.; Li, Q.; Wang-Gillam, A.; Yu, J.; Lim, K.H. Tumor-Stroma IL1β-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer. Cancer Res. 2018, 78, 1700–1712. [Google Scholar] [CrossRef]
- Melchionna, R.; Spada, S.; Di Modugno, F.; D’Andrea, D.; Di Carlo, A.; Panetta, M.; Mileo, A.M.; Sperduti, I.; Antoniani, B.; Gallo, E.; et al. The actin modulator hMENA regulates GAS 6- AXL axis and pro-tumor cancer/stromal cell cooperation. EMBO Rep. 2020, 21, e50078. [Google Scholar] [CrossRef]
- Vennin, C.; Mélénec, P.; Rouet, R.; Nobis, M.; Cazet, A.S.; Murphy, K.J.; Herrmann, D.; Reed, D.A.; Lucas, M.C.; Warren, S.C.; et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun. 2019, 10, 3637. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Oon, C.; Kothari, A.; Horton, W.; Link, J.; Sears, R.C.; Sherman, M.H. Acidic fibroblast growth factor underlies microenvironmental regulation of MYC in pancreatic cancer. J. Exp. Med. 2020, 217, e20191805. [Google Scholar] [CrossRef] [PubMed]
- Duluc, C.; Moatassim-Billah, S.; Chalabi-Dchar, M.; Perraud, A.; Samain, R.; Breibach, F.; Gayral, M.; Cordelier, P.; Delisle, M.; Bousquet-Dubouch, M.; et al. Pharmacological targeting of the protein synthesis mTOR/4E- BP 1 pathway in cancer-associated fibroblasts abrogates pancreatic tumour chemoresistance. EMBO Mol. Med. 2015, 7, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yang, L.; Baddour, J.; Achreja, A.; Bernard, V.; Moss, T.; Nagrath, D. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife 2016, 5, e10250. [Google Scholar] [CrossRef]
- Bhagat, T.D.; Von Ahrens, D.; Dawlaty, M.; Zou, Y.; Baddour, J.; Achreja, A.; Zhao, H.; Yang, L.; Patel, B.; Kwak, C.; et al. Lactate-mediated epigenetic reprogramming regulates formation of human pancreatic cancer-associated fibroblasts. eLife 2019, 8, e50663. [Google Scholar] [CrossRef] [PubMed]
- Rhee, I.; Bachman, K.E.; Park, B.H.; Jair, K.-W.; Yen, R.-W.C.; Schuebel, K.E.; Cui, H.; Feinberg, A.P.; Lengauer, C.; Kinzler, K.W.; et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature 2002, 416, 552–556. [Google Scholar] [CrossRef]
- Williams, K.; Christensen, J.; Helin, K. DNA methylation: TET proteins-guardians of CpG islands? EMBO Rep. 2011, 13, 28–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Recouvreux, M.V.; Jung, M.; Galenkamp, K.M.O.; Li, Y.; Zagnitko, O.; Commisso, C. Macropinocytosis in Cancer-Associated Fibroblasts Is Dependent on CaMKK2/ARHGEF2 Signaling and Functions to Support Tumor and Stromal Cell Fitness. Cancer Discov. 2021, 11, 1808–1825. [Google Scholar] [CrossRef]
- Kerk, S.A.; Lin, L.; Myers, A.L.; Sutton, D.J.; Andren, A.; Sajjakulnukit, P.; Lyssiotis, C.A. Metabolic requirement for GOT2 in pancreatic cancer depends on environmental context. eLife 2022, 11, e73245. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Tu, B.; Li, H.; Pang, H.; Zhang, N.; Fan, M.; Bai, J.; Wang, W.; Shu, Z.; DuFort, C.C.; et al. Cancer-associated fibroblasts employ NUFIP1-dependent autophagy to secrete nucleosides and support pancreatic tumor growth. Nat. Cancer 2022, 3, 945–960. [Google Scholar] [CrossRef]
- Jalan-Sakrikar, N.; Anwar, A.; Yaqoob, U.; Gan, C.; Lagnado, A.B.; Wixom, A.Q.; Jurk, D.; Huebert, R.C. Telomere dysfunction promotes cholangiocyte senescence and biliary fibrosis in primary sclerosing cholangitis. JCI Insight 2023, 8, e170320. [Google Scholar] [CrossRef] [PubMed]
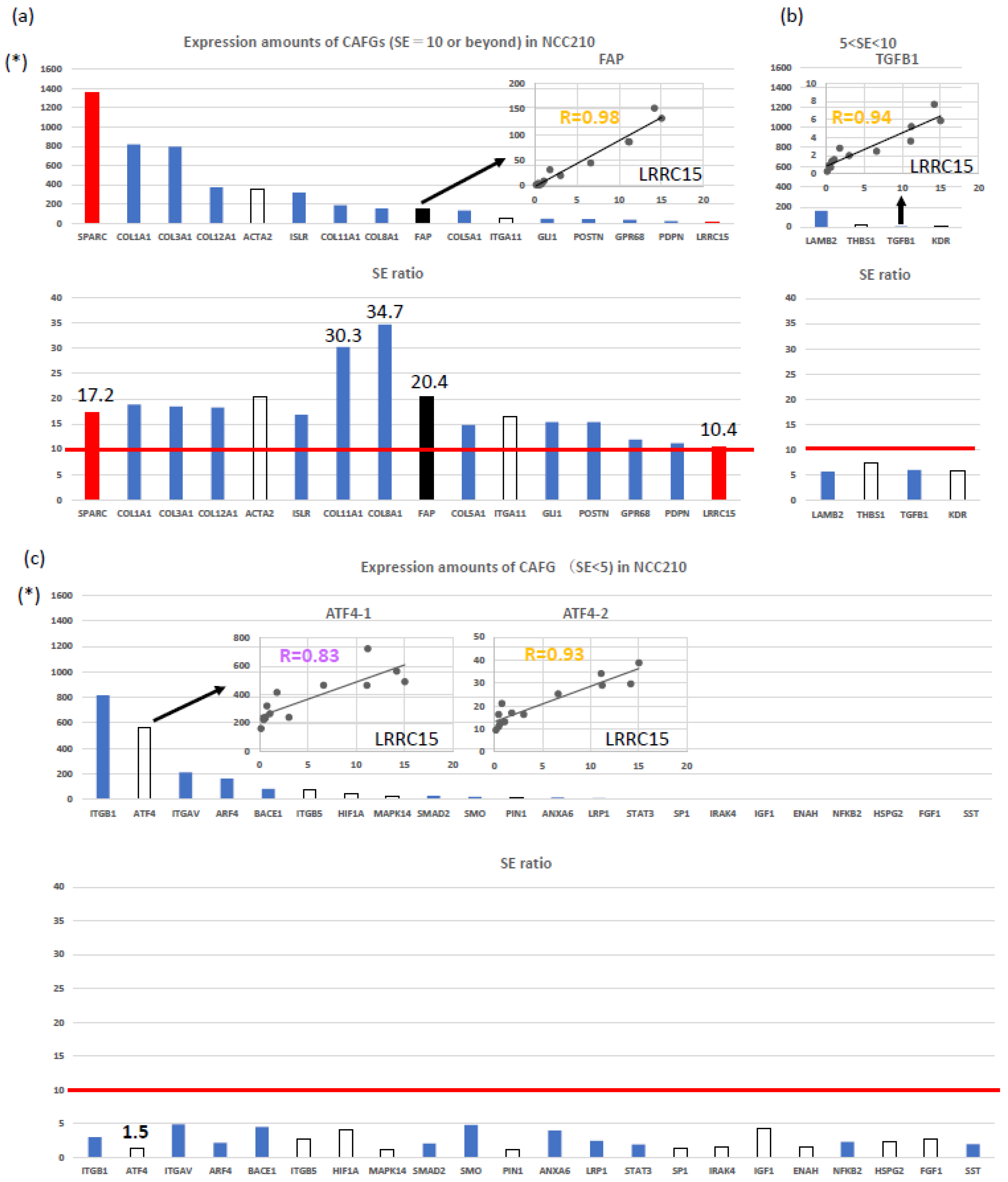
| Expression Order | SYMBOL | NCC210 | R Index with fCAFG (LRCC15) | SE Ratio | Figure 1a |
|---|---|---|---|---|---|
| Order of expression amounts | LRRC15 | 14 | 1.00 | 10.5 | Yes |
| 26 | CMTM3 | 179 | 0.99 | 13.6 | |
| 25 | LOXL1 | 182 | 0.98 | 18.3 | |
| 34 | FAP | 152 | 0.98 | 20.4 | Yes |
| 10 | COL3A1 | 797 | 0.98 | 18.5 | Yes |
| 5 | COL1A2 | 942 | 0.98 | 21.9 | |
| 47 | MXRA5 | 57 | 0.98 | 14.1 | |
| 2 | SPARC | 1356 | 0.97 | 17.2 | Yes |
| 30 | TAGLN | 162 | 0.97 | 19.6 | |
| 21 | INHBA | 280 | 0.97 | 20.9 | |
| 43 | COL8A2 | 65 | 0.97 | 21.2 | |
| 49 | C1QTNF5 | 48 | 0.97 | 14.0 | |
| 9 | COL1A1 | 819 | 0.96 | 18.9 | Yes |
| 11 | CTSK | 682 | 0.96 | 17.2 | |
| 52 | POSTN | 44 | 0.96 | 15.4 | Yes |
| 27 | RAB34 | 174 | 0.95 | 10.7 | |
| 8 | C1S | 853 | 0.95 | 21.4 | |
| 20 | AEBP1 | 284 | 0.95 | 23.1 | |
| 45 | SSPN | 60 | 0.95 | 12.4 | |
| 12 | COL5A2 | 674 | 0.95 | 18.4 | |
| 1 | IGFBP7 | 1443 | 0.95 | 11.6 | |
| 51 | GLI1 | 47 | 0.95 | 16.9 | Yes |
| 32 | AL359062 (COL8A1) | 153 | 0.95 | 34.7 | Yes |
| 4 | FBLN1 | 1004 | 0.95 | 30.4 | |
| 41 | CFHR1 | 75 | 0.94 | 17.6 | |
| 48 | AK022110 (GPX8) | 56 | 0.94 | 15.9 | |
| 17 | ISLR | 318 | 0.94 | 16.8 | Yes |
| 42 | FBLN2 | 65 | 0.94 | 17.7 | |
| 16 | DKK3 | 365 | 0.94 | 13.9 | |
| 33 | SERPINF1 | 152 | 0.94 | 11.7 | |
| 3 | SPON2 | 1194 | 0.94 | 12.0 | |
| 19 | PMP22 | 287 | 0.93 | 12.4 | |
| 36 | COL5A1 | 132 | 0.93 | 14.8 | Yes |
| 50 | COX7A1 | 48 | 0.93 | 15.7 | |
| 22 | ANTXR1 | 247 | 0.93 | 24.3 | |
| 39 | CR603437 (GNB4) | 79 | 0.93 | 12.9 | |
| 13 | MXRA8 | 460 | 0.93 | 19.3 | |
| 18 | COL6A3 | 298 | 0.93 | 16.2 | |
| 14 | NNMT | 442 | 0.93 | 20.6 | |
| 37 | C16orf30 (TMEM204) | 130 | 0.93 | 14.3 | |
| 40 | TSPAN4 | 78 | 0.93 | 13.0 | |
| 15 | COL12A1 | 374 | 0.93 | 18.3 | Yes |
| 35 | PRKCDBP (CAVIN3) | 137 | 0.92 | 12.6 | |
| 23 | C1R | 243 | 0.92 | 17.8 | |
| 28 | C10orf10 (DEPP1) | 169 | 0.92 | 14.3 | |
| 29 | MYLK | 162 | 0.92 | 17.2 | |
| 24 | COL11A1 | 188 | 0.92 | 30.3 | Yes |
| 6 | THBS2 | 893 | 0.91 | 30.3 | |
| 38 | HOPX | 104 | 0.91 | 17.5 | |
| 31 | RAB31 | 159 | 0.91 | 16.8 | |
| 46 | BHLHB3 | 58 | 0.91 | 10.6 | |
| 53 | ITIH5 | 44 | 0.91 | 12.3 | |
| 54 | PLXND1 | 43 | 0.90 | 10.8 | |
| 7 | LUM | 879 | 0.90 | 18.3 | |
| 44 | MEG3 | 61 | 0.90 | 22.2 |
| Molecular Markers | Function | Roles in PDAC Progression | |
|---|---|---|---|
| myCAFs | ACTA2, COL8A1, COL11A1 | TGFB1 pathway activation | immune suppression |
| iCAFs | COL14A1, PDPN | SASP (senescence-associated) secretion phenotypes, chemoresistance | hypoxia response, immune mobilization |
| POSTN CAFs | POSTN | macrophage interaction | tumor cell aggressiveness |
| apCAFs | MHC class II, CD74 | Treg mobilization | immune suppression |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, K.; Kumamoto, Y. CAFs-Associated Genes (CAFGs) in Pancreatic Ductal Adenocarcinoma (PDAC) and Novel Therapeutic Strategy. Int. J. Mol. Sci. 2024, 25, 6003. https://doi.org/10.3390/ijms25116003
Yamashita K, Kumamoto Y. CAFs-Associated Genes (CAFGs) in Pancreatic Ductal Adenocarcinoma (PDAC) and Novel Therapeutic Strategy. International Journal of Molecular Sciences. 2024; 25(11):6003. https://doi.org/10.3390/ijms25116003
Chicago/Turabian StyleYamashita, Keishi, and Yusuke Kumamoto. 2024. "CAFs-Associated Genes (CAFGs) in Pancreatic Ductal Adenocarcinoma (PDAC) and Novel Therapeutic Strategy" International Journal of Molecular Sciences 25, no. 11: 6003. https://doi.org/10.3390/ijms25116003
APA StyleYamashita, K., & Kumamoto, Y. (2024). CAFs-Associated Genes (CAFGs) in Pancreatic Ductal Adenocarcinoma (PDAC) and Novel Therapeutic Strategy. International Journal of Molecular Sciences, 25(11), 6003. https://doi.org/10.3390/ijms25116003







